Abstract
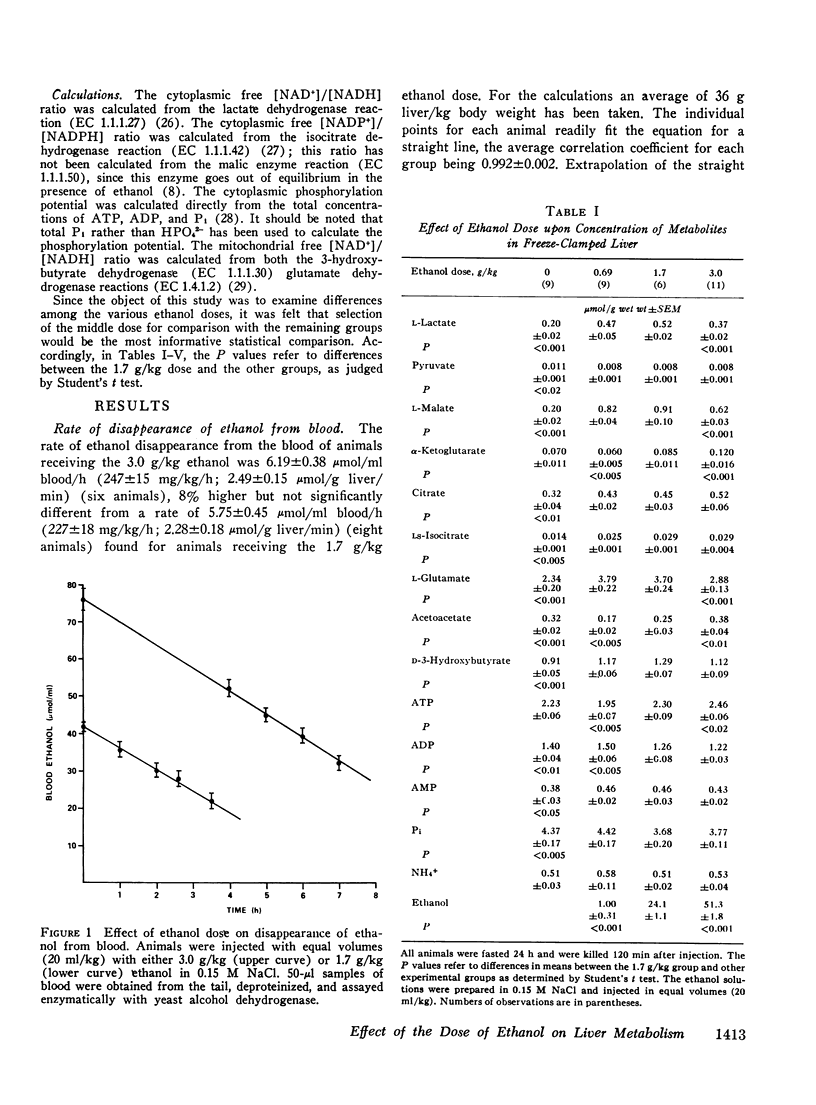
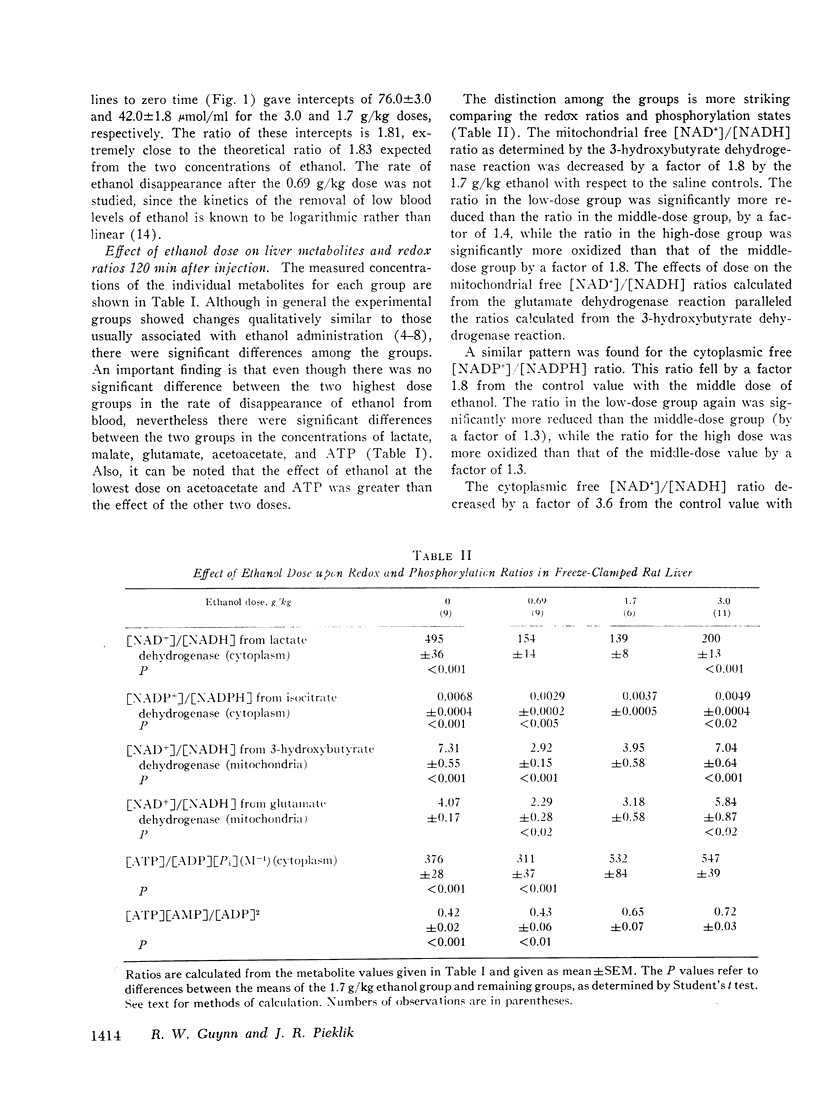
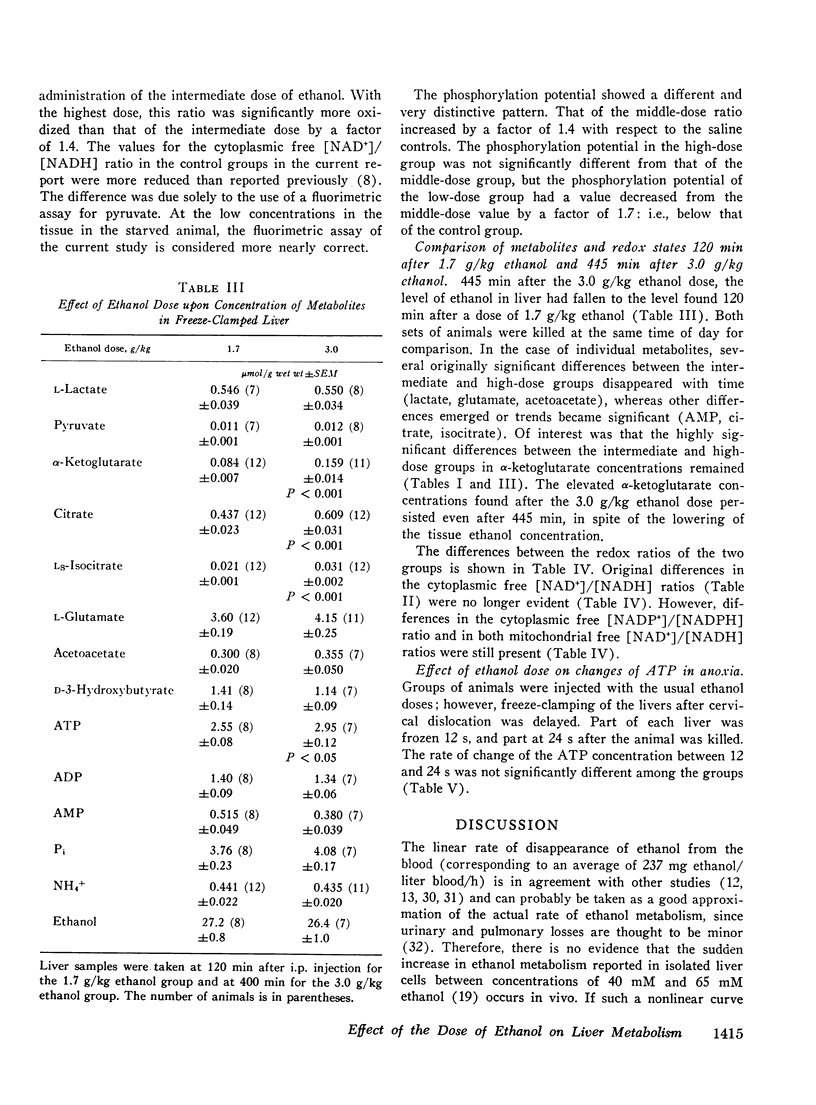
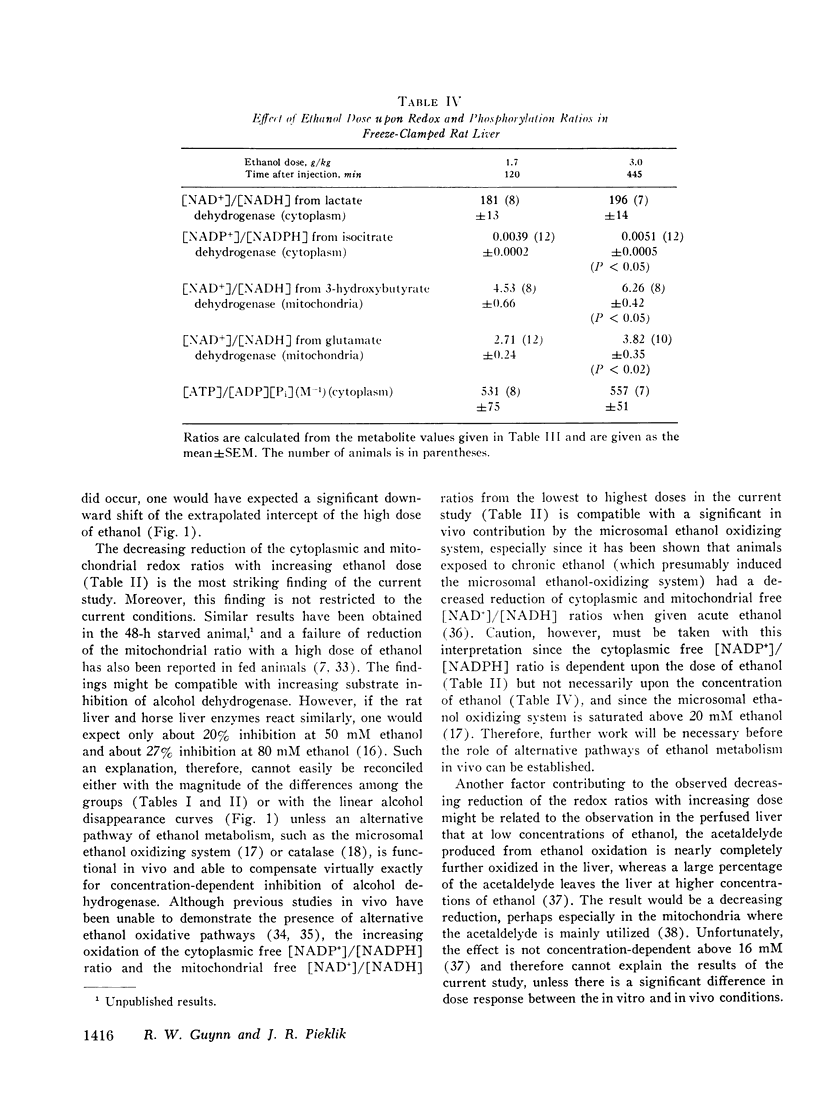
The dose dependence of the acute effects of ethanol upon liver intermediary metabolism in vivo has been demonstrated in rats. Ethanol was given i.p. in doses of 0.69, 1.7, and 3.0 g/kg in equal volumes (20 ml/kg). The liver was freeze-clamped 120 min after injection, and multiple metabolites were measured in the perchloric acid extract of the tissue. Each group showed a significantly different pattern of metabolites, redox states, and phosphorylation potentials although the rate of ethanol disappearance, at least between the two highest dose groups, was not significantly different. The mitochondrial free [NAD+]/[NADH] ratios and the cytoplasmic free [NADP+]/[NADPH] ratio were paradoxically most reduced with the lowest dose of ethanol and became progressively more oxidized with increasing dose. Once established, the differences in these ratios between the groups tended to persist with time, relatively independent of the concentration of ethanol. In a somewhat different pattern, the phosphorylation potential ([ATP]/[ADP][P1]) remained at the control level in the low-dose group but was significantly elevated in the two higher-dose groups. The results, therefore, show distinct and complicated dose-dependent patterns of intermediary metabolism that cannot be explained completely by any one hypothesis but that imply significant dose-dependent effects of ethanol upon intermediary metabolism not directly related to NADH production.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AULL J. C., Jr, KINARD F. W., ROBERTS W. J., Jr Rate of metabolism of ethanol in the rat. Am J Physiol. 1956 Sep;186(3):380–382. doi: 10.1152/ajplegacy.1956.186.3.380. [DOI] [PubMed] [Google Scholar]
- Bernstein J., Videla L., Israel Y. Metabolic alterations produced in the liver by chronic ethanol administration. Changes related to energetic parameters of the cell. Biochem J. 1973 Jun;134(2):515–521. doi: 10.1042/bj1340515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel K., Dickinson F. M. The kinetics and mechanism of liver alcohol dehydrogenase with primary and secondary alcohols as substrates. Biochem J. 1966 Jul;100(1):34–46. doi: 10.1042/bj1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domschke S., Domschke W., Lieber C. S. Hepatic redox state: attenuation of the acute effects of ethanol induced by chronic ethanol consumption. Life Sci. 1974 Oct 1;15(7):1327–1334. doi: 10.1016/0024-3205(74)90314-2. [DOI] [PubMed] [Google Scholar]
- Folbergrová J., Passonneau J. V., Lowry O. H., Schulz D. W. Glycogen, ammonia and related metabolities in the brain during seizures evoked by methionine sulphoximine. J Neurochem. 1969 Feb;16(2):191–203. doi: 10.1111/j.1471-4159.1969.tb05937.x. [DOI] [PubMed] [Google Scholar]
- Grunnet N., Quistorff B., Thieden H. I. Rate-limiting factors in ethanol oxidation by isolated rat-liver parenchymal cells. Effect of ethanol concentration, fructose, pyruvate and pyrazole. Eur J Biochem. 1973 Dec 3;40(1):275–282. doi: 10.1111/j.1432-1033.1973.tb03195.x. [DOI] [PubMed] [Google Scholar]
- Grunnet N., Thieden H. I. The effect of ethanol concentration upon in vivo metabolite levels of rat liver. Life Sci II. 1972 Oct 22;11(20):983–993. doi: 10.1016/0024-3205(72)90030-6. [DOI] [PubMed] [Google Scholar]
- Guynn R. W., Veloso D., Harris R. L., Lawson J. W., Veech R. L. Ethanol administration and the relationship of malonyl-coenzyme A concentrations to the rate of fatty acid synthesis in rat liver. Biochem J. 1973 Nov;136(3):639–647. doi: 10.1042/bj1360639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guynn R. W., Veloso D., Veech R. L. Enzymic determination of inorganic phosphate in the presence of creatine phosphate. Anal Biochem. 1972 Jan;45(1):277–285. doi: 10.1016/0003-2697(72)90028-0. [DOI] [PubMed] [Google Scholar]
- Hems D. A., Brosnan J. T. Effects of ischaemia on content of metabolites in rat liver and kidney in vivo. Biochem J. 1970 Nov;120(1):105–111. doi: 10.1042/bj1200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel Y., Kalant H., Laufer I. Effects of ethanol on na, K, mg-stimulated microsomal ATPase activity. Biochem Pharmacol. 1965 Dec;14(12):1803–1814. doi: 10.1016/0006-2952(65)90270-4. [DOI] [PubMed] [Google Scholar]
- Khanna J. M., Kalant H. Effect of inhibitors and inducers of drug metabolism on ethanol metabolism in vivo. Biochem Pharmacol. 1970 Jun;19(6):2033–2041. doi: 10.1016/0006-2952(70)90300-x. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Freedland R. A., Hems R., Stubbs M. Inhibition of hepatic gluconeogenesis by ethanol. Biochem J. 1969 Mar;112(1):117–124. doi: 10.1042/bj1120117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Perkins J. R. The physiological role of liver alcohol dehydrogenase. Biochem J. 1970 Jul;118(4):635–644. doi: 10.1042/bj1180635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A. The effects of ethanol on the metabolic activities of the liver. Adv Enzyme Regul. 1968;6:467–480. doi: 10.1016/0065-2571(68)90029-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Lieber C. S., DeCarli L. M. Hepatic microsomal ethanol-oxidizing system. In vitro characteristics and adaptive properties in vivo. J Biol Chem. 1970 May 25;245(10):2505–2512. [PubMed] [Google Scholar]
- Lindros K. O. Interference of ethanol and sorbitol with hepatic ketone body metabolism in normal, hyper- and hypothyroid rats. Eur J Biochem. 1970 Mar 1;13(1):111–116. doi: 10.1111/j.1432-1033.1970.tb00905.x. [DOI] [PubMed] [Google Scholar]
- Lindros K. O., Vihma R., Forsander O. A. Utilization and metabolic effects of acetaldehyde and ethanol in the perfused rat liver. Biochem J. 1972 Feb;126(4):945–952. doi: 10.1042/bj1260945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makar A. B., Mannering G. J. Kinetics of ethanol metabolism in the intact rat and monkey. Biochem Pharmacol. 1970 Jun;19(6):2017–2022. doi: 10.1016/0006-2952(70)90298-4. [DOI] [PubMed] [Google Scholar]
- OWENS A. H., Jr, MARSHALL E. K., Jr The metabolism of ethyl alcohol in the rat. J Pharmacol Exp Ther. 1955 Nov;115(3):360–370. [PubMed] [Google Scholar]
- Parrilla R., Okawa K., Lindros K. O., Zimmerman U. J., Kobayashi K., Williamson J. R. Functional compartmentation of acetaldehyde oxidation in rat liver. J Biol Chem. 1974 Aug 10;249(15):4926–4933. [PubMed] [Google Scholar]
- Roach M. K., Khan M., Knapp M., Reese W. N., Jr Ethanol metabolism in vivo and the role of hepatic microsomal ethanol oxidation. Q J Stud Alcohol. 1972 Sep;33(3):751–755. [PubMed] [Google Scholar]
- Rubin E., Lieber C. S. Experimental alcoholic hepatitis: a new primate model. Science. 1973 Nov 16;182(4113):712–713. doi: 10.1126/science.182.4113.712. [DOI] [PubMed] [Google Scholar]
- Rubin E., Lieber C. S. Fatty liver, alcoholic hepatitis and cirrhosis produced by alcohol in primates. N Engl J Med. 1974 Jan 17;290(3):128–135. doi: 10.1056/NEJM197401172900303. [DOI] [PubMed] [Google Scholar]
- Thurman R. G., Ley H. G., Scholz R. Hepatic microsomal ethanol oxidation. Hydrogen peroxide formation and the role of catalase. Eur J Biochem. 1972 Feb;25(3):420–430. doi: 10.1111/j.1432-1033.1972.tb01711.x. [DOI] [PubMed] [Google Scholar]
- Van Harken D. R., Mannering G. J. Ethanol metabolism in the isolated, perfused rat liver. Biochem Pharmacol. 1969 Oct;18(10):2759–2766. doi: 10.1016/0006-2952(69)90183-x. [DOI] [PubMed] [Google Scholar]
- Veech R. L., Eggleston L. V., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide phosphate in the cytoplasm of rat liver. Biochem J. 1969 Dec;115(4):609–619. doi: 10.1042/bj1150609a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veech R. L., Guynn R., Veloso D. The time-course of the effects of ethanol on the redox and phosphorylation states of rat liver. Biochem J. 1972 Apr;127(2):387–397. doi: 10.1042/bj1270387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veech R. L., Raijman L., Krebs H. A. Equilibrium relations between the cytoplasmic adenine nucleotide system and nicotinamide-adenine nucleotide system in rat liver. Biochem J. 1970 Apr;117(3):499–503. doi: 10.1042/bj1170499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veloso D., Passonneau J. V., Veech R. L. The effects of intoxicating doses of ethanol upon intermediary metabolism in rat brain. J Neurochem. 1972 Nov;19(11):2679–2686. doi: 10.1111/j.1471-4159.1972.tb01327.x. [DOI] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R., Scholz R., Browning E. T., Thurman R. G., Fukami M. H. Metabolic effects of ethanol in perfused rat liver. J Biol Chem. 1969 Sep 25;244(18):5044–5054. [PubMed] [Google Scholar]


