Abstract
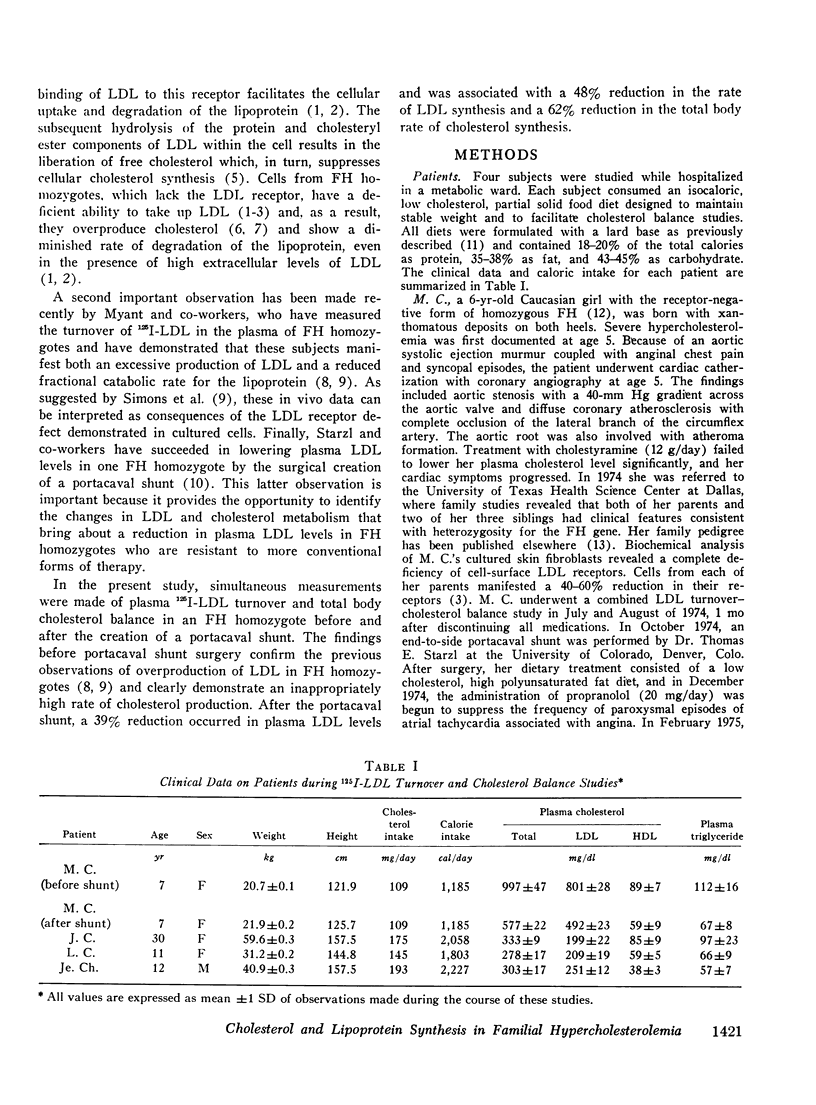
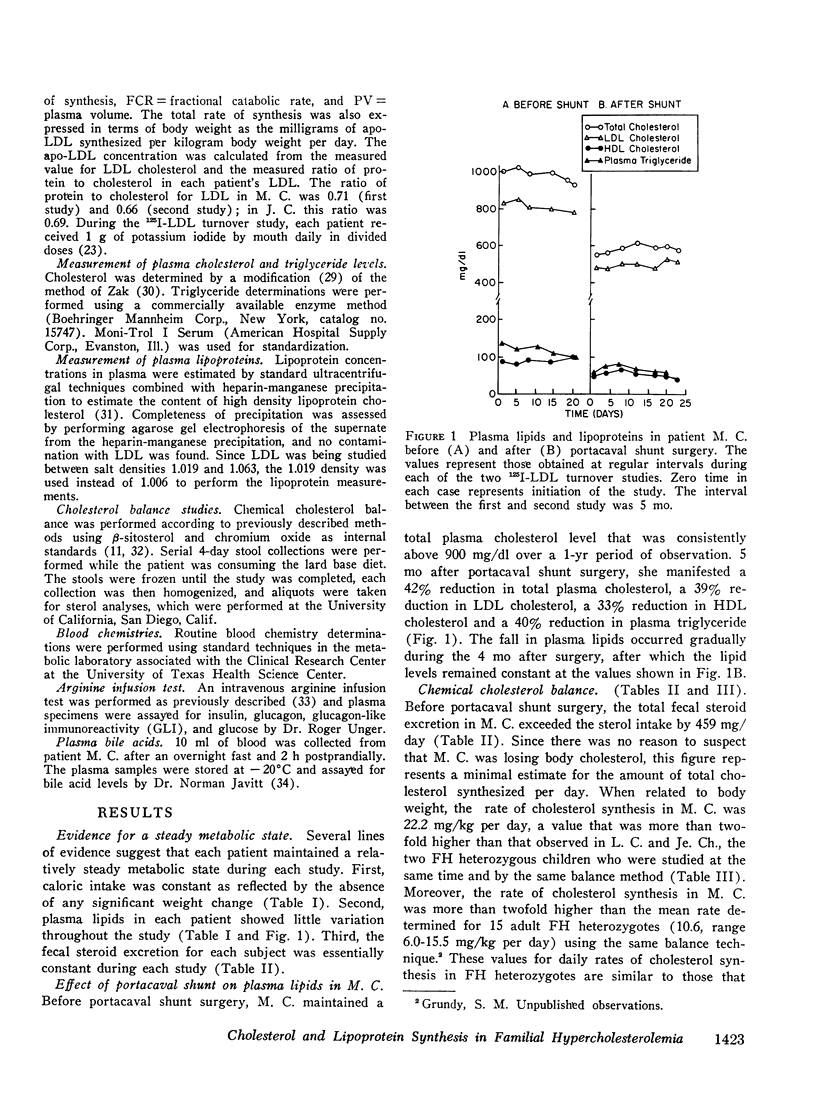
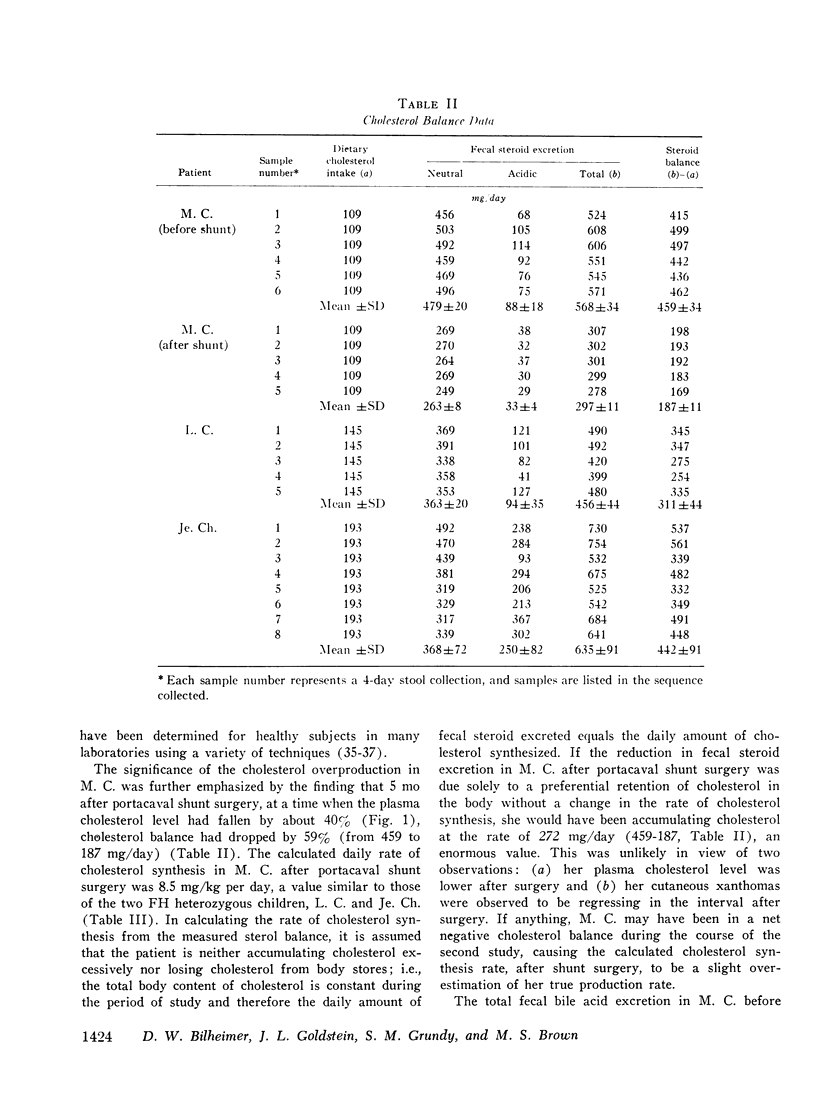
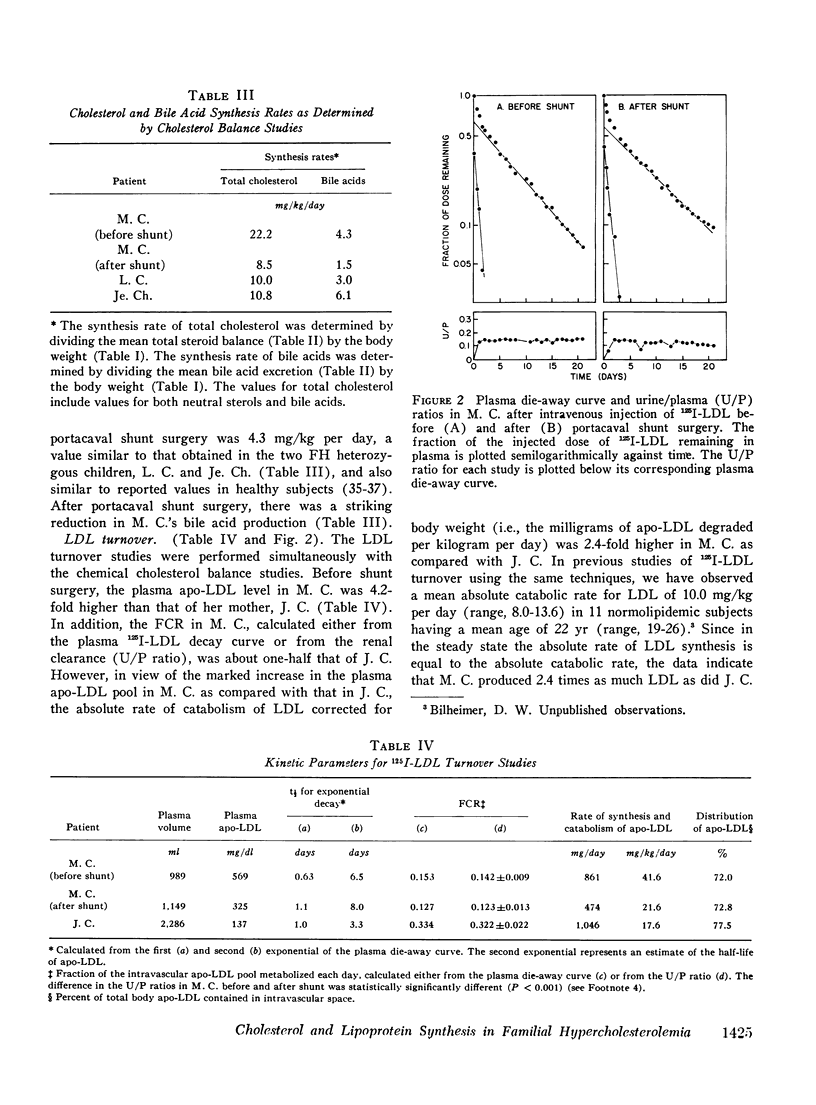
The turnover of 125I-labeled low density lipoprotein (LDL) and the total body balance of cholestrol were studied in a 6-yr-old girl with the homozygous form of familial hypercholesterolemia (FH) before and after the surgical creation of an end-to-side portacaval shunt. The results were compared with those of similar studies simultaneously performed in untreated patients with the heterozygous form of FH and with the results of earlier studies performed on normolipidemic subjects. Before shunt surgery, the rate of synthesis of LDL in the FH homozygote (mg/kg per day) was fourfold higher than in normolipidemic subjects and twofold higher than in her heterozygous mother. The fractional catabolic rate for LDL in the homozygote was decreased to 33% of normal control values. The rate of cholesterol synthesis, estimated by chemical sterol balance, was higher in the FH homozygote than in two FH heterozygotes of similar age studied simultaneously. When considered in relation to the markedly elevated level of plasma cholesterol, the observed rate of cholesterol synthesis in the FH homozygote was inappropriately elevated. Bile acid production was normal in all three children. 5 mo after shunt surgery, the rate of LDL synthesis in the homozygote had declined by 48% as compared with the preoperative value, and this caused a 39% drop in the plasma LDL cholesterol level despite a 17% reduction in the fractional catabolic rate of the lipoprotein. The rate of cholesterol synthesis fell by 62% as compared with the preoperative value. The findings of an inappropriately elevated rate of production of both cholesterol and LDL as well as a reduced fractional catabolic rate for the lipoprotein in the untreated FH homozygote are consistent with results of studies in cultured fibroblasts indicating that the primary genetic defect in FH involves a deficiency in a cell-surface receptor for LDL that regulates both cholesterol synthesis and LDL degradation. Although the mechanism for the decline in production of cholesterol and LDL after portacaval shunt surgery is unknown, it was observed that these changes were associated with marked increases in the plasma concentrations of bile acids and glucagon.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Cholesterol ester formation in cultured human fibroblasts. Stimulation by oxygenated sterols. J Biol Chem. 1975 May 25;250(10):4025–4027. [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Receptor-dependent hydrolysis of cholesteryl esters contained in plasma low density lipoprotein. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2925–2929. doi: 10.1073/pnas.72.8.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Feb 10;249(3):789–796. [PubMed] [Google Scholar]
- Brown M. S., Faust J. R., Goldstein J. L. Role of the low density lipoprotein receptor in regulating the content of free and esterified cholesterol in human fibroblasts. J Clin Invest. 1975 Apr;55(4):783–793. doi: 10.1172/JCI107989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Expression of the familial hypercholesterolemia gene in heterozygotes: mechanism for a dominant disorder in man. Science. 1974 Jul 5;185(4145):61–63. doi: 10.1126/science.185.4145.61. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Familial hypercholesterolemia: defective binding of lipoproteins to cultured fibroblasts associated with impaired regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity. Proc Natl Acad Sci U S A. 1974 Mar;71(3):788–792. doi: 10.1073/pnas.71.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Expression of the familial hypercholesterolemia gene in heterozygotes: model for a dominant disorder in man. Trans Assoc Am Physicians. 1974;87:120–131. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Familial hypercholesterolemia. A genetic regulatory defect in cholesterol metabolism. Am J Med. 1975 Feb;58(2):147–150. doi: 10.1016/0002-9343(75)90563-x. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Familial hypercholesterolemia: identification of a defect in the regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity associated with overproduction of cholesterol. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2804–2808. doi: 10.1073/pnas.70.10.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Dana S. E., Brunschede G. Y., Brown M. S. Genetic heterogeneity in familial hypercholesterolemia: evidence for two different mutations affecting functions of low-density lipoprotein receptor. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1092–1096. doi: 10.1073/pnas.72.3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr, Salen G. Interruption of the enterohepatic circulation of bile acids in man: comparative effects of cholestyramine and ileal exclusion on cholesterol metabolism. J Lab Clin Med. 1971 Jul;78(1):94–121. [PubMed] [Google Scholar]
- Grundy S. M. Effects of polyunsaturated fats on lipid metabolism in patients with hypertriglyceridemia. J Clin Invest. 1975 Feb;55(2):269–282. doi: 10.1172/JCI107930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch F. T. Practical methods for plasma lipoprotein analysis. Adv Lipid Res. 1968;6:1–68. [PubMed] [Google Scholar]
- Kaplowitz N., Kok E., Javitt N. B. Postprandial serum bile acid for the detection of hepatobiliary disease. JAMA. 1973 Jul 16;225(3):292–293. [PubMed] [Google Scholar]
- Khachadurian A. K., Kawahara F. S. Cholesterol synthesis by cultured fibroblasts: decreased feedback inhibition in familial hypercholesterolemia. J Lab Clin Med. 1974 Jan;83(1):7–15. [PubMed] [Google Scholar]
- LEFFLER H. H., McDOUGALD C. H. Estimation of cholesterol in serum by means of improved technics. Tech Bull Regist Med Technol. 1963 Feb;33:19–23. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langer T., Strober W., Levy R. I. The metabolism of low density lipoprotein in familial type II hyperlipoproteinemia. J Clin Invest. 1972 Jun;51(6):1528–1536. doi: 10.1172/JCI106949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B., Myant N. B. Studies in the metabolism of cholesterol in subjects with normal plasma cholesterol levels and in patients with essential hypercholesterolaemia. Clin Sci. 1967 Apr;32(2):201–213. [PubMed] [Google Scholar]
- MATTHEWS C. M. The theory of tracer experiments with 131I-labelled plasma proteins. Phys Med Biol. 1957 Jul;2(1):36–53. doi: 10.1088/0031-9155/2/1/305. [DOI] [PubMed] [Google Scholar]
- Miettinen T. A. Cholesterol metabolism in patients with coronary heart disease. Ann Clin Res. 1971 Dec;3(6):313–322. [PubMed] [Google Scholar]
- Moutafis C. D., Myant N. B. The metabolism of cholesterol in two hypercholesterolaemic patients treated with cholestyramine. Clin Sci. 1969 Oct;37(2):443–454. [PubMed] [Google Scholar]
- Myant N. B. The regulation of cholesterol metabolism as related to familial hypercholesterolaemia. Sci Basis Med Annu Rev. 1970:230–259. [PubMed] [Google Scholar]
- Noble R. P. Electrophoretic separation of plasma lipoproteins in agarose gel. J Lipid Res. 1968 Nov;9(6):693–700. [PubMed] [Google Scholar]
- Quintão E., Grundy S. M., Ahrens E. H., Jr An evaluation of four methods for measuring cholesterol absorption by the intestine in man. J Lipid Res. 1971 Mar;12(2):221–232. [PubMed] [Google Scholar]
- RADDING C. M., STEINBERG D. Studies on the synthesis and secretion of serum lipoproteins by rat liver slices. J Clin Invest. 1960 Oct;39:1560–1569. doi: 10.1172/JCI104177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichl D., Simons L. A., Myant N. B. The metabolism of low-density lipoprotein in a patient with familial hyperbetalipoproteinaemia. Clin Sci Mol Med. 1974 Dec;47(6):635–638. doi: 10.1042/cs0470635. [DOI] [PubMed] [Google Scholar]
- STEINFELD J. L., PATON R. R., FLICK A. L., MILCH R. A., BEACH F. E., TABERN D. L. Distribution and degradation of human serum albumin labeled with I 131 by different techniques. Ann N Y Acad Sci. 1957 Aug 30;70(1):109–121. doi: 10.1111/j.1749-6632.1957.tb35382.x. [DOI] [PubMed] [Google Scholar]
- Simons L. A., Reichl D., Myant N. B., Mancini M. The metabolism of the apoprotein of plasma low density lipoprotein in familial hyperbetalipoproteinaemia in the homozygous form. Atherosclerosis. 1975 Mar-Apr;21(2):283–298. doi: 10.1016/0021-9150(75)90087-8. [DOI] [PubMed] [Google Scholar]
- Starzl T. E., Putnam C. W., Chase H. P., Porter K. A. Portacaval shunt in hyperlipoproteinaemia. Lancet. 1973 Oct 27;2(7835):940–944. doi: 10.1016/s0140-6736(73)92599-3. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Aguilar-Parada E., Müller W. A., Eisentraut A. M. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970 Apr;49(4):837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAK B. Simple rapid microtechnic for serum total cholesterol. Am J Clin Pathol. 1957 May;27(5):583–588. doi: 10.1093/ajcp/27.5_ts.583. [DOI] [PubMed] [Google Scholar]


