Abstract
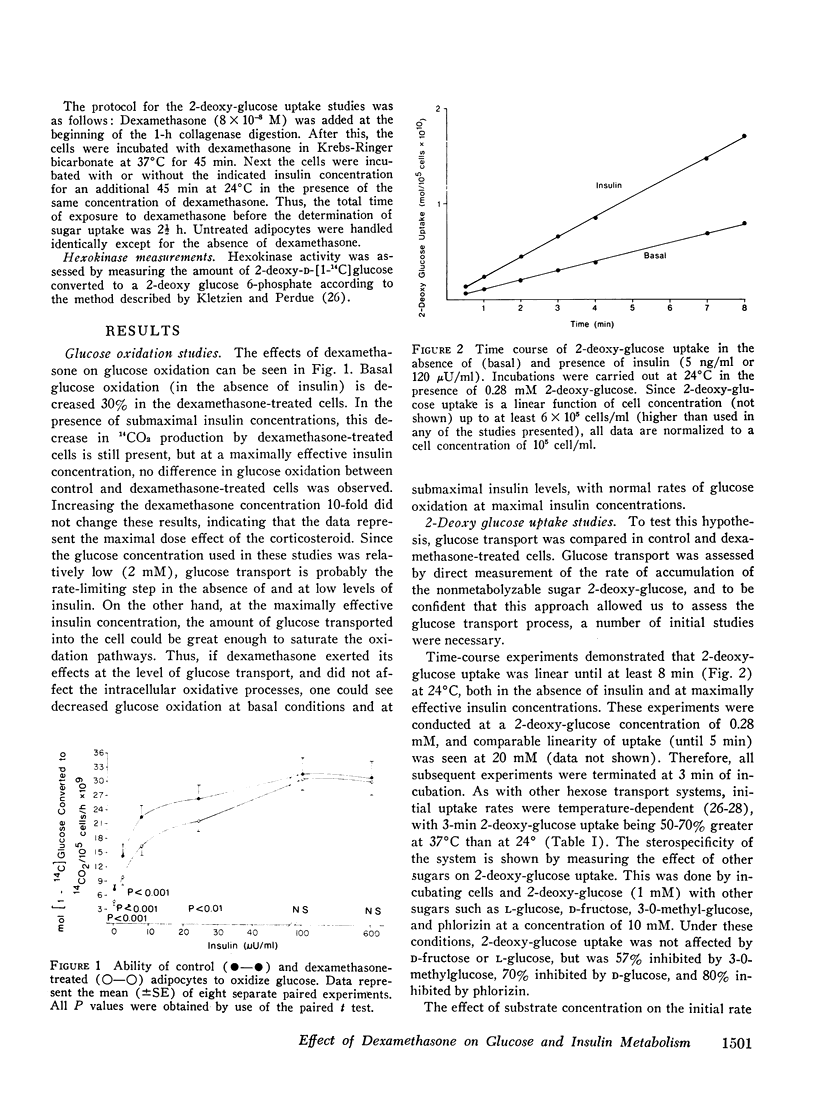
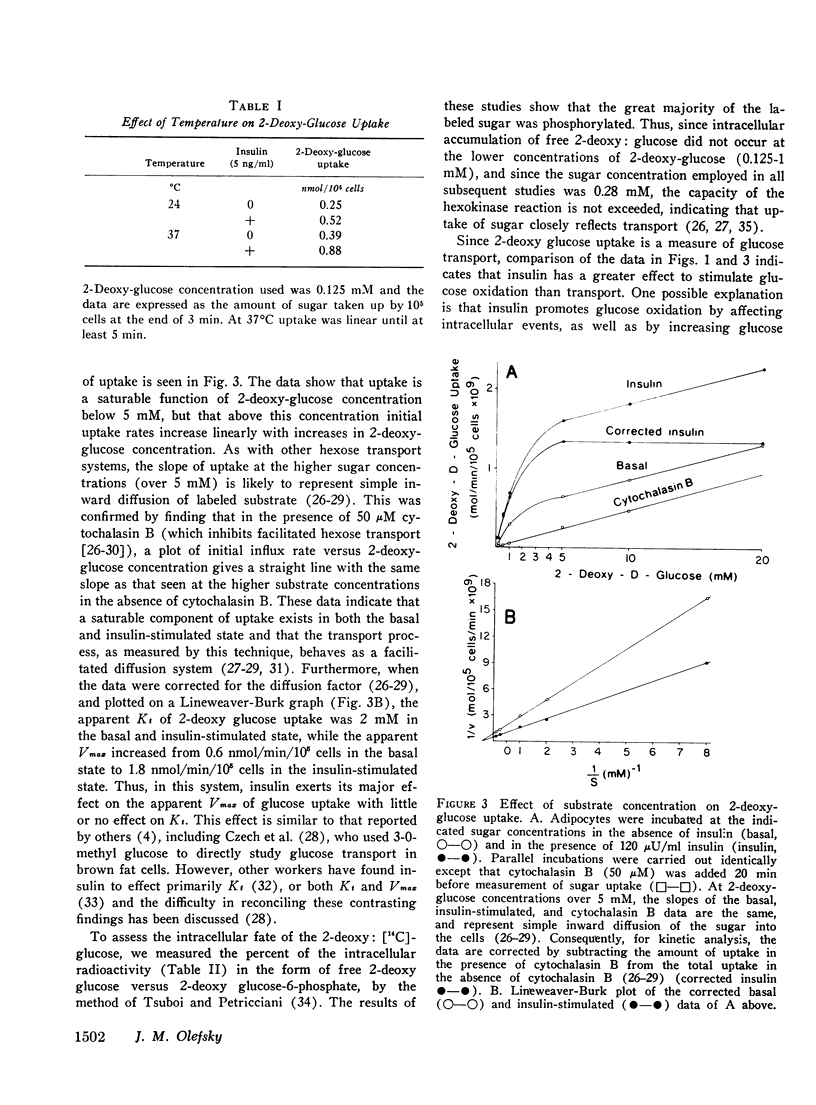
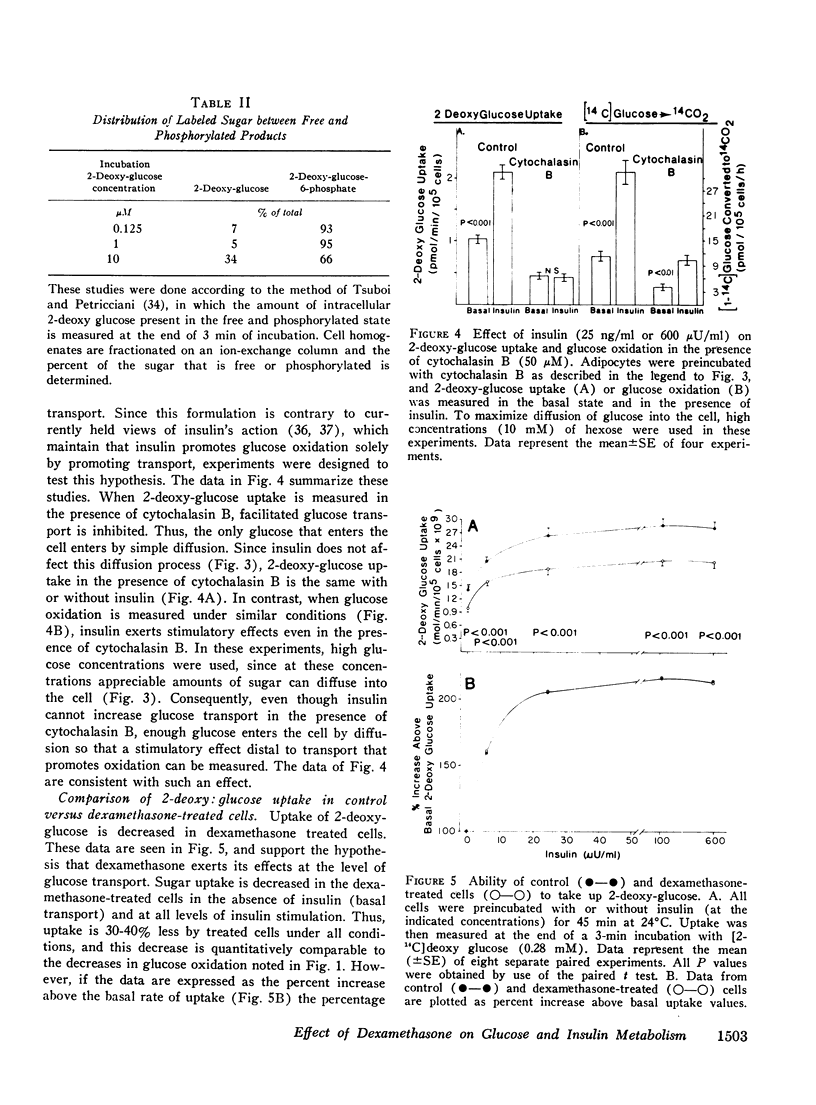
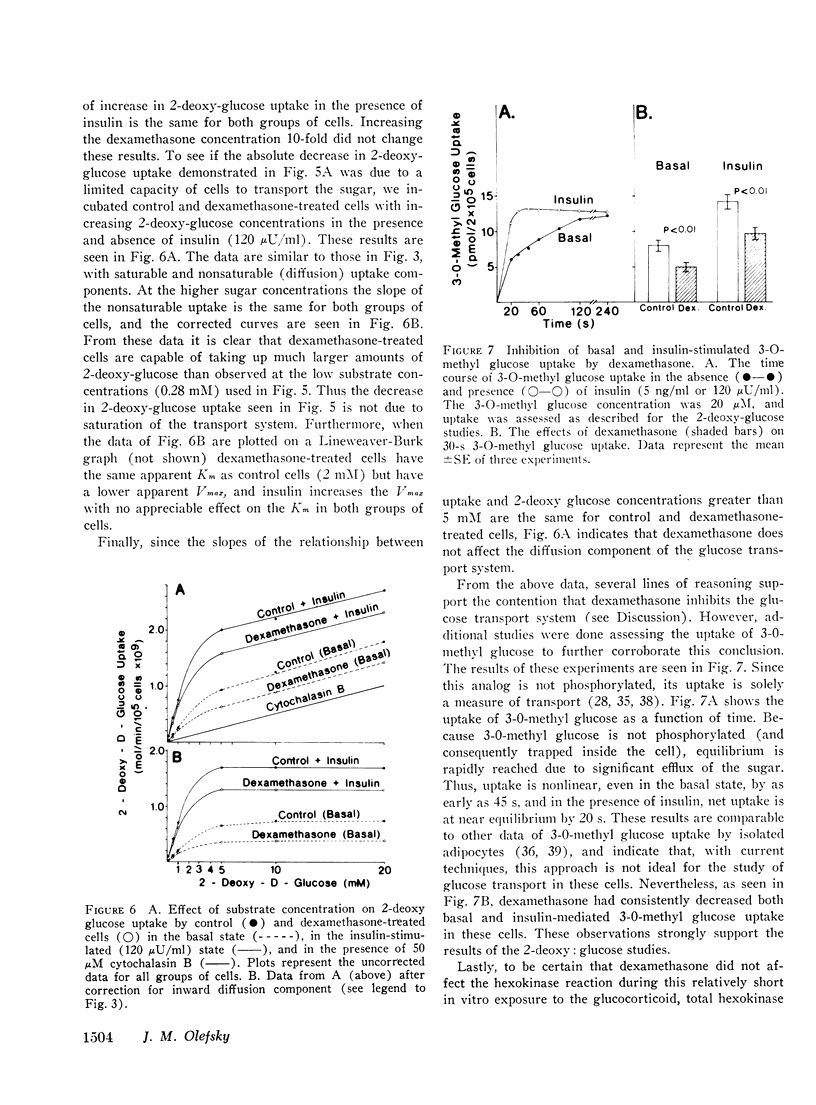
We have studied the in vitro effects of dexamethasone on isolated rat adipocytes at concentrations of dexamethasone therapeutically achieved in man. Glucose oxidation, glucose transport, and insulin binding were assessed. In dexamethasone-treated cells, glucose oxidation was decreased by 30-40% both in the absence of insulin (basal state) and at low insulin levels (less than 25 mu/ML). At maximally effective insulin levels (over 100 muU/ml) no differences existed between control and treated cells. If glucose transport were the rate-limiting step for glucose oxidation in the basal state and at low (submaximal) insulin levels, but not at maximally effective insulin concentrations, then these data could be explained by postulating that dexamethasone has a direct affect on glucose transport and does not affect intracellular oxidative pathways. We tested this hypothesis by directly assessing glucose transport in dexamethasone-treated cells. Glucose transport was assessed by measuring the uptake of [14C]2-deoxy glucose. These studies demonstrated a 30-40% decrease in 2-deoxy glucose uptake by treated cells both in the basal state and at all insulin concentrations. Thus, a direct glucocorticoid effect on the glucose transport system seems to account for the decreased ability of dexamethasone-treated cells to oxidize glucose. Since dexamethasone treatment leads to decreased insulin binding to adipocytes in vivo, we examined the possibility that the in vitro decreases in insulin-mediated glucose transport could be due to decreased insulin receptors. Insulin binding to control and treated adipocytes was measured, and no differences were found. Therefore, in cntrast to previously reported in vivo studies, adipocytes treated in vitro with dexamethasone retain a normal ability to bind insulin. Thus, these studies suggest that all of the in vitro effects of dexamethasone on glucose oxidation are due to direct inhibition of the glucose transport system.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett V., O'Keefe E., Cuatrecasaş P. Mechanism of action of cholera toxin and the mobile receptor theory of hormone receptor-adenylate cyclase interactions. Proc Natl Acad Sci U S A. 1975 Jan;72(1):33–37. doi: 10.1073/pnas.72.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein R. S., Kipnis D. M. Regulation of rat hexokinase isoenzymes. II. Effects of growth hormone and dexamethasone. Diabetes. 1973 Dec;22(12):923–931. doi: 10.2337/diab.22.12.923. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L., Pohl S. L., Kaumann A. J. Receptors and acceptors: a necessary distinction in hormone binding studies. Adv Cyclic Nucleotide Res. 1974;4(0):239–281. [PubMed] [Google Scholar]
- CONN J. W., FAJANS S. S. Influence of adrenal cortical steroids on carbohydrate metabolism in man. Metabolism. 1956 Mar;5(2):114–127. [PubMed] [Google Scholar]
- CROFFORD O. B., RENOLD A. E. GLUCOSE UPTAKE BY INCUBATED RAT EPIDIDYMAL ADIPOSE TISSUE. CHARACTERISTICS OF THE GLUCOSE TRANSPORT SYSTEM AND ACTION OF INSULIN. J Biol Chem. 1965 Aug;240:3237–3244. [PubMed] [Google Scholar]
- CROFFORD O. B., RENOLD A. E. GLUCOSE UPTAKE BY INCUBATED RAT EPIDIDYMAL ADIPOSE TISSUE. RATE-LIMITING STEPS AND SITE OF INSULIN ACTION. J Biol Chem. 1965 Jan;240:14–21. [PubMed] [Google Scholar]
- Chang K. J., Cuatrecasas P. Adenosine triphosphate-dependent inhibition of insulin-stimulated glucose transport in fat cells. Possible role of membrane phosphorylation. J Biol Chem. 1974 May 25;249(10):3170–3180. [PubMed] [Google Scholar]
- Czech M. P., Fain J. N. Antagonism of insulin action on glucose metabolism in white fat cells by dexamethasone. Endocrinology. 1972 Aug;91(2):518–522. doi: 10.1210/endo-91-2-518. [DOI] [PubMed] [Google Scholar]
- Czech M. P., Lawrence J. C., Jr, Lynn W. S. Hexose transport in isolated brown fat cells. A model system for investigating insulin action on membrane transport. J Biol Chem. 1974 Sep 10;249(17):5421–5427. [PubMed] [Google Scholar]
- Czech M. P., Lynn D. G., Lynn W. S. Cytochalasin B-sensitive 2-deoxy-D-glucose transport in adipose cell ghosts. J Biol Chem. 1973 May 25;248(10):3636–3641. [PubMed] [Google Scholar]
- Di Girolamo M., Mendlinger S., Fertig J. W. A simple method to determine fat cell size and number in four mammalian species. Am J Physiol. 1971 Sep;221(3):850–858. doi: 10.1152/ajplegacy.1971.221.3.850. [DOI] [PubMed] [Google Scholar]
- FAIN J. N. EFFECTS OF DEXAMETHASONE AND 2-DEOXY-D-GLUCOSE ON FRUCTOSE AND GLUCTOSE METABOLISM BY INCUBATED ADIPOSE TISSUE. J Biol Chem. 1964 Apr;239:958–962. [PubMed] [Google Scholar]
- Feldman D., Funder J. W., Edelman I. S. Subcellular mechanisms in the action of adrenal steroids. Am J Med. 1972 Nov;53(5):545–560. doi: 10.1016/0002-9343(72)90152-0. [DOI] [PubMed] [Google Scholar]
- Fisher R. B., Gilbert J. C. The effect of insulin on the kinetics of pentose permeation of the rat heart. J Physiol. 1970 Sep;210(2):297–304. doi: 10.1113/jphysiol.1970.sp009211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Monoiodoinsulin: demonstration of its biological activity and binding to fat cells and liver membranes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):400–408. doi: 10.1016/0006-291x(71)90767-4. [DOI] [PubMed] [Google Scholar]
- Gammeltoft S., Gliemann J. Binding and degradation of 125I-labelled insulin by isolated rat fat cells. Biochim Biophys Acta. 1973 Aug 17;320(1):16–32. doi: 10.1016/0304-4165(73)90161-x. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Gorden P., Roth J., Archer J. A., Buell D. N. Characteristics of the human lymphocyte insulin receptor. J Biol Chem. 1973 Mar 25;248(6):2202–2207. [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliemann J., Osterlind K., Vinten J., Gammeltoft S. A procedure for measurement of distribution spaces in isolated fat cells. Biochim Biophys Acta. 1972 Nov 24;286(1):1–9. doi: 10.1016/0304-4165(72)90082-7. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hatanaka M. Transport of sugars in tumor cell membranes. Biochim Biophys Acta. 1974 Apr 29;355(1):77–104. doi: 10.1016/0304-419x(74)90008-0. [DOI] [PubMed] [Google Scholar]
- Hirsch J., Gallian E. Methods for the determination of adipose cell size in man and animals. J Lipid Res. 1968 Jan;9(1):110–119. [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F. Sugar transport in chick embryo fibroblasts. I. A functional change in the plasma membrane associated with the rate of cell growth. J Biol Chem. 1974 Jun 10;249(11):3366–3374. [PubMed] [Google Scholar]
- Kono T., Barham F. W. The relationship between the insulin-binding capacity of fat cells and the cellular response to insulin. Studies with intact and trypsin-treated fat cells. J Biol Chem. 1971 Oct 25;246(20):6210–6216. [PubMed] [Google Scholar]
- LEBOEUF B., RENOLD A. E., CAHILL G. F., Jr Studies on rat adipose tissue in vitro. IX. Further effects cortisol on glucose metabolism. J Biol Chem. 1962 Apr;237:988–991. [PubMed] [Google Scholar]
- Livingston J. N., Lockwood D. H. Direct measurements of sugar uptake in small and large adipocytes from young and adult rats. Biochem Biophys Res Commun. 1974 Dec 11;61(3):989–996. doi: 10.1016/0006-291x(74)90253-8. [DOI] [PubMed] [Google Scholar]
- Meikle A. W., Lagerquist L. G., Tyler F. H. Apparently normal pituitary-adrenal suppressibility in Cushing's syndrome: dexamethasone metabolism and plasma levels. J Lab Clin Med. 1975 Sep;86(3):472–478. [PubMed] [Google Scholar]
- Olefsky J. M., Jen P., Reaven G. M., Alto P. Insulin binding to isolated human adipocytes. Diabetes. 1974 Jul;23(7):565–571. doi: 10.2337/diab.23.7.565. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Johnson J., Liu F., Jen P., Reaven G. M. The effects of acute and chronic dexamethasone administration on insulin binding to isolated rat hepatocytes and adipocytes. Metabolism. 1975 Apr;24(4):517–527. doi: 10.1016/0026-0495(75)90076-1. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Reaven G. M. Effects of age and obesity on insulin binding to isolated adipocytes. Endocrinology. 1975 Jun;96(6):1486–1498. doi: 10.1210/endo-96-6-1486. [DOI] [PubMed] [Google Scholar]
- Olefsky J., Reaven G. M. The human lymphocyte: a model for the study of insulin-receptor interaction. J Clin Endocrinol Metab. 1974 Apr;38(4):554–560. doi: 10.1210/jcem-38-4-554. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Richey D. P. Transport of nucleosides, nucleic acid bases, choline and glucose by animal cells in culture. Biochim Biophys Acta. 1974 Dec 16;344(3-4):263–305. doi: 10.1016/0304-4157(74)90010-0. [DOI] [PubMed] [Google Scholar]
- RIDDICK F. A., Jr, REISLER D. M., KIPNIS D. M. The sugar transport system in striated muscle. Effect of growth hormone, hydrocortisone and alloxan diabetes. Diabetes. 1962 May-Jun;11:171–178. [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Renner E. D., Plagemann P. G., Bernlohr R. W. Permeation of glucose by simple and facilitated diffusion by Novikoff rat hepatoma cells in suspension culture and its relationship to glucose metabolism. J Biol Chem. 1972 Sep 25;247(18):5765–5776. [PubMed] [Google Scholar]
- Rousseau G. G., Baxter J. D., Tomkins G. M. Glucocorticoid receptors: relations between steroid binding and biological effects. J Mol Biol. 1972 Jun 14;67(1):99–115. doi: 10.1016/0022-2836(72)90389-0. [DOI] [PubMed] [Google Scholar]
- Tsuboi K. K., Petricciani J. C. Concentrative accumulation (active transport) of 2-deoxy-D-glucose in primate fibroblasts. Biochem Biophys Res Commun. 1975 Feb 3;62(3):587–593. doi: 10.1016/0006-291x(75)90439-8. [DOI] [PubMed] [Google Scholar]
- WICK A. N., DRURY D. R., NAKADA H. I., WOLFE J. B. Localization of the primary metabolic block produced by 2-deoxyglucose. J Biol Chem. 1957 Feb;224(2):963–969. [PubMed] [Google Scholar]
- Weber M. J. Hexose transport in normal and in Rous sarcoma virus-transformed cells. J Biol Chem. 1973 May 10;248(9):2978–2983. [PubMed] [Google Scholar]
- Yorke R. E. The influence of dexamethasone on adipose tissue metabolism in vitro. J Endocrinol. 1967 Nov;39(3):329–343. doi: 10.1677/joe.0.0390329. [DOI] [PubMed] [Google Scholar]



