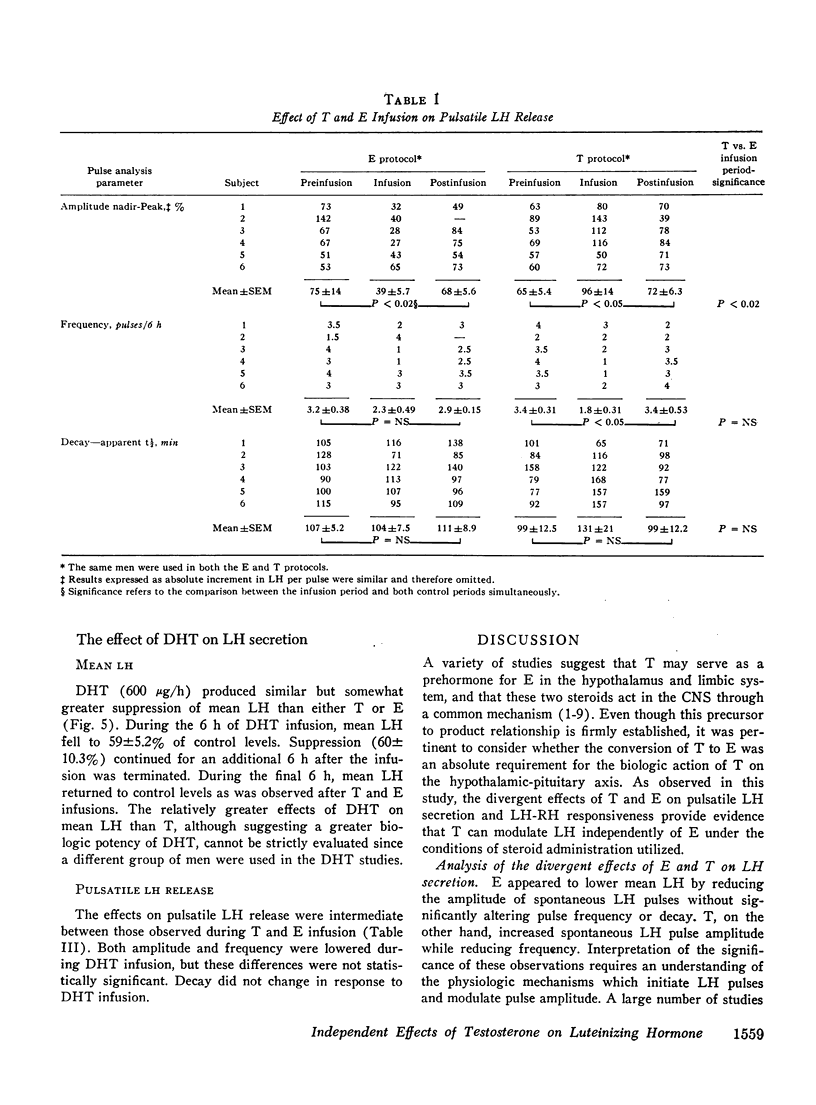
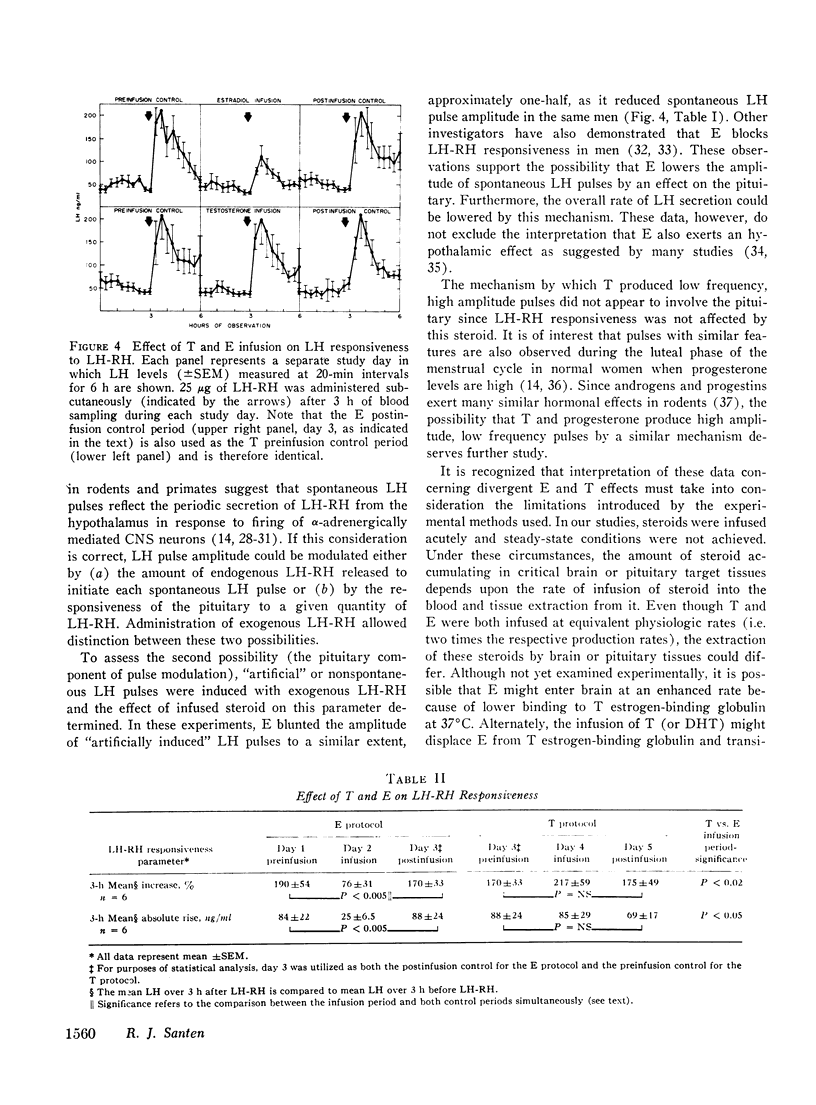
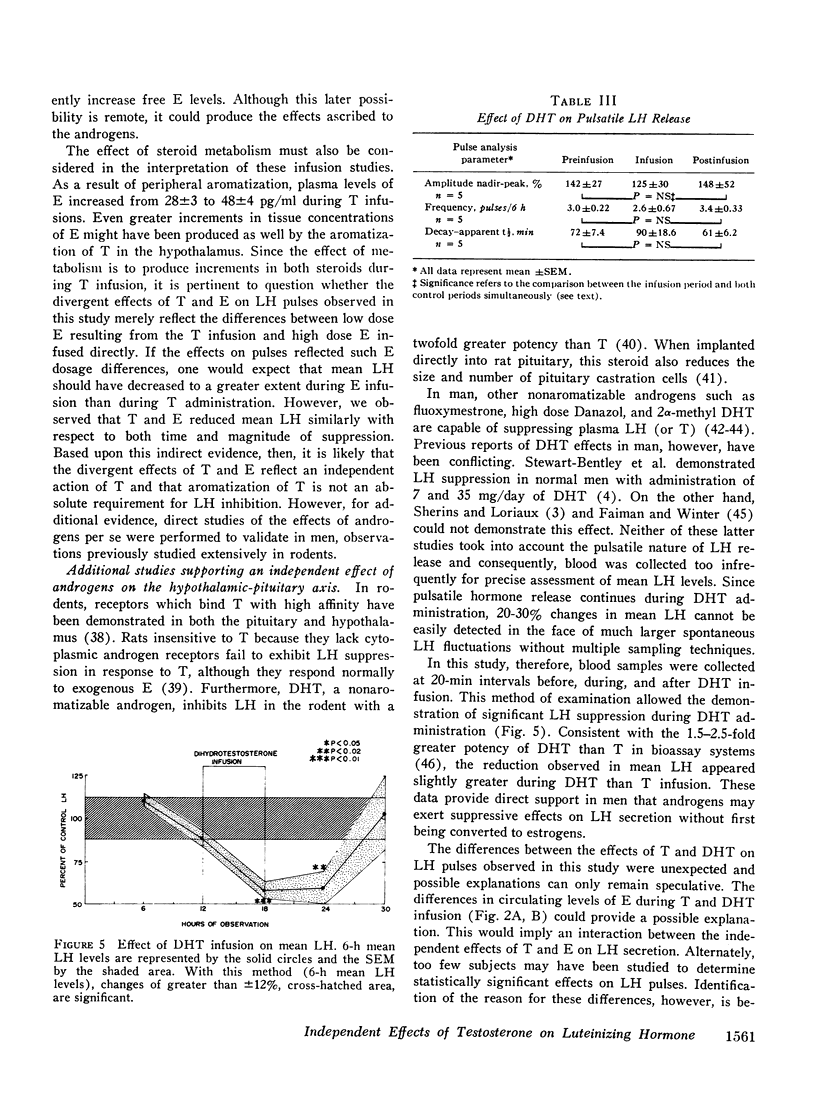
Abstract
A variety of studies in man and animals demonstrate that testosterone (T) is aromatized to estradiol (E) in the hypothalamus and limbic system. These observations suggested the possibility that conversion to E is an absolute requirement for the biologic activity of T on the hypothalamic-pituitary axis. Since this hypothesis implies a common mechanism of action of these two steroids, the demonstration of divergent effects of T and E on luteinizing hormone (LH) secretion would exclude this possibility. To test this hypothesis, the actions of T and E on three separate aspects of LH release (mean LH, pulsatile LH secretion, and responsiveness to LH-releasing hormone [LH-RH]) were contrasted. T and E, infused at two times their respective production rates into normal men, reduced mean LH levels similarly during 6 h of steroid infusion and for 6 h thereafter. However, these steroids exerted different effects on pulsatile secretion. E reduced the amplitude of spontaneous LH pulse from pre- and postinfusion control levels of 75+/-14 and 68+/-5.6% (SEM) to 39+/-5.7%. In contrast, T increased pulse amplited to 96+/-14% and decreased pulse frequency from basal levels of 3.4+/-0.31 to 1.8+/-0.31 pulses/6h. The site of suppressive action was determined by administering 25 microgms of LH-RH to the same men during T and E infusions and during three additional control periods without steroid administration. LH-RH produced similar 170-190% increments in serum LH during the three control periods and during T infusion. In contrast, E markedly blunted (76+/-31%, p less than 0.005) the LH response to LH-RH. Under the conditions of acute steroid infusion at doses (utilized in these experiments) producing similar inhibition of mean LH, E but not T acted directly on the pituitary to diminish LH-RH responsiveness. As further support that androgens can act without conversion to estrogens, the effects of a nonaromatizable androgen, dihydrotestosterone (DHT), on mean LH levels were studied. DHT, infused at the same rate as T, suppressed mean LH to a similar but somewhat greater extent than T. Since T and E produced divergent effects on LH secretion and a nonaromatizable androgen, DHT, suppressed mean LH, aromatization is not a necessary prerequisite for the action of androgens on the hypothalamic-pituitary axis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird D., Horton R., Longcope C., Tait J. F. Steroid prehormones. Perspect Biol Med. 1968 Spring;11(3):384–421. doi: 10.1353/pbm.1968.0053. [DOI] [PubMed] [Google Scholar]
- Bardin C. W., Mahoudeau J. A. Dynamics of androgen metabolism in women with hirsutism. Ann Clin Res. 1970 Dec;2(4):251–262. [PubMed] [Google Scholar]
- Bardin C. W., Ross G. T., Lipsett M. B. Site of action of clomiphene citrate in men: a study of the pituitary-Leydig cell axis. J Clin Endocrinol Metab. 1967 Nov;27(11):1558–1564. doi: 10.1210/jcem-27-11-1558. [DOI] [PubMed] [Google Scholar]
- Bartke A., Steele R. E., Musto N., Caldwell B. V. Fluctuations in plasma testosterone levels in adult male rats and mice. Endocrinology. 1973 Apr;92(4):1223–1228. doi: 10.1210/endo-92-4-1223. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A. N., Dierschke D. J., Yamaji T., Knobil E. The pharmacologic blockade of the circhoral mode of LH secretion in the ovariectomized rhesus monkey. Endocrinology. 1972 Mar;90(3):778–786. doi: 10.1210/endo-90-3-778. [DOI] [PubMed] [Google Scholar]
- Blake C. A., Sawyer C. H. Effects of hypothalamic deafferentation on the pulsatile rhythm in plasma concentrations of luteinizing hormone in ovariectomized rats. Endocrinology. 1974 Mar;94(3):730–736. doi: 10.1210/endo-94-3-730. [DOI] [PubMed] [Google Scholar]
- Boyar R., Perlow M., Hellman L., Kapen S., Weitzman E. Twenty-four hour pattern of luteinizing hormone secretion in normal men with sleep stage recording. J Clin Endocrinol Metab. 1972 Jul;35(1):73–81. doi: 10.1210/jcem-35-1-73. [DOI] [PubMed] [Google Scholar]
- Bremner W. J., Paulsen C. A. Two pools of luteinizing hormone in the human pituitary: evidence from constant administration of luteinizing hormone-releasing hormone. J Clin Endocrinol Metab. 1974 Nov;39(5):811–815. doi: 10.1210/jcem-39-5-811. [DOI] [PubMed] [Google Scholar]
- Cole E. N., Boyns A. R., Llewelyn D. E. Proceedings: Acute effects of gonadal steroids on pituitary sensitivity in healthy men. J Endocrinol. 1974 Nov;63(2):49P–49P. [PubMed] [Google Scholar]
- DAVIS T. E., LIPSETT M. B., KORENMAN S. G. SUPPRESSION OF TESTOSTERONE PRODUCTION BY PHYSIOLOGIC DOSES OF 2-ALPHA-METHYLDIHYDROTESTOSTERONE PROPIONATE. J Clin Endocrinol Metab. 1965 Apr;25:476–479. doi: 10.1210/jcem-25-4-476. [DOI] [PubMed] [Google Scholar]
- Dufau M. L., Dulmanis A., Catt K. J., Hudson B. Measurement of plasma estradiol-17beta by competitive binding assay employing pregnancy plasma. J Clin Endocrinol Metab. 1970 Mar;30(3):351–356. doi: 10.1210/jcem-30-3-351. [DOI] [PubMed] [Google Scholar]
- Faiman C., Winter J. S. The control of gonadotropin secretion in complete testicular feminization. J Clin Endocrinol Metab. 1974 Oct;39(4):631–638. doi: 10.1210/jcem-39-4-631. [DOI] [PubMed] [Google Scholar]
- GORSKI R. A., BARRACLOUGH C. A. EFFECTS OF LOW DOSAGES OF ANDROGEN ON THE DIFFERENTIATION OF HYPOTHALAMIC REGULATORY CONTROL OF OVULATION IN THE RAT. Endocrinology. 1963 Aug;73:210–216. doi: 10.1210/endo-73-2-210. [DOI] [PubMed] [Google Scholar]
- Gorski R. A. Modification of ovulatory mechanisms by postnatal administration of estrogen to the rat. Am J Physiol. 1963 Nov;205(5):842–844. doi: 10.1152/ajplegacy.1963.205.5.842. [DOI] [PubMed] [Google Scholar]
- Ito T., Horton R. The source of plasma dihydrotestosterone in man. J Clin Invest. 1971 Aug;50(8):1621–1627. doi: 10.1172/JCI106650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley T. R., Bogdanove E. M. Direct feedback of androgens: localized effects of intrapituitary implants of androgens on gonadotrophic cells and hormone stores. Endocrinology. 1973 Dec;93(6):1398–1409. doi: 10.1210/endo-93-6-1398. [DOI] [PubMed] [Google Scholar]
- Krieger D. T., Ossowski R., Fogel M., Allen W. Lack of circaidan periodicity of human serum FSH and LH levels. J Clin Endocrinol Metab. 1972 Oct;35(4):619–623. doi: 10.1210/jcem-35-4-619. [DOI] [PubMed] [Google Scholar]
- Lee P. A., Jaffe R. B., Midgley A. R., Jr, Kohen F., Niswinder G. D. Regulation of human gonadotropins. 8. Suppression of serum LH and FSH in adult males following exogenous testosterone administration. J Clin Endocrinol Metab. 1972 Nov;35(5):636–641. doi: 10.1210/jcem-35-5-636. [DOI] [PubMed] [Google Scholar]
- Libertun C., Cooper K. J., Fawcett C. P., McCann S. M. Effects of ovariectomy and steroid treatment on hypophyseal sensitivity to purified LH-releasing factor (LRF). Endocrinology. 1974 Feb;94(2):518–525. doi: 10.1210/endo-94-2-518. [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Pfaff D. W. Factors influencing sex hormone uptake by rat brain regions. I. Effects of neonatal treatment, hypophysectomy, and competing steroid on estradiol uptake. Brain Res. 1970 Jun 30;21(1):1–16. doi: 10.1016/0006-8993(70)90016-8. [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Pfaff D. W., Zigmond R. E. Factors influencing sex hormone uptake by rat brain regions. 3. Effects of competing steroids on testosterone uptake. Brain Res. 1970 Jun 30;21(1):29–38. doi: 10.1016/0006-8993(70)90018-1. [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Pfaff D. W., Zigmond R. E. Factors influencing sex hormone uptake by rat brain regions. II. Effects of neonatal treatment and hypophysectomy on testosterone uptake. Brain Res. 1970 Jun 30;21(1):17–28. doi: 10.1016/0006-8993(70)90017-x. [DOI] [PubMed] [Google Scholar]
- Mowszowicz I., Bieber D. E., Chung K. W., Bullock L. P., Bardin C. W. Synandrogenic and antiandrogenic effect of progestins: comparison with nonprogestational antiandrogens. Endocrinology. 1974 Dec;95(6):1589–1599. doi: 10.1210/endo-95-6-1589. [DOI] [PubMed] [Google Scholar]
- Naess O., Attramadal A., Aakvaag A. Androgen binding proteins in the anterior pituitary, hypothalamus, preoptic area and brain cortex of the rat. Endocrinology. 1975 Jan;96(1):1–9. doi: 10.1210/endo-96-1-1. [DOI] [PubMed] [Google Scholar]
- Naftolin F., Ryan K. J., Petro Z. Aromatization of androstenedione by limbic system tissue from human foetuses. J Endocrinol. 1971 Dec;51(4):795–796. doi: 10.1677/joe.0.0510795. [DOI] [PubMed] [Google Scholar]
- Naftolin F., Ryan K. J., Petro Z. Aromatization of androstenedione by the anterior hypothalamus of adult male and female rats. Endocrinology. 1972 Jan;90(1):295–298. doi: 10.1210/endo-90-1-295. [DOI] [PubMed] [Google Scholar]
- Naftolin F., Ryan K. J., Petro Z. Aromatization of androstenedione by the diencephalon. J Clin Endocrinol Metab. 1971 Aug;33(2):368–370. doi: 10.1210/jcem-33-2-368. [DOI] [PubMed] [Google Scholar]
- Naftolin F., Yen S. S., Tsai C. C. Rapid cycling of plasma gonadotrophins in normal men as demonstrated by frequent sampling. Nat New Biol. 1972 Mar 22;236(64):92–93. doi: 10.1038/newbio236092a0. [DOI] [PubMed] [Google Scholar]
- Nankin H. R., Troen P. Overnight patterns of serum luteinizing hormone in normal men. J Clin Endocrinol Metab. 1972 Nov;35(5):705–710. doi: 10.1210/jcem-35-5-705. [DOI] [PubMed] [Google Scholar]
- Nankin H. R., Troen P. Repetitive luteinizing hormone elevations in serum of normal men. J Clin Endocrinol Metab. 1971 Sep;33(3):558–560. doi: 10.1210/jcem-33-3-558. [DOI] [PubMed] [Google Scholar]
- Rebar R., Yen S. S., VandenBerg G., Naftolin F., Ehara Y., Engblom S., Ryan K. J., Amoss M., Guillemin R. Gonadotropin responses to synthetic LRF: dose-response relationship in men. J Clin Endocrinol Metab. 1973 Jan;36(1):10–16. doi: 10.1210/jcem-36-1-10. [DOI] [PubMed] [Google Scholar]
- Reddy V. V., Naftolin F., Ryan K. J. Aromatization in the central nervous system of rabbits: effects of castration and hormone treatment. Endocrinology. 1973 Feb;92(2):589–594. doi: 10.1210/endo-92-2-589. [DOI] [PubMed] [Google Scholar]
- Robertson H. A., Smeaton T. C., Durnford R. A method for the extraction, separation and estimation of unconjugated estrone, estradiol-17 and estradiol-17 in plasma. Steroids. 1972 Dec;20(6):651–667. doi: 10.1016/0039-128x(72)90049-9. [DOI] [PubMed] [Google Scholar]
- Ryan K. J., Naftolin F., Reddy V., Flores F., Petro Z. Estrogen formation in the brain. Am J Obstet Gynecol. 1972 Oct 15;114(4):454–460. doi: 10.1016/0002-9378(72)90204-9. [DOI] [PubMed] [Google Scholar]
- Santen R. J., Bardin C. W. Episodic luteinizing hormone secretion in man. Pulse analysis, clinical interpretation, physiologic mechanisms. J Clin Invest. 1973 Oct;52(10):2617–2628. doi: 10.1172/JCI107454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyler L. E., Jr, Reichlin S. Episodic secretion of luteinizing hormone-releasing factor (LRF) in the human. J Clin Endocrinol Metab. 1974 Sep;39(3):471–478. doi: 10.1210/jcem-39-3-471. [DOI] [PubMed] [Google Scholar]
- Seyler L. E., Jr, Reichlin S. Feedback regulation of circulating LRF concentrations in men. J Clin Endocrinol Metab. 1974 Nov;39(5):906–912. doi: 10.1210/jcem-39-5-906. [DOI] [PubMed] [Google Scholar]
- Sherins R. J., Bullock L., Gay V. L., Vanha-Perttula T., Bardin C. W. Plasma LH and FSH levels in the androgen insensitive pseudohermaphroditic rat: responses to steroid administration. Endocrinology. 1971 Mar;88(3):763–770. doi: 10.1210/endo-88-3-763. [DOI] [PubMed] [Google Scholar]
- Sherins R. J., Gandy H. M., Thorslund T. W., Paulsen C. A. Pituitary and testicular function studies. I. Experience with a new gonadal inhibitor, 17-alpha-pregn-4-en-20-yno-(2,3-d)isoxazol-17-ol (Danazol). J Clin Endocrinol Metab. 1971 Apr;32(4):522–531. doi: 10.1210/jcem-32-4-522. [DOI] [PubMed] [Google Scholar]
- Sherins R. J., Loriaux D. L. Studies of the role of sex steroids in the feedback control of FSH concentrations in men. J Clin Endocrinol Metab. 1973 May;36(5):886–893. doi: 10.1210/jcem-36-5-886. [DOI] [PubMed] [Google Scholar]
- Stewart-Bentley M., Odell W., Horton R. The feedback control of luteinizing hormone in normal adult men. J Clin Endocrinol Metab. 1974 Apr;38(4):545–553. doi: 10.1210/jcem-38-4-545. [DOI] [PubMed] [Google Scholar]
- Stumpf W. E. Estrogen-neurons and estrogen-neuron systems in the periventricular brain. Am J Anat. 1970 Oct;129(2):207–217. doi: 10.1002/aja.1001290209. [DOI] [PubMed] [Google Scholar]
- Swerdloff R. S., Walsh P. C., Odell W. D. Control of LH and FSH secretion in the male: Evidence that aromatization of androgens to estradiol is not required for inhibition of gonadotropin secretion. Steroids. 1972 Jul;20(1):13–22. doi: 10.1016/0039-128x(72)90115-8. [DOI] [PubMed] [Google Scholar]
- Yen S. S., Tsai C. C., Naftolin F., Vandenberg G., Ajabor L. Pulsatile patterns of gonadotropin release in subjects with and without ovarian function. J Clin Endocrinol Metab. 1972 Apr;34(4):671–675. doi: 10.1210/jcem-34-4-671. [DOI] [PubMed] [Google Scholar]
- zur Mühlen A von, Köbberling J. Effect of testosterone on the LH and FSH release induced by LH-releasing factor (LRF) in normal men. Horm Metab Res. 1973 Jul;5(4):266–270. doi: 10.1055/s-0028-1093944. [DOI] [PubMed] [Google Scholar]



