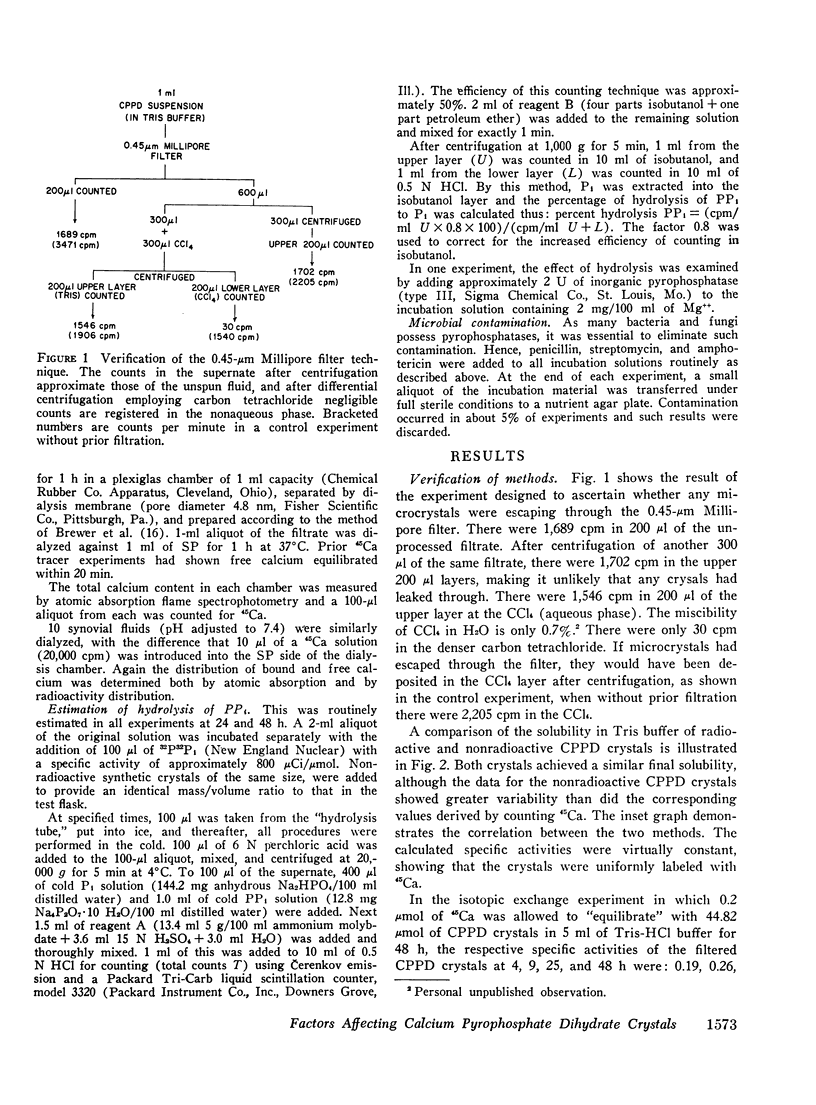
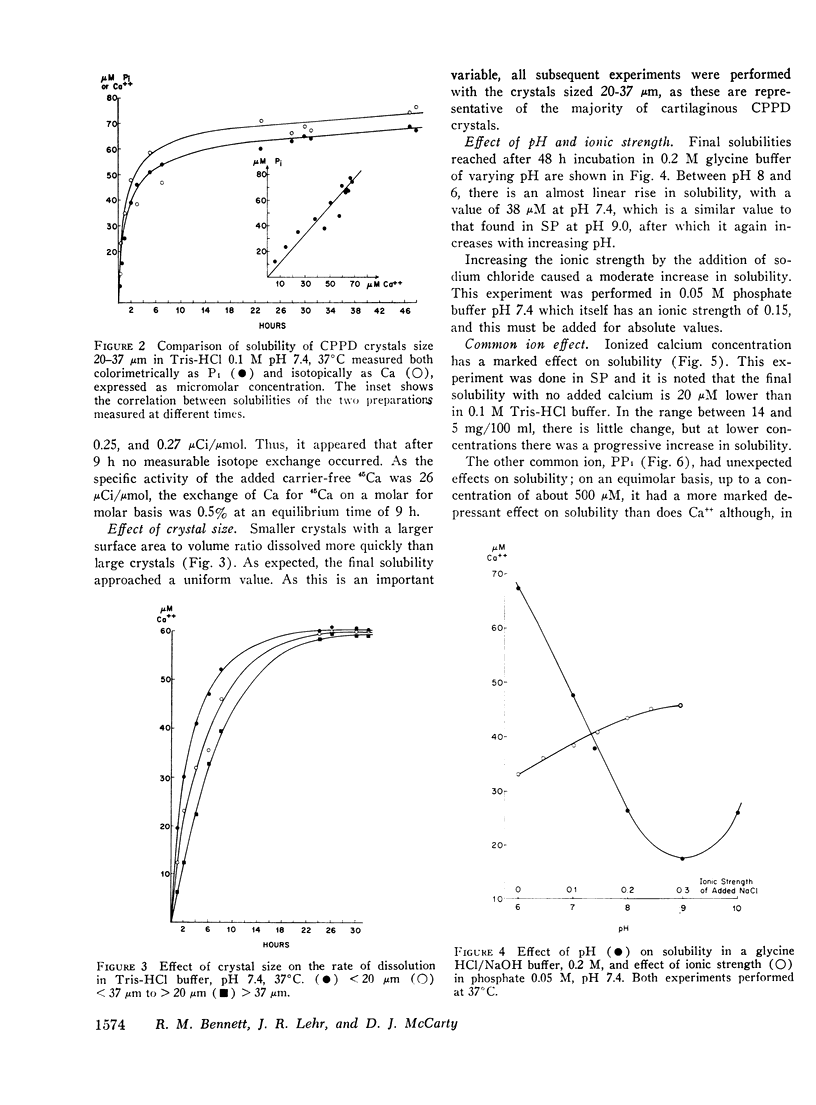
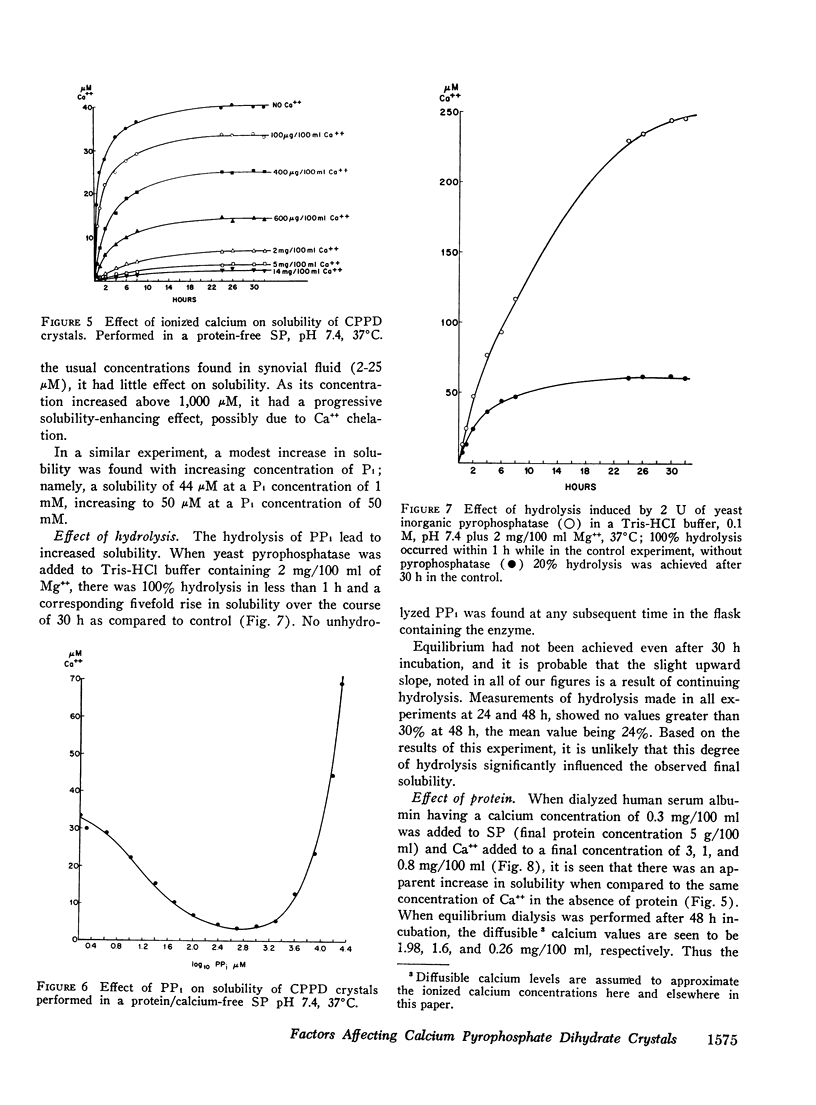
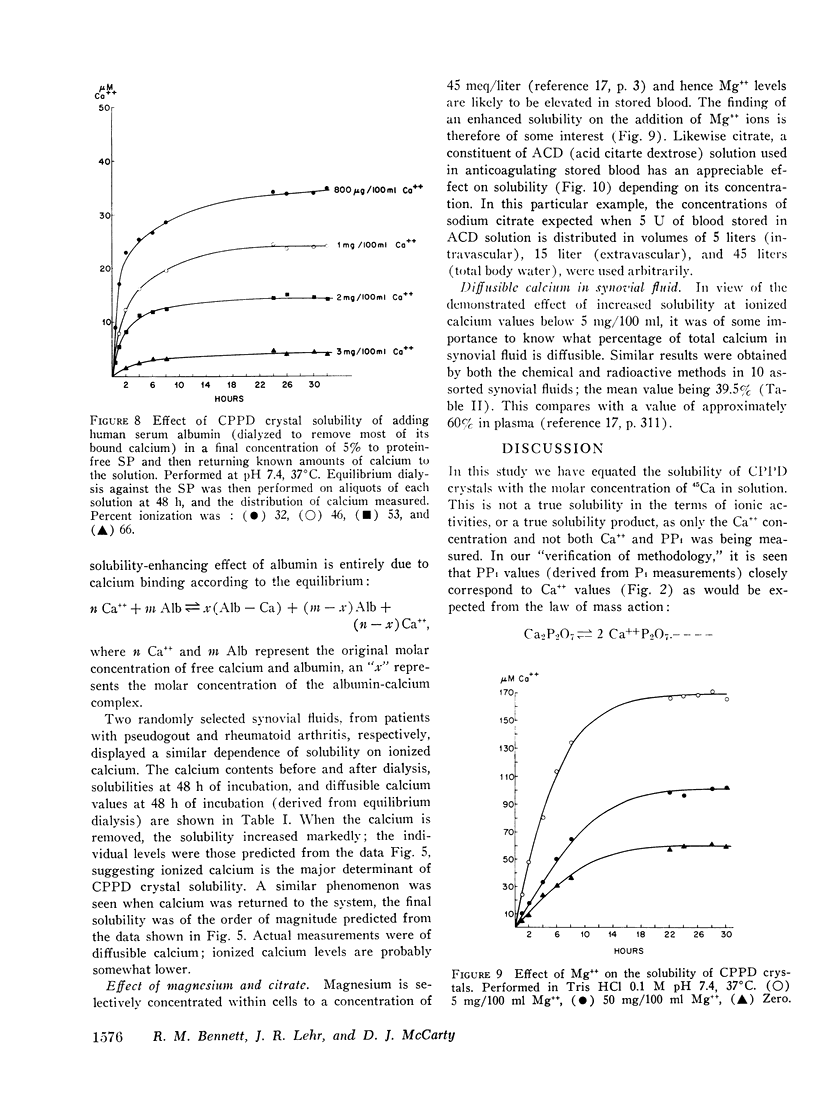
Abstract
The solubility of triclinic calcium pyrophosphate dihydrate (CPPD) crystals was measured under varying conditions using 45Ca-labeled crystals, expressing solubility as micromoles per liter of 45Ca in solution. In a 0.1-M Tris-HC1 buffer pH 7.4, the solubility of accurately sized CPPD crystals (37-20mum) was 60muM with maximal solubility being attained after about 8 h incubation at 37degreeC. Reduction in crystal size, decrease in pH, increase in ionic strength, Mg++, citrate, and albumin all increased solubility. The most marked effects on solubility occurred when changing the calcium concentration or by enzymatic hydrolysis of inoganic pyrophosphate to orthophosphate. It was found that decreasing the ionized calcium level below 5 mg/100 ml resulted in a progressive enhancement of solubility. The observed solubility-enhancing effects of albumin could be explained solely on its calcium-binding ability and thereby, altered ionized calcium level. Diffusible calcium in synovial fluid was only 40% of the total calcium concentration, which means most joint fluids are normally near the critical concentration of 5 mg/100 ml of ionized calcium, below which solubility is enhanced. During surgery, especially parathyroidectomy, calcium levels fall, favoring dissolution of CPPD crystals. We speculate that the slight decrease in crystal size during dissolution frees them from their cartilaginous mold, resulting in a dose-dependent inflammatory reaction as they are "shed" into the joint space. Crystal shedding may be reinforced by the modest fall in joint fluid pH accompanying the inflammatory response.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman R. D., Muniz O. E., Pita J. C., Howell D. S. Articular chondrocalcinosis. Microanalysis of pyrophosphate (PPi) in synovial fluid and plasma. Arthritis Rheum. 1973 Mar-Apr;16(2):171–178. doi: 10.1002/art.1780160206. [DOI] [PubMed] [Google Scholar]
- BYWATERS E. G., DIXON A. S., SCOTT J. T. Joint lesions of hyperparathyroidism. Ann Rheum Dis. 1963 May;22:171–187. doi: 10.1136/ard.22.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilezikian J. P., Connor T. B., Aptekar R., Freijanes J., Aurbach G. D., Pachas W. N., Wells S. A., Decker J. L. Pseudogout after parathyroidectomy. Lancet. 1973 Mar 3;1(7801):445–446. doi: 10.1016/s0140-6736(73)91876-x. [DOI] [PubMed] [Google Scholar]
- CLOWES G. H., Jr, SIMEONE F. A. Acute hypocalcemia in surgical patients. Ann Surg. 1957 Oct;146(4):530–541. doi: 10.1097/00000658-195710000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerlain M., McCarty D. J., Silcox D. C., Jung A. Inorganic pyrophosphate pool size and turnover rate in arthritic joints. J Clin Invest. 1975 Jun;55(6):1373–1381. doi: 10.1172/JCI108056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. P., Gilbert P., Jr, Griffin M. J. Alkaline inorganic pyrophosphatase activity of mammalian-cell alkaline phosphatase. Biochem J. 1967 Oct;105(1):155–161. doi: 10.1042/bj1050155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton R. H., Moss D. W. Alkaline orthophosphatase and inorganic pyrophosphatase activities in human serum. Nature. 1967 May 20;214(5090):842–843. doi: 10.1038/214842a0. [DOI] [PubMed] [Google Scholar]
- Hamilton E., Williams R., Barlow K. A., Smith P. M. The arthropathy of idiopathic haemochromatosis. Q J Med. 1968 Jan;37(145):171–182. [PubMed] [Google Scholar]
- McCarty D. J. Selected aspects of synovial membrane physiology. Arthritis Rheum. 1974 May-Jun;17(3):289–296. doi: 10.1002/art.1780170314. [DOI] [PubMed] [Google Scholar]
- McCarty D. J., Solomon S. D., Warnock M. L., Paloyan E. Inorganic pyrophosphate concentrations in the synovial fluid of arthritic patients. J Lab Clin Med. 1971 Aug;78(2):216–229. [PubMed] [Google Scholar]
- O'Duffy J. D. Hypophosphatasia associated with calcium pyrophosphate dihydrate deposits in cartilage. Report of a case. Arthritis Rheum. 1970 Jul-Aug;13(4):381–388. doi: 10.1002/art.1780130404. [DOI] [PubMed] [Google Scholar]
- O'Duffy J. D. Pseudogout syndrome in hospital patients. JAMA. 1973 Oct 1;226(1):42–44. [PubMed] [Google Scholar]
- Phelps P., Steele A. D., McCarty D. J., Jr Compensated polarized light microscopy. Identification of crystals in synovial fluids from gout and pseudogout. JAMA. 1968 Feb 12;203(7):508–512. doi: 10.1001/jama.203.7.508. [DOI] [PubMed] [Google Scholar]
- Russell R. G., Bisaz S., Fleisch H., Currey H. L., Rubinstein H. M., Dietz A. A., Boussina I., Micheli A., Fallet G. Inorganic pyrophosphate in plasma, urine, and synovial fluid of patients with pyrophosphate arthropathy (chondrocalcinosis or pseudogout). Lancet. 1970 Oct 31;2(7679):899–902. doi: 10.1016/s0140-6736(70)92070-2. [DOI] [PubMed] [Google Scholar]
- Schumacher H. R., Phelps P. Sequential changes in human polymorphonuclear leukocytes after urate crystal phagocytosis. An electron microscopic study. Arthritis Rheum. 1971 Jul-Aug;14(4):513–526. doi: 10.1002/art.1780140411. [DOI] [PubMed] [Google Scholar]
- Silcox D. C., McCarty D. J., Jr Elevated inorganic pyrophosphate concentrations in synovial fluids in osteoarthritis and pseudogout. J Lab Clin Med. 1974 Apr;83(4):518–531. [PubMed] [Google Scholar]



