Abstract
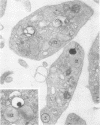
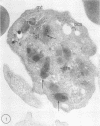
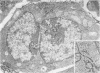
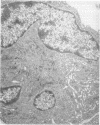
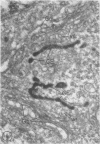
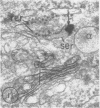
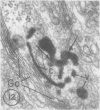
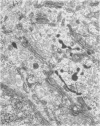
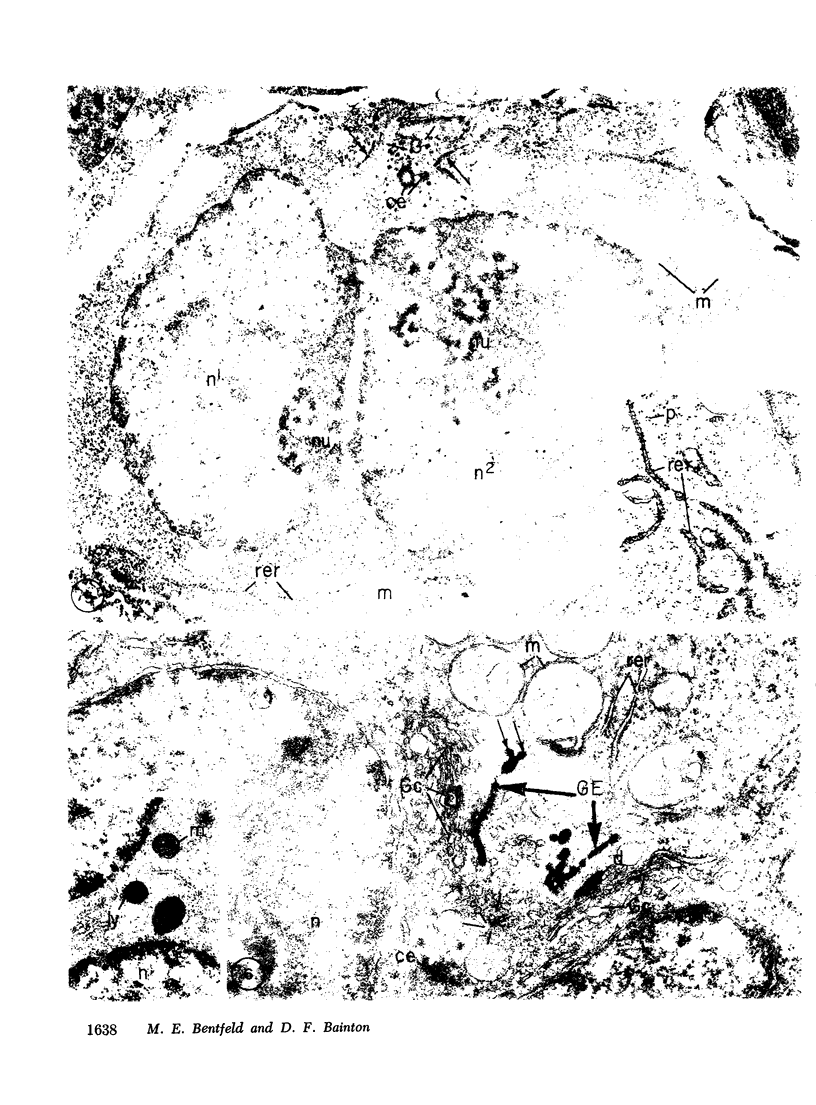
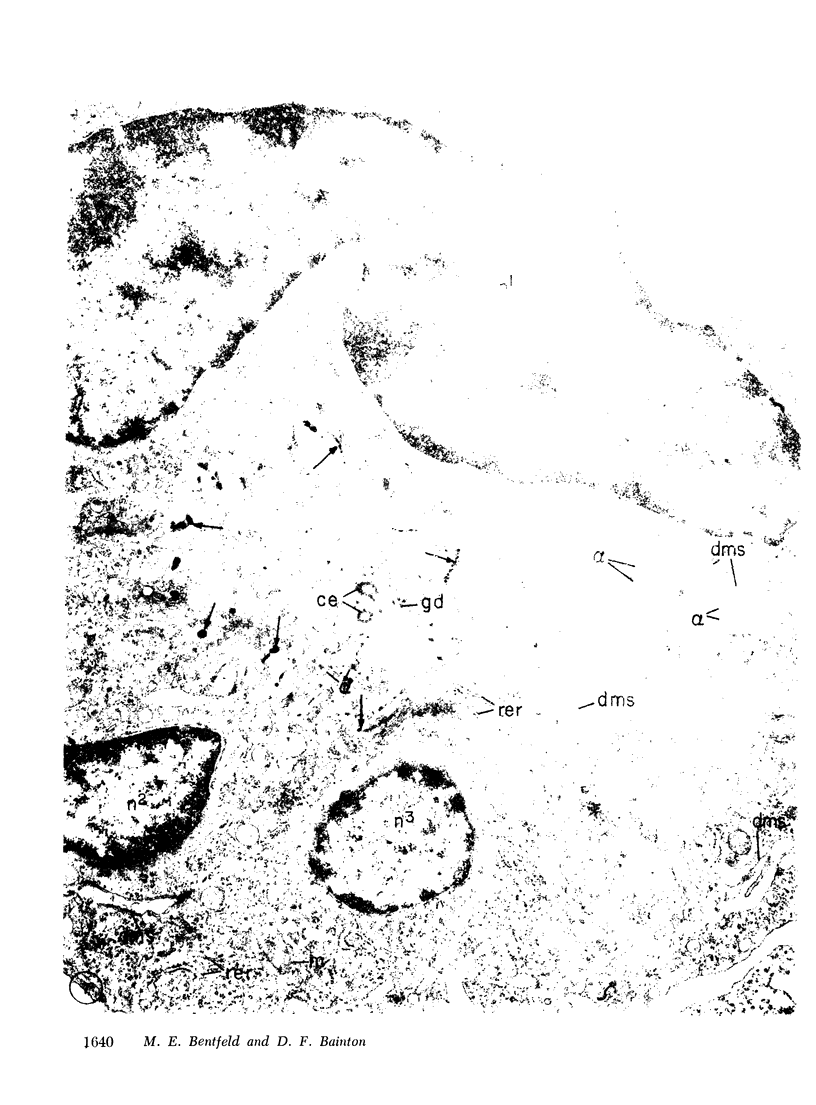
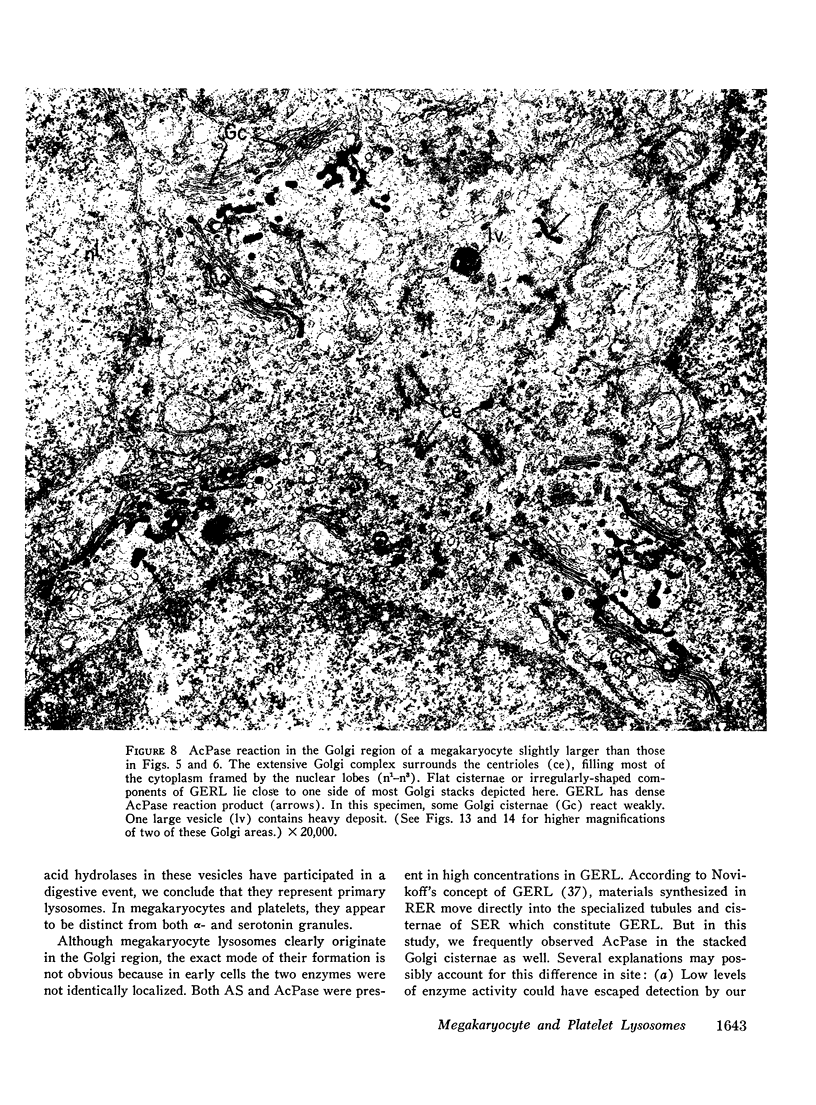
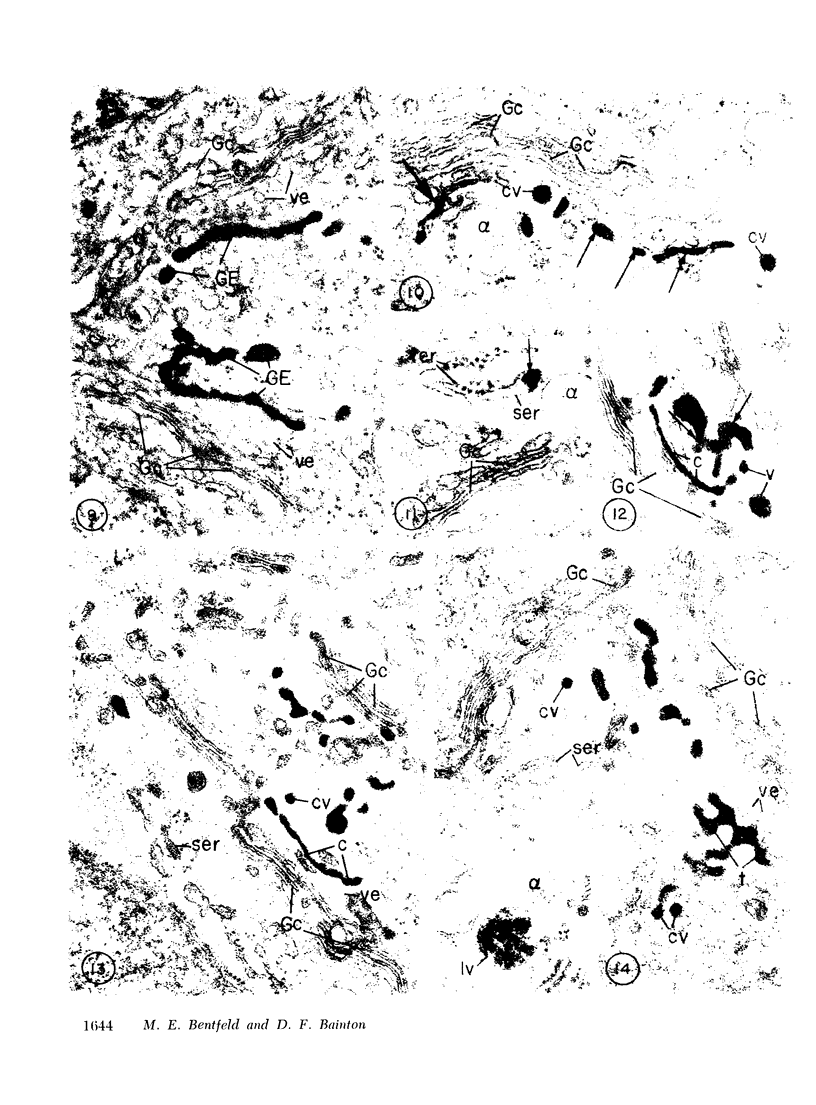
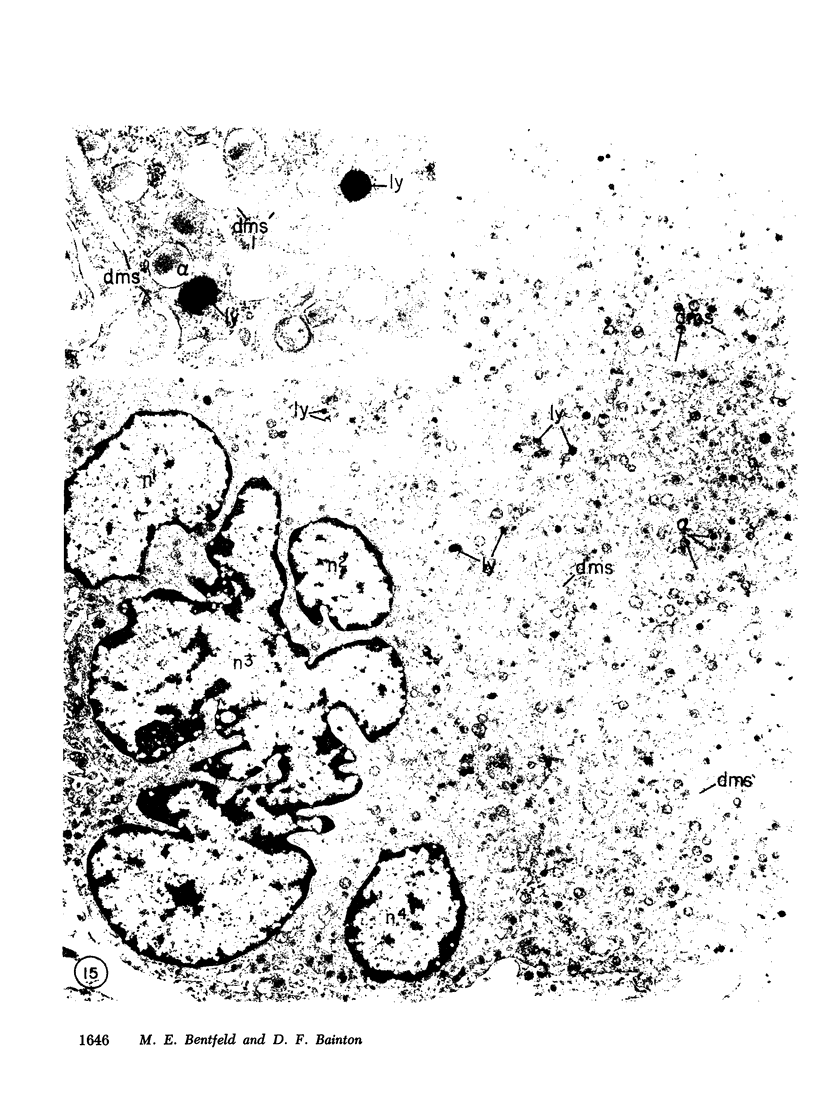
Platelets secrete lysosmal enzymes during the "platelet release reaction" early in clot formation. This study was undertaken to identify primary lysosomes of platelets and to detemine their origin in megakaryocytes. Using electron microscopy and cytochemistry, we localized two lysosomal enzymes, arylsulfatase and acid phosphatase, in megakaryocytes and platelets of normal and thrombocytopenic rats. In platelets and mature megakaryocytes, reaction product for both enzymes is confined to vesicles measuring 175-250 nm. These vesicles, which are primary lysosmes, first appear in the earliest recognizable megakaryocytes and increase in number during cellular maturation. In immature and maturing megakaryocytes, arylsulfatase and acid phosphatase can also be demonstrated in an organell similar to GERL (Golgi-endoplasmic reticulumlysosome), i.e., single smooth-surfaced cisternal with associated vesicles near the stacked Golgi cisternae. Scant reaction product for acid phosphatase is also sometimes seen in Golgi cisternae and endoplasmic reticulum. No reaction product was found in alpha-granules at any stage of megakaryocyte maturation, nor in alpha- or serotonin granules of platelets. Thus, our findings indicate that the primay lysosomes of megakaryocytes and platelets are small vesicles derived from GERL early in megakaryocyte differentiation. They can be indentified only after cytochemical staining and are distinct from both alpha- and serotonin granules.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baggiolini M., Hirsch J. G., De Duve C. Further biochemical and morphological studies of granule fractions from rabbit heterophil leukocytes. J Cell Biol. 1970 Jun;45(3):586–597. doi: 10.1083/jcb.45.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. F., Farquhar M. G. Differences in enzyme content of azurophil and specific granules of polymorphonuclear leukocytes. I. Histochemical staining of bone marrow smears. J Cell Biol. 1968 Nov;39(2):286–298. doi: 10.1083/jcb.39.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. F., Farquhar M. G. Differences in enzyme content of azurophil and specific granules of polymorphonuclear leukocytes. II. Cytochemistry and electron microscopy of bone marrow cells. J Cell Biol. 1968 Nov;39(2):299–317. doi: 10.1083/jcb.39.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak I. J., May B., Hassler R. Electron microscopical demonstration of acid phosphatase in blood platelets. Z Zellforsch Mikrosk Anat. 1969;96(4):641–648. doi: 10.1007/BF00973339. [DOI] [PubMed] [Google Scholar]
- Behnke O. An electron microscope study of the megacaryocyte of the rat bone marrow. I. The development of the demarcation membrane system and the platelet surface coat. J Ultrastruct Res. 1968 Sep;24(5):412–433. doi: 10.1016/s0022-5320(68)80046-2. [DOI] [PubMed] [Google Scholar]
- Behnke O., Emmersen J. Structural identification of thrombosthenin in rat megakaryocytes. Scand J Haematol. 1972;9(2):130–137. doi: 10.1111/j.1600-0609.1972.tb00921.x. [DOI] [PubMed] [Google Scholar]
- Behnke O. Nonspecific deposition of lead in experiments on fine structural localization of enzymatic activity of rat blood platelets. J Histochem Cytochem. 1966 May;14(5):432–433. doi: 10.1177/14.5.432. [DOI] [PubMed] [Google Scholar]
- Behnke O. The morphology of blood platelet membrane systems. Ser Haematol. 1970;3(4):3–16. [PubMed] [Google Scholar]
- Boutry J. M., Nivikoff A. B. Cytochemical studies on golgi apparatus, GERL, and lysosomes in neurons of dorsal root ganglia in mice. Proc Natl Acad Sci U S A. 1975 Feb;72(2):508–512. doi: 10.1073/pnas.72.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekman M. J., Westmoreland N. P., Cohen P. An improved method for isolating alpha granules and mitochondria from human platelets. J Cell Biol. 1974 Feb;60(2):507–519. doi: 10.1083/jcb.60.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. B. Glycogen distribution in rat blood platelets. Am J Pathol. 1973 Aug;72(2):241–252. [PMC free article] [PubMed] [Google Scholar]
- Day H. J., Solum N. O. Fibrinogen associated with subcellular platelet particles. Scand J Haematol. 1973;10(2):136–143. doi: 10.1111/j.1600-0609.1973.tb00050.x. [DOI] [PubMed] [Google Scholar]
- Decker R. S. Lysosomal packaging in differentiating and degenerating anuran lateral motor column neurons. J Cell Biol. 1974 Jun;61(3):599–612. doi: 10.1083/jcb.61.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen E. R. Centriole morphogenesis in developing ciliated epithelium of the mouse oviduct. J Cell Biol. 1971 Oct;51(1):286–302. doi: 10.1083/jcb.51.1.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droller M. J. An electron microscope study of the time course of platelet nucleotide, calcium, and acid phosphatase secretion. Lab Invest. 1974 Aug;31(2):197–205. [PubMed] [Google Scholar]
- EBBE S., STOHLMAN F., Jr MEGAKARYOCYTOPOIESIS IN THE RAT. Blood. 1965 Jul;26:20–35. [PubMed] [Google Scholar]
- Ebbe S., Stohlman F., Jr, Donovan J., Overcash J. Megakaryocyte maturation rate in thrombocytopenic rats. Blood. 1968 Nov;32(5):787–795. [PubMed] [Google Scholar]
- Farquhar M. G., Bergeron J. J., Palade G. E. Cytochemistry of Golgi fractions prepared from rat liver. J Cell Biol. 1974 Jan;60(1):8–25. doi: 10.1083/jcb.60.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfischer S. The cytochemical demonstration of lysosomal aryl sulfatase activity by light and electron microscopy. J Histochem Cytochem. 1965 Jul-Aug;13(6):520–523. doi: 10.1177/13.6.520. [DOI] [PubMed] [Google Scholar]
- Harker L. A. Kinetics of thrombopoiesis. J Clin Invest. 1968 Mar;47(3):458–465. doi: 10.1172/JCI105742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmsen H., Day H. J., Stormorken H. The blood platelet release reaction. Scand J Haematol Suppl. 1969;8:3–26. [PubMed] [Google Scholar]
- Holmsen H., Day H. J. The selectivity of the thrombin-induced platelet release reaction: subcellular localization of released and retained constituents. J Lab Clin Med. 1970 May;75(5):840–855. [PubMed] [Google Scholar]
- Holtzman E., Dominitz R. Cytochemical studies of lysosomes, golgi apparatus and endoplasmic reticulum in secretion and protein uptake by adrenal medulla cells of the rat. J Histochem Cytochem. 1968 May;16(5):320–336. doi: 10.1177/16.5.320. [DOI] [PubMed] [Google Scholar]
- JONES O. P. Origin of megakaryocyte granules from Golgi vesicles. Anat Rec. 1960 Oct;138:105–113. doi: 10.1002/ar.1091380204. [DOI] [PubMed] [Google Scholar]
- Kaulen H. D., Gross R. The differentiation of acid phosphatases of human blood platelets. Thromb Diath Haemorrh. 1971 Oct 31;26(2):353–361. [PubMed] [Google Scholar]
- MacPherson G. G. Development of megakaryocytes in bone marrow of the rat: an analysis by electron microscopy and high resolution autoradiography. Proc R Soc Lond B Biol Sci. 1971 Mar 16;177(1047):265–274. doi: 10.1098/rspb.1971.0027. [DOI] [PubMed] [Google Scholar]
- MacPherson G. G. Synthesis and localization of sulphated mucopolysaccharide in megakaryocytes and platelets of the rat, an anlysis by electron-microscope autoradiography. J Cell Sci. 1972 May;10(3):705–717. doi: 10.1242/jcs.10.3.705. [DOI] [PubMed] [Google Scholar]
- Marcus A. J., Zucker-Franklin D., Safier L. B., Ullman H. L. Studies on human platelet granules and membranes. J Clin Invest. 1966 Jan;45(1):14–28. doi: 10.1172/JCI105318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. H., Carson F. L., Race G. J. Calcium-containing platelet granules. J Cell Biol. 1974 Mar;60(3):775–777. doi: 10.1083/jcb.60.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern E., Walter E., Weber E. Functional aspects of lysosomal organelles in blood platelets. Z Zellforsch Mikrosk Anat. 1971;118(2):283–296. doi: 10.1007/BF00341571. [DOI] [PubMed] [Google Scholar]
- Murata F., Hardin J. H., Spicer S. S. Coexistence of acid phosphatase and acid mucosubstance in the nucleoid of human blood platelet granules. Histochemie. 1973 Jun 29;35(4):319–329. doi: 10.1007/BF00310671. [DOI] [PubMed] [Google Scholar]
- Nichols B. A., Bainton D. F., Farquhar M. G. Differentiation of monocytes. Origin, nature, and fate of their azurophil granules. J Cell Biol. 1971 Aug;50(2):498–515. doi: 10.1083/jcb.50.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff P. M., Novikoff A. B., Quintana N., Hauw J. J. Golgi apparatus, GERL, and lysosomes of neurons in rat dorsal root ganglia, studied by thick section and thin section cytochemistry. J Cell Biol. 1971 Sep;50(3):859–886. doi: 10.1083/jcb.50.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell T. T., Jr, Jackson C. W. Polyploidy and maturation of rat megakaryocytes. Blood. 1968 Jul;32(1):102–110. [PubMed] [Google Scholar]
- Paulus J. M. DNA metabolism and development of organelles in guinea-pig megakaryocytes: a combined ultrastructural, autoradiographic and cytophotometric study. Blood. 1970 Mar;35(3):298–311. [PubMed] [Google Scholar]
- Pletscher A., Da Prada M., Berneis K. H., Tranzer J. P. New aspects on the storage of 5-hydroxytryptamine in blood platelets. Experientia. 1971 Sep 15;27(9):993–1002. doi: 10.1007/BF02138839. [DOI] [PubMed] [Google Scholar]
- RODMAN N. F., Jr, MASON R. G., BRINKHOUS K. M. SOME PATHOGENETIC MECHANISMS OF WHITE THROMBUS FORMATION: AGGLUTINATION AND SELF-DESTRUCTION OF THE PLATELET. Fed Proc. 1963 Nov-Dec;22:1356–1365. [PubMed] [Google Scholar]
- Sato T., Herman L., Chandler J. A., Stracher A., Detwiler T. C. Localization of a thrombin-sensitive calcium pool in platelets. J Histochem Cytochem. 1975 Feb;23(2):103–106. doi: 10.1177/23.2.1167875. [DOI] [PubMed] [Google Scholar]
- Seeman P. M., Palade G. E. Acid phosphatase localization in rabbit eosinophils. J Cell Biol. 1967 Sep;34(3):745–756. doi: 10.1083/jcb.34.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel A., Burri P. H., Weibel E. R., Bettex-Galland M., Lüscher E. F. Density gradient centrifugation and electron microscopic characterization of subcellular fractions from human blood platelets. Thromb Diath Haemorrh. 1971 Jun 30;25(2):252–267. [PubMed] [Google Scholar]
- Siegel A., Lüscher E. F. Non-identity of the alpha-granules of human blood platelets with typical lysosomes. Nature. 1967 Aug 12;215(5102):745–747. doi: 10.1038/215745a0. [DOI] [PubMed] [Google Scholar]
- Spicer S. S., Greene W. B., Hardin J. H. Ultrastructural localization of acid mucosubstance and antimonate-precipitable cation in human and rabbit platelets and megakaryocytes. J Histochem Cytochem. 1969 Dec;17(12):781–792. doi: 10.1177/17.12.781. [DOI] [PubMed] [Google Scholar]
- THIERY J. P., BESSIS M. Mécanisme de la plaquettogénèse; etude in vitro par la microcinématographie. Rev Hematol. 1956 Apr-May;11(2):162–174. [PubMed] [Google Scholar]
- Walter E., Morgenstern E., Weber E. Lysosomal and microsomal acid phosphatase in blood platelets. Naturwissenschaften. 1971 Nov;58(11):575–575. doi: 10.1007/BF00598738. [DOI] [PubMed] [Google Scholar]
- Wetzel B. K., Spicer S. S., Horn R. G. Fine structural localization of acid and alkaline phosphatases in cells of rabbit blood and bone marrow. J Histochem Cytochem. 1967 Jun;15(6):311–334. doi: 10.1177/15.6.311. [DOI] [PubMed] [Google Scholar]
- White J. G. Interaction of membrane systems in blood platelets. Am J Pathol. 1972 Feb;66(2):295–312. [PMC free article] [PubMed] [Google Scholar]
- White J. G. Uptake of latex particles by blood platelets: phagocytosis or sequestration? Am J Pathol. 1972 Dec;69(3):439–458. [PMC free article] [PubMed] [Google Scholar]
- ZUCKER M. B., BORELLI J. A survey of some platelet enzymes and functions; the platelets as the source of normal serum acid glycerophosphatase. Ann N Y Acad Sci. 1958 Oct 13;75(1):203–213. doi: 10.1111/j.1749-6632.1958.tb36867.x. [DOI] [PubMed] [Google Scholar]
- ZUCKER M. B., BORRELLI J. Platelets as a source of serum acid nitrophenylphosphatase. J Clin Invest. 1959 Jan 1;38(1 Pt 1):148–154. doi: 10.1172/JCI103784. [DOI] [PMC free article] [PubMed] [Google Scholar]