Abstract
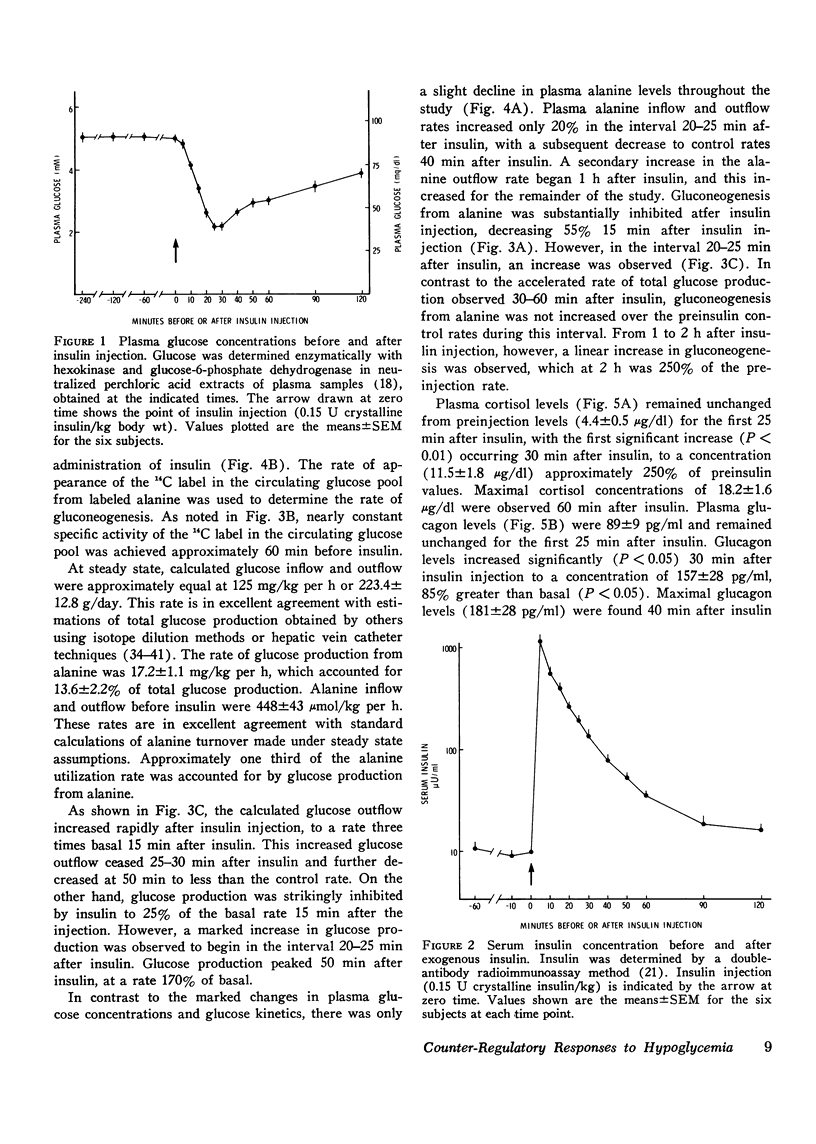
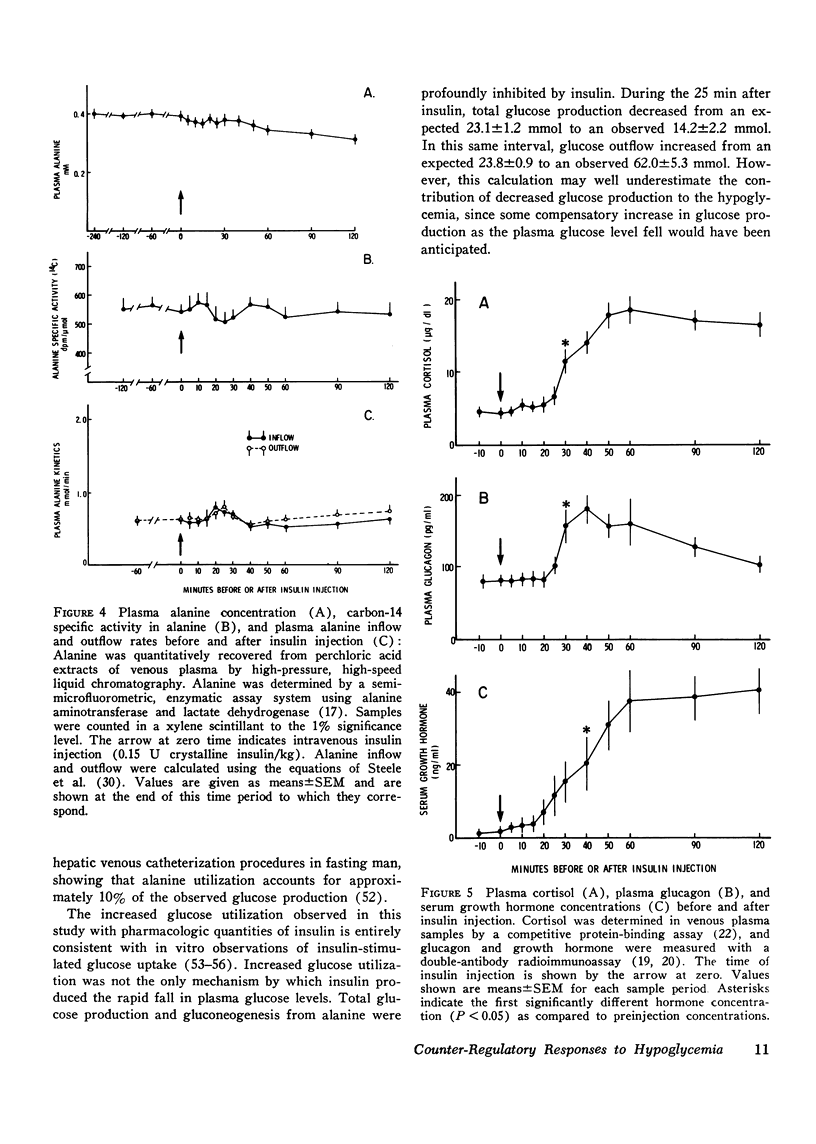
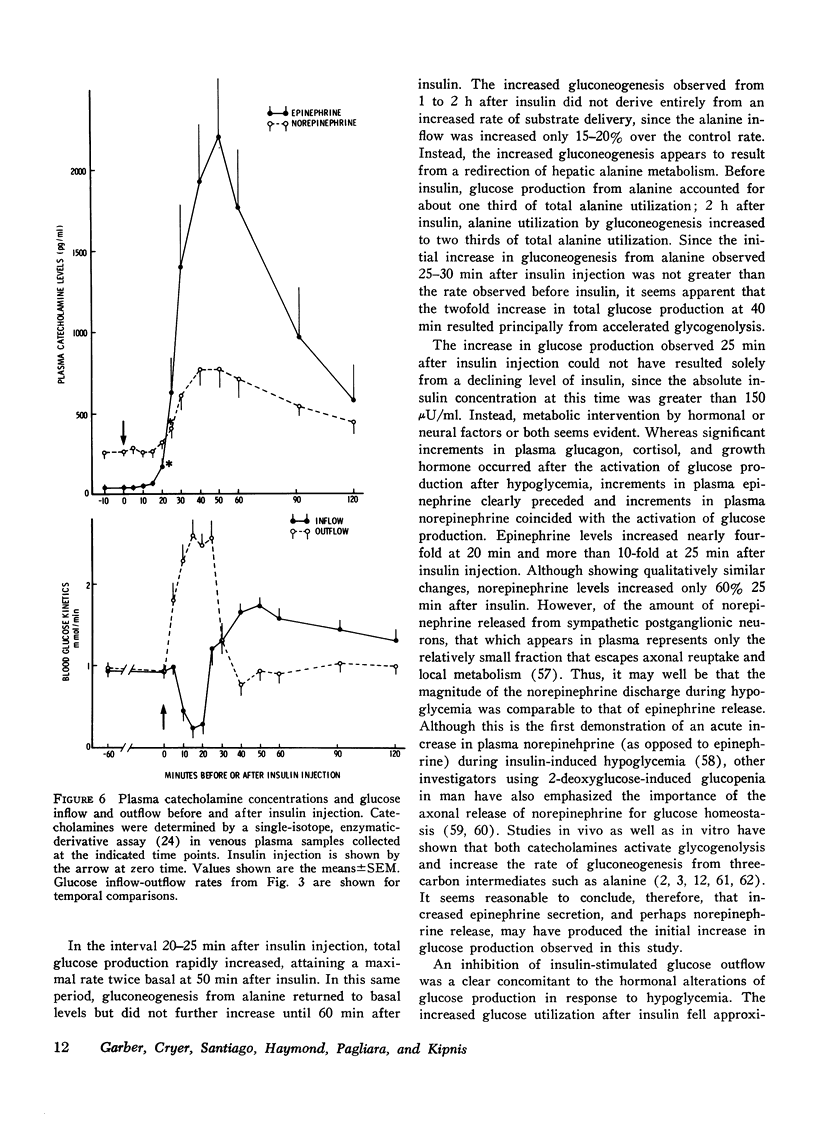
Sequential determinations of glucose outflow and inflow, and rates of gluconeogenesis from alanine, before, during and after insulin-induced hypoglycemia were obtained in relation to alterations in circulating epinephrine, norepinephrine, glucagon, cortisol, and growth hormone in six normal subjects. Insulin decreased the mean (+/-SEM) plasma glucose from 89+/-3 to 39+/-2 mg/dl 25 min after injection, but this decline ceased despite serum insulin levels of 153+/-22 mul/ml. Before insulin, glucose inflow and outflow were constant averaging 125.3+/-7.1 mg/kg per h. 15 min after insulin, mean glucose outflow increased threefold, but then decreased at 25 min, reaching a rate 15% less than the preinsulin rate. Glucose inflow decreased 80% 15 min after insulin, but increased at 25 min, reaching a maximum of twice the basal rate. Gluconeogenesis from alanine decreased 68% 15 min after insulin, but returned to preinsulin rates at 25 min, and remained constant for the next 25 min, after which it increased linearly. A fourfold increase in mean plasma epinephrine was found 20 min after insulin, with maximal levels 50 times basal. Plasma norepinephrine concentrations first increased significantly at 25 min after insulin, whereas significantly increased levels of cortisol and glucagon occurred at 30 min, and growth hormone at 40 min after insulin. Thus, insulin-induced hypoglycemia in man results from both a decrease in glucose production and an increase in glucose utilization. Accelerated glycogenolysis produced much of the initial, posthypoglycemic increment in glucose production. The contribution of glycogenolysis decreased with time, while that of gluconeogenesis from alanine increased. Of the hormones studied, only the increments in plasma catecholamines preceded or coincided with the measured increase in glucose production after hypoglycemia. It therefore seems probable that adrenergic mechanisms play a major role in the initiation of counter-regulatory responses to insulin-induced hypoglycemia in man.
Full text
PDF

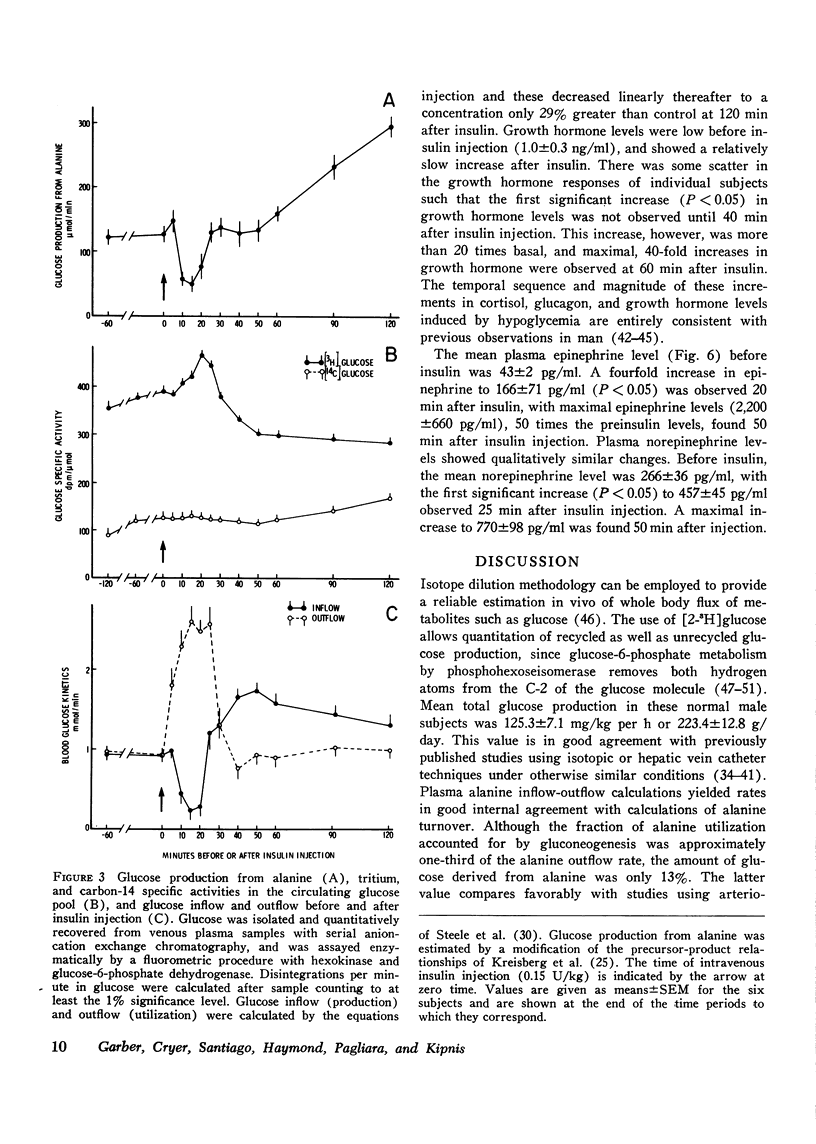



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlborg G., Felig P., Hagenfeldt L., Hendler R., Wahren J. Substrate turnover during prolonged exercise in man. Splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. J Clin Invest. 1974 Apr;53(4):1080–1090. doi: 10.1172/JCI107645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod J., Weinshilboum R. Catecholamines. N Engl J Med. 1972 Aug 3;287(5):237–242. doi: 10.1056/NEJM197208032870508. [DOI] [PubMed] [Google Scholar]
- Balasse E. O., Neef M. A. Influence of nicotinic acid on the rates of turnover and oxidation of plasma glucose in man. Metabolism. 1973 Sep;22(9):1193–1204. doi: 10.1016/0026-0495(73)90207-2. [DOI] [PubMed] [Google Scholar]
- Blackard W. G., Heidingsfelder S. A. Adrenergic receptor control mechanism for growth hormone secretion. J Clin Invest. 1968 Jun;47(6):1407–1414. doi: 10.1172/JCI105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair J. B., Cook D. E., Lardy H. A. Influence of glucagon on the metabolism of xylitol and dihydroxyacetone in the isolated perfused rat liver. J Biol Chem. 1973 May 25;248(10):3601–3607. [PubMed] [Google Scholar]
- Bowen H. F., Moorhouse J. A. Glucose turnover and disposal in maturity-onset diabetes. J Clin Invest. 1973 Dec;52(12):3033–3045. doi: 10.1172/JCI107502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodows R. G., Pi-Sunyer, Campbell R. G. Sympathetic control of hepatic glycogenolysis during glucopenia in man. Metabolism. 1975 May;24(5):617–624. doi: 10.1016/0026-0495(75)90141-9. [DOI] [PubMed] [Google Scholar]
- Brodows R. G., Pi-Sunyer F. X., Campbell R. G. Neural control of counter-regulatory events during glucopenia in man. J Clin Invest. 1973 Aug;52(8):1841–1844. doi: 10.1172/JCI107366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill G. F., Jr The Banting Memorial Lecture 1971. Physiology of insulin in man. Diabetes. 1971 Dec;20(12):785–799. doi: 10.2337/diab.20.12.785. [DOI] [PubMed] [Google Scholar]
- Chambaut A. M., Eboué-Bonis D., Hanoune J., Clauser H. Antagonistic actions between dibutyryl adenosine-3',5'-cyclic monophosphate and insulin on the metabolism of the surviving rat diaphragm. Biochem Biophys Res Commun. 1969 Feb 7;34(3):283–290. doi: 10.1016/0006-291x(69)90829-8. [DOI] [PubMed] [Google Scholar]
- Clark M. G., Kneer N. M., Bosch A. L., Lardy H. A. The fructose 1,6-diphosphatase-phosphofructokinase substrate cycle. A site of regulation of hepatic gluconeogenesis by glucagon. J Biol Chem. 1974 Sep 25;249(18):5695–5703. [PubMed] [Google Scholar]
- Cowan J. S., Hetenyi G., Jr Glucoregulatory responses in normal and diabetic dogs recorded by a new tracer method. Metabolism. 1971 Apr;20(4):360–372. doi: 10.1016/0026-0495(71)90098-9. [DOI] [PubMed] [Google Scholar]
- Cryer P. E., Santiago J. V., Shah S. Measurement of norepinephrine and epinephrine in small volumes of human plasma by a single isotope derivative method: response to the upright posture. J Clin Endocrinol Metab. 1974 Dec;39(6):1025–1029. doi: 10.1210/jcem-39-6-1025. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Gluconeogenesis. Metabolism. 1972 Oct;21(10):945–990. doi: 10.1016/0026-0495(72)90028-5. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Park C. R. Control of gluconeogenesis in liver. II. Effects of glucagon, catecholamines, and adenosine 3',5'-monophosphate on gluconeogenesis in the perfused rat liver. J Biol Chem. 1968 Aug 25;243(16):4189–4196. [PubMed] [Google Scholar]
- Exton J. H., Park C. R. The stimulation of gluconeogenesis from lactate by epinephrine, glucagon, cyclic 3',5'-adenylate in the perfused rat liver. Pharmacol Rev. 1966 Mar;18(1):181–188. [PubMed] [Google Scholar]
- Exton J. H., Robison G. A., Sutherland E. W., Park C. R. Studies on the role of adenosine 3',5'-monophosphate in the hepatic actions of glucagon and catecholamines. J Biol Chem. 1971 Oct 25;246(20):6166–6177. [PubMed] [Google Scholar]
- Felig P., Marliss E., Pozefsky T., Cahill G. F., Jr Amino acid metabolism in the regulation of gluconeogenesis in man. Am J Clin Nutr. 1970 Jul;23(7):986–992. doi: 10.1093/ajcn/23.7.986. [DOI] [PubMed] [Google Scholar]
- Felig P., Owen O. E., Wahren J., Cahill G. F., Jr Amino acid metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):584–594. doi: 10.1172/JCI106017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg S. E., Merimee T. J. Acute metabolic effects of human growth hormone. Diabetes. 1974 Jun;23(6):499–504. doi: 10.2337/diab.23.6.499. [DOI] [PubMed] [Google Scholar]
- Garber A. J., Bier D. M., Cryer P. E., Pagliara A. S. Hypoglycemia in compensated chronic renal insufficiency. Substrate limitation of gluconeogenesis. Diabetes. 1974 Dec;23(12):982–986. doi: 10.2337/diab.23.12.982. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Karam J. H., Forsham P. H. Stimulation of glucagon secretion by epinephrine in man. J Clin Endocrinol Metab. 1973 Sep;37(3):479–481. doi: 10.1210/jcem-37-3-479. [DOI] [PubMed] [Google Scholar]
- Greenwood F. C., Landon J. Assessment of hypothalamic pituitary function in endocrine disease. J Clin Pathol. 1966 May;19(3):284–292. doi: 10.1136/jcp.19.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood F. C., Landon J., Stamp T. C. The plasma sugar, free fatty acid, cortisol, and growth hormone response to insulin. I. In control subjects. J Clin Invest. 1966 Apr;45(4):429–436. doi: 10.1172/JCI105357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERMAN M. S., RAMEY E. R. Epinephrine action on glucose uptake by rat diaphragm; effect of ionic composition. Am J Physiol. 1960 Aug;199:226–228. doi: 10.1152/ajplegacy.1960.199.2.226. [DOI] [PubMed] [Google Scholar]
- Hammerstedt R. H. The use of Dowex-1-borate to separate 3HOH from 2-3H-glucose. Anal Biochem. 1973 Nov;56(1):292–293. doi: 10.1016/0003-2697(73)90192-9. [DOI] [PubMed] [Google Scholar]
- Hanson R. W., Garber A. J. Phosphoenolpyruvate carboxykinase. I. Its role in gluconeogenesis. Am J Clin Nutr. 1972 Oct;25(10):1010–1021. doi: 10.1093/ajcn/25.10.1010. [DOI] [PubMed] [Google Scholar]
- Hetenyi G., Jr, Norwich K. H. Validity of the rates of production and utilization of metabolites as determined by tracer methods in intact animals. Fed Proc. 1974 Jul;33(7):1841–1848. [PubMed] [Google Scholar]
- Hornbrook K. R. Adrenergic receptors for metabolic responses in the liver. Fed Proc. 1970 Jul-Aug;29(4):1381–1385. [PubMed] [Google Scholar]
- Hue L., Hers H. G. On the use of (3H, 14C)labelled glucose in the study of the so-called "futile cycles" in liver and muscle. Biochem Biophys Res Commun. 1974 Jun 4;58(3):532–539. doi: 10.1016/s0006-291x(74)80453-5. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr, Allen M., Borkow I. Estimation of glucose turnover in the dog with glucose-2-T and glucose-U- 14 C. Am J Physiol. 1972 Mar;222(3):710–712. doi: 10.1152/ajplegacy.1972.222.3.710. [DOI] [PubMed] [Google Scholar]
- Iversen J. Adrenergic receptors and the secretion of glucagon and insulin from the isolated, perfused canine pancreas. J Clin Invest. 1973 Sep;52(9):2102–2116. doi: 10.1172/JCI107395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. E., Das N. M., Butcher F. R., Fain J. N. The regulation of gluconeogenesis in isolated rat liver cells by glucagon, insulin, dibutyryl cyclic adenosine monophosphate, and fatty acids. J Biol Chem. 1972 May 25;247(10):3229–3235. [PubMed] [Google Scholar]
- KIPNIS D. M., CORI C. F. Studies of tissue permeability. III. The effect of insulin on pentose uptake by the diaphragm. J Biol Chem. 1957 Feb;224(2):681–693. [PubMed] [Google Scholar]
- KIPNIS D. M., HELMREICH E., CORI C. F. Studies of tissue permeability. IV. The distribution of glucose between plasma and muscle. J Biol Chem. 1959 Jan;234(1):165–170. [PubMed] [Google Scholar]
- Karl I. E., Pagliara A. S., Kipnis D. M. A microfluorometric enzymatic assay for the determination of alanine and pyruvate in plasma and tissues. J Lab Clin Med. 1972 Sep;80(3):434–441. [PubMed] [Google Scholar]
- Katz J., Dunn A., Chenoweth M., Golden S. Determination of synthesis, recycling and body mass of glucose in rats and rabbits in vivo 3H-and 14C-labelled glucose. Biochem J. 1974 Jul;142(1):171–183. doi: 10.1042/bj1420171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Rostami H., Dunn A. Evaluation of glucose turnover, body mass and recycling with reversible and irreversible tracers. Biochem J. 1974 Jul;142(1):161–170. doi: 10.1042/bj1420161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneer N. M., Bosch A. L., Clark M. G., Lardy H. A. Glucose inhibition of epinephrine stimulation of hepatic gluconeogenesis by blockade of the alpha-receptor function. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4523–4527. doi: 10.1073/pnas.71.11.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisberg R. A., Pennington L. F., Boshell B. R. Lactate turnover and gluconeogenesis in normal and obese humans. Effect of starvation. Diabetes. 1970 Jan;19(1):53–63. doi: 10.2337/diab.19.1.53. [DOI] [PubMed] [Google Scholar]
- Kreisberg R. A., Siegal A. M., Owen W. C. Glucose-lactate interrelationships: effect of ethanol. J Clin Invest. 1971 Jan;50(1):175–185. doi: 10.1172/JCI106471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leichter S. B., Pagliara A. S., Grieder M. H., Pohl S., Rosai J., Kipnis D. M. Uncontrolled diabetes mellitus and hyperglucagonemia associated with an islet cell carcinoma. Am J Med. 1975 Feb;58(2):285–293. doi: 10.1016/0002-9343(75)90579-3. [DOI] [PubMed] [Google Scholar]
- Lindsey C. A., Faloona G. R., Unger R. H. PLasma glucagon levels during rapid exsanguination with and without adrenergic blockade. Diabetes. 1975 Apr;24(4):313–316. [PubMed] [Google Scholar]
- MYERS J. D. Net splanchnic glucose production in normal man and in various disease states. J Clin Invest. 1950 Nov;29(11):1421–1429. doi: 10.1172/JCI102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet L. E., Exton J. H., Park C. R. Control of gluconeogenesis from amino acids in the perfused rat liver. J Biol Chem. 1969 Oct 25;244(20):5713–5723. [PubMed] [Google Scholar]
- Miller T. B., Jr, Larner J. Mechanism of control of hepatic glycogenesis by insulin. J Biol Chem. 1973 May 25;248(10):3483–3488. [PubMed] [Google Scholar]
- Murphy B. E. Some studies of the protein-binding of steroids and their application to the routine micro and ultramicro measurement of various steroids in body fluids by competitive protein-binding radioassay. J Clin Endocrinol Metab. 1967 Jul;27(7):973–990. doi: 10.1210/jcem-27-7-973. [DOI] [PubMed] [Google Scholar]
- Nakagawa K., Horiuchi Y., Mashimo K. Further studies on the relation between growth hormone and corticotrophin secretion in insulin-induced hypoglycemia. J Clin Endocrinol Metab. 1971 Feb;32(2):188–191. doi: 10.1210/jcem-32-2-188. [DOI] [PubMed] [Google Scholar]
- Nakai Y., Imura H., Yoshimi T., Matsukura S. Adrenergic control mechanism for ACTH secretion in man. Acta Endocrinol (Copenh) 1973 Oct;74(2):263–270. doi: 10.1530/acta.0.0740263. [DOI] [PubMed] [Google Scholar]
- Owen O. E., Felig P., Morgan A. P., Wahren J., Cahill G. F., Jr Liver and kidney metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):574–583. doi: 10.1172/JCI106016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrilla R., Goodman M. N., Toews C. J. Effect of glucagon: insulin ratios on hepatic metabolism. Diabetes. 1974 Sep;23(9):725–731. doi: 10.2337/diab.23.9.725. [DOI] [PubMed] [Google Scholar]
- Passon P. G., Peuler J. D. A simplified radiometric assay for plasma norepinephrine and epinephrine. Anal Biochem. 1973 Feb;51(2):618–631. doi: 10.1016/0003-2697(73)90517-4. [DOI] [PubMed] [Google Scholar]
- Paul P., Bortz W. M. Turnover and oxidation of plasma glucose in lean and obese humans. Metabolism. 1969 Jul;18(7):570–584. doi: 10.1016/0026-0495(69)90091-2. [DOI] [PubMed] [Google Scholar]
- Porte D., Jr, Williams R. H. Inhibition of insulin release by norepinephrine in man. Science. 1966 May 27;152(3726):1248–1250. doi: 10.1126/science.152.3726.1248. [DOI] [PubMed] [Google Scholar]
- SCHALCH D. S., PARKER M. L. A SENSITIVE DOUBLE ANTIBODY IMMUNOASSAY FOR HUMAN GROWTH HORMONE IN PLASMA. Nature. 1964 Sep 12;203:1141–1142. doi: 10.1038/2031141a0. [DOI] [PubMed] [Google Scholar]
- SHRAGO E., LARDY H. A., NORDLIE R. C., FOSTER D. O. METABOLIC AND HORMONAL CONTROL OF PHOSPHOENOLPYRUVATE CARBOXYKINASE AND MALIC ENZYME IN RAT LIVER. J Biol Chem. 1963 Oct;238:3188–3192. [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- STEELE R., WALL J. S., DE BODO R. C., ALTSZULER N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol. 1956 Sep;187(1):15–24. doi: 10.1152/ajplegacy.1956.187.1.15. [DOI] [PubMed] [Google Scholar]
- Searle G. L., Gulli R., Cavalieri R. R. Effect of Phenformin in nondiabetic humans. Estimation of glucose turnover rate and Cori cycle activity. Metabolism. 1969 Feb;18(2):148–154. doi: 10.1016/0026-0495(69)90109-7. [DOI] [PubMed] [Google Scholar]
- Sherline P., Lynch A., Glinsmann W. H. Cyclic AMP and adrenergic receptor control of rat liver glycogen metabolism. Endocrinology. 1972 Sep;91(3):680–690. doi: 10.1210/endo-91-3-680. [DOI] [PubMed] [Google Scholar]
- Steele R., Rostami H., Altszuler N. A two-compartment calculator for the dog glucose pool in the nonsteady state. Fed Proc. 1974 Jul;33(7):1869–1876. [PubMed] [Google Scholar]
- Toivola P. T., Gale C. C., Goodner C. J., Werrbach J. H. Central -adrenergic regulation of growth hormone and insulin. Hormones. 1972;3(4):192–213. [PubMed] [Google Scholar]
- Toivola P. T., Gale C. C. Stimulation of growth release by microinjection of norepinephrine into hypothalamus of baboons. Endocrinology. 1972 Apr;90(4):895–902. doi: 10.1210/endo-90-4-895. [DOI] [PubMed] [Google Scholar]
- Tolbert M. E., Butcher F. R., Fain J. N. Lack of correlation between catecholamine effects on cyclic adenosine 3':5'-monophosphate and gluconeogenesis in isolated rat liver cells. J Biol Chem. 1973 Aug 25;248(16):5686–5692. [PubMed] [Google Scholar]
- UI M. ACTION OF EPINEPHRINE ON MUSCLE GLUCOSE UPTAKE DEPENDING ON CA++ AND PHOSPHATE. Am J Physiol. 1965 Aug;209:359–364. doi: 10.1152/ajplegacy.1965.209.2.359. [DOI] [PubMed] [Google Scholar]
- UI M. BLOCKAGE OF EPINEPHRINE-INDUCED HYPERGLYCEMIA DURING EXPOSURE TO SIMULATED ALTITUDES. Am J Physiol. 1965 Aug;209:353–358. doi: 10.1152/ajplegacy.1965.209.2.353. [DOI] [PubMed] [Google Scholar]
- UNGER R. H., EISENTRAUT A. M., McCALL M. S., MADISON L. L. Measurements of endogenous glucagon in plasma and the influence of blood glucose concentration upon its secretion. J Clin Invest. 1962 Apr;41:682–689. doi: 10.1172/JCI104525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALAAS O., WALAAS E. Effect of epinephrine on rat diaphragm. J Biol Chem. 1950 Dec;187(2):769–776. [PubMed] [Google Scholar]
- Wahren J., Felig P., Cerasi E., Luft R. Splanchnic and peripheral glucose and amino acid metabolism in diabetes mellitus. J Clin Invest. 1972 Jul;51(7):1870–1878. doi: 10.1172/JCI106989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Paetkau V., Lardy H. A. Paths of carbon in gluconeogenesis and lipogenesis. 3. The role and regulation of mitochondrial processes involved in supplying precursors of phosphoenolpyruvate. J Biol Chem. 1966 Jun 10;241(11):2523–2532. [PubMed] [Google Scholar]
- Weir G. C., Knowlton S. D., Martin D. B. Glucagon secretion from the perfused rat pancreas. Studies with glucose and catecholamines. J Clin Invest. 1974 Dec;54(6):1403–1412. doi: 10.1172/JCI107887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis L. S., Narahara H. T. Regulation of cell membrane permeability in skeletal muscle. I. Action of insulin and trypsin on the transport system for sugar. J Biol Chem. 1969 Jun 10;244(11):3084–3091. [PubMed] [Google Scholar]



