Abstract
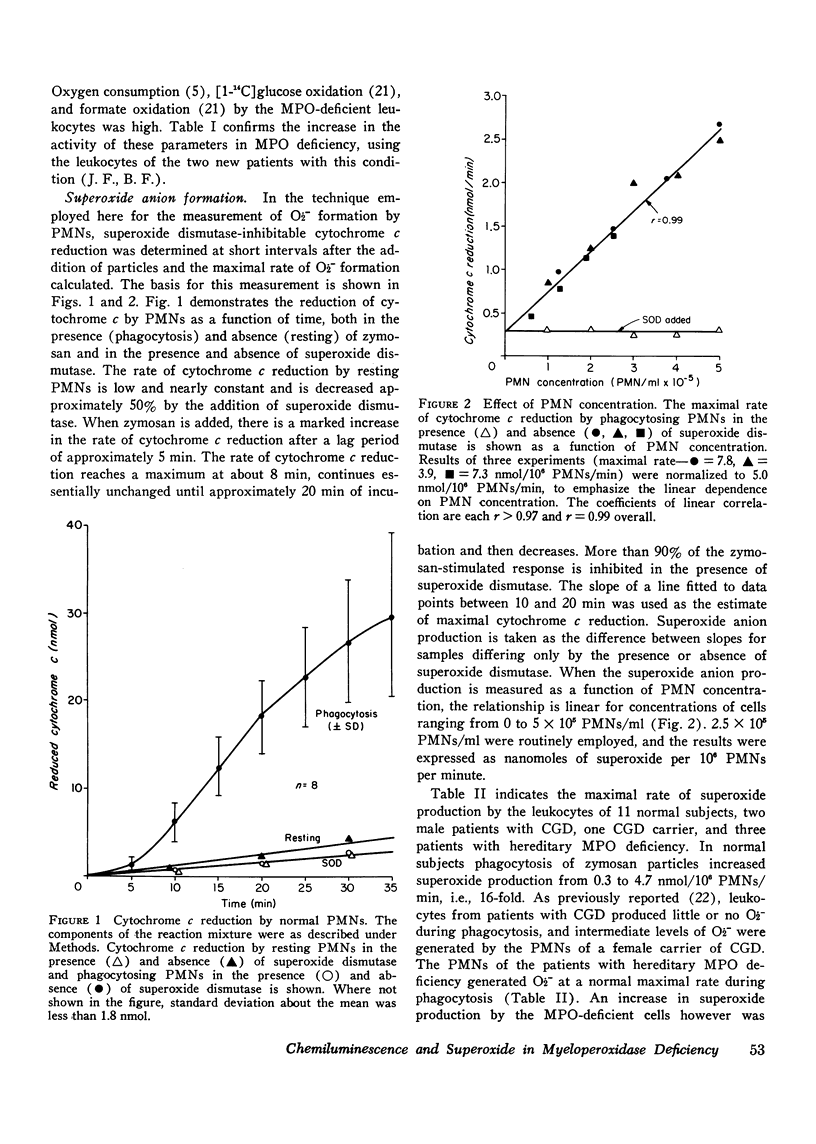
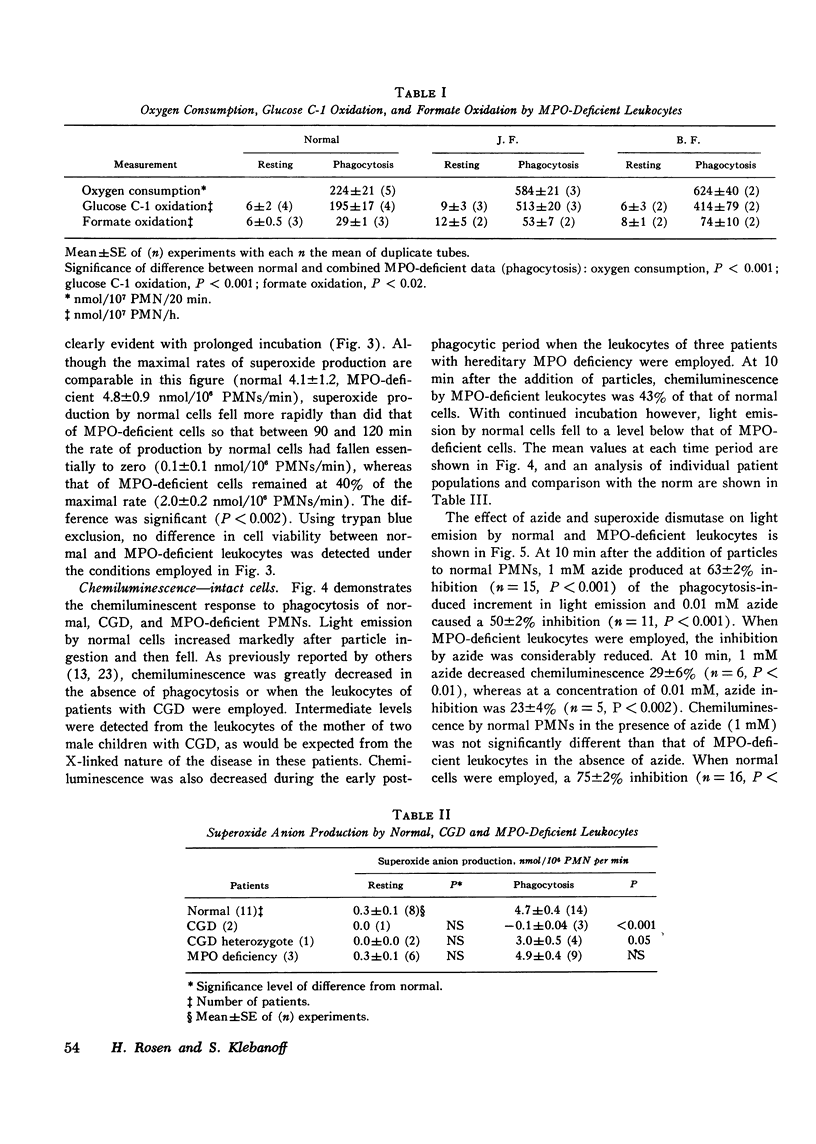
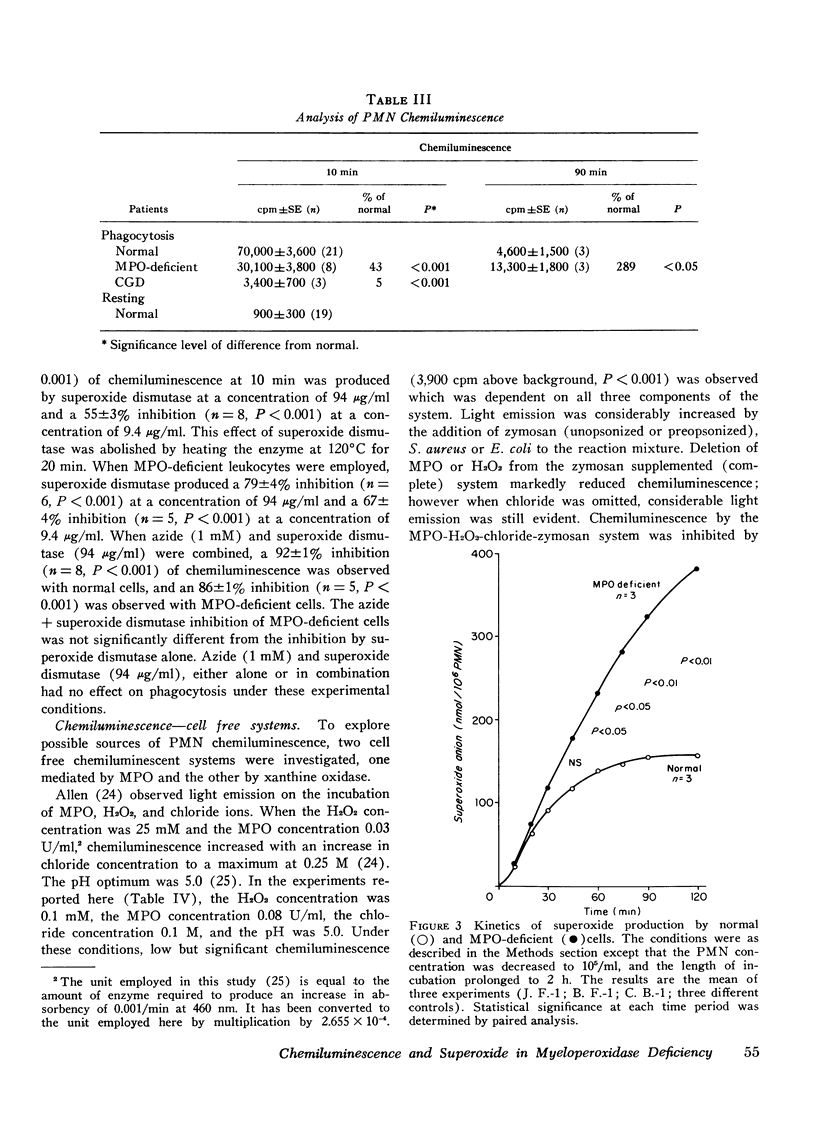
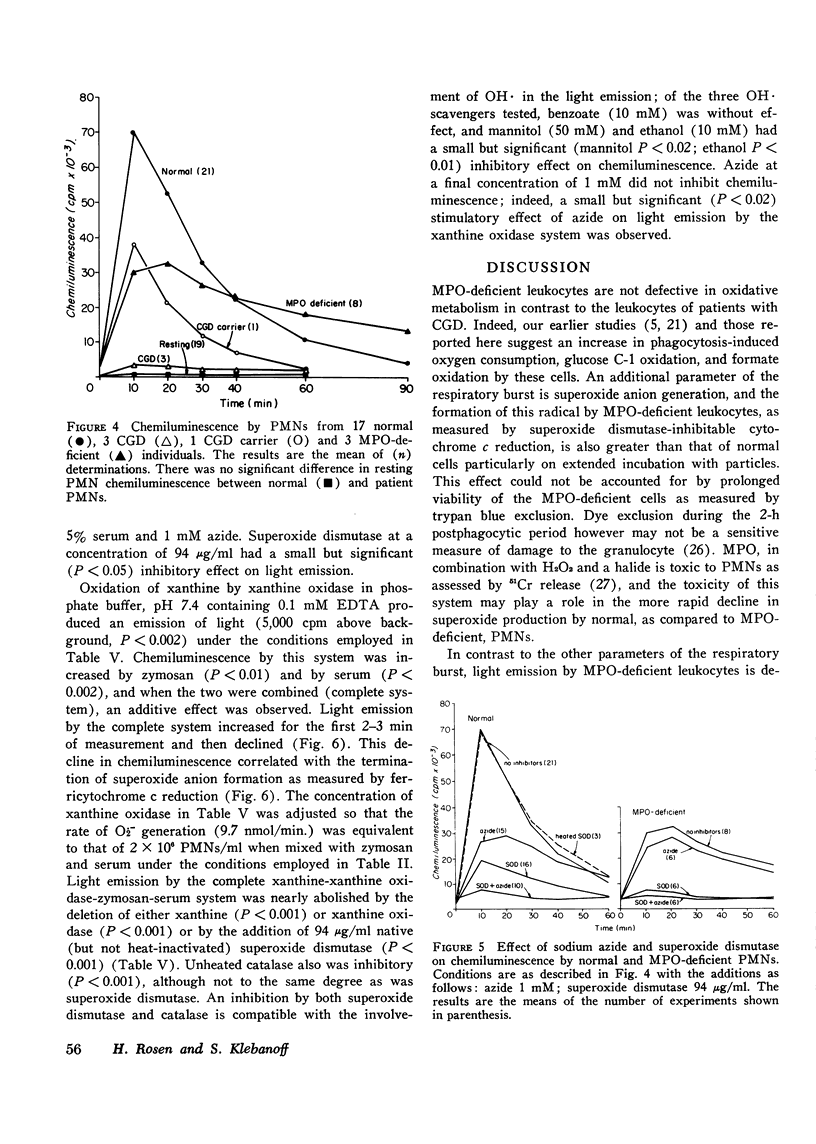
The role of superoxide anion- and myeloperoxidase-dependent reactions in the light emission by phagocytosing polymorphonuclear leukocytes has been investigated using leukocytes that lack myeloperoxidase, inhibitors (azide, superoxide dismutase), and model systems. Our earlier finding that oxygen consumption, glucose C-1 oxidation, and formate oxidation are greater in polymorphonuclear leukocytes that lack myeloperoxidase than in normal cells during phagocytosis has been confirmed with leukocytes from two newly described myeloperoxidase-deficient siblings. Although the maximal rate of superoxide anion production by myeloperoxidase-deficient leukocytes is not significantly different from that of normal cells, superoxide production falls off less rapidly with time so that with prolonged incubation, it is greater in myeloperoxidase-deficient than in normal cells. Chemiluminescence by myeloperoxidase-deficient leukocytes during the early postphagocytic period however is decreased. Light emission by normal leukocytes is strongly inhibited by both superoxide dismutase and azide, whereas that of myeloperoxidase-deficient leukocytes, while still strongly inhibited by superoxide dismutase is considerably less sensitive to azide. Zymosan, the phagocytic particle employed in the intact cell system, considerably increased the chemiluminescence of a cell-free superoxide-H2O2 generating system (xanthine-xanthine oxidase) and a system containing myeloperoxidase, H2O2, and chloride. Light emission by the xanthine oxidase model system is strongly inhibited by superoxide dismutase and is not inhibited by azide, whereas the myeloperoxidase-dependent model system is strongly inhibited by azide but only slightly inhibited by superoxide dismutase. These findings suggest that light emission by phagocytosing polymorphonuclear leukocytes is dependent on both myeloperoxidase-catalyzed reactions and the superoxide anion, and involves in part the excitation of the ingested particle. These studies are discussed in relation to the role of the superoxide anion and chemiluminescence in the microbicidal activity of the polymorphonuclear leukocyte.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agro' A. F., Giovagnoli C., De Sole P., Calabrese L., Rotilio G., Mondovi' B. Erythrocuprein and singlet oxygen. FEBS Lett. 1972 Mar 15;21(2):183–185. doi: 10.1016/0014-5793(72)80132-7. [DOI] [PubMed] [Google Scholar]
- Allen R. C. Halide dependence of the myeloperoxidase-mediated antimicrobial system of the polymorphonuclear leukocyte in the phenomenon of electronic excitation. Biochem Biophys Res Commun. 1975 Apr 7;63(3):675–683. doi: 10.1016/s0006-291x(75)80437-2. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Allen R. C. The role of PH in the chemiluminescent response of the myeloperoxidase-halide-HOOH antimicrobial system. Biochem Biophys Res Commun. 1975 Apr 7;63(3):684–691. doi: 10.1016/s0006-291x(75)80438-4. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Yevich S. J., Orth R. W., Steele R. H. The superoxide anion and singlet molecular oxygen: their role in the microbicidal activity of the polymorphonuclear leukocyte. Biochem Biophys Res Commun. 1974 Oct 8;60(3):909–917. doi: 10.1016/0006-291x(74)90401-x. [DOI] [PubMed] [Google Scholar]
- Arneson R. M. Substrate-induced chemiluminescence of xanthine oxidase and aldehyde oxidase. Arch Biochem Biophys. 1970 Feb;136(2):352–360. doi: 10.1016/0003-9861(70)90205-5. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J., Einstein A. B., Fefer A. Peroxidase-H2O2-halide system: Cytotoxic effect on mammalian tumor cells. Blood. 1975 Feb;45(2):161–170. [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Biological defense mechanisms. The effect of bacteria and serum on superoxide production by granulocytes. J Clin Invest. 1974 Jun;53(6):1662–1672. doi: 10.1172/JCI107717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Whitten D. M., Babior B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974 Mar 14;290(11):593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):35–97. doi: 10.1002/9780470122860.ch2. [DOI] [PubMed] [Google Scholar]
- GREENLEE L., FRIDOVICH I., HANDLER P. Chemiluminescence induced by operation of iron-flavoproteins. Biochemistry. 1962 Sep;1:779–783. doi: 10.1021/bi00911a008. [DOI] [PubMed] [Google Scholar]
- Goda K., Kimura T., Thayer A. L., Kees K., Schaap A. P. Singlet molecular oxygen in biological systems: non-quenching of singlet oxygen-mediated chemiluminescence by superoxide dismutase. Biochem Biophys Res Commun. 1974 Jun 4;58(3):660–666. doi: 10.1016/s0006-291x(74)80469-9. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Yost F. J., Jr, Fridovich I. Superoxide dismutases of Escherichia coli: intracellular localization and functions. J Bacteriol. 1973 Sep;115(3):987–991. doi: 10.1128/jb.115.3.987-991.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson E. K., Fridovich I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: chemiluminescence and peroxidation. Biochemistry. 1975 Dec 2;14(24):5299–5303. doi: 10.1021/bi00695a011. [DOI] [PubMed] [Google Scholar]
- Hodgson E. K., Fridovich I. The production of superoxide radical during the decomposition of potassium peroxochromate(V). Biochemistry. 1974 Aug 27;13(18):3811–3815. doi: 10.1021/bi00715a030. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg E. W., 3rd, Fridovich I. Superoxide, hydrogen peroxide, and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J Biol Chem. 1975 Nov 25;250(22):8812–8817. [PubMed] [Google Scholar]
- Khan A. U. Singlet molecular oxygen from superoxide anion and sensitized fluorescence of organic molecules. Science. 1970 Apr 24;168(3930):476–477. doi: 10.1126/science.168.3930.476. [DOI] [PubMed] [Google Scholar]
- King M. M., Lai E. K., McCay P. B. Singlet oxygen production associated with enzyme-catalyzed lipid peroxidation in liver microsomes. J Biol Chem. 1975 Aug 25;250(16):6496–6502. [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Klebanoff S. J., Hamon C. B. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc. 1972 Aug;12(2):170–196. [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase: contribution to the microbicidal activity of intact leukocytes. Science. 1970 Sep 11;169(3950):1095–1097. doi: 10.1126/science.169.3950.1095. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Pincus S. H. Hydrogen peroxide utilization in myeloperoxidase-deficient leukocytes: a possible microbicidal control mechanism. J Clin Invest. 1971 Oct;50(10):2226–2229. doi: 10.1172/JCI106718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinsky N. I. Singlet excited oxygen as a mediator of the antibacterial action of leukocytes. Science. 1974 Oct 25;186(4161):363–365. doi: 10.1126/science.186.4161.363. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Hanifin J., Cline M. J. Defective bactericidal activity in myeloperoxidase-deficient human neutrophils. Nature. 1969 Jul 5;223(5201):78–79. doi: 10.1038/223078a0. [DOI] [PubMed] [Google Scholar]
- MASSEY V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta. 1959 Jul;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- Michelson A. M. Is singlet oxygen a substrate for superoxide dismutase? No. FEBS Lett. 1974 Aug 15;44(1):97–100. doi: 10.1016/0014-5793(74)80314-5. [DOI] [PubMed] [Google Scholar]
- Nilsson R., Kearns D. R. Role of singlet oxygen in some chemiluminescence and enzyme oxidation reactions. J Phys Chem. 1974 Aug 15;78(17):1681–1683. doi: 10.1021/j100610a001. [DOI] [PubMed] [Google Scholar]
- Paschen W., Weser U. Letter: Singlet oxygen decontaminating activity of erythrocuprein (superoxide dismutase). Biochim Biophys Acta. 1973 Nov 15;327(1):217–222. doi: 10.1016/0005-2744(73)90120-4. [DOI] [PubMed] [Google Scholar]
- Paschen W., Weser U. Problems concerning the biochemical action of superoxide dismutase (erythrocuprein). Hoppe Seylers Z Physiol Chem. 1975 Jun;356(6):727–737. doi: 10.1515/bchm2.1975.356.s1.727. [DOI] [PubMed] [Google Scholar]
- Salin M. L., McCord J. M. Free radicals and inflammation. Protection of phagocytosine leukocytes by superoxide dismutase. J Clin Invest. 1975 Nov;56(5):1319–1323. doi: 10.1172/JCI108208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjernholm R. L., Allen R. C., Steele R. H., Waring W. W., Harris J. A. Impaired chemiluminescence during phagocytosis of opsonized bacteria. Infect Immun. 1973 Feb;7(2):313–314. doi: 10.1128/iai.7.2.313-314.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb L. S., Keele B. B., Jr, Johnston R. B., Jr Inhibition of phagocytosis-associated chemiluminescence by superoxide dismutase. Infect Immun. 1974 Jun;9(6):1051–1056. doi: 10.1128/iai.9.6.1051-1056.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weening R. S., Wever R., Roos D. Quantitative aspects of the production of superoxide radicals by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):245–252. [PubMed] [Google Scholar]
- Wilson T., Hastings J. W. Chemical and biological aspects of singlet excited molecular oxygen. Photophysiology. 1970;5:49–95. [PubMed] [Google Scholar]
- Yost F. J., Jr, Fridovich I. Superoxide radicals and phagocytosis. Arch Biochem Biophys. 1974 Apr 2;161(2):395–401. doi: 10.1016/0003-9861(74)90320-8. [DOI] [PubMed] [Google Scholar]



