Abstract
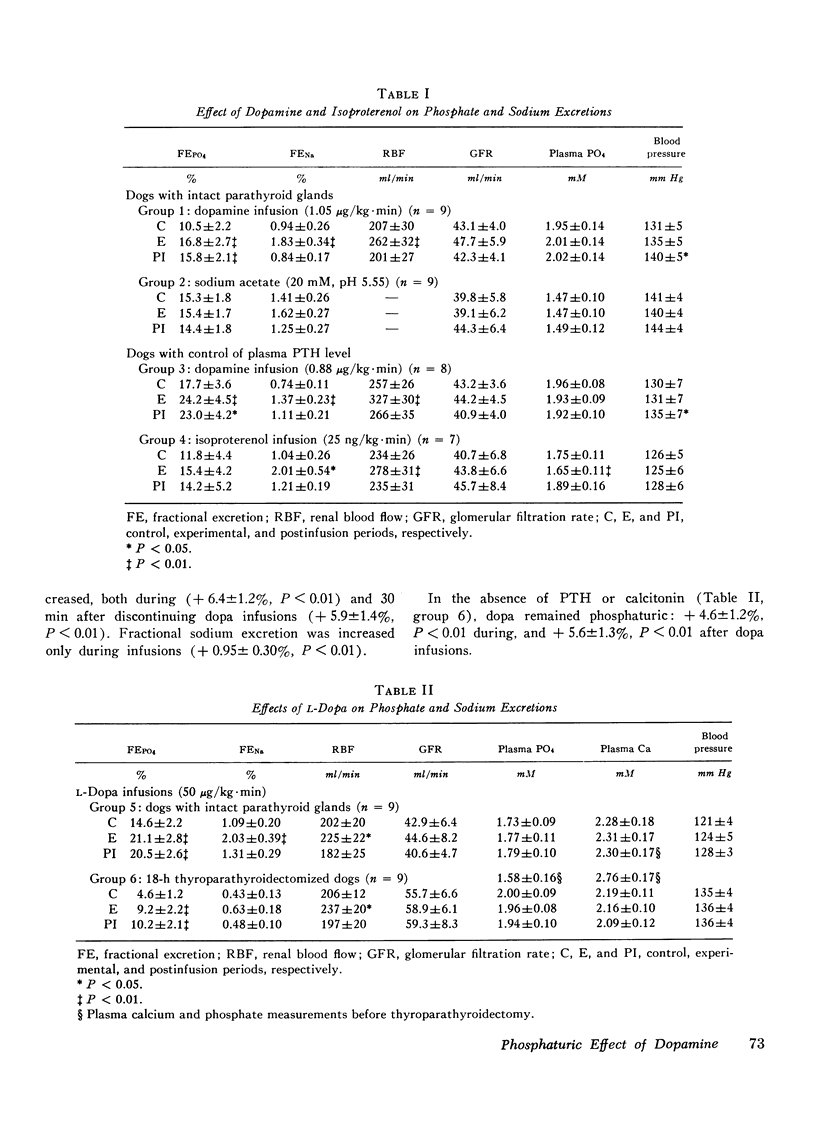
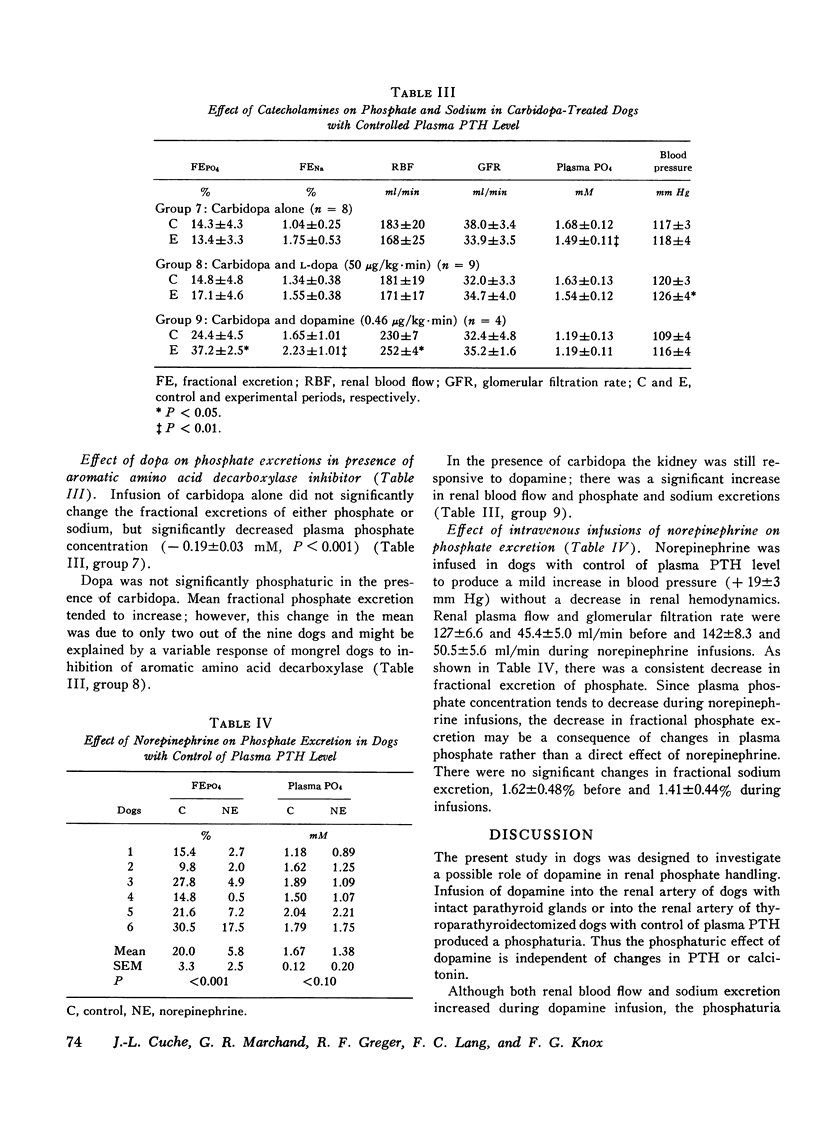
A possible role for dopamine in phosphate handling by the dog kidney was investigated by intrarenal artery infusions of dopamine. Dopamine increased fractional phosphate excretion both in the presence and absence of control of parathyroid hormone and calcitonin. In addition, dopamine increased both renal blood flow and sodium excretion, however, the phosphaturia was independent of these changes; since 30 min after completion of dopamine infusion, renal blood flow and sodium excretion returned to control levels and phosphate excretion remained elevated. For comparison, the vasodilator isoproterenol increased renal blood flow and sodium excretion without a significant change in fractional phosphate excretion. Thus, the phosphaturic effect of dopamine is probably independent of its vasodilator effect. The phosphaturic effect of dopamine could not be accounted for by subsequent conversion to norepinephrine, since norepinephrine was antiphosphaturic in the dog. The effect of endogenous dopamine on renal phosphate excretion was investigated by intrarenal infusion of the precursor dopa. Dopa was phosphaturic both in the presence and absence of parathyroid hormone and calcitonin. In dogs pretreated with carbidopa, which blocks conversion of dopa to dopamine, dopa was no longer phosphaturic, although the kidney remained responsive to dopamine. It is postulated that dopamine may play a role in the intrarenal regulation of phosphate excretion.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLASCHKO H., CHRUSCIEL T. L. The decarboxylation of amino acids related to tyrosine and their awakening action in reserpine-treated mice. J Physiol. 1960 May;151:272–284. doi: 10.1113/jphysiol.1960.sp006437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLSSON A. EVIDENCE FOR A ROLE OF DOPAMINE IN EXTRAPYRAMIDAL FUNCTIONS. Acta Neuroveg (Wien) 1964 Oct 2;26:484–493. doi: 10.1007/BF01252144. [DOI] [PubMed] [Google Scholar]
- DAVIS V. E., AWAPARA J. A method for the determination of some amino acid decarboxylases. J Biol Chem. 1960 Jan;235:124–127. [PubMed] [Google Scholar]
- FUHR J., KACZMARCZYK J., KRUTTGEN C. D. Eine einfache colorimetrische Methode zur Inulinbestimmung für Nieren-Clearance-Untersuchungen bei Stoffwechselgesunden und Diabetikern. Klin Wochenschr. 1955 Aug 1;33(29-30):729–730. doi: 10.1007/BF01473295. [DOI] [PubMed] [Google Scholar]
- Fischer J. A., Blum J. W., Binswanger U. Acute parathyroid hormone response to epinephrine in vivo. J Clin Invest. 1973 Oct;52(10):2434–2440. doi: 10.1172/JCI107434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg L. I. Cardiovascular and renal actions of dopamine: potential clinical applications. Pharmacol Rev. 1972 Mar;24(1):1–29. [PubMed] [Google Scholar]
- Kukreja S. C., Hargis G. K., Bowser E. N., Henderson W. J., Fisherman E. W., Williams G. A. Role of adrenergic stimuli in parathyroid hormone secretion in man. J Clin Endocrinol Metab. 1975 Mar;40(3):478–481. doi: 10.1210/jcem-40-3-478. [DOI] [PubMed] [Google Scholar]
- LOVENBERG W., WEISSBACH H., UDENFRIEND S. Aromatic L-amino acid decarboxylase. J Biol Chem. 1962 Jan;237:89–93. [PubMed] [Google Scholar]
- MOREY E. R., KENNY A. D. EFFECTS OF CATECHOLAMINES ON URINARY CALCIUM AND PHOSPHORUS IN INTACT AND PARATHYROIDECTOMIZED RATS. Endocrinology. 1964 Jul;75:78–85. doi: 10.1210/endo-75-1-78. [DOI] [PubMed] [Google Scholar]
- MURPHY G. F., SOURKES T. L. The action of antidecarboxylases on the conversion of 3,4-dihydroxyphenylalanine to dopamine in vivo. Arch Biochem Biophys. 1961 May;93:338–343. doi: 10.1016/0003-9861(61)90276-4. [DOI] [PubMed] [Google Scholar]
- Osborne M. W., Wenger J. J., Willems W. The cardiovascular pharmacology of L(--)-dopa: peripheral and central effects. J Pharmacol Exp Ther. 1971 Sep;178(3):517–528. [PubMed] [Google Scholar]
- Romero J. A., Lytle L. D., Ordonez L. A., Wurtman R. J. Effects of L-dopa administration of the concentrations of dopa, dopamine and norepinephrine in various rat tissues. J Pharmacol Exp Ther. 1973 Jan;184(1):67–72. [PubMed] [Google Scholar]
- Schneider E. G., Strandhoy J. W., Willis L. R., Knox F. G. Relationship between proximal sodium reabsorption and excretion of calcium, magnesium and phosphate. Kidney Int. 1973 Dec;4(6):369–376. doi: 10.1038/ki.1973.133. [DOI] [PubMed] [Google Scholar]
- Vickers S., Stuart E. K., Bianchine J. R., Hucker H. B., Jaffe M. E., Rhodes R. E., Vandenheuvel W. J. Metabolism of carbidopa (1-(-)-alpha-hydrazino-3,4-dihydroxy-alpha-methylhydrocinnamic acid monohydrate), an aromatic amino acid decarboxylase inhibitor, in the rat, rhesus monkey, and man. Drug Metab Dispos. 1974 Jan-Feb;2(1):9–22. [PubMed] [Google Scholar]
- WEGMANN A. DETERMINATION OF 3-HYDROXYTYRAMINE AND DOPA IN VARIOUS ORGANS OF DOG AFTER DOPA-INFUSION. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1963 Nov 8;246:184–190. doi: 10.1007/BF00245004. [DOI] [PubMed] [Google Scholar]
- Watanabe A. M., Parks L. C., Kopin I. J. Modification of the cardiovascular effects of L-dopa by decarboxylase inhibitors. J Clin Invest. 1971 Jun;50(6):1322–1328. doi: 10.1172/JCI106611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. S. Improved method for the automatic determination of serum inorganic phosphate. J Clin Pathol. 1966 Jul;19(4):397–399. doi: 10.1136/jcp.19.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]


