Abstract
Idiopathic pulmonary fibrosis (IPF) is a complex disease with poorly understood etiology. Previously, we reported upregulation of matrix metalloproteinase 7 (MMP7) in both lung and peripheral blood of IPF patients. Here we report evidence for genetic correlation of plasma levels and promoter polymorphisms (rs11568818 and rs11568819) of MMP7 in a well-characterized IPF cohort. Both the AA genotype of rs11568818 and the CT genotype of rs11568819 were found to be significantly associated with higher MMP7 plasma levels. These associations were observed only in IPF patients and not in healthy controls. The G-to-A transition of rs11568818 resulted in a novel binding site for the forkhead box A2 (FOXA2) transcription factor, a key regulator of embryonic lung development and proper function of the mature lung. In vitro, this transition led to increased sensitivity of the MMP7 promoter to FOXA2. In IPF lungs, FOXA2 was localized in the nucleus of epithelial cells that expressed MMP7 in the cytoplasm. These results suggest that increased sensitivity of the polymorphic MMP7 promoter to FOXA2 provides one of the genetic bases for the upregulation of MMP7 in IPF.
Keywords: biomarker, single nucleotide polymorphism, outcome, genetic basis, forkhead box A2
idiopathic pulmonary fibrosis (IPF) is a devastating disease characterized by expansion of fibroblasts/myofibroblasts and by excessive accumulation of extracellular matrix resulting in progressive and severe distortion of the pulmonary architecture (1, 22). Although the etiology of IPF is unknown, a number of environmental exposures have been associated with elevated risk for IPF development (3, 4, 13, 24, 38). In particular, chronic exposure to cigarette smoke is considered a major risk factor for IPF (4, 24, 38).
Matrix metalloproteinase 7 (MMP7) is produced by the epithelium of lung, liver, and breast and is involved in cancer invasion, epithelial response to injury, tissue repair, and disease outcomes in cancers (28, 29, 50, 51). Lung gene expression microarray studies (26, 41, 58) have consistently identified MMP7 to be one of the most upregulated genes in IPF lungs compared with normal lungs and to lung tissues of other interstitial lung diseases. MMP7 was strongly expressed in alveolar and bronchiolar epithelial cells of IPF lungs, and MMP7(−/−) null mice were relatively protected from bleomycin-induced lung fibrosis, suggesting that it is an important regulator of pulmonary fibrosis (58). Upregulation of MMP7 was also observed in bronchoalveolar lavage fluid and peripheral blood of IPF subjects (14, 37). Peripheral blood upregulation of MMP7 was detected only in IPF patients and not in patients with either sarcoidosis or chronic obstructive pulmonary disease (37). However, very little is known about the molecular basis of the MMP7 upregulation in both lung tissues and peripheral blood.
The promoter of MMP7 has been extensively studied. Two single nucleotide polymorphisms (SNPs) were identified in the promoter with transitions of A-to-G at −181 (rs11568818) and C-to-T at −153 (rs11568819) relative to the transcription start site (21). These SNPs are associated with decreased coronary arterial dimensions among hypercholesterolemic patients and with disease progression in patients with colorectal carcinoma and breast cancer (5, 16, 21). With the upregulation of MMP7 in IPF and the presence of promoter variations, we hypothesized that the promoter variations mediate the increased expression of MMP7 in human IPF. To address this hypothesis we correlated the promoter variants and plasma levels of MMP7 in a well-characterized IPF cohort. We further analyzed the functional significance of these variants in MMP7 promoter activity and provided evidence for allele-specific regulation of MMP7 by FOXA2.
METHODS
Patient population.
This study was approved by the Institutional Review Board for Human Subject Research at the University of Pittsburgh. One-hundred thirty-six IPF patients from the Simmons Center for Interstitial Lung Diseases at the University of Pittsburgh Medical Center were included. Diagnosis of IPF was supported by clinical, physiologic, and high-resolution computed tomography and was corroborated by a typical surgical biopsy in 80 of the 136 patients (58.8%) that showed typical findings of usual interstitial pneumonia (22). The patients fulfilled the criteria of the American Thoracic Society and European Respiratory Society for the diagnosis of IPF (11). Patients with known causes of interstitial lung disease were excluded. The controls consisted of 166 unrelated healthy subjects with no self-reported lung diseases randomly recruited from the University of Pittsburgh Medical Center. To control for population stratification, only US non-Hispanic white patients were included for this study.
Analysis of MMP7 levels in peripheral blood.
After informed consent, peripheral blood samples were obtained from study subjects using standard venopuncture and BD vacutainer tubes. Citrated plasma samples were collected within 1 h and stored at −80°C immediately. Plasma levels of MMP7 were analyzed using Fluorokine MAP Human MMP kit or ELISA (R&D Systems, Minneapolis, MN).
Genotyping of the MMP7 promoter polymorphisms.
The rs11568818 and rs11568819 SNPs were genotyped using PCR-based restriction fragment length polymorphism analysis (21) or Taqman SNP analysis using primer/probe sets-specific for rs11568818 (C_27852953_10) and rs11568819 (C_32018637_10) and 7900 HT DNA analyzer (Applied Biosystems, Foster City, CA).
EMSA.
Nuclear proteins were prepared from colorectal adenocarcinoma cells, HT-29. For EMSA, 5 μg nuclear protein were incubated with 32P (5′-end)-labeled double-stranded oligonucleotides (10 fmol), 5′-AAAATCCTTTGAAAGAC(A/G)AATACATTGTGTG-3′ for rs11568818 (8). DNA-protein complexes were separated by PAGE and detected by autoradiography.
Chromatin immunoprecipitation assay.
A549 cells were cultured under normal condition to ∼80–90% confluence. Cells were cross-linked with 1% formaldehyde for 10 min and harvested for fragmentation using sonication. Chromatin immunoprecipitation (ChIP) assay was performed using a goat polyclonal anti-FOXA2 antibody and goat normal IgG (Santa Cruz). The precipitated DNA fragments were used for specific amplification of MMP7 promoter (−300 to +50) using PCR and a primer pair listed below.
Generation of the MMP7 promoter luciferase constructs.
A 350-bp DNA sequence (−300 to +50) of MMP7 haplotype-specific promoter was amplified from genomic DNA by PCR using primers 5′-GGTACCATAATGTCCTGAATG-3′ and 5′-TAGCTGCCGTCCAGAGA-3′ and cloned into the pGL2basic vector (Promega, Madison, WI). Three of the four possible haplotypes between the rs11568818 and rs11568819, AC, GC, and GT, were cloned since the AT haplotype was not observed in the study population. The haplotype AC-, GC-, and GT-specific luciferase clones were confirmed by DNA sequencing and designated as MMP7-AC, MMP7-GC, and MMP7-GT, respectively.
Generation of the rs11568818 allele-specific luciferase constructs.
Three concatemers of a 30-bp oligonucleotide consisting of 23-bp-specific for rs11568818 and 7 nonspecific linker sequences [5′-CTTTGAAAGAC(A/G)AATACATTTGcgtatct-3′] were cloned into the pGL2-TATA. Since there were putative FOXA binding sites upstream of the luciferase gene in the pGL2basic vector, we have replaced the upstream sequence with a minimum TATA sequence to generate the pGL2-TATA. The rs11568818A- and rs11568818G-specific clones were confirmed by DNA sequencing.
Primary human airway epithelial cells.
Primary human airway epithelial cells were cultured from excess pathologic tissue after lung transplantation and organ donation, under a protocol approved by the University of Pittsburgh Institutional Review Board, as previously described (12). The cells were isolated and cultured using the method described previously (31) with the following modifications. For the in vitro luciferase assays, cells were maintained in bronchial epithelial growth medium (Lonza, Basel, Switzerland) and plated onto Type VI human placental collagen-treated tissue culture flasks. Cells in 80–90% confluence were used for subsequent seeding and in vitro luciferase assays.
Cell culture and in vitro luciferase assays.
The A549, HT-29, and H292 cells were cultured, respectively, in Ham's F-12K, DMEM, and RPMI-1640 media supplemented with 10% FBS and penicillin/streptomycin (Invitrogen, Carlsbad, CA). The cells were seeded at 1–2 × 105 cells/well in 24-well culture plates 16 h before the transfection with Lipofectamine 2000 (Invitrogen). Cotransfection assays were performed at different ratios of the luciferase constructs and the plasmids expressing the rat FOXA2 gene under the control of CMV promoter (a generous gift from Dr. Markus Stoffel, Rockefeller University, New York). The cells were harvested at 48 h posttransfection and analyzed using luciferase assay system (Promega). Plasmids pGL2basic and pGL2control were used for negative and positive controls, respectively.
Western blot analysis.
Lung tissues were obtained from excess pathologic tissue after lung transplantation and organ donation as described above (12). Twenty micrograms of total protein isolated from parenchyma of the lung tissues were resolved by SDS-PAGE, transferred to nitrocellulose membrane, and analyzed using standard method. Rabbit polyclonal anti-FOXA2 (Aviva Systems Biology, San Diego, CA) and mouse monoclonal anti-β-Actin (Sigma) antibodies were used. Semiquantification of the Western blots was performed using ImageJ program (http://rsbweb.nih.gov/ij/index.html). The expression level of FOXA2 in the lung tissue relative to β-actin was computed.
Immunoflorescence analysis.
Frozen sections of lung tissues from IPF patients were analyzed for protein expression as described previously (9, 32). Mouse anti-MMP7 (Millipore, Billerica, MA), rabbit anti-β-catenin (Santa Cruz), and goat anti-FOXA2 (Santa Cruz) antibodies were used.
In silico functional analysis.
The Transcription Element Search Software (TESS: http://www.cbil.upenn.edu/tess) was used to assess potentially functional significance of rs11568818.
Statistical analysis.
We calculated site-specific allele frequencies by gene counting and tested for departures from Hardy-Weinberg equilibrium using χ2-testing in SAS Genetics version 9.1. In the case-control association study, allele and genotypes of single SNPs as well as haplotypes were tested for association with IPF. To conduct the haplotype analysis, we used PHASE (version 2.1; Ref. 44) to infer haplotypes from the unphased SNPs and coded the resulting haplotypes according to the number of copies present for each participant. Case-control analysis of overall haplotype distribution was performed using 2 × m contingency tables with a value of P < 0.05 considered to indicate statistical significance. Linear regression was used to test for association between genotypes and plasma MMP7 levels. Analysis of covariance was used to assess dominant and recessive models. All models were adjusted for age, gender, and smoking status and performed using SAS version 9.1 (SAS Institute, Cary, NC) and the R environment for data analysis and graphics (19).
Differences in haplotype-specific luciferase reporter activities of basal and FOXA2 coexpressed cells were analyzed by linear regression with FOXA2 dose as an independent variable, zero indicating the basal state, and P < 0.05 considered statistically significant. Paired t-test was used to analyze the differences in rs11568818 allele-specific luciferase reporter construct activities of basal and FOXA2-coexpressed cells. The Wilcoxon rank sum test was used for the comparison of FOXA2 expression level in IPF and normal lung. A P < 0.05 was considered statistically significant.
RESULTS
Case-control analysis of the MMP7 promoter variants and IPF.
The demographic and clinical characteristics of the IPF cohort are summarized in Table 1. Both rs11568818 and rs11568819 met the expectations of Hardy-Weinberg equilibrium (p > 0.1). Linkage disequilibrium between these two SNPs was analyzed (r2 = 0.062; D′ = 0.916). Three of four possible haplotypes were observed: AC, GC, and GT. In a case-control association analysis, we did not detect any significant association with IPF for allele, genotype, or haplotype of these two variants (Table 2).
Table 1.
Demographic and clinical characteristics of the IPF cohort
| Variable | IPF (n = 136) | Control (n = 166) |
|---|---|---|
| Age | ||
| Year | 67.3 ± 8.2 | 48.7 ± 16.1 |
| Gender | ||
| Male, % | 97 (71.3%) | 71 (42.8%) |
| Smoking | ||
| Current, % | 2 (1.5%) | 21 (12.7%) |
| Former, % | 98 (72.0%) | 47 (28.3%) |
| Never, % | 36 (26.5%) | 98 (59.0%) |
| FVC, %predicted* | 61.7 ± 19.6 | NA |
| DLCO, % predicted† | 45.0 ± 17.3 | NA |
IPF, Idiopathic pulmonary fibrosis; FVC, forced vital capacity; DLCO, diffusing capacity for carbon monoxide.
Based on 117 subjects and measurements within 4 mo of blood draw date.
Based on 105 subjects with available measurements within 4 mo of blood draw date.
Table 2.
Case-control analysis of MMP7 promoter variants in IPF
| Variable | IPF (n = 136) | Control (n = 166) |
|---|---|---|
| rs11568818 | ||
| Minor allele (G) | 108 (39.7%) | 141 (42.5%) |
| Allelic Association (P value) | 0.4923 | |
| Genotype | ||
| AA | 44 (32.4%) | 53 (31.9%) |
| AG | 76 (55.9%) | 85 (51.2%) |
| GG | 16 (11.8%) | 28 (16.9%) |
| Genotype Association (P value) | 0.4389 | |
| rs11568819 | ||
| Minor allele (T) | 11 (4.0%) | 18 (5.4%) |
| Allelic Association (P value) | 0.4308 | |
| Genotype | ||
| CC | 125 (91.9%) | 148 (89.2%) |
| CT | 11 (8.1%) | 18 (10.8%) |
| TT | 0 (0%) | 0 (0%) |
| Genotype Association (P value)* | 0.4406 | |
| Haplotype | ||
| AC | 164 (60.3%) | 191 (57.6%) |
| GC | 97 (35.7%) | 123 (37.0%) |
| GT | 11 (4.0%) | 18 (5.4%) |
| Haplotype Association (P value) | 0.6505 | |
| AC/AC | 44 (32.4%) | 53 (31.9%) |
| AC/GC | 69 (50.7%) | 76 (45.8%) |
| AC/GT | 7 (5.2%) | 19 (11.5%) |
| GC/GC | 12 (8.8%) | 9 (5.4%) |
| GC/GT | 4 (2.9%) | 9 (5.4%) |
| Overall Haplotype Association (P value)† | 0.1746 | |
MMP7, matrix metalloproteinase 7.
Association was performed based on CC and CT using Fisher exact test.
Based on χ2-analysis with four degrees of freedom.
Association of promoter variants with plasma levels of MMP7 in IPF.
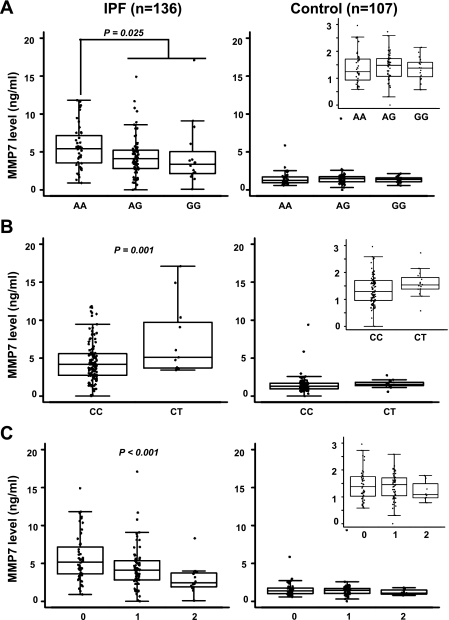
We identified significant associations of MMP7 plasma levels with both genotypes and haplotypes of rs11568818 and rs11568819 in patients with IPF (Fig. 1). All of these associations remained statistically significant after adjustment for age, gender, and smoking status (Table 3). Since the distributions of haplotypes AC and GT were 100% concordant with rs11568818 and rs11568819 genotypes, associations between these two haplotypes and plasma MMP7 levels were detected at the same significance levels as for the genotypes. Additionally, the GC haplotype exhibited a significant dose-dependent correlation with MMP7 plasma levels (Fig. 1C and Table 3). No significant association was detected between the promoter variants and plasma levels of MMP7 in the control population based on 107 subjects with available plasma samples (Table 3).
Fig. 1.
Correlations of plasma level and promoter variant of matrix metalloproteinase 7 (MMP7) in idiopathic pulmonary fibrosis (IPF) and controls. MMP7 plasma levels were analyzed in a subset of IPF (n = 136) and controls (n = 107) with available plasma samples. Correlations of MMP7 plasma level with rs11568818 genotype (A), rs11568819 genotype (B), and copy number of GC haplotype (C) were analyzed using linear regression analysis. Genotypes of each single nucleotide polymorphism (SNP) and the copy numbers of the GC haplotype are shown on the x-axis. Significant P values are labeled (P < 0.05). Same scale for MMP7 levels is used for both IPF and controls. Insets: expanded scale for the lower plasma concentrations of MMP7 is included for each of the analyses associated with the control group.
Table 3.
Association of promoter variants with plasma levels of MMP7
| Variants | MMP7 Plasma Levels (SE), ng/ml | P Value* | ||
|---|---|---|---|---|
| IPF | ||||
| SNP | ||||
| rs11568818 | AA | AG | GG | 0.025 (0.022)§ |
| (n = 44) | (n = 76) | (n = 16) | ||
| 5.62 (0.45) | 4.35 (0.34) | 4.41 (0.74) | ||
| rs11568819 | CC | CT | 0.001 (0.001)† | |
| (n = 125) | (n = 11) | |||
| 4.52 (0.26) | 7.57 (0.87) | |||
| Haplotype‡ | 0 | 1 | 2 | |
| AC | (n = 16) | (n = 76) | (n = 44) | 0.025 (0.022)§ |
| 4.41 (0.74) | 4.35 (0.34) | 5.62 (0.45) | ||
| GC | (n = 51) | (n = 73) | (n = 12) | <0.001 (<0.001) |
| 5.75 (0.40) | 4.40 (0.34) | 2.81 (0.84) | ||
| GT | (n = 125) | (n = 11) | 0.001 (0.001)† | |
| 4.52 (0.26) | 7.57 (0.87) | |||
| Control | ||||
| SNP | ||||
| rs11568818 | AA | AG | GG | 0.934 (0.961)§ |
| (n = 17) | (n = 53) | (n = 370) | ||
| 1.33 (0.24) | 1.56 (0.14) | 1.50 (0.17) | ||
| rs11568819 | CC) | CT | 0.721 (0.676)† | |
| (n = 91 | (n = 16) | |||
| 1.48 (0.11) | 1.61 (0.26) | |||
| Haplotype‡ | 0 | 1 | 2 | |
| AC | (n = 17) | (n = 53) | (n = 370) | 0.934 (0.961)§ |
| 1.33 (0.24) | 1.56 (0.14) | 1.50 (0.17) | ||
| GC | (n = 44) | (n = 55) | (n = 8) | 0.591 (0.516) |
| 1.52 (0.15) | 1.56 (0.14) | 1.04 (0.36) | ||
| GT | (n = 91) | (n = 16) | 0.721 (0.676)† | |
| 1.48 (0.11) | 1.61 (0.26) | |||
SNP, single nucleotide polymorphism.
P values with age, gender, and smoking status are presented in parentheses.
For correlation of CC and CT genotypes or zero and one copy of GT haplotype with MMP7 plasma levels.
Zero, one, and two copies of the specific haplotype.
P values comparing AA to AG + GG genotypes, or 0 to 1 or 2 copies of AC haplotype.
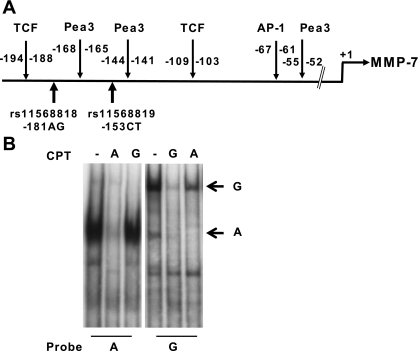
Allele-specific nuclear complex formation for the rs11568818.
To determine whether the promoter SNPs were functionally relevant to MMP7 gene expression, we first analyzed immediate sequences associated with these SNPs for nuclear protein binding. A different major nuclear protein complex was detected for each of the rs11568818 alleles using allele-specific probes (Fig. 2B, complex A and complex G) and nuclear extracts of HT-29 cells, a cell line constitutively over expressing MMP7 (7). Allele-specific probe change did not completely abolish the complexes specific for the reciprocal allele-specific probes, as both complexes were observed with either probe. Self- and cross-competition with excess 40 and 80 fold unlabeled probes confirmed the specificity of these two complexes. In addition, the A probe was much more efficient than G probe in allele-specific competition with reciprocal probes. For rs11568819, we detected multiple protein complexes with nuclear proteins of HT-29 cells in both allele-specific probes. However, no difference was detected between the two alleles (data not shown).
Fig. 2.
Allele-specific nuclear protein complex formation of the MMP7 promoter. A: schematic of the known transcription factor binding sites for the proximal MMP7 promoter. B: allele-specific nuclear protein complex formation of the rs11568818AG SNP. EMSA were performed using nuclear proteins isolated from HT-29 cells and oligonucleotides specific for the rs11568818A and G alleles. Competitors (CPT) of unlabeled probes at excess of 80-fold concentration compared with the labeled probes were included in the reactions for self competition and reciprocal competition of the 2 alleles. The nuclear protein complexes specific for A and G allele are labeled as A and G, respectively.
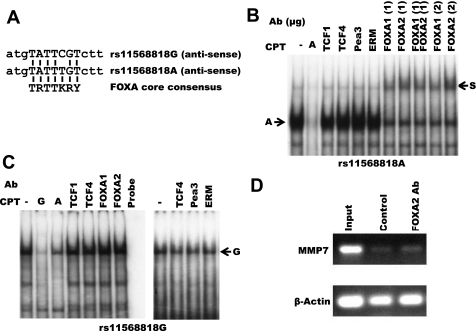
Allele-specific binding of the FOXA transcription factors to rs11568818.
Since the nuclear complex formation of rs11568819-specific probes was not allele specific in the in vitro system, we focused subsequent functional analysis on rs11568818 only. EMSA with antibodies specific for known transcription factors of MMP7, TCF1, TCF4, ERM, or PEA3, did not alter either the complex A or complex G formed with nuclear proteins of HT-29 cells (Fig. 3, B and C) (7, 10). Using TESS, we discovered that the G-to-A transition in the rs11568818 SNP created a novel binding site for the FOXA transcription factors (Fig. 3A). EMSA with antibody specific for FOXA1 and FOXA2 demonstrated mobility shift of complex A but not complex G (Fig. 3, B and C). However, the A-specific complex was not completely supershifted by either FOXA1 or FOXA2 alone or in combination. In addition, using ChIP with FOXA2-specific antibody, we detected enhanced binding of FOXA2 protein to the MMP7 promoter of A549 cells (Fig. 3D).
Fig. 3.
Allele-specific binding of the forkhead box A2 (FOXA2) transcription factors to the rs11568818 SNP and FOXA2 binding to the MMP7 promoter. A: alignment of the rs11568818 sequences with known FOXA binding core consensus. B and C: supershift analyses of the rs11568818A or G allele-specific nuclear protein complexes. Nuclear proteins of HT-29 cells and antibodies (Ab) specific for TCF1, TCF4, Pea3, ERM, FOXA1, and FOXA2 were used. For each antibody, 1 μg of antibody was used for each binding reaction prior to the electrophoretic analysis. FOXA1 and FOXA2 binding reactions were also carried out using 2 μg of antibodies and a combination of 1 μg FOXA1 and 1 μg FOXA2 for the A-specific oligonucleotide. The rs11568818A- and G-specific nuclear protein complexes and the supershifted nuclear protein complex are labeled as A, G, and S, respectively. Competitors (CPT) of unlabeled rs11568818A and rs11568818G probes were included and labeled as A and G, respectively. Reagent controls of labeled oligonucleotides are labeled as Probe. Two independent experiments were performed for C. D: chromatin immunoprecipitation (ChIP) analysis of FOXA2 binding to the MMP7 promoter in A549 cells. MMP7 promoter was amplified using input DNA, precipitated DNA fragments of control goat normal IgG, or FOXA2-specific antibody using PCR (labeled as input, control and FOXA2 Ab, respectively). PCR amplification of β-Actin was used as an internal control.
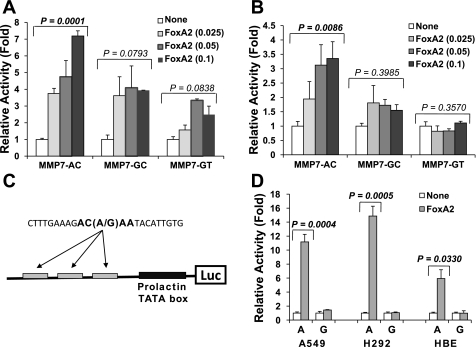
Differential regulation of the haplotype-specific MMP7 promoters by FOXA2.
Since FOXA2 plays a major role in embryonic lung development as well as proper function of the mature lung, we further analyzed the FOXA2 effects on MMP7 promoter haplotype-specific activity using a transient expression system (46, 47). Cotransfection of the MMP7 haplotype-specific luciferase reporters with plasmid expressing the rat FOXA2 at 1:0.025 molar ratio (reporter:transactivator) consistently demonstrated a 3- to 4-fold (in A549) or 1.5- to 2-fold (in HT-29) activation for the AC and GC haplotypes (Fig. 4, A and B). In contrast, at higher molar ratios (1:0.05 and 1:0.1), additional upregulation was only observed for the rs11568818A-specific AC haplotype not for the rs11568818G-specific GC or GT haplotypes in both cell types. Significant correlation of promoter activation with increased input of FOXA2 expression plasmid was observed for haplotype AC in both A549 and HT-29 cells. Only a marginal dose effect of FOXA2 was detected in A549 cells for either haplotype GC or GT. In HT-29 cells, no statistically significant FOXA2 dose effect was detected in either haplotype GC or GT.
Fig. 4.
Haplotype and allele-specific regulation of the MMP7 promoter by FOXA2. A and B: transactivation of the MMP7 haplotype-specific luciferase reporter constructs by FOXA2 in A549 (A) and HT-29 (B). C: schematic of the minimum rs11568818 allele-specific luciferase reporter constructs. D: allele-specific transactivation of the rs11568818A by FOXA2 in A549, H292, and primary airway epithelial cells. Haplotype and allele-specific constructs are indicated. For each transfection, the amounts of specific MMP7 reporter constructs and total DNA amounts were kept in constant. Expression plasmid of rat FOXA2 was added based on reporter-to-transactivator molar ratios as indicated for A-C (for D, 1:0.25 was used). Plasmid pBluescript was used as carrier DNA. In each experiment, a minimum of 2 duplicate transfections were performed. Relative promoter activity changes (in fold) in FOXA2 coexpressed cells compared with reporter alone are shown. SDs are calculated based on duplicate (A and B) or quadruplicate (D) transfections.
rs11568818A allele-dependent regulation of MMP7 promoter by FOXA2.
We further analyzed putative FOXA2 binding sites for the MMP7 promoter constructs in addition to the rs11568818A. We identified four additional putative FOXA2 binding sites within the 350-bp promoter fragment and upstream sequences of the luciferase gene in the pGL2basic plasmid vector. To specifically analyze the effects of FOXA2 on rs11568818 without confounding effects from the other putative FOXA2 binding sites, additional luciferase reporter plasmids driven by only three copies of the minimal 23 bp DNA sequences containing rs11568818 were constructed. The upstream sequence of the pGLbasic plasmid vector was replaced with a minimum TATA element from the prolactin promoter (Fig. 4C). We consistently observed 10- to 20-fold activation of the rs11568818A-specific reporter when cotransfected with FOXA2 at 1:0.25 reporter-to-transactivator molar ratio in A549 (P = 0.0004) and another lung carcinoma cell line H292 cells (P = 0.0005). In contrast, FOXA2 induced the rs11568818G-specific reporter activity to less than twofold (Fig. 4D). A dose-dependent transactivation of the rs11568818A-specific reporter by FOXA2 was observed with different reporter-to-activator molar ratios. In primary airway epithelial cells isolated from explanted human lungs, cotransfection of the FOXA2 at 1:0.25 reporter to activator molar ratio transactivated the A allele-specific promoter more than fivefold (P = 0.033) while no transactivation was observed in the G allele-specific promoter (Fig. 4D).
FOXA2 expression and colocalization with MMP7 in epithelial cells of IPF lung.
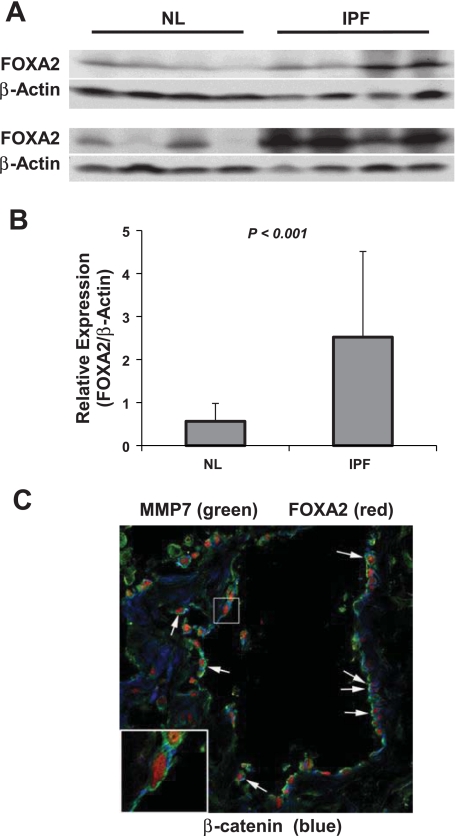
Expression level of FOXA2 protein in the parenchyma of IPF and normal lung was analyzed using Western blot. Approximately fivefold higher expression of FOXA2 protein was detected in IPF lung compared with normal lung tissue (Fig. 5, A and B). Since minimal expression of MMP7 was detected in normal lung tissues compared with IPF lungs (58), we analyzed the coexpression of MMP7 and FOXA2 in IPF lungs using the immunofluorescence confocal technique. Both proteins were expressed in the epithelial cells of IPF lungs with the FOXA2 and MMP7 expressed in the nucleus and cytoplasm, respectively. In contrast, β-catenin, a known transcriptional activator of MMP7, was not localized to the nucleus of the MMP7 expressing cells (Fig. 5C).
Fig. 5.
Expression of FOXA2 in IPF and normal lungs. A: FOXA2 expression was analyzed using normal lung (NL) and IPF lung tissues using Western blot. β-Actin was analyzed to demonstrate protein loading B: semiquantification of FOXA2 expression in the lung. β-Actin was used as an internal control. Relative expression of FOXA2 protein was determined using FOXA2/β-Actin for each sample. C: colocalization of FOXA2 and MMP7 in IPF lungs. Expression of FOXA2, MMP7, and β-catenin was analyzed in IPF lungs by immunofluorescent confocal microscopy. Donkey anti-goat Cy3 (Jackson ImmunoResearch), anti-mouse Alexa 488 (Molecular Probes), and anti-rabbit Cy5 (Jackson ImmunoResearch) were used for the fluorescent staining of FOXA2, MMP7, and β-catenin, respectively. Arrows indicate epithelial cells expressing nuclear FOXA2 and cytoplasmic MMP7.
DISCUSSION
Although the etiology and pathogenic mechanisms of IPF remain largely unknown, biomarkers of disease development have been reported for predicting disease progression and outcomes. These include chemokine (C-C motif) ligand 18 (CCL18), MMP7, MMP1, KL6, and surfactant protein A (SFTPA; Refs. 23, 30, 35, 37, 52). In this study, we identified significant associations between rs11568818AA and rs11568819CT genotypes and elevated plasma levels of MMP7 using a well-defined IPF cohort. When we assessed the functionality of rs11568818, we discovered that the G-to-A transition created a novel cis-regulatory element for FOXA transcription factors. We further demonstrated rs11568818A allele-specific regulation by FOXA2 in transient expression systems. We also showed that nuclear FOXA2 specifically correlates with epithelial cell expression of MMP7 in IPF lungs. Taken together our results suggest that increased sensitivity of the polymorphic MMP7 promoter to FOXA2 provides a mechanism for elevated peripheral blood expression of MMP7 in IPF. To our knowledge, this is the first study demonstrating a molecular and genetic basis for a peripheral blood biomarker in IPF.
Our data suggest that the MMP7 gene plays a role in IPF disease progression and outcomes. Both rs11568818AA and rs11568819CT are significantly associated with elevated MMP7 plasma levels, which are in turn associated with poor outcome in IPF (36). MMP7 has previously been implicated in cancer outcomes and in epithelial response to injury (28, 29, 51). The lack of significant differences in either allele or genotype or haplotype frequencies between IPF and control suggests that these MMP7 variants may not predispose to the disease but rather modify disease progression and outcomes of IPF patients. The upregulation of MMP7 in plasma and lung tissues is specific to IPF and not observed in other advanced lung diseases including chronic obstructive pulmonary disease, sarcoidosis (37), and hypersensitivity pneumonitis (41). MMP7 is expressed in abnormal alveolar epithelial cells of IPF lungs (14). Elevated levels of MMP7 in bronchoalveolar lavage have already been associated with IPF disease progression (30).
Allele-specific transactivation by FOXA2 leads to elevated MMP7 gene expression in IPF. Before this study, MMP7 was known to be induced by members of the Ets family and by TCF4/β-catenin (10); however, this is the first time that MMP7 has been shown to be a FOXA2 target gene. The fact that only 32% of IPF cases exhibited the rs11568818AA genotype in their MMP7 gene suggests that MMP7 can be upregulated in IPF via mechanisms other than allele-specific transactivation by FOXA2. Elevated plasma levels of MMP7 were also associated with rs11568819CT genotype in IPF, although the allele-specific transcription factor(s) remain to be identified. In the current study, we detected higher FOXA2 expression in the parenchyma of IPF lung compared with normal (Fig. 5B). This result suggests that the elevated plasma levels of MMP7 may attribute to the enhanced FOXA2 expression in the distal lung of IPF. In this respect, FOXA2 may resemble the abnormal expression of MUC5B, a normal proximal airway protein expressed in the abnormal epithelium in the distal lung of IPF (34). A hallmark of IPF is distal lung bronchiolization due to abnormal activation of epithelial differentiation, a normal process of development to which FOXA2 is essential (18, 27, 42, 45–47, 49).
We (55) recently showed in vitro that FOXA2 was among the genes downregulated by treatment of A549 cells with transforming growth factor-β1 (TGF-β1), an important mediator in the development of IPF (15, 43). Our current study thus suggests that FOXA2 activation in IPF lungs may be via TGF-β1-independent pathways. Interestingly, Yu et al. (53, 54) observed induction of FOXA2 expression by Wnt/β-catenin activation in prostate tissues using a mouse model. Wnt/β-catenin signaling pathway is involved in the epithelial cell injury and hyperplasia and abnormal epithelial-mesenchymal transition associated with IPF (25). Alternatively, TGF-β1 may differentially affect FOXA2 expression during the disease course of IPF.
The unique associations of rs11568818 and rs11568819 with plasma MMP7 levels in IPF have led to several important functional discoveries that support the biological significance of these associations. Identifying the associations were only the starting point for our investigation into the regulatory mechanisms of MMP7 induction in IPF. FOXA2 is important in tissue-specific gene expression and organ development and regulates expression of SFTPB, SFTPC, SFTPD, CCSP, and thyroid transcription factor-1 (TTF-1) gene (6, 17, 20, 39, 57). Evaluation of additional genes affected by FOXA2 transcriptional regulation is likely to provide exciting insights regarding the mechanisms of FOXA2-dependent effects in fibrotic and malignant diseases. Mutations in SFTPC, telomerase, and SFTPA2 have been identified in familial cases of pulmonary fibrosis (2, 33, 48). Association of a SNP in the MUC5B promoter with IPF was recently reported for both familial and sporadic cases of IPF (40, 56). It will be important to determine whether FOXA2, a transcription factor important for lung epithelial gene expression, regulates MUC5B expression. Demonstrating rs11568818A allele-specific binding of FOXA2 to the MMP7 promoter in primary lung epithelial cells and relating that allele-specific binding to MMP7 expression levels in those cells will provide additional evidence for allele-specific activation of MMP7 by FOXA2. Similarly, direct comparison of MMP7 activity in peripheral blood between IPF and control subjects will also provide insight into the role of MMP7 in IPF development. Furthermore, studies aimed at dissecting functional consequences of MMP7 upregulation in IPF patients and the role of MMP7 in other fibrotic diseases are important and warranted.
FUNDING
This work was supported, in part, by the Dorothy P. and Richard P. Simmons Endowment; the National Institutes of Health Grants R01-HL-095397, P50-HL-0894932, and R01-LM-009657 (to N. Kaminski); and the Pulmonary Fibrosis Foundation (a Young Investigator Award to Y. Zhang). The funding institutions have not been involved in study design, data collection, interpretation, or preparation of this manuscript.
DISCLOSURES
M. Selman participates in a Steering Committee of Boehringer/Ingelheim regarding a new drug for the treatment of IPF. N. Kaminski is a consultant to Stromedix and Sanofti Aventis and in the past received investigator initiated grants from Biogen Idec and Centocor and is an inventor on several patent applications. None of these relationships have a connection with this article.
AUTHOR CONTRIBUTIONS
Author contributions: T.R., K.F.G., Y.P.D., A.P., A.M.k.C., M.S., N.K., and Y.Z. conception and design of research; T.R., C.P., Y.C., Y.P.D., A.P., and Y.Z. analyzed data; T.R., K.F.G., Y.P.D., A.P., S.C.W., A.M.k.C., M.S., J.M.P., N.K., and Y.Z. interpreted results of experiments; T.R., Y.P.D., and Y.Z. prepared figures; T.R., Y.P.D., A.P., A.M.k.C., M.S., N.K., and Y.Z. edited and revised manuscript; T.R., C.P., Y.C., K.F.G., Y.P.D., A.P., S.C.W., A.M.k.C., M.S., J.M.P., N.K., and Y.Z. approved final version of manuscript; C.P., Y.C., K.F.G., S.C.W., J.M.P., and Y.Z. performed experiments; Y.Z. drafted manuscript.
ACKNOWLEDGMENTS
We thank Mattie Porter, Yanxia Chu, and Inna Loutev for technical support; Drs. Joseph Locker, Dean Sheppard, Robert Ferrell, James Dauber, Shai Izraeli, and Nir Friedman for valuable discussion and critical review of the manuscript.
REFERENCES
- 1.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 161: 646–664, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA, III, Lansdorp PM, Greider CW, Loyd JE. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med 356: 1317–1326, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Baumgartner KB, Samet JM, Coultas DB, Stidley CA, Hunt WC, Colby TV, Waldron JA. Occupational and environmental risk factors for idiopathic pulmonary fibrosis: a multicenter case-control study. Collaborating Centers. Am J Epidemiol 152: 307–315, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 155: 242–248, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Beeghly-Fadiel A, Long JR, Gao YT, Li C, Qu S, Cai Q, Zheng Y, Ruan ZX, Levy SE, Deming SL, Snoddy JR, Shu XO, Lu W, Zheng W. Common MMP-7 polymorphisms and breast cancer susceptibility: a multistage study of association and functionality. Cancer Res 68: 6453–6459, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohinski RJ, Di Lauro R, Whitsett JA. The lung-specific surfactant protein B gene promoter is a target for thyroid transcription factor 1 and hepatocyte nuclear factor 3, indicating common factors for organ-specific gene expression along the foregut axis. Mol Cell Biol 14: 5671–5681, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T. β-Catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol 155: 1033–1038, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buratowski SC, Chodosh LA. Mobility shift DNA-binding assay using gel electrophoresis. In: Current Protocols in Molecular Biology, edited by Ausubel F. and others. New York: Wiley, 1996, p. 12.2.1–12.2.11 [DOI] [PubMed] [Google Scholar]
- 9.Clark RS, Kochanek PM, Watkins SC, Chen M, Dixon CE, Seidberg NA, Melick J, Loeffert JE, Nathaniel PD, Jin KL, Graham SH. Caspase-3 mediated neuronal death after traumatic brain injury in rats. J Neurochem 74: 740–753, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Crawford HC, Fingleton B, Gustavson MD, Kurpios N, Wagenaar RA, Hassell JA, Matrisian LM. The PEA3 subfamily of Ets transcription factors synergizes with beta-catenin-LEF-1 to activate matrilysin transcription in intestinal tumors. Mol Cell Biol 21: 1370–1383, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demedts M, Costabel U. ATS/ERS international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Eur Respir J 19: 794–796, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Devor DC, Bridges RJ, Pilewski JM. Pharmacological modulation of ion transport across wild-type and ΔF508 CFTR-expressing human bronchial epithelia. Am J Physiol Cell Physiol 279: C461–C479, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Enomoto T, Usuki J, Azuma A, Nakagawa T, Kudoh S. Diabetes mellitus may increase risk for idiopathic pulmonary fibrosis. Chest 123: 2007–2011, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Fujishima S, Shiomi T, Yamashita S, Yogo Y, Nakano Y, Inoue T, Nakamura M, Tasaka S, Hasegawa N, Aikawa N, Ishizaka A, Okada Y. Production and activation of matrix metalloproteinase 7 (matrilysin 1) in the lungs of patients with idiopathic pulmonary fibrosis. Arch Pathol Lab Med 134: 1136–1142 [DOI] [PubMed] [Google Scholar]
- 15.Gauldie J, Bonniaud P, Sime P, Ask K, Kolb M. TGF-beta, Smad3 and the process of progressive fibrosis. Biochem Soc Transact 35: 661–664, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Ghilardi G, Biondi ML, Erario M, Guagnellini E, Scorza R. Colorectal carcinoma susceptibility and metastases are associated with matrix metalloproteinase-7 promoter polymorphisms. Clin Chem 49: 1940–1942, 2003 [DOI] [PubMed] [Google Scholar]
- 17.He Y, Crouch EC, Rust K, Spaite E, Brody SL. Proximal promoter of the surfactant protein D gene: regulatory roles of AP-1, forkhead box, and GT box binding proteins. J Biol Chem 275: 31051–31060, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Hromas R, Costa R. The hepatocyte nuclear factor-3/forkhead transcription regulatory family in development, inflammation, and neoplasia. Crit Rev Oncol Hematol 20: 129–140, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Ihaka RGR. R: a language for data analysis and graphics. J Comput Graphic Stat 5: 299–314, 1996 [Google Scholar]
- 20.Ikeda K, Shaw-White JR, Wert SE, Whitsett JA. Hepatocyte nuclear factor 3 activates transcription of thyroid transcription factor 1 in respiratory epithelial cells. Mol Cell Biol 16: 3626–3636, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jormsjo S, Whatling C, Walter DH, Zeiher AM, Hamsten A, Eriksson P. Allele-specific regulation of matrix metalloproteinase-7 promoter activity is associated with coronary artery luminal dimensions among hypercholesterolemic patients. Arterioscler Thromb Vasc Biol 21: 1834–1839, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Katzenstein AL, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med 157: 1301–1315, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Kinder BW, Brown KK, McCormack FX, Ix JH, Kervitsky A, Schwarz MI, King TE., Jr Serum surfactant protein-A is a strong predictor of early mortality in idiopathic pulmonary fibrosis. Chest 135: 1557–1563, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King TE, Jr, Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med 164: 1171–1181, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Konigshoff M, Balsara N, Pfaff EM, Kramer M, Chrobak I, Seeger W, Eickelberg O. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLos One 3: e2142, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konishi K, Gibson KF, Lindell KO, Richards TJ, Zhang Y, Dhir R, Bisceglia M, Gilbert S, Yousem SA, Song JW, Kim DS, Kaminski N. Gene expression profiles of acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 180: 167–175, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai E, Clark KL, Burley SK, Darnell JE., Jr Hepatocyte nuclear factor 3/fork head or “winged helix” proteins: a family of transcription factors of diverse biologic function. Proc Natl Acad Sci USA 90: 10421–10423, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 111: 635–646, 2002 [DOI] [PubMed] [Google Scholar]
- 29.McGuire JK, Li Q, Parks WC. Matrilysin (matrix metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium. Am J Pathol 162: 1831–1843, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKeown S, Richter AG, O'Kane C, McAuley DF, Thickett DR. MMP expression and abnormal lung permeability are important determinants of outcome in IPF. Eur Respir J 33: 77–84, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Myerburg MM, Harvey PR, Heidrich EM, Pilewski JM, Butterworth MB. Acute regulation of the epithelial sodium channel in airway epithelia by proteases and trafficking. Am J Respir Cell Mol Biol 43: 712–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ning W, Li CJ, Kaminski N, Feghali-Bostwick CA, Alber SM, Di YP, Otterbein SL, Song R, Hayashi S, Zhou Z, Pinsky DJ, Watkins SC, Pilewski JM, Sciurba FC, Peters DG, Hogg JC, Choi AM. Comprehensive gene expression profiles reveal pathways related to the pathogenesis of chronic obstructive pulmonary disease. Proc Natl Acad Sci USA 101: 14895–14900, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nogee LM, Dunbar AE, 3rd, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med 344: 573–579, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Plantier L, Crestani B, Wert SE, Dehoux M, Zweytick B, Guenther A, Whitsett JA. Ectopic respiratory epithelial cell differentiation in bronchiolised distal airspaces in idiopathic pulmonary fibrosis. Thorax 66: 651–657, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Prasse A, Probst C, Bargagli E, Zissel G, Toews GB, Flaherty KR, Olschewski M, Rottoli P, Muller-Quernheim J. Serum CC-chemokine ligand 18 concentration predicts outcome in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 179: 717–723, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Richards TJ, Kaminski N, Baribaud F, Flavin S, Brodmerkel C, Horowitz D, Li K, Choi J, Vuga LJ, Lindell KO, Klesen M, Zhang Y, Gibson KF. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 185: 67–76, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosas IO, Richards TJ, Konishi K, Zhang Y, Gibson K, Lokshin AE, Lindell KO, Cisneros J, Macdonald SD, Pardo A, Sciurba F, Dauber J, Selman M, Gochuico BR, Kaminski N. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med 5: e93, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryu JH, Colby TV, Hartman TE, Vassallo R. Smoking-related interstitial lung diseases: a concise review. Eur Respir J 17: 122–132, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Sawaya PL, Luse DS. Two members of the HNF-3 family have opposite effects on a lung transcriptional element; HNF-3 alpha stimulates and HNF-3 beta inhibits activity of region I from the Clara cell secretory protein (CCSP) promoter. J Biol Chem 269: 22211–22216, 1994 [PubMed] [Google Scholar]
- 40.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, Fingerlin TE, Zhang W, Gudmundsson G, Groshong SD, Evans CM, Garantziotis S, Adler KB, Dickey BF, du Bois RM, Yang IV, Herron A, Kervitsky D, Talbert JL, Markin C, Zhang L, Park J, Crews AL, Slifer SH, Xu H, Auerbach S, Roy MG, Lin J, Hennessy CE, Schwarz M, Schwartz DA. A common polymorphism in the putative promoter of MUC5B is associated with familial interstitial pneumonia (FIP) and idiopathic pulmonary fibrosis (IPF). N Engl J Med 2011 [Google Scholar]
- 41.Selman M, Pardo A, Barrera L, Estrada A, Watson SR, Wilson K, Aziz N, Kaminski N, Zlotnik A. Gene expression profiles distinguish idiopathic pulmonary fibrosis from hypersensitivity pneumonitis. Am J Respir Crit Care Med 173: 188–198, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Selman M, Pardo A, Kaminski N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs? PLoS Med 5: e62, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest 100: 768–776, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet 68: 978–989, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wan H, Dingle S, Xu Y, Besnard V, Kaestner KH, Ang SL, Wert S, Stahlman MT, Whitsett JA. Compensatory roles of Foxa1 and Foxa2 during lung morphogenesis. J Biol Chem 280: 13809–13816, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Wan H, Kaestner KH, Ang SL, Ikegami M, Finkelman FD, Stahlman MT, Fulkerson PC, Rothenberg ME, Whitsett JA. Foxa2 regulates alveolarization and goblet cell hyperplasia. Development 131: 953–964, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Wan H, Xu Y, Ikegami M, Stahlman MT, Kaestner KH, Ang SL, Whitsett JA. Foxa2 is required for transition to air breathing at birth. Proc Natl Acad Sci USA 101: 14449–14454, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Kuan PJ, Xing C, Cronkhite JT, Torres F, Rosenblatt RL, DiMaio JM, Kinch LN, Grishin NV, Garcia CK. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet 84: 52–59, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitsett JA, Haitchi HM, Maeda Y. Intersections between pulmonary development and disease. Am J Respir Crit Care Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson CL, Matrisian LM. Matrilysin: an epithelial matrix metalloproteinase with potentially novel functions. Int J Biochem Cell Biol 28: 123–136, 1996 [DOI] [PubMed] [Google Scholar]
- 51.Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, Lopez-Boado YS, Stratman JL, Hultgren SJ, Matrisian LM, Parks WC. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286: 113–117, 1999 [DOI] [PubMed] [Google Scholar]
- 52.Yokoyama A, Kondo K, Nakajima M, Matsushima T, Takahashi T, Nishimura M, Bando M, Sugiyama Y, Totani Y, Ishizaki T, Ichiyasu H, Suga M, Hamada H, Kohno N. Prognostic value of circulating KL-6 in idiopathic pulmonary fibrosis. Respirology 11: 164–168, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Yu X, Wang Y, DeGraff DJ, Wills ML, Matusik RJ. Wnt/beta-catenin activation promotes prostate tumor progression in a mouse model. Oncogene 30: 1868–1879, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu X, Wang Y, Jiang M, Bierie B, Roy-Burman P, Shen MM, Taketo MM, Wills M, Matusik RJ. Activation of beta-Catenin in mouse prostate causes HGPIN and continuous prostate growth after castration. Prostate 69: 249–262, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Handley D, Kaplan T, Yu H, Bais AS, Richards T, Pandit KV, Zeng Q, Benos PV, Friedman N, Eickelberg O, Kaminski N. High throughput determination of TGFbeta1/SMAD3 targets in A549 lung epithelial cells. PLos One 6: e20319, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y, Noth I, Garcia JGN, Kaminski N. A variant in the promoter of MUC5B and idiopathic pulmonary fibrosis. N Engl J Med 364: 1576–1577, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou L, Lim L, Costa RH, Whitsett JA. Thyroid transcription factor-1, hepatocyte nuclear factor-3beta, surfactant protein B, C, and Clara cell secretory protein in developing mouse lung. J Histochem Cytochem 44: 1183–1193, 1996 [DOI] [PubMed] [Google Scholar]
- 58.Zuo F, Kaminski N, Eugui E, Allard J, Yakhini Z, Ben-Dor A, Lollini L, Morris D, Kim Y, DeLustro B, Sheppard D, Pardo A, Selman M, Heller RA. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci USA 99: 6292–6297, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]