Abstract
Bronchopulmonary dysplasia (BPD) is characterized by simplified alveolarization and arrested vascular development of the lung with associated evidence of endothelial dysfunction, inflammation, increased oxidative damage, and iron deposition. Heme oxygenase-1 (HO-1) has been reported to be protective in the pathogenesis of diseases of inflammatory and oxidative etiology. Because HO-1 is involved in the response to oxidative stress produced by hyperoxia and is critical for cellular heme and iron homeostasis, it could play a protective role in BPD. Therefore, we investigated the effect of HO-1 in hyperoxia-induced lung injury using a neonatal transgenic mouse model with constitutive lung-specific HO-1 overexpression. Hyperoxia triggered an increase in pulmonary inflammation, arterial remodeling, and right ventricular hypertrophy that was attenuated by HO-1 overexpression. In addition, hyperoxia led to pulmonary edema, hemosiderosis, and a decrease in blood vessel number, all of which were markedly improved in HO-1 overexpressing mice. The protective vascular response may be mediated at least in part by carbon monoxide, due to its anti-inflammatory, antiproliferative, and antiapoptotic properties. HO-1 overexpression, however, did not prevent alveolar simplification nor altered the levels of ferritin and lactoferrin, proteins involved in iron binding and transport. Thus the protective mechanisms elicited by HO-1 overexpression primarily preserve vascular growth and barrier function through iron-independent, antioxidant, and anti-inflammatory pathways.
Keywords: vasculogenesis, vascular permeability, vascular leak, endothelial cells, carbon monoxide
bronchopulmonary dysplasia (BPD) is a debilitating disorder that affects thousands of premature newborns every year (2). The etiology likely involves a combination of factors, such as developmental immaturity, inflammation, mechanical injury, and similar conditions that may result in a reduced capacity of the lung to repair (20). The pathology of BPD is characterized by decreased alveolarization and vascularization of the developing lung, persistent inflammation, and parenchymal fibrosis (18). In addition to these structural abnormalities, neonates with BPD are susceptible to developing pulmonary hypertension (PH), which can be triggered at least partly by endothelial dysfunction; PH is defined as an increase in resistance to blood flow due to impaired lung vessel growth and remodeling, eventually leading to right ventricular hypertrophy (RVH) and, in some patients, RV failure and death (1, 41). Although improved neonatal care has decreased the rate of respiratory morbidity in later childhood, little advances have been made to find therapies that cure or prevent pulmonary disease in these patients.
Heme oxygenase-1 (HO-1) is an enzyme that is induced by a variety of stressors, including its substrate heme, and regulated by an array of agents and conditions aimed to protect against oxidative damage (26). Cellular metabolism of the prooxidant heme molecule by HO-1 produces biliverdin, carbon monoxide (CO), and iron (26). HO-1 deficiency in humans manifests evidence of oxidative injury and severe endothelial damage (50), while HO-1 null mice exhibit reduced resistance to oxidative stress with excess iron deposition (34). In the lung, HO-1 is induced in alveolar epithelial cells (23), alveolar macrophages (16), as well as endothelial and smooth muscle cells of the pulmonary vasculature (46) in response to an inciting stimulus.
Exposure of neonatal rodents to mild hyperoxia induces a pattern of lung injury similar to the one described in immature human lungs. These features include alveolar simplification, inflammation, and vascular remodeling (11, 49). Importantly, mice and rats exhibit at birth a saccular stage of lung development that is completed after 2 wk of postnatal alveolarization, thus closely resembling the stage of lung development in premature infants between 26 and 28 wk gestation requiring intensive care. In rodents, HO-1 expression is induced during lung maturation and following exposure to hyperoxia, consistent with adaptation to extrauterine conditions of increased oxidative stress and inflammation (13, 40).
Damage to the microvascular endothelium of the lung and subsequent disruption of the alveolar-capillary unit caused by exposure to hyperoxia plays a crucial role in mediating oxidative stress-induced injury. Release of erythrocyte-derived heme evident as hemorrhage and hemolysis increases cellular susceptibility to oxidative damage mediated by polymorphonuclear leukocytes and free radicals. The role of HO-1 in lung heme and iron metabolism may be particularly relevant in mitigating lung injury induced by hemorrhage. However, transient HO-1 induction can only have a temporizing effect and only continuous overexpression of this protein could provide long-lasting protection. Previous data from our laboratory (27, 45) reported a protective function of HO-1 in hypoxia-induced pulmonary hypertension and inflammation in adult mice. Moreover, the mechanisms by which HO-1 appears to modulate vascular and inflammatory responses depend at least on one gaseous product of HO-1 enzymatic action, CO, since its exogenous application to hypoxic animal models ameliorates both pulmonary arteriolar remodeling and elevations in RV systolic pressure by regulating reactivity of the lung vasculature (47).
In this study, we investigated the pulmonary effects of HO-1 in an experimental model of BPD by using a transgenic (TG) approach that targets the constitutive overexpression of HO-1 in pulmonary epithelial cells (27). HO-1 overexpressing mouse pups exposed to hyperoxia showed reduced inflammation, thinner alveolar septa, decreased hemorrhage and edema, and preservation of pulmonary vessel density, compared with wild-type (WT) neonates. In addition, TG animals manifested decreased lung vascular remodeling and reduced RVH, two key components of PH. The vascular effects of HO-1 overexpression were mimicked by administration of CO, since the inhaled gas was capable of decreasing both vascular and RV remodeling. Taken together, these results suggest an important role for pulmonary HO-1 production in protecting the developing pulmonary vasculature from hyperoxia-induced injury.
MATERIALS AND METHODS
Mice and hyperoxic exposure.
All animal experimental protocols were approved by the Animal Care and Use Committee of the Children's Hospital Boston. Pregnant HO-1 TG mice that constitutively overexpress HO-1 in alveolar type II epithelial cells under the control of surfactant protein C promoter and non-TG (FVB) mice were raised in the animal facility. These TG mice have been previously characterized by our group and shown to have constitutive 4- to 6-fold increased lung HO-1 protein levels and 2.5-fold higher enzymatic activity measured by bilirubin production and by inference, CO release, compared with their non-TG littermates (27). Neonatal pups were pooled and exposed to 75% O2 with or without 250 ppm CO (1 h daily) in a plexiglass chamber or to 21% O2 beginning at birth and continuing for 14 days (room air, n = 50; hyperoxia, n = 67; and hyperoxia with CO, n = 10). Ventilation was adjusted by an Oxycycler controller (Biospherix, Lacona, NY) to remove CO2 so that it did not exceed 5,000 ppm (0.5%). Ammonia was removed by ventilation and activated charcoal filtration through an air purifier. Dams were rotated from hyperoxia to room air every 24 to 48 h to prevent excessive oxygen toxicity to the adult mice. Each litter consisted of <12 pups to control for the effect of litter size on nutrition and growth. At the end of the exposure period, mice were killed for lung histology and protein analysis (n = 51), collection of bronchoalveolar lavage (BAL) fluid (n = 42), or analysis of pulmonary edema (n = 24).
Histology and immunohistochemistry.
Following anesthesia with pentobarbital (60 mg/kg ip), mice were perfused through the aorta at a constant pressure of 25 cm H2O with PBS. Subsequently, lungs were inflated and fixed with an intratracheal injection of 4% paraformaldehyde and postfixed overnight at 4°C. Tissues were paraffin embedded, sectioned to 5 μm thickness, and stained with hematoxylin and eosin. To visualize the pulmonary medial arterial wall of pulmonary vessels, lung sections were immunostained with a monoclonal anti-α-smooth muscle actin (α-SMA) antibody at a dilution of 1:125 (14). To quantify the density of pulmonary vessels, a polyclonal anti-human von Willebrand Factor (vWF) antibody (Chemicon International, Temecula, CA) was employed as an endothelial marker at a dilution of 1:400. To examine endogenous HO-1 staining, a rabbit polyclonal antibody to rat HO-1 (StressGen; Victoria, British Columbia, Canada) was diluted to 1:400. Stained sections were visualized under light microscopy (Nikon Eclipse 80i; Tokyo, Japan), and images were captured using a digital camera (DXM1200F; Nikon). Morphometric analysis was performed as described previously (5) in ×100 magnification hematoxylin and eosin stained lung sections. The software package Metamorph v.6.2r (Universal Imaging) was used to analyze tissue and vessel morphometry. Briefly, the volume density of alveolar wall tissue was determined by a point-counting method using a computer-generated 30 × 30 grid superimposed to each image. To measure mean linear intercept, a grid with parallel lines spaced at 58 μm was then overlaid onto the image, and the length of each chord, defined by the intercept with alveolar walls, was measured. The thickness of the alveolar septum was calculated by measuring the fiber breadth (area/length). To quantify vessel density, the number of vWF-positive vessels per high-power field was counted at a magnification of ×200. For measurements of medial thickness index, α-SMA-stained tissue sections were captured at ×400 magnification. The thickness of the vessel wall was calculated using the following equation: medial thickness index: [(areaext − areaint)/areaext] × 100, where areaext and areaint are the areas within the external and internal boundaries of the α-SMA layer, respectively.
To quantify the degree of RVH, hearts were removed and weighed with the RV dissected and weighed separately from the interventricular septum (S) and left ventricle (LV). As an indicator of RVH, we determined Fulton's index as the ratio of RV/S + LV.
Quantitative PCR.
Total RNA from total lung was isolated using the mirVANA RNA isolation kit (Ambion, Austin Texas). One-microgram aliquots of RNA were used for cDNA synthesis (Superscript III; Invitrogen, Carlsbad, CA), primed with oligo dT, and subsequently amplified using specific oligonucleotide primers based on the human HO-1 sequence: fwd: 5′-GCSAGTCAGGCAGAGGGTGATA-3′ and rev: 5′-AGCCTGGGAGCGGGTGTTGAG-3′; α-tubulin served as housekeeping gene: fwd: 5′- TGACAGAATTCCAGACCAACC-3′ and rev: 5′-CTGCTACAGAAAGCTGCTCGT-3′. Annealing was carried out at 60°C for 30 s, extension at 72°C for 30 s, and denaturation at 95°C for 30 s for 40 cycles. Analysis of the fold change was performed based on the Pfaffl method (33).
Protein extraction and Western blot analysis.
Lung samples were homogenized with lysis buffer containing 10 mM Tris·HCl pH 8.0, 10 mM EDTA, 140 mM NaCl, 1% TritonX-100, 1% Na deoxycholate, and 0.1% SDS supplemented with protease inhibitors (Complete Protease Inhibitor Cocktail; Roche, Basel, Switzerland). Protein samples were electrophoresed on 10–12% denaturing polyacrylamide gels before transfer to 0.45-μm PVDF membranes (Millipore, Billerica, MA), and the membranes were probed with polyclonal rabbit anti-human ferritin (Dako Cytomation; 1:1,000), and rabbit anti-human lactoferrin antibodies (1:500; Sigma, St. Louis, MO), or rabbit anti-human-HO-1 (1:5,000; Stressgen). After incubation with primary antibodies, the membranes were incubated with peroxidase-conjugated anti-rabbit secondary antibody used at a 1:10,000 dilution for an hour at room temperature. Immunoreactive bands were visualized by application of ECL substrate (Thermo Scientific, Waltham, MA), and the blots were developed according to the manufacturer's instructions.
Bronchoalveolar lavage and protein determination.
Mice were anesthetized as above, and the tracheas were cannulated with a 23-G Stub Luer adapter, caudal to the larynx. Lungs were lavaged three times with 250–350 μl of Hank's balanced buffer containing 10 mM EDTA and 1 mM HEPES and centrifuged at 400 g for 5 min. Total white cell count was determined on Kimura-stained preparations using a hemocytometer chamber. Differential cell count for neutrophils and macrophages within the BAL fluid was performed using HEMA 3 staining kit (Fisher Scientific, Pittsburgh, PA).
Statistical analysis.
All values are expressed as means ± SD. Comparison between different groups was performed by one-way ANOVA followed by Tukey's multiple comparison test using GraphPad Prism (5.0; GraphPad, La Jolla, CA). Significance was considered at P values <0.05.
RESULTS
Expression of surfactant protein C driven HO-1 following hyperoxia.
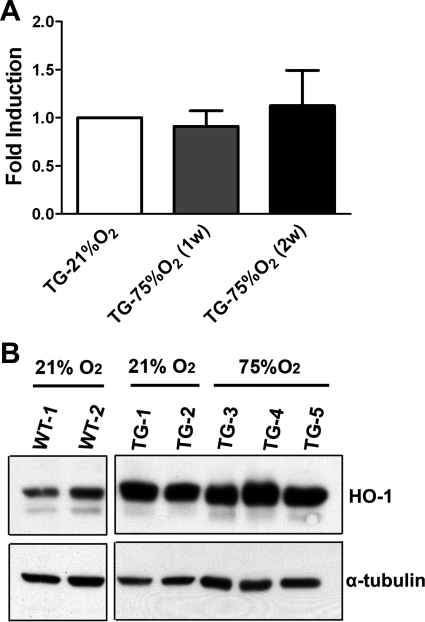
Expression levels of HO-1 mRNA in the lungs of TG mice exposed to 1 or 2 wk of hyperoxia after birth were not statistically different from the levels found in the lungs of normoxic mice (Fig. 1A), as determined by quantitative PCR. Furthermore, using an antibody that detects both human and murine HO-1, we detected elevated baseline levels of HO-1 protein in the lungs of TG mice exposed to room air compared with WT mice. Exposure of TG animals to hyperoxia for 2 wk did not further increase lung HO-1 levels (Fig. 1B), confirming constitutive HO-1 transgene overexpression that is not modified by hyperoxia.
Fig. 1.
Expression of heme oxygenase-1 (HO-1) in transgenic mice is not affected by hyperoxic exposure. A: mRNA levels of human HO-1 were assessed through quantitative PCR in total lung at 1 and 2 wk (w) following hyperoxia. Values are shown relative to normoxia. B: Western blot analysis of HO-1 protein in whole lungs from neonatal wild-type (WT) mice (21% O2) and transgenic (TG) mice after 2 wk of exposure to room air (21% O2) or hyperoxia (75%O2) after birth. Note that the antibody used detects both the constitutive human HO-1 and the endogenous murine HO-1. α-Tubulin was used as an internal control.
HO-1-produced CO decreases neutrophil accumulation in the hyperoxic lung.
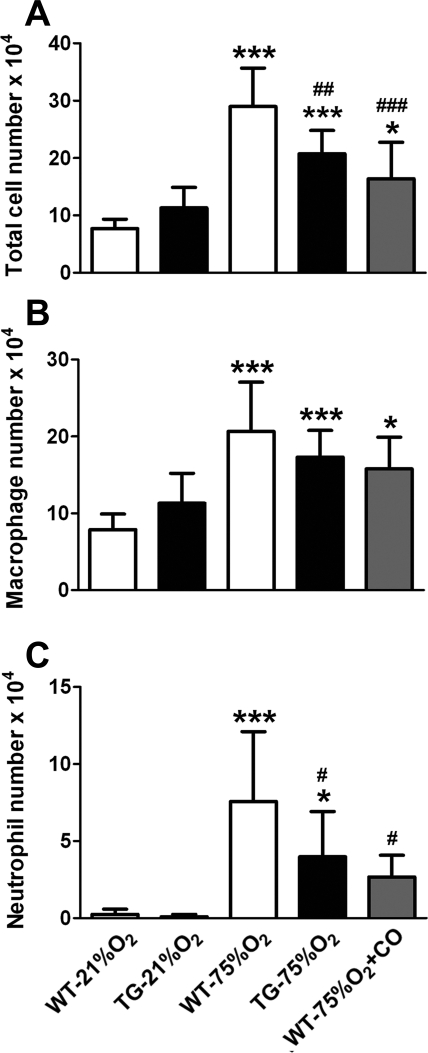
Neonatal animals were exposed to 75% O2 as described in materials and methods. Following 14 days of hyperoxia exposure, the total number of inflammatory cells in BAL fluid of WT mice increased >3.5-fold compared with normoxic WT littermates (P < 0.001), as shown in Fig. 2A. This recruitment of macrophages and neutrophils in BAL fluid collected from neonatal lungs exposed to hyperoxia was observed in both WT and TG groups. However, the expression of the HO-1 transgene in developing lung parenchyma decreased the influx of inflammatory cells to ∼70% of that found in the hyperoxic WT group (P < 0.01). There was no statistically significant reduction in the number of macrophages collected in the BAL fluid of TG hyperoxic mice (Fig. 2B); however, a pronounced reduction in the number of neutrophils was evident (P < 0.05; Fig. 2C). Exogenously administered CO elicited a similar reduction in the total cell count compared with WT hyperoxic group (P < 0.001; Fig. 2A). This reduction could also be accounted by a preferential decrease in the number of neutrophils (Fig. 2C) in the presence of inhaled CO (P < 0.05). The efficacy of CO in decreasing neutrophil accumulation in WT mice paralleled the efficacy of HO-1 overexpression in TG mice.
Fig. 2.
Hyperoxia-induced inflammatory cell influx in neonatal mouse lungs is attenuated by HO-1 overexpression and carbon monoxide (CO) inhalation. Total (A) and differential cell counts (B and C) were performed in bronchoalveolar lavage (BAL) fluid after 2 wk of exposure to room air (21% O2) or hyperoxia (75% O2) revealing a modest effect on the number of macrophages (B) and a significant decrease in the number of neutrophils (C) in hyperoxic HO-1 overexpressing mice and mice receiving inhaled CO, compared with WT controls (n = 10 mice per group). *P < 0.05, ***P < 0.001 vs. WT-21% O2; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. WT-75% O2.
HO-1 TG mice are protected from RVH, vessel wall remodeling, and blood vessel loss following hyperoxia.
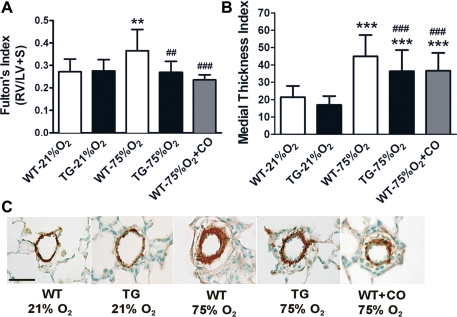
Following 2 wk of hyperoxia exposure, WT mice developed RVH, manifested as an increase in Fulton's Index of 127% above values observed in normoxic neonates, as shown in Fig. 3A (P < 0.01). Hyperoxia exposure did not produce a significant increase in Fulton's index in TG mice compared with their room air control littermates. Moreover, the exogenous application of CO to hyperoxic WT mice achieved the same attenuating effect on RVH similar to the HO-1 TG group.
Fig. 3.
HO-1 overexpression and inhaled CO diminished the development of pulmonary vascular remodeling resulting from hyperoxia exposure. A: neonatal WT mice exposed for two wk to 75% O2 develop right ventricular hypertrophy (RVH) that is significantly reduced in HO-1 TG and CO-treated mice (250 ppm, 1 h daily; n = 15–18 mice per group). B: vascular remodeling of pulmonary arterioles was also partially but significantly decreased in TG and CO-treated mice compared with WT mice following 2 wk of hyperoxic exposure (n = 4–6 mice per group). C: Representative paraffin sections from lungs stained with α-smooth muscle actin for the visualization of medial wall thickness of pulmonary arterioles (25- to 75-μm diameter) shows a decreased smooth muscle layer thickening in HO-1 TG and CO receiving mice compared with WT mice following exposure to 75% O2. RV, right ventricle; LV, left ventricle; S, septum. **P < 0.01; ***P < 0.001 vs. WT-21% O2; ##P < 0.01, ###P < 0.001 vs. WT-75% O2. Scale bar in C = 30 μm.
Because RVH is often a result of pulmonary vascular changes that involve either a reduction of vessel density and/or abnormal muscularization of peripheral vessels, we determined the degree of pulmonary vessel wall remodeling in mice exposed to hyperoxia (Fig. 3, B and C). As shown in Fig. 3B, medial thickness index of vessels in WT mice exposed to 75% O2 increased more than twofold compared with littermates in room air (P < 0.001). Exposure of TG mice to hyperoxia also resulted in increased medial thickness index; however, the remodeling exhibited in TG hyperoxic mice was significantly reduced compared with that present in the WT hyperoxic group (P < 0.001). Inhaled CO similarly attenuated the effects of hyperoxia, with a significant 20% reduction in vessel wall remodeling compared with the WT hyperoxic group that did not receive inhaled CO (P < 0.001).
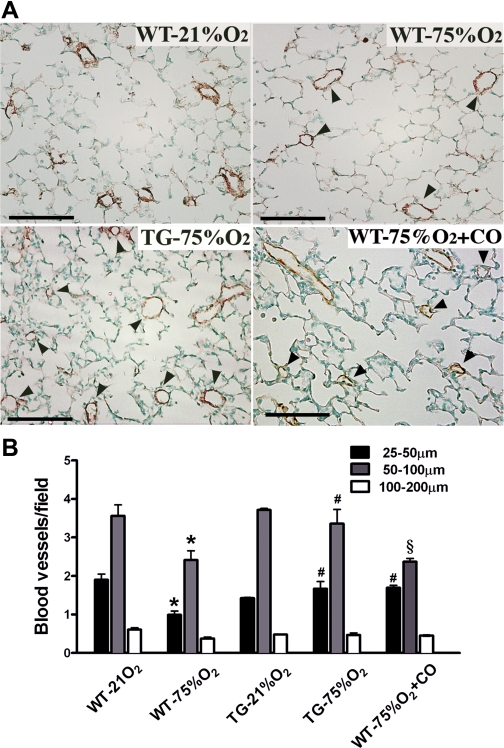
To determine the contribution of HO-1 overexpression to the preservation of the pulmonary microvasculature, blood vessel density was also assessed at the end of the 2-wk hyperoxic period. Histological analysis of tissue slides obtained from each experimental group and stained for vWF revealed a clear loss of small caliber vessels but preservation of those with larger diameter (Fig. 4A). The number of vessels per field with a diameter ranging between 25–50 μm and 50–100 μm was reduced in WT hyperoxic mice compared with normoxic littermates (P < 0.001 for 25- to 50-μm vessels and, P < 0.001 for 50- to 100-μm vessels in room air vs. hyperoxia, respectively), as shown in Fig. 4B. However, in TG mice, the blood vessel density was preserved to normoxic levels and was significantly different from WT pups exposed to hyperoxia (P < 0.001 for 25 to 50-μm vessels and P < 0.001 for 50- to 100-μm size). In addition, inhaled CO led to preservation of the small caliber vessel number (P < 0.001 vs. WT), while the number of intermediate-sized vessels remained unchanged after hyperoxia exposure compared with WT littermates (Fig. 4B).
Fig. 4.
Blood vessel loss after hyperoxic exposure is preserved by HO-1 overexpression and CO inhalation. A: lung sections stained for von Willebrand Factor (vWF), a marker of endothelial cells, revealed a greater loss of pulmonary blood vessels (arrowheads) per high-power field in lung sections from WT mice compared with HO-1 TG and CO-treated mice exposed to 75% O2. Scale bar = 100 μm. B: quantification of the number of small (25–50 μm), intermediate (50–100 μm), and large (100–200 μm) pulmonary vessels. Note that 75% O2 exposure in WT mice decreased the number of all three categories of vessels, while HO-1 overexpression preserved vessels of all sizes to numbers comparable to 21% O2-exposed mice (n = 3–4 mice per group) and CO treatment maintained the number of only the small vessels equivalent to those in normoxic mice. *P < 0.001 vs. WT-21% O2; #P < 0.001 vs. WT-75% O2; §P < 0.001 vs. TG −75% O2.
HO-1 overexpression ameliorates hyperoxia-induced damage to alveolar structures.
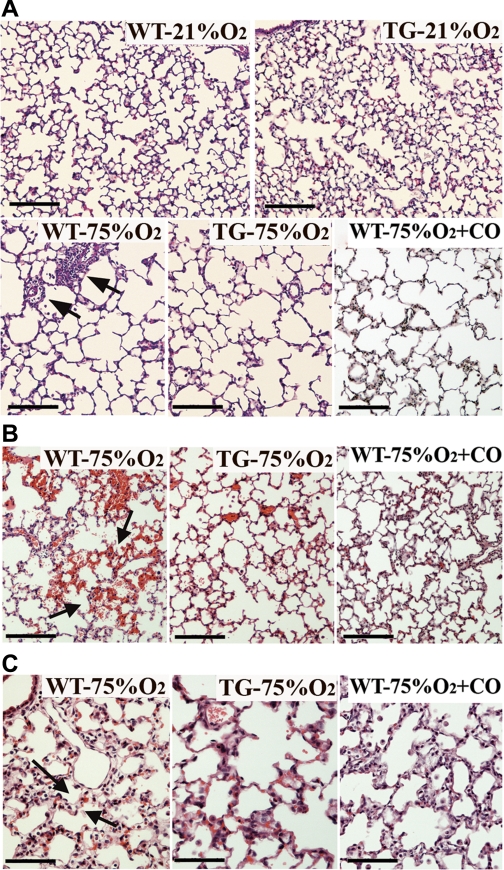
The comparison of body and lung weights at the end of the experimental period revealed no differences between room air and mice exposed to hyperoxia, irrespective of their genotype (Table 1). Detailed morphometric analysis of lung tissue sections from WT, TG, and CO-treated mice exposed to hyperoxia demonstrated a two to threefold increase in average alveolar size and an increase in septal thickness compared with room air exposed mice. However, hyperoxic TG and CO-treated mice exhibited significantly less septal thickening than WT mice (Table 1 and Fig. 5C, arrows). The volume density of alveolar wall tissue significantly decreased (P < 0.001), while the mean linear intercept increased (P < 0.05) after hyperoxia exposure. Overexpression of HO-1 did not prevent this effect, while inhaled CO had a minimal but statistically significant effect on both parameters (P < 0.05). In contrast, the overall blood vessel density decreased by 40% in WT hyperoxic mice compared with normoxic littermates but this decrease was prevented in TG hyperoxic and CO-treated neonates, although it did not reach statistical significance in the latter group (Table 1). Thus the protective effects of epithelial HO-1 overexpression are imparted specifically on the lung vasculature but not the lung parenchyma. Consistently, microscopic examination of the lungs exposed to 75% O2 showed that neonatal WT, TG, and CO-treated animals exhibited enlarged and simplified distal air spaces with fewer septa (Fig. 5A, bottom). A higher alveolar insult in the WT group of mice was supported histologically by an increased neutrophilic invasion that contributes to hypercellularity (Fig. 5A, bottom left, arrows), entrapment of red blood cells in tissue sections from WT hyperoxia-exposed mice (Fig. 5B, left, arrows) and the presence of thickened alveolar septa indicative of pulmonary edema (Fig. 5C, left, arrows). The corresponding TG and CO-treated mice manifested significantly decreased inflammatory cell infiltrates, less hemorrhage, and markedly decreased septal thickness (Fig. 5 and Table 1).
Table 1.
Lung morphometric analysis
| Normoxia (21 O2%) |
Hyperoxia (75 O2%) |
||||
|---|---|---|---|---|---|
| WT | TG | WT | TG | WT + CO | |
| Body wt, g | 7.86 ± 0.96 | 8.04 ± 1.88 | 7.49 ± 0.45 | 7.79 ± 0.72 | ND |
| Lung wt, mg | 155.5 ± 14.5 | 150.4 ± 7.30 | 162.0 ± 15.7 | 171.4 ± 11.7 | ND |
| Alveolar size average, μm2 | 1,146 ± 245.3 | 1,304 ± 491.7 | 4,432 ± 755† | 4,573 ± 614† | 4,337 ± 577† |
| Septal thickness, μm | 4.52 ± 0.29 | 4.19 ± 0.17 | 5.22 ± 0.46† | 4.90 ± 0.44*§ | 4.94 ± 0.32*§ |
| VDawt | 1.39 ± 0.14 | 1.41 ± 0.21 | 0.90 ± 0.12† | 0.94 ± 0.14† | 1.076 ± 0.12†‡ |
| MLI, μm | 25.28 ± 2.78 | 25.32 ± 2.86 | 40.83 ± 7.32* | 40.84 ± 5.86* | 37.88 + 5.22*‡ |
| BV density, vessels/mm2 | 4.29 ± 0.34 | 3.98 ± 0.04 | 2.67 ± 0.26† | 3.89 ± 0.42§ | 3.20 ± 0.11* |
Values are means ± SD of 4–6 animals examined.
MLI, mean linear intercept; ND, not determined; VDawt, volume density of alveolar wall tissue; BV density, blood vessel density; TG, transgenic; CO, carbon monoxide.
P < 0.01,
P < 0.001 vs. normoxic wild-type (WT) group.
P < 0.05,
P < 0.001 vs. hyperoxic WT group.
Fig. 5.
Lung histological examination showing the effects of hyperoxia, HO-1 overexpression and CO inhalation on alveolarization, hemorrhage, and edema. A: paraffin lung sections stained with hematoxylin and eosin demonstrated increased alveolar size and reduced septation in WT, HO-1 TG, and CO-treated mice exposed to hyperoxia (75%O2) 2 wk after birth, compared with normoxic (21% O2) mice. WT lungs also exhibited abundant perivascular mononuclear infiltration (arrows). B: areas of hemorrhage (seen as mild to moderate accumulation of red blood cells in the pulmonary parenchyma) as well as thickened alveolar walls, indicative of pulmonary edema (C, arrows) were abundant in hematoxylin and eosin-stained lung sections from WT mice exposed to 75% O2 but less common in lung sections from similarly exposed HO-1 TG and CO-treated mice (n = 4–6 mice per group). Scale bars in A and B = 100 μm and in C = 50 μm.
HO-1 overexpression ameliorates pulmonary hemorrhage and edema.
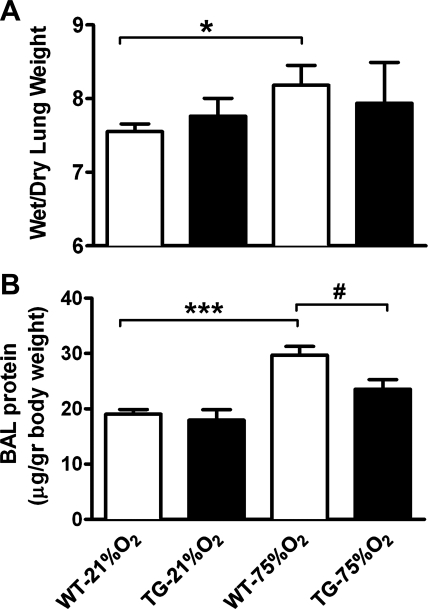
Because features of hemorrhage and edema are indicators of endothelial barrier dysfunction in response to high levels of oxygen, we began to investigate the role of HO-1 in preventing increases in endothelial permeability and leakage. We determined pulmonary endothelial permeability by assessing wet-to-dry lung weight ratios. As shown in Fig. 6A, hyperoxia caused a significant increase in the wet-to-dry lung weight ratio of neonatal mice (P < 0.05). This increase in the wet-to-dry lung weight ratio was absent in the hyperoxic TG group and not statistically different from normoxia. Similarly, the protein content in BAL fluid of WT mice caused by hyperoxic exposure significantly increased in hyperoxia (P < 0.001), while the same treatment induced a smaller increase in BAL protein content in the TG group above the protein content in normoxia, which was significantly lower than WT hyperoxia but not significantly different from normoxia (Fig. 6B). Although permeability was not assessed in lungs from CO-treated neonates, histological examination of tissue sections demonstrated signs of reduced pulmonary edema (Fig. 5C, right).
Fig. 6.
HO-1 TG mice exhibit decreased pulmonary edema and have lower BAL fluid protein content following hyperoxia. A: weight ratio of the wet-to-dry lung (n = 5–7) was significantly increased in WT neonatal mice 2 wk following 75% O2 exposure but not in HO-1 TG mice. B: total protein concentration in BAL fluid (n = 6–9) significantly increased in the WT hyperoxic group but not in the hyperoxic TG group compared with normoxic mice. *P < 0.05, ***P < 0.001 vs. WT-21% O2; #P < 0.05 vs. WT-75% O2.
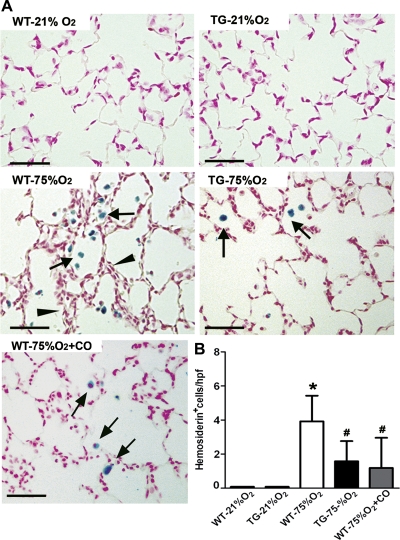
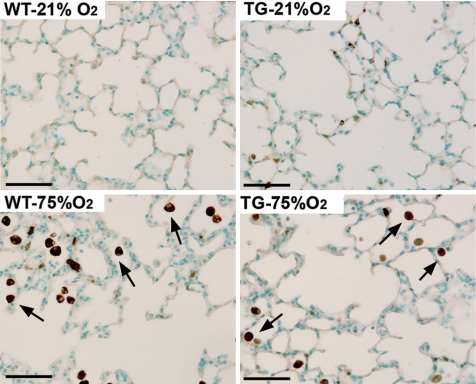
To quantify the degree of parenchymal hemorrhage, we stained lung sections from room air and hyperoxia-exposed neonatal mice with Perls Prussian blue. Macrophages loaded with free iron were detected in all hyperoxic groups as clusters of cells in close proximity with thickened alveolar septal areas while they were not detected in normoxic lungs from either WT or TG mice (Fig. 7A). However, the total number of iron-laden cells per high-power field present in hyperoxia-exposed WT mice was 2.5 (P < 0.05) and 3.3 times higher (P < 0.05) than the numbers detected in hyperoxic TG and CO-treated groups, respectively (Fig. 7B).
Fig. 7.
Hemosiderosis induced by hyperoxia exposure is attenuated by HO-1 overexpression and CO inhalation. A: lung sections from WT, TG, and CO-treated mice stained with Perls Prussian blue iron revealed areas of severe hemosiderosis (arrows) in proximity to thickened alveolar walls (arrowheads) in WT mice exposed to 75% O2. Only moderate hemosiderosis was observed in hyperoxic HO-1 transgenic and CO-treated mice. B: hemosiderosis was quantified by counting iron-laden cells per high-power field in lung sections from each experimental group (n = 8). *P < 0.05 vs. WT- 21% O2; #P < 0.05 vs. WT-75% O2. Scale bar = 50 μm.
Ferritin activity is upregulated in hyperoxic WT lungs.
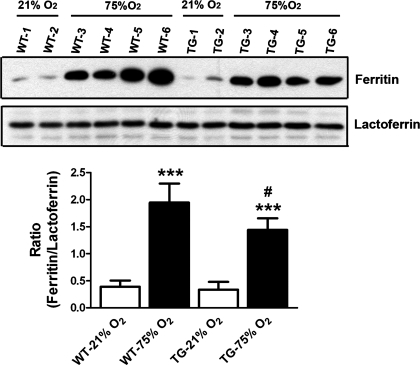
To investigate whether iron metabolism upon exposure to hyperoxia is differentially regulated by overexpression of epithelial HO-1, we determined the levels of two proteins, ferritin and lactoferrin, known to participate in processes of iron sequestration and transport, respectively (for a review, see Ref. 44), in alveolar macrophages and pulmonary epithelial cells. Quantification of ferritin levels in lung homogenates by Western blot (Fig. 8) showed that overexpression of HO-1 did not affect ferritin expression at baseline. Following hyperoxia, ferritin was induced in both genotypes; however, this induction was more pronounced in the WT neonatal mice compared with the TG group, providing a correlation with the higher levels of hemosiderosis evident in hyperoxic WT lungs. Examination of ferritin immunostaining in the lung revealed that expression of this protein is mostly restricted to alveolar macrophages (data not shown). In contrast, the levels of lactoferrin, which transports iron for sequestration into ferritin, did not show induction by hyperoxia in either of the examined genotypes. Thus the observed increased ratio of ferritin/lactoferrin indicates that hyperoxia causes upregulation of mechanisms leading to increased cellular iron storage in the absence of enhanced iron transport. This ratio was statistically, but modestly, lower in TG mice exposed to hyperoxia (Fig. 8).
Fig. 8.
Induction of ferritin by heme-released iron is increased in hyperoxic WT mice. Representative Western blot of ferritin and lactoferrin proteins in whole lungs of neonatal mice from WT and HO-1 TG groups following 2 wk of room air (21% O2) or hyperoxia (75%O2) after birth. Quantification of the bands by densitometry revealed a significant increase in the ratio of ferritin to lactoferrin levels in WT hyperoxic mice compared with normoxia or to the TG group. Forty- to fifty-microliter aliquots of lung homogenates were electrophoresed and immunoblotted with anti-ferritin or anti-lactoferrin (see materials and methods). ***P < 0.001 vs. WT-21% O2; #P < 0.05 vs. WT-75% O2.
We wanted to confirm that the induction of ferritin is coupled to endogenous HO-1 induction in the macrophages and, therefore, the direct result of the liberation of iron from heme. Similarly to the iron-binding protein (data not shown), immunostaining for endogenous HO-1 revealed mouse HO-1 expression to be restricted to alveolar macrophages after hyperoxia exposure and to be more abundant in WT compared with TG lungs (Fig. 9), thus indicating a correlation among increased heme metabolism, amount of free iron, and ferritin induction in the macrophage.
Fig. 9.
Hyperoxia-induced HO-1 expression is mainly restricted to pulmonary alveolar macrophages. Immunohistochemistry of paraffin lung sections with an antibody against murine HO-1 demonstrated minimal staining in mice that were exposed to room air (21% O2), whereas immunostaining was intense in macrophages (arrows) of both genotypes exposed to hyperoxia (75% O2) with significantly fewer stained macrophages in the hyperoxic HO-1 TG lungs, compared with WT hyperoxia. The evident epithelial HO-1 signal in the TG lung was due to cross-reactivity with the human HO-1 transgene expressed in type II epithelial cells. Scale bar = 50 μm.
DISCUSSION
The mechanisms triggering oxygen-induced lung injury in BPD infants involve disruption of the pulmonary endothelial cell barrier and increase of vascular permeability with the invasion of airspaces by polymorphonuclear neutrophils with associated alveolar hypoplasia and reduced capillary development (8). In this study, we sought to investigate whether HO-1 can provide alveolar or vascular protection in a model of developmental lung injury induced by hyperoxia. In addition, we were interested in examining whether the effects of HO-1 could be mediated at least in part by the anti-inflammatory and antiapoptotic molecule CO or whether the enzymatic degradation of the prooxidant free heme and consequent metabolism to iron and induction of ferritin also contributed to these beneficial effects. The study of mechanisms of HO-1 protection in the developing lung is relevant because the expression of this inducible gene is maximal during alveolarization (13), and its deficiency in this critical phase disrupts normal lung growth (51). In addition, the mechanisms by which a high concentration of oxygen induces pulmonary damage indicate that hyperoxic injury is at least partially iron mediated (15, 36). Free iron, which catalyzes the production of toxic reactive oxygen species, can be detected in preterm infants (39). HO-1 overexpression has been reported to provide cellular protection against oxidant injury in vitro (24), to attenuate hyperoxia-induced lung damage, and to increase survival in response to lethal hyperoxia in adult rodents (30). HO-1 has also been reported to provide cellular protection against heme-mediated toxicity (3). However, the salutary action of HO-1 overexpression has not been examined in a model of oxygen-induced lung injury during neonatal development, a model relevant to oxygen-induced lung injury in BPD infants.
The present study shows that constitutive overexpression of HO-1 in lung epithelial cells of neonatal mice exposed to prolonged hyperoxia ameliorates lung inflammation, pulmonary arterial remodeling, and RVH. It markedly reduces thickening of alveolar septa (pulmonary edema and interstitial inflammation) and preserves vessel density, thereby ameliorating the histological injury of BPD. The effects of HO-1 overexpression on lung inflammation were seen predominantly as a reduction in the number of neutrophils with a less pronounced effect on macrophages found in BAL fluid. In the developing lung, hyperoxia exposure produces a sustained inflammation that starts early after exposure and continues for the duration of the exposure (12). It has been documented that this sustained inflammatory cascade and the production of cytokines by target cells lead to structural abnormalities and remodeling of the vasculature of the neonatal lung (5, 10, 35). Our laboratory (25, 27, 45) has previously reported protection in adult hypoxia-induced inflammation and PH by lung-specific constitutive or inducible overexpression of HO-1. Therefore, a beneficial effect of the anti-inflammatory actions of HO-1 in developmental oxygen-induced injury could include amelioration of RVH and vascular remodeling. The present study demonstrates complete prevention of cardiac hypertrophy due to hyperoxia with a partial amelioration of lung vascular remodeling, as shown by α-SMA staining. It also demonstrates increased pulmonary blood vessel number in HO-1 overexpressing lungs that is comparable to normoxic controls, suggesting decreased impairment of vessel growth. Vascular wall remodeling in newborn animals exposed to a prohypertensive stimulus has been reported to be more severe than in older animals with vessels manifesting more pronounced medial wall thickening (19, 42) that, as our study shows, can be partially prevented by HO-1. It is thus conceivable that, in our model, it is the preservation of normal lung vessel number in the HO-1 TG lung rather than the partially reduced muscle hypertrophy that is the key determinant of lower vascular resistance leading to the prevention of RVH under hyperoxia. The protective effects of HO-1 in the vasculature may be due in part to the properties of its enzymatic product, CO. Inhaled CO treatment of hyperoxia-exposed neonatal mice resulted in decreased number of inflammatory cells in BAL fluid, attenuated pulmonary arteriolar muscularization, and prevention of RVH with significant preservation of pulmonary blood vessel number. Although we have not examined the mechanisms by which CO confers vascular protection in this injury model, previous reports by us and others (28, 29, 47) suggest an inhibitory effect of CO on vascular smooth muscle cell (VSMC) proliferation and migration as well as potent anti-inflammatory properties of this gaseous molecule limiting lung inflammation (31, 45).
The lung vascular effects of HO-1 may also reside in its endothelial cell-specific protective properties, as HO-1 deficiency in the human results in severe and persistent endothelial damage, with marked signs of iron deposition (50). Hyperoxia-induced lung injury involves endothelial leak, extravasation of hemoglobin and heme into the interstitium and alveolar space, and an increase in the prooxidant state of the lung. Free heme impacts endothelial cell survival and propagates cell injury by inducing the activation of endothelial cells, which results in the recruitment of leukocytes (6). In the present study, HO-1 overexpression preserved endothelial integrity and improved hyperoxia-induced pulmonary edema as demonstrated by a decrease in protein content in BAL fluid and a lower wet-to-dry lung ratio as well as reduced iron deposition in alveolar macrophages, clear features of vascular leakage, and endothelial dysfunction. However, despite reduced endothelial damage achieved by HO-1 overexpression, we only observed a minor improvement of the alveolar structures. Although HO-1 is considered to have a cytoprotective role, the remarkable accumulation of reactive iron during prolonged hyperoxia may overwhelm the lung's defenses and increase the susceptibility to oxygen injury, as suggested in other models of iron accumulation (22, 43). We have observed an induction in ferritin and HO-1 protein expression in the lungs of WT mice exposed to hyperoxia. However, TG mice exhibited lower expression of both proteins, suggesting that this is a secondary effect of the hyperoxic insult and a consequence of heme load. In addition, the expression of lactoferrin, another iron binding protein with antioxidant properties (4), was also not affected by HO-1 overexpression, suggesting that endogenous alveolar macrophage HO-1 and ferritin serve as markers of tissue injury and their expression is reduced in TG mice by the endothelial protection afforded by epithelial HO-1 overexpression. Therefore, HO-1 overexpression in the lungs of TG mice likely protects against early lung injury by preventing endothelial damage and vascular leak and limiting the excess amount of heme that reaches the alveolar space, while in the WT mice excess heme from vascular leak probably overwhelms the binding capacity of alveolar ferritin and propagates more extensive endothelial damage. We can thus speculate that lung tissue and macrophage HO-1 activities work in sequence to counteract the toxic effect of hyperoxic injury, with tissue HO-1 activity being the first line of defense protecting vascular integrity and macrophage HO-1 limiting the injurious effects of excess heme. Although the immunolocalization studies performed here do not allow us to quantify the contribution of endothelial heme breakdown to the total lung heme catabolism, it is likely small since the upregulation of endogenous HO-1 and ferritin expression in the lung vasculature appears to be minimal and is predominantly evident in alveolar macrophages. Previous studies (15) indicate that hyperoxia alone results in a relatively small induction of HO-1 expression in endothelial cells. In this context, HO-1 overexpression in the TG mice could preserve endothelial integrity by means that are completely independent of the ability of the enzyme to degrade large amounts of heme but, rather, depend on one or more of its catalytic products exerting vasculoprotective effects. For instance, biliverdin, previously reported by our laboratory to protect against hypoxia-induced RV failure and oxidative injury (47), is a potent antioxidant molecule that confers vascular cytoprotection against insults that increase reactive oxygen species and cell death (7) acting to maintain an intact barrier function (17). CO also initiates intracellular signaling pathways related to endothelial cell survival, mimicking the effects of HO-1 induction (38). Although the present study does not elucidate the molecular and cellular mechanisms by which CO, potentially resulting from increased HO-1 enzymatic activity in the TGs, may ameliorate the effect of hyperoxia, they likely involve modulation of early response genes that signal the deleterious effects of oxidative stress and inflammation. Gaseous CO is highly diffusible, thereby capable of transmitting signals between different cell types in the pulmonary microvasculature. In this context, epithelial-derived CO may inhibit the proliferation of VSMCs following exposure to hyperoxia by stimulating cGMP levels in a paracrine manner and by modulating the levels of important cell cycle genes that have been previously found to be regulated by cGMP levels, like E2F1 and p21cip1 (29, 32). Alternatively, it may increase the endothelial cGMP content to preserve barrier function and decrease the endothelial expression of mitogens and growth factors acting on VSMC proliferation, similar to the effects of CO in the vessel wall during hypoxia (28). In addition, CO has a vascular protective mechanism against induced apoptosis of endothelial cells. The protective effect of CO against different apoptotic pathways has been found to be associated with the enhanced expression of various antiapoptotic genes that depend in part on activation of MAPK signaling (9, 48). The elucidation of the downstream pathways activated by CO, which is released from the enzymatic activity of the HO-1 transgene, requires further investigation but likely involves molecular targets that modulate endothelial-SMC interactions in the pulmonary microvasculature. Recent clinical studies (for a review, see Ref. 37) have demonstrated a CO-dependent protection in numerous animal models of disease and are currently investigating the potential use of inhaled CO as a treatment for inflammatory lung diseases. In addition, as an alternative to CO inhalation, pharmacologic application of CO-releasing molecules may provide an additional therapeutic avenue as a safer and effective modality of administration.
Overall, we have observed a modest protective effect of HO-1 overexpression on hyperoxia-induced alveolar injury represented by a significant reduction specifically in the degree of septal thickening, indicative of less pulmonary interstitial edema and inflammation. In lung-recovery models of experimental BPD, alveolar enlargement persists even after removing the injury and it has been suggested that this may be partly due to sustained impairment resulting from a signaling molecule induced in the early phases of hyperoxic injury (21), which may not be modulated by HO-1. Our results indicate that the protective action of HO-1 can be dampened in conditions where excessive heme and iron overload further propagate endothelial injury caused by high oxygen tension, such as occurs in BPD. Alternatively, HO-1 may not have an effect on the pulmonary epithelium to promote alveolarization. Instead, the results provided by this study point to a selective, vasculoprotective role of HO-1 in the lung's defense against inflammation, endothelial cell damage, and vascular leak, as well as preservation of blood vessel number, processes critical to normal lung development that are significantly altered by hyperoxia. These findings highlight the importance of developing agents that halt endothelial injury in conditions of heme overload, such as BPD.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants RO1-HL-055454 and RO1-HL-085446 (S. Kourembanas and S. A. Mitsialis).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.F.-G., S.A.M., and S.K. conception and design of research; A.F.-G. and X.L. performed experiments; A.F.-G., S.A.M., and S.K. analyzed data; A.F.-G., S.A.M., and S.K. interpreted results of experiments; A.F.-G. prepared figures; A.F.-G. drafted manuscript; A.F.-G., S.A.M., X.L., and S.K. approved final version of manuscript; S.A.M. and S.K. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Sarah Gately for expert assistance in preparation of this manuscript.
REFERENCES
- 1. Abman SH. Impaired vascular endothelial growth factor signaling in the pathogenesis of neonatal pulmonary vascular disease. Adv Exp Med Biol 661: 323– 335, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Abman SH, Mourani PM, Sontag M. Bronchopulmonary dysplasia: a genetic disease. Pediatrics 122: 658– 659, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Abraham NG, Lavrovsky Y, Schwartzman ML, Stoltz RA, Levere RD, Gerritsen ME, Shibahara S, Kappas A. Transfection of the human heme oxygenase gene into rabbit coronary microvessel endothelial cells: protective effect against heme and hemoglobin toxicity. Proc Natl Acad Sci USA 92: 6798– 6802, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Actor JK, Hwang SA, Kruzel ML. Lactoferrin as a natural immune modulator. Curr Pharm Des 15: 1956– 1973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA, Kourembanas S. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med 180: 1122– 1130, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Balla J, Balla G, Jeney V, Kakuk G, Jacob HS, Vercellotti GM. Ferriporphyrins and endothelium: a 2-edged sword-promotion of oxidation and induction of cytoprotectants. Blood 95: 3442– 3450, 2000 [PubMed] [Google Scholar]
- 7. Baranano DE, Rao M, Ferris CD, Snyder SH. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci USA 99: 16093– 16098, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhandari V. Hyperoxia-derived lung damage in preterm infants. Semin Fetal Neonatal Med 15: 223– 229, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brouard S, Berberat PO, Tobiasch E, Seldon MP, Bach FH, Soares MP. Heme oxygenase-1-derived carbon monoxide requires the activation of transcription factor NF-kappaB to protect endothelial cells from tumor necrosis factor-alpha-mediated apoptosis. J Biol Chem 277: 17950– 17961, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Bry K, Hogmalm A, Backstrom E. Mechanisms of inflammatory lung injury in the neonate: Lessons from a transgenic mouse model of bronchopulmonary dysplasia. Semin Perinatol 34: 211– 221, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Dauger S, Ferkdadji L, Saumon G, Vardon G, Peuchmaur M, Gaultier C, Gallego J. Neonatal exposure to 65% oxygen durably impairs lung architecture and breathing pattern in adult mice. Chest 123: 530– 538, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Deng H, Mason SN, Auten RL., Jr Lung inflammation in hyperoxia can be prevented by antichemokine treatment in newborn rats. Am J Respir Crit Care Med 162: 2316– 2323, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Dennery PA, Lee CS, Ford BS, Weng YH, Yang G, Rodgers PA. Developmental expression of heme oxygenase in the rat lung. Pediatr Res 53: 42– 47, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Fernandez-Gonzalez A, Kourembanas S, Wyatt TA, Mitsialis SA. Mutation of murine adenylate kinase 7 underlies a primary ciliary dyskinesia phenotype. Am J Respir Cell Mol Biol 40: 305– 313, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fogg S, Agarwal A, Nick HS, Visner GA. Iron regulates hyperoxia-dependent human heme oxygenase 1 gene expression in pulmonary endothelial cells. Am J Respir Cell Mol Biol 20: 797– 804, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Harju T, Soini Y, Paakko R, Kinnula VL. Up-regulation of heme oxygenase-i in alveolar macrophages of newly diagnosed asthmatics. Respir Med 96: 418– 423, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Hayashi S, Takamiya R, Yamaguchi T, Matsumoto K, Tojo SJ, Tamatani T, Kitajima M, Makino N, Ishimura Y, Suematsu M. Induction of heme oxygenase-1 suppresses venular leukocyte adhesion elicited by oxidative stress: Role of bilirubin generated by the enzyme. Circ Res 85: 663– 671, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Hayes D, Jr, Feola DJ, Murphy BS, Shook LA, Ballard HO. Pathogenesis of bronchopulmonary dysplasia. Respiration 79: 425– 436, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Kelly DA, Hislop AA, Hall SM, Haworth SG. Relationship between structural remodeling and reactivity in pulmonary resistance arteries from hypertensive piglets. Pediatr Res 58: 525– 530, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Kinsella JP, Greenough A, Abman SH. Bronchopulmonary dysplasia. Lancet 367: 1421– 1431, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Kunig AM, Balasubramaniam V, Markham NE, Morgan D, Montgomery G, Grover TR, Abman SH. Recombinant human VEGF treatment enhances alveolarization after hyperoxic lung injury in neonatal rats. Am J Physiol Lung Cell Mol Physiol 289: L529– L535, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Lee JY, Keep RF, He Y, Sagher O, Hua Y, Xi G. Hemoglobin and iron handling in brain after subarachnoid hemorrhage and the effect of deferoxamine on early brain injury. J Cereb Blood Flow Metab 30: 1793– 1803, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee PJ, Alam J, Sylvester SL, Inamdar N, Otterbein L, Choi AMK. Regulation of heme oxygenase-1 expression in vivo and in vitro in hyperoxic lung injury. Am J Respir Cell Mol Biol 14: 556– 568, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Lee PJ, Alam J, Wiegand GW, Choi AMK. Overexpression of heme oxygenase-1 in human pulmonary epithelial cells results in cell growth arrest and increased resistance to hyperoxia. Proc Natl Acad Sci USA 93: 10393– 10398, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liang OD, Mitsialis SA, Chang MS, Vergadi E, Lee C, Aslam M, Fernandez-Gonzalez A, Liu X, Baveja R, Kourembanas S. Mesenchymal stromal cells expressing heme oxygenase-1 reverse pulmonary hypertension. Stem Cells 29: 99– 107, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maines MD. The heme oxygenase system: A regulator of second messenger gases. Annu Rev Pharmacol Toxicol 37: 517– 554, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Minamino T, Christou H, Hsieh CM, Liu Y, Dhawan V, Abraham NG, Perrella MA, Mitsialis SA, Kourembanas S. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci USA 98: 8798– 8803, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morita T, Kourembanas S. Endothelial cell expression of vasoconstrictors and growth factors is regulated by smooth muscle cell-derived carbon monoxide. J Clin Invest 96: 2676– 2682, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morita T, Mitsialis SA, Koike H, Liu Y, Kourembanas S. Carbon monoxide controls the proliferation of hypoxic vascular smooth muscle cells. J Biol Chem 272: 32804– 32809, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Otterbein LE, Kolls JK, Mantell LL, Cook JL, Alam J, Choi AMK. Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxia-induced lung injury. J Clin Invest 103: 1047– 1054, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Otterbein LE, Mantell LL, Choi AMK. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol Lung Cell Mol Physiol 276: L688– L694, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Otterbein LE, Zuckerbraun BS, Haga M, Liu F, Song R, Usheva A, Stachulak C, Bodyak N, Smith RN, Csizmadia E, Tyagi S, Akamatsu Y, Flavell RJ, Billiar TR, Tzeng E, Bach FH, Choi AM, Soares MP. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat Med 9: 183– 190, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA 94: 10919– 10924, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ryan RM, Ahmed Q, Lakshminrusimha S. Inflammatory mediators in the immunobiology of bronchopulmonary dysplasia. Clin Rev Allergy Immunol 34: 174– 190, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Ryan TP, Krzesicki RF, Blakeman DP, Chin JE, Griffin RL, Richards IM, Aust SD, Petry TW. Pulmonary ferritin: differential effects of hyperoxic lung injury on subunit mRNA levels. Free Radic Biol Med 22: 901– 908, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Ryter SW, Choi AM. Heme oxygenase-1/carbon monoxide: from metabolism to molecular therapy. Am J Respir Cell Mol Biol 41: 251– 260, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ryter SW, Morse D, Choi AM. Carbon monoxide: to boldly go where no has gone before. Sci STKE 2004: RE6– RE15, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Saugstad OD. Bronchopulmonary dysplasia-oxidative stress and antioxidants. Semin Neonatol 8: 39– 49, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Stanford SJ, Hislop AA, Oltmanns U, Nabel EG, Sang H, Haworth SG, Mitchell JA. Transition from placental to air breathing stimulates haem-oxygenase-1 expression without functional consequence for pulmonary vascular adaptation in pigs and mice. Br J Pharmacol 144: 467– 476, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Steinhorn RH. Neonatal pulmonary hypertension. Pediatr Crit Care Med 11: S79– S84, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stenmark KR, Aldashev AA, Orton EC, Durmowicz AG, Badesch DB, Parks WC, Mecham RP, Voelkel NF, Reeves JT. Cellular adaptation during chronic neonatal hypoxic pulmonary hypertension. Am J Physiol Heart Circ Physiol 261: H097– H104, 1991 [DOI] [PubMed] [Google Scholar]
- 43. Suttner DM, Dennery PA. Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J 13: 1800– 1809, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Turi JL, Yang F, Garrick MD, Piantadosi CA, Ghio AJ. The iron cycle and oxidative stress in the lung. Free Radic Biol Med 36: 850– 857, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Vergadi E, Chang MS, Lee C, Liang OD, Liu X, Fernandez-Gonzalez A, Mitsialis SA, Kourembanas S. Early macrophage recruitment and alternative activation are critical for the later development of hypoxia-induced pulmonary hypertension. Circulation 123: 1986– 1995, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Visner GA, Fogg S, Nick HS. Hyperoxia-responsive proteins in rat pulmonary microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 270: L517– L525, 1996 [DOI] [PubMed] [Google Scholar]
- 47. Vitali SH, Mitsialis SA, Liang OD, Liu X, Fernandez-Gonzalez A, Christou H, Wu X, McGowan FX, Kourembanas S. Divergent cardiopulmonary actions of heme oxygenase enzymatic products in chronic hypoxia. PLos One 4: e5978, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang X, Wang Y, Kim HP, Nakahira K, Ryter SW, Choi AM. Carbon monoxide protects against hyperoxia-induced endothelial cell apoptosis by inhibiting reactive oxygen species formation. J Biol Chem 282: 1718– 1726, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol Lung Cell Mol Physiol 275: L110– L117, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest 103: 129– 135, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhuang T, Zhang M, Zhang H, Dennery PA, Lin QS. Disrupted postnatal lung development in heme oxygenase-1 deficient mice. Respir Res 11: 142– 151, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]