Abstract
While the factors that regulate the onset and progression of idiopathic pulmonary fibrosis (IPF) are incompletely understood, recent investigations have revealed that endoplasmic reticulum (ER) stress and activation of the unfolded protein response (UPR) are prominent in alveolar epithelial cells in this disease. Initial observations linking ER stress and IPF were made in cases of familial interstitial pneumonia (FIP), the familial form of IPF, in a family with a mutation in surfactant protein C (SFTPC). Subsequent studies involving lung biopsy specimens revealed that ER stress markers are highly expressed in the alveolar epithelium in IPF and FIP. Recent mouse modeling has revealed that induction of ER stress in the alveolar epithelium predisposed to enhanced lung fibrosis after treatment with bleomycin, which is mediated at least in part by increased alveolar epithelial cell (AEC) apoptosis. Emerging data also indicate that ER stress in AECs could impact fibrotic remodeling by altering inflammatory responses and inducing epithelial-mesenchymal transition. Although the cause of ER stress in IPF remains unknown, common environmental exposures such as herpesviruses, inhaled particulates, and cigarette smoke induce ER stress and are candidates for contributing to AEC dysfunction by this mechanism. Together, investigations to date suggest that ER stress predisposes to AEC dysfunction and subsequent lung fibrosis. However, many questions remain regarding the role of ER stress in initiation and progression of lung fibrosis, including whether ER stress or the UPR could be targeted for therapeutic benefit.
Keywords: familial interstitial pneumonia, herpesvirus, interstitial lung disease, surfactant protein-C, unfolded protein response
endoplasmic reticulum (ER) stress is caused by conditions that perturb the processing and folding of proteins, resulting in the accumulation of misfolded proteins in the ER and activation of the unfolded protein response (UPR; Ref. 68). Several diseases have been linked to misfolded proteins, and over the past decade accumulating evidence suggests a role for ER stress in idiopathic pulmonary fibrosis (IPF; Refs. 29, 35). Initial observations for such an association between ER stress and interstitial lung disease came from evaluation of families with surfactant protein C (SFTPC) mutation associated familial interstitial pneumonia (FIP; Refs. 61, 78). Interestingly, even in the absence of SFTPC mutations, FIP and sporadic IPF are characterized by prominent ER stress in the alveolar epithelial cells (AECs; Refs. 29, 35). Furthermore, cell and mouse modeling of ER stress provides evidence that increased ER stress may be a key component in disease pathogenesis (33). Here, we will review general aspects of ER stress and the UPR and then describe implications of ER stress and the UPR in the pathogenesis of progressive lung fibrosis.
ER Stress and the UPR
The ER is involved in proper folding of membrane and secreted proteins, production of steroids, synthesis of lipids, storage and production of glycogen, and calcium homeostasis (39). Secreted proteins are initially delivered to the ER as an unfolded polypeptide chain. These polypeptides are correctly folded into functional three-dimensional conformations, assembled and glycosylated, and then proceed through the secretory pathway. In normal conditions, folding of proteins in the ER is assisted by chaperone proteins such as immunoglobulin heavy-chain-binding protein (BiP), also known as glucose regulated protein-78 (GRP78). However, when a cell is under stress due to factors such as calcium depletion, metabolic stress, reduced energy stores, elevated protein synthesis, or expression of mutant proteins, activation of the UPR can occur (46). The UPR is designed to improve protein folding, maintain cellular homeostasis, and prevent cell death from accumulation of misfolded proteins that can aggregate and interfere with basic cellular functions (24, 37, 68, 70). The UPR functions through mechanisms that reduce protein translation, increase expression of metabolism and redox proteins, enhance ER chaperone production, and promote protein degradation (11, 18, 19, 30, 36, 38, 44). When these UPR mechanisms fail or if ER stress is too severe, it may lead to growth arrest and cell death through apoptosis.
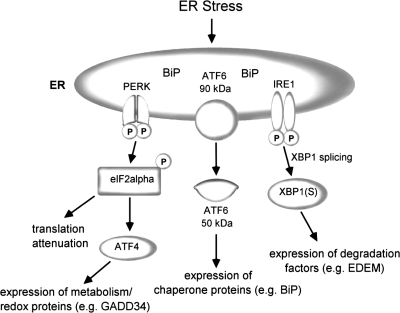
The UPR pathways are governed by three ER transmembrane proteins: PKR-like endoplasmic reticulum kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring enzyme 1 (IRE-1; Refs. 24, 68) (Fig. 1). In the unstressed state, these three proteins are bound by BiP and maintained in an inactive state (45). BiP/GRP78 is the predominant ER chaperone that belongs to the family of heat shock proteins and facilitates protein folding in the ER. Correctly assembled proteins are released from BiP and are transported to the Golgi apparatus. When abnormally folded or improperly assembled proteins remain bound to BiP, they are retained within the ER or degraded. With protein accumulation in the ER, BiP is sequestered away from the three sensors (6), allowing them to assume active conformations and initiate signaling cascades designed to protect the cell from ER stress (68).
Fig. 1.
Schematic illustration of endoplasmic reticulum (ER) stress and the unfolded protein response (UPR). ATF, activating transcription factor; BiP, immunoglobulin heavy-chain-binding protein [glucose regulated protein-78 (GRP78)]; EDEM, ER degradation enhancing α-mannosidase-like protein; eIF2α, eukaryotic initiation factor 2α; GADD34, growth arrest and DNA damage protein 34; IRE, inositol-requiring enzyme 1 (IRE-1); PERK, PKR-like ER kinase; XBP-1, X-box binding protein 1. [Adapted from Isler et al. (24) with permission from the American Society for microbiology.]
PERK senses accumulation of misfolded proteins in the ER and, once activated, undergoes autophosphorylation and dimerization (6, 41). The activated form of PERK phosphorylates and inactivates its only identified target, the α-subunit of eukaryotic translational initiation factor 2 (eIF2α). In all eukaryotic cells, initiation of protein synthesis requires the eIF2 complex and phosphorylation of eIF2α inhibits the initiation of protein synthesis. eIF2α phosphorylation also regulates ATF4-dependent expression of ATF3 and C/EBP homologous protein (CHOP), genes involved in amino acid metabolism, and genes that promote gluthatione biosynthesis (11, 18, 19, 19, 30, 44). In states of chronic ER stress, ATF4-dependent induction of growth arrest and DNA damage inducible gene 34 (GADD34) has been shown to dephosphorylate eIF2α, allowing translational recovery (62).
When BiP is released from ATF6 during ER stress, site 1 and site 2 proteases cleave ATF6 (71, 88), releasing the cytoplasmic domain into the cytosol. The cleaved domain migrates into the nucleus where it binds to cis-acting ER stress response elements (ERSE) and activates the transcription of ER protein-folding chaperones such as BiP, GRP94, calreticulin, calnexin, and protein disulfide isomerase (PDI; Refs. 66, 83, 88, 91). ATF6 exists in two isoforms, ATF6α and ATF6β, in mammalian cells and also induces the expression of X-box binding protein 1 (XBP-1), a transcription factor activated by IRE-1 (38, 91).
IRE-1 is a transmembrane protein that has intrinsic serine/threonine kinase and endonuclease activity that is activated in response to ER stress. In mammals two homologues of IRE-1 exist, IRE1α and IRE1β, which homodimerize upon release from BiP. Dimerized IRE-1 possesses an RNase domain, which cleaves a 26-nucleotide intron sequence from the transcription factor XBP-1. Spliced XBP-1 translocates to the nucleus and binds to ERSE (different from ATF6-binding site) and promotes the transcription of ER associated degradation (ERAD) target genes such as ER degradation enhancing α-mannosidase-like protein (EDEM; Refs. 1, 50). Interestingly, sustained IRE-1 kinase activation can cause oligomerization of RNase domains of IRE-1, resulting in relaxed specificity of the RNase (16). This change allows degradation of many ER-localized mRNAs (in addition to XBP-1) and has the potential to alter the outcome of the ER stress response.
Induction of the ERAD pathway is an important component of the ER stress response. The exact mechanism by which ERAD recognizes and degrades misfolded proteins is not clear but is divided into five steps: 1) recognition of misfolded proteins by EDEM, PDI, or BIP; 2) translocation of the misfolded proteins across the ER membrane into the cytoplasm through translocons mediated via Sec61; 3) ubiquitination of the misfolded proteins by a sequence of enzymatic reactions mediated by E1 (ubiquitin activating enzyme), E2 (ubiquitin conjugating enzyme), and E3 (ubiquitin ligase; Refs. 28, 90); 4) deglycosylation; and 5) transport into the proteasome for degradation (36, 38, 90).
All three UPR pathway sensors are activated to attenuate ER stress and protect the cell. However, prolonged or severe ER stress can result in cellular apoptosis through both extrinsic and intrinsic pathways involving calcium influx from the ER to the cytosol as well as signaling cascades initiated by the UPR through activation of ER bound caspase-4 (and its mouse homolog, caspase-12; Refs. 21, 63, 68, 77). Caspase-12 is activated under ER stress and mediates cell death pathways through caspase-3 and caspase-9 (92). Another transcription factor involved in ER stress induced apoptosis is CHOP, which is activated by all three UPR pathways, as ATF4, ATF6, and XBP-1 all have binding sites in the CHOP gene promoter (17, 43). c-Jun NH2-terminal kinase (JNK) has also been implicated in UPR-mediated apoptosis (58, 81) The manner by which JNK leads to apoptosis is not yet clearly determined, but a mitochondrial mechanism through the BCL2 family, release of cytochrome c into cytoplasm from mitochondria, formation of apoptotic proteasome activating factor 1 (Apaf1), and cleavage of procaspases 3, 6, and 7 have been reported (31). Another mechanism through which cells cope with ER stress is autophagy (23), a cellular response to degrade long-lived proteins that accumulate in the ER, occurring through activation of the lysosomal pathway. Several studies (64, 68) have linked ER stress to autophagy. However, it is not clear whether ER stress-mediated autophagy protects cells from undergoing apoptosis, as some data (13, 14, 65, 76) suggest that it also induces cell death.
ER Stress in Familial and Sporadic IPF
ER stress and the UPR have been associated with a number of diseases, including Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis, diabetes mellitus, cystic fibrosis, and more recently FIP and sporadic IPF. IPF is characterized pathologically as usual interstitial pneumonia (UIP). FIP is most commonly associated with UIP on lung biopsy, but lung pathology associated with other idiopathic interstitial pneumonias can be seen in some affected family members (72). FIP accounts for 2–20% of cases of IPF, depending on different reports (22, 42, 51, 82).
In 2001, Nogee et al. (61) first reported a mutation in the carboxy-terminal region of surfactant protein-C (SFTPC) that resulted in deletion of exon 4 and its 37 amino acids (Δexon4) in an infant with nonspecific interstial pneumonia whose mother had desquamative interstitial pneumonia. Subsequently, our group (78) described a different SFTPC mutation that resulted in the substitution of glutamine for leucine at amino acid position 188 (L188Q) of pro-SP-C in a kindred with 11 adults with UIP and 3 children with nonspecific interstial pneumonia (78). Since then, SFTPC mutations have been described in other pediatric cases of interstitial lung disease (60) and adult cases of FIP (82). SFTPC transcription and translation results in a 197 amino acid precursor protein (pro-SP-C; Ref. 86) that enters the ER where folding of the carboxy-terminal region is performed followed by processing through the secretory pathway until mature SP-C protein is packaged in the lamellar body before secretion into the alveolar space (59, 86). In vitro studies (4, 5, 26, 53) reveal that mutations in the carboxy-terminal region of SFTPC result in abnormal processing and protein misfolding, with accumulation in the ER. Modeling of the Δexon4 and L188Q mutations in vitro demonstrated that these mutant forms of pro-SP-C cause ER stress in AEC lines, including human A549 cells (35, 52, 53), mouse lung epithelial cells (MLE12; Ref. 35), and rat lung epithelial cells (RLE6TN; Ref. 74). In addition to SFTPC, mutations in SFTPA2, one of the two isoforms of surfactant protein-A, have been linked to FIP (84). In 2009, Wang et al. (84) reported two different FIP families in which rare missense mutations were identified in SFTPA2, one resulting in the substitution of valine for glycine at codon 231 (G231V) and the other in the substitution of serine for phenylalanine at codon 198 (F198S). When both of these mutations were expressed in A549 cells, the mutant surfactant protein-A forms were retained within the ER, leading to ER stress and UPR pathway activation (48, 84), suggesting a similar role for ER stress in SFTPA2 mutation associated pulmonary fibrosis to that seen with SFTPC mutations.
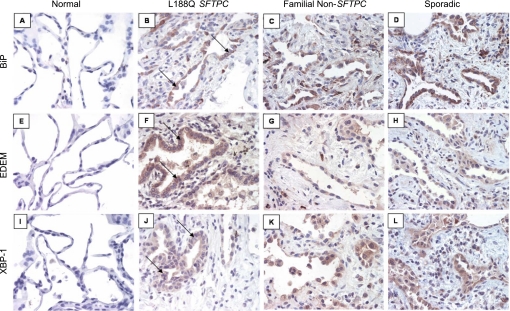
While frequently encountered in childhood ILD, SFTPC mutations are rare in IPF (34). Nevertheless, their association with FIP highlights the importance of AECs in the initiation and early pathogenesis of IPF since surfactant proteins are produced in the lungs exclusively by type II AECs (61, 78). Investigation of the mechanisms involved in SFTPC mutation associated FIP led our group and others to evaluate the role of ER stress in other forms of IPF. In 2008, we (35) reported immunohistochemistry for markers of ER stress and UPR activation on lung tissue from FIP patients with the L188Q SFTPC mutation, FIP patients without SFTPC mutations, and sporadic IPF patients. We found that AECs lining areas of lung fibrosis were positive for BiP, EDEM, and XBP-1 expression in all three categories (Fig. 2). Similarly, Korfei et al. (29) showed that AECs lining areas of fibrosis in IPF had ER stress. Thus ER stress was identified in IPF lungs even in the absence of SFTPC mutations.
Fig. 2.
Immunohistochemistry (IHC) for components of the UPR in idiopathic pulmonary fibrosis (IPF). Lung tissue was obtained from a normal lung (A, E, and I), individuals with usual interstitial pneumonia (UIP) on biopsy from the L188Q SFTPC mutation family (B, F, and J), an individual with familial pulmonary fibrosis with UIP by biopsy without an SFTPC mutation (C, G, and K), and an individual with sporadic IPF/UIP (D, H, and L). IHC for BiP (A), EDEM (E), and XBP-1 (I) revealed little or no staining in normal lung. IHC for BiP (B), EDEM (F), and XBP-1 (J) in L188Q SFTPC-associated UIP revealed positive staining in epithelial cells lining areas of affected lung (arrows). In familial UIP in the absence of SFTPC mutations and sporadic IPF/UIP, immunostaining for BiP, EDEM, and XBP-1 was also noted in the lining epithelial cells. All images are magnification of ×200. Immunostaining controls with secondary antibody only were negative. [From Lawson et al. (35)].
Potential Mechanisms Linking ER Stress and Lung Fibrosis
Following these important observations suggesting a role for ER stress in FIP and IPF, several studies have highlighted potential mechanisms by which ER stress may contribute to the development and progression of lung fibrosis.
AEC injury and apoptosis.
When considering how ER stress may impact disease pathogenesis, most studies have focused on ER stress-induced apoptosis. In vitro experiments with expression of both the Δexon4 and L188Q SFTPC mutations have revealed that ER stress is accompanied by increased AEC death (35, 52, 53). In evaluations of the Δexon4 and L188Q SFTPC mutations, Mulugeta et al. (52, 53) noted that increased AEC death was associated with increased caspase activity, specifically through a caspase-4 (caspase-12) mechanism.
To better evaluate for potential mechanistic relationships between ER stress and lung fibrosis, mouse models have been employed. In 2003, Bridges et al. (10) reported a transgenic mouse with expression of the SFTPCΔexon4 mutation under the human SFTPC promoter during lung development. These mice had abnormal lung morphogenesis resulting in fetal death, with findings supporting protein misfolding and aberrant surfactant processing (10). Subsequently, we developed a transgenic model based on the tet-on system to conditionally express mutant L188Q SFTPC exclusively in type II AECs. In the absence of doxycycline, these mice grew and developed normally. Once in adulthood, L188Q SFTPC expression in these mice resulted in ER stress in type II AECs but no evidence of increased apoptosis in the lungs or fibrosis (33). However, following low-dose bleomycin, greater lung fibrosis, enhanced AEC death, and increased caspase pathway activation were observed with mutant SFTPC expression. As a confirmatory model for effects of ER stress, we exposed wild-type mice to tunicamycin, an agent commonly used to induce ER stress, followed by low dose bleomycin. As with L188Q SFTPC expression, tunicamycin induced ER stress in AECs but did not cause lung fibrosis. However, in combination with bleomycin, tunicamycin-treated mice developed enhanced lung fibrosis and increased AEC apoptosis.
In human IPF lung tissue, Korfei et al. (29) showed apoptotic pathway activation in the same AECs lining areas of fibrosis that prominently expressed ER stress markers. Thus these cell, animal, and human studies suggest that ER stress may impact disease pathogenesis by leading to enhanced AEC injury and death.
Inflammation.
In some systems, ER stress and inflammation are interconnected by UPR pathways through activation of JNK, p38 mitogen-activated protein kinases, and NF-κB (67). In AECs, Maquire et al. (47) analyzed the Δexon4 SFTPC mutation in vitro and noted NF-κB pathway activation. However, their investigations of the L188Q SFTPC mutation did not find such an association. Our in vivo mouse modeling did not reveal enhanced lung inflammation with mutant L188Q SFTPC expression (33). Although interactions between the UPR and inflammatory signaling pathways could impact fibrotic remodeling, the inflammatory effects of ER stress likely vary considerably depending on the cause, severity, and duration of ER stress.
Regulation of cell phenotype.
A variety of studies (27, 75, 87) have suggested that epithelial cells in the lungs and other organs can contribute to fibrosis by undergoing epithelial-mesenchymal transition (EMT). Ulianich et al. (80) first demonstrated that ER stress induces thyroid epithelial cells to undergo EMT. In lung AEC lines, we recently reported that RLE6TN cells expressing mutant L188Q SFTPC or following exposure to tunicamycin develop ER stress accompanied by EMT (74). Zhong et al. (93) found a similar relationship with the Δexon4 SFTPC mutation, as well as tunicamycin. In our studies, we (74) observed that inhibition of smad2 and src signaling, whether through small molecule inhibition or small interfering RNA targeting, attenuated ER stress-induced EMT. Furthermore, small interfering RNA silencing of IRE1α attenuated the effects of ER stress on smad2 and src pathway activation, suggesting that this UPR arm was involved in ER stress-induced EMT (74). Taken together, these studies suggest that induction of ER stress may lead to a profibrotic AEC phenotype through EMT. Recently, a study by Baek et al. (2) revealed that UPR activation may also be involved in myofibroblast differentiation of lung fibroblasts, extending this potential association between ER stress and profibrotic cell phenotypes. Ultimately, these observations will require in vivo animal modeling to elucidate their significance.
Potential Causes of ER Stress in IPF
As described above, a combination of cell culture studies, animal models, and human pathological evaluation suggests that ER stress has a role in lung fibrosis. While mutant pro-SP-C is the underlying culprit in SFTPC mutation-mediated FIP, the cause of ER stress in other families with FIP and sporadic IPF remains unknown. Certainly, aberrant protein processing (including surfactant) remains a possibility and a target for future investigations. It is also possible that AECs, in their attempt to regenerate the alveolar epithelium after injury, may inherently be under ER stress and UPR activation in their hyperplastic state secondary to intrinsic factors such as increased metabolic demand, thus perpetuating an ongoing injury-remodeling cycle. Finally, exogenous exposures could contribute to ER stress, including herpesviruses, cigarette smoke, and inhaled particulates, all of which have been shown to induce ER stress in lung epithelium (24, 25, 32, 49).
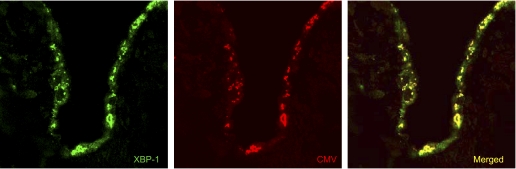
Studies from several groups have implicated herpesviruses as potentially important in the pathogenesis of IPF. Increased titers of antibodies to cytomegalovirus and Epstein Barr virus have been noted in IPF patients (89). Herpesvirus antigens can be detected in AECs lining areas of fibrosis in IPF lung biopsy samples but not in lung epithelium of controls (15, 35, 73). Interestingly, herpesviruses are known to cause ER stress and activate the UPR (24). We observed that herpesvirus proteins (Epstein Barr virus, cytomegalovirus, and Kaposi's sarcoma herpesvirus) were expressed in AECs lining areas of fibrosis in IPF and that these viral proteins colocalized with ER stress markers (Fig. 3; Ref. 35). Taken together, available data suggest that herpesviruses are involved in the pathogenesis of IPF and raise questions as to whether they could exert their effects through ER stress.
Fig. 3.
Colocalization of XBP-1 and cytomegalovirus (CMV) late antigens in alveolar epithelial cells from an individual with UIP. Confocal laser scanning microscopy with Z-stack imaging was performed on lung tissue sections. Immunofluorescence for XBP-1 (green) and CMV (red) identify expression in epithelial cells lining areas of fibrosis. On dual fluorescence imaging in a single Z-stack plane, coexpression of these proteins (yellow) is detected in alveolar epithelium. Magnification = ×200. [From Lawson et al. (35).]
Exposure to inhaled particulate matter has been associated with a variety of local and systemic conditions, including pulmonary fibrosis, chronic obstructive pulmonary disease, and cardiovascular disease (40). Inhaled environmentally derived fine particulates have been shown to activate several profibrotic intracellular signaling pathways that could contribute to lung fibrosis (8). Furthermore, recent in vitro and in vivo studies (32, 85) have demonstrated that particulate matter induces ER stress and activates all three arms of the UPR pathway in lung epithelial cells and promotes apoptosis.
Cigarette smoke has been associated with the development of both IPF (3) and FIP (72). Several studies have found that cigarette smoke exposure can activate the UPR. In vitro exposure of Swiss 3T3 cells to aqueous extracts of cigarette smoke was associated with PERK and ATF4 activation, resulting in upregulation of BiP and other ER stress associated genes (9, 20). Additional evaluations in normal and malignant lung epithelial cells similarly revealed that expression of UPR-related genes was induced by cigarette smoke exposure (25). Together, a variety of studies have shown that environmental stimuli relevant to lung fibrosis can cause ER stress and UPR activation. Whether any or all of these environmental factors impact fibrotic remodeling through ER stress induction is an area that requires further study.
Effects of Aging on ER Stress Responses in the Lungs
Aging has long been considered a central player in many chronic diseases and recently has been implicated as a major cofactor in the development of IPF (12, 69), with evidence suggesting that the aged lung is at greater risk for the development of lung fibrosis following injury (57, 69). Interestingly, aging leads to both a decline in protein folding ability in cells and in impaired ability of UPR responses to maintain cellular homeostasis in the setting of ER stress (54, 55). With aging, studies have demonstrated increased proapoptotic signaling because of impaired UPR responses (56) and age-related impairments in ubiquitin-proteosome-mediated degradation pathways (7). Recently, Torres-Gonzalez et al. (79) demonstrated that infection with murine gamma herpesvirus 68 (MHV68) results in the development of lung fibrosis in aged mice, whereas young mice do not develop lung fibrosis. After MHV68 infection, aged mice developed greater ER stress in the AEC population as evidenced by increased BiP expression and increased XBP1 splicing, as well as increased AEC apoptosis, compared with young mice. The results of this study support the possibility that aging of the lung leads to diminished ability of the AEC population to maintain homeostasis in the setting of ER stressors. Taken together, these observations raise questions about the degree to which age-related impairments in UPR responses may contribute to IPF pathogenesis, consistent with speculation that IPF is a disease of aging in the lungs.
Unanswered Questions and Future Directions
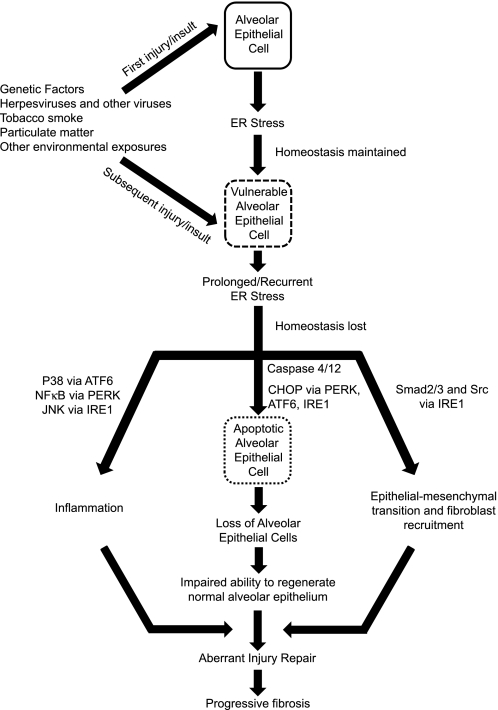
While increasing evidence suggests a role for ER stress in IPF, the mechanisms behind such an association need to be further delineated. Given the experimental evidence to date, we speculate that ER stress leads to a vulnerable AEC population that is then highly sensitive to additional environmental insults, which together lead to aberrancies in epithelial repair processes, thus propagating fibrosis (Fig. 4). Many questions remain in determining the role for ER stress in IPF. Specifically, it will be important to determine which environmental insults lead to ER stress in human lungs and why induction of ER stress alone does not cause lung fibrosis in experimental animals. Although type II AECs appear to be the primary target for ER stress in lung fibrosis, other cells, including fibroblasts, could regulate fibrosis through UPR activation. Determining which UPR pathways are most important and which ER stress induced cellular phenotypes (inflammatory, apoptotic, or EMT) regulate fibrotic remodeling will be important for designing effective therapies to limit ER stress effects on lung fibrosis. Given that the UPR is designed to protect the cell, it is quite possible that trying to attenuate the ER stress response might have deleterious effects. Thus it may be better to develop agents that improve protein processing or block downstream signaling. To help address these and many other questions and better understand the role that ER stress plays in IPF, additional work is needed. In vitro studies have the potential to define specific targets or pathways of interest, but ultimately animal modeling will likely provide the best chance for delineating mechanistic relationships and will provide opportunities for testing new therapeutic strategies.
Fig. 4.
Schematic of proposed mechanisms by which ER stress may contribute to the development of pulmonary fibrosis. CHOP, C/EBP homologous protein.
Conclusions
With this review, we provide a glimpse into the potential roles that ER stress and UPR pathway activation may play in the pathogenesis of IPF. Taken together, several lines of evidence now suggest a prominent role for ER stress in IPF, supporting the concept that dysfunctional AECs facilitate the progression of lung fibrosis. An improved understanding of factors involved in UPR pathway activation has the potential to suggest new therapeutic avenues to pursue for this devastating disease with limited therapies.
GRANTS
This manuscript was supported by National Heart, Lung, and Blood Institute Grants HL-085317, HL-092870, HL-085406, and HL105479 and the Francis Families Foundation. H. Tanjore is a Parker B. Francis Fellow in Pulmonary Research.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.T., T.S.B., and W.E.L. conception and design of research; H.T., T.S.B., and W.E.L. performed experiments; H.T., T.S.B., and W.E.L. analyzed data; H.T., T.S.B., and W.E.L. interpreted results of experiments; H.T., T.S.B., and W.E.L. prepared figures; H.T., T.S.B., and W.E.L. drafted manuscript; H.T., T.S.B., and W.E.L. edited and revised manuscript; H.T., T.S.B., and W.E.L. approved final version of manuscript.
REFERENCES
- 1. Back SH, Lee K, Vink E, Kaufman RJ. Cytoplasmic IRE1alpha-mediated XBP1 mRNA splicing in the absence of nuclear processing and endoplasmic reticulum stress. J Biol Chem 281: 18691–18706, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Baek HA, Kim DS, Park HS, Jang KY, Kang MJ, Lee DG, Moon WS, Chae HJ, Chung MJ. Involvement of endoplasmic reticulum stress in myofibroblastic differentiation of lung fibroblasts. Am J Respir Cell Mol Biol 2011. August 18 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3. Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 155: 242–248, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Beers MF, Lomax CA, Russo SJ. Synthetic processing of surfactant protein C by alevolar epithelial cells. The COOH terminus of proSP-C is required for post-translational targeting and proteolysis. J Biol Chem 273: 15287–15293, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Beers MF, Mulugeta S. Surfactant protein C biosynthesis and its emerging role in conformational lung disease. Annu Rev Physiol 67: 663–696, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2: 326–332, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Bodas M, Min T, Vij N. Early-age-related changes in proteostasis augment immunopathogenesis of sepsis and acute lung injury. PLos One 5: e15480, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bonner JC. Lung fibrotic responses to particle exposure. Toxicol Pathol 35: 148–153, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Bosio A, Knorr C, Janssen U, Gebel S, Haussmann HJ, Muller T. Kinetics of gene expression profiling in Swiss 3T3 cells exposed to aqueous extracts of cigarette smoke. Carcinogenesis 23: 741–748, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Bridges JP, Wert SE, Nogee LM, Weaver TE. Expression of a human surfactant protein C mutation associated with interstitial lung disease disrupts lung development in transgenic mice. J Biol Chem 278: 52739–52746, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Brush MH, Weiser DC, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol Cell Biol 23: 1292–1303, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Castriotta RJ, Eldadah BA, Foster WM, Halter JB, Hazzard WR, Kiley JP, King TE, Jr, Horne FM, Nayfield SG, Reynolds HY, Schmader KE, Toews GB, High KP. Workshop on idiopathic pulmonary fibrosis in older adults. Chest 138: 693–703, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ding WX, Ni HM, Gao W, Hou YF, Melan MA, Chen X, Stolz DB, Shao ZM, Yin XM. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J Biol Chem 282: 4702–4710, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Ding WX, Ni HM, Gao W, Yoshimori T, Stolz DB, Ron D, Yin XM. Linking of autophagy to ubiquitin-proteasome system is important for the regulation of endoplasmic reticulum stress and cell viability. Am J Pathol 171: 513–524, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Egan JJ, Stewart JP, Hasleton PS, Arrand JR, Carroll KB, Woodcock AA. Epstein-Barr virus replication within pulmonary epithelial cells in cryptogenic fibrosing alveolitis. Thorax 50: 1234–1239, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, Backes BJ, Oakes SA, Papa FR. IRE1 kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell 138: 562–575, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell 7: 1153–1163, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5: 897–904, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11: 619–633, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Hengstermann A, Muller T. Endoplasmic reticulum stress induced by aqueous extracts of cigarette smoke in 3T3 cells activates the unfolded-protein-response-dependent PERK pathway of cell survival. Free Radic Biol Med 44: 1097–1107, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, Manabe T, Yamagishi S, Bando Y, Imaizumi K, Tsujimoto Y, Tohyama M. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J Cell Biol 165: 347–356, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hodgson U, Laitinen T, Tukiainen P. Nationwide prevalence of sporadic and familial idiopathic pulmonary fibrosis: evidence of founder effect among multiplex families in Finland. Thorax 57: 338–342, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoyer-Hansen M, Jaattela M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ 14: 1576–1582, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Isler JA, Skalet AH, Alwine JC. Human cytomegalovirus infection activates and regulates the unfolded protein response. J Virol 79: 6890–6899, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jorgensen E, Stinson A, Shan L, Yang J, Gietl D, Albino AP. Cigarette smoke induces endoplasmic reticulum stress and the unfolded protein response in normal and malignant human lung cells. BMC Cancer 8: 229, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keller A, Steinhilber W, Schafer KP, Voss T. The C-terminal domain of the pulmonary surfactant protein C precursor contains signals for intracellular targeting. Am J Respir Cell Mol Biol 6: 601–608, 1992 [DOI] [PubMed] [Google Scholar]
- 27. Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA 103: 13180–13185, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kincaid MM, Cooper AA. ERADicate ER stress or die trying. Antioxid Redox Signal 9: 2373–2387, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, Koch M, Lang G, Fink L, Bohle RM, Seeger W, Weaver TE, Guenther A. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 178: 838–846, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krishnamoorthy T, Pavitt GD, Zhang F, Dever TE, Hinnebusch AG. Tight binding of the phosphorylated alpha subunit of initiation factor 2 (eIF2alpha) to the regulatory subunits of guanine nucleotide exchange factor eIF2B is required for inhibition of translation initiation. Mol Cell Biol 21: 5018–5030, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuo WH, Chen JH, Lin HH, Chen BC, Hsu JD, Wang CJ. Induction of apoptosis in the lung tissue from rats exposed to cigarette smoke involves p38/JNK MAPK pathway. Chem Biol Interact 155: 31–42, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Laing S, Wang G, Briazova T, Zhang C, Wang A, Zheng Z, Gow A, Chen AF, Rajagopalan S, Chen LC, Sun Q, Zhang K. Airborne particulate matter selectively activates endoplasmic reticulum stress response in the lung and liver tissues. Am J Physiol Cell Physiol 299: C736–C749, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lawson WE, Cheng DS, Degryse AL, Tanjore H, Polosukhin VV, Xu XC, Newcomb DC, Jones BR, Roldan J, Lane KB, Morrisey EE, Beers MF, Yull FE, Blackwell TS. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc Natl Acad Sci USA 108: 10562–10567, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lawson WE, Grant SW, Ambrosini V, Womble KE, Dawson EP, Lane KB, Markin C, Renzoni E, Lympany P, Thomas AQ, Roldan J, Scott TA, Blackwell TS, Phillips JA, III, Loyd JE, du Bois RM. Genetic mutations in surfactant protein C are a rare cause of sporadic cases of IPF. Thorax 59: 977–980, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, Lane KB, Blackwell TR, Xu C, Markin C, Ware LB, Miller GG, Loyd JE, Blackwell TS. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol 294: L1119–L1126, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23: 7448–7459, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 35: 373–381, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Lee K, Tirasophon W, Shen X, Michalak M, Prywes R, Okada T, Yoshida H, Mori K, Kaufman RJ. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev 16: 452–466, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lin JH, Walter P, Yen TS. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol 3: 399–425, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ling SH, van Eeden SF. Particulate matter air pollution exposure: role in the development and exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 4: 233–243, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu CY, Schroder M, Kaufman RJ. Ligand-independent dimerization activates the stress response kinases IRE1 and PERK in the lumen of the endoplasmic reticulum. J Biol Chem 275: 24881–24885, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Loyd JE. Pulmonary fibrosis in families. Am J Respir Cell Mol Biol 29: S47–S50, 2003 [PubMed] [Google Scholar]
- 43. Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol 318: 1351–1365, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Ma Y, Hendershot LM. Delineation of a negative feedback regulatory loop that controls protein translation during endoplasmic reticulum stress. J Biol Chem 278: 34864–34873, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Ma Y, Hendershot LM. ER chaperone functions during normal and stress conditions. J Chem Neuroanat 28: 51–65, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Ma Y, Hendershot LM. The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer 4: 966–977, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Maguire JA, Mulugeta S, Beers MF. Endoplasmic reticulum stress induced by surfactant protein c BRICHOS mutants promotes proinflammatory signaling by epithelial cells. Am J Respir Cell Mol Biol 44: 404–414, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maitra M, Wang Y, Gerard RD, Mendelson CR, Garcia CK. Surfactant protein A2 mutations associated with pulmonary fibrosis lead to protein instability and endoplasmic reticulum stress. J Biol Chem 285: 22103–22113, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Malhotra D, Thimmulappa R, Vij N, Navas-Acien A, Sussan T, Merali S, Zhang L, Kelsen SG, Myers A, Wise R, Tuder R, Biswal S. Heightened endoplasmic reticulum stress in the lungs of patients with chronic obstructive pulmonary disease: the role of Nrf2-regulated proteasomal activity. Am J Respir Crit Care Med 180: 1196–1207, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50. Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol 18: 716–731, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Marshall RP, Puddicombe A, Cookson WO, Laurent GJ. Adult familial cryptogenic fibrosing alveolitis in the United Kingdom. Thorax 55: 143–146, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mulugeta S, Nguyen V, Russo SJ, Muniswamy M, Beers MF. A surfactant protein C precursor protein BRICHOS domain mutation causes endoplasmic reticulum stress, proteasome dysfunction, and caspase 3 activation. Am J Respir Cell Mol Biol 32: 521–530, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mulugeta S, Maguire JA, Newitt JL, Russo SJ, Kotorashvili A, Beers MF. Misfolded BRICHOS SP-C mutant proteins induce apoptosis via caspase-4- and cytochrome c-related mechanisms. Am J Physiol Lung Cell Mol Physiol 293: L720–L729, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Naidoo N. ER and aging–protein folding and the ER stress response. Ageing Res Rev 8: 150–159, 2009 [DOI] [PubMed] [Google Scholar]
- 55. Naidoo N. The endoplasmic reticulum stress response and aging. Rev Neurosci 20: 23–37, 2009 [DOI] [PubMed] [Google Scholar]
- 56. Naidoo N, Ferber M, Master M, Zhu Y, Pack AI. Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling. J Neurosci 28: 6539–6548, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Naik PK, Moore BB. Viral infection and aging as cofactors for the development of pulmonary fibrosis. Expert Rev Respir Med 4: 759–771, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nishitoh H, Saitoh M, Mochida Y, Takeda K, Nakano H, Rothe M, Miyazono K, Ichijo H. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol Cell 2: 389–395, 1998 [DOI] [PubMed] [Google Scholar]
- 59. Nogee LM. Abnormal expression of surfactant protein C and lung disease. Am J Respir Cell Mol Biol 26: 641–644, 2002 [DOI] [PubMed] [Google Scholar]
- 60. Nogee LM, Dunbar AE, III, Wert S, Askin F, Hamvas A, Whitsett JA. Mutations in the surfactant protein C gene associated with interstitial lung disease. Chest 121: 20S–21S, 2002 [DOI] [PubMed] [Google Scholar]
- 61. Nogee LM, Dunbar AE, III, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med 344: 573–579, 2001 [DOI] [PubMed] [Google Scholar]
- 62. Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2+. J Cell Biol 153: 1011–1022, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Obeng EA, Boise LH. Caspase-12 and caspase-4 are not required for caspase-dependent endoplasmic reticulum stress-induced apoptosis. J Biol Chem 280: 29578–29587, 2005 [DOI] [PubMed] [Google Scholar]
- 64. Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 26: 9220–9231, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Oh S, Lim S. Endoplasmic reticulum stress-mediated autophagy/apoptosis induced by capsaicin and dihydrocapsaicin (DHC) is regulated by the extent of JNK/ERK activation in WI38 lung epithelial fibroblast cells. J Pharmacol Exp Ther 329: 112–122, 2009 [DOI] [PubMed] [Google Scholar]
- 66. Okada T, Yoshida H, Akazawa R, Negishi M, Mori K. Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J 366: 585–594, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rath E, Haller D. Inflammation and cellular stress: a mechanistic link between immune-mediated and metabolically driven pathologies. Eur J Nutr 50: 219–233, 2011 [DOI] [PubMed] [Google Scholar]
- 68. Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem 74: 739–789, 2005 [DOI] [PubMed] [Google Scholar]
- 69. Selman M, Rojas M, Mora AL, Pardo A. Aging and interstitial lung diseases: unraveling an old forgotten player in the pathogenesis of lung fibrosis. Semin Respir Crit Care Med 31: 607–617, 2010 [DOI] [PubMed] [Google Scholar]
- 70. Shang J. Quantitative measurement of events in the mammalian unfolded protein response. Methods 35: 390–394, 2005 [DOI] [PubMed] [Google Scholar]
- 71. Shen J, Prywes R. Dependence of site-2 protease cleavage of ATF6 on prior site-1 protease digestion is determined by the size of the luminal domain of ATF6. J Biol Chem 279: 43046–43051, 2004 [DOI] [PubMed] [Google Scholar]
- 72. Steele MP, Speer MC, Loyd JE, Brown KK, Herron A, Slifer SH, Burch LH, Wahidi MM, Phillips JA, III, Sporn TA, McAdams HP, Schwarz MI, Schwartz DA. Clinical and pathologic features of familial interstitial pneumonia. Am J Respir Crit Care Med 172: 1146–1152, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stewart JP, Egan JJ, Ross AJ, Kelly BG, Lok SS, Hasleton PS, Woodcock AA. The detection of Epstein-Barr virus DNA in lung tissue from patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 159: 1336–1341, 1999 [DOI] [PubMed] [Google Scholar]
- 74. Tanjore H, Cheng DS, Degryse AL, Zoz DF, Abdolrasulnia R, Lawson WE, Blackwell TS. Alveolar epithelial cells undergo epithelial-to-mesenchymal transition in response to endoplasmic reticulum stress. J Biol Chem 286: 30972–30980, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tanjore H, Xu XC, Polosukhin VV, Degryse AL, Li B, Han W, Sherrill TP, Plieth D, Neilson EG, Blackwell TS, Lawson WE. Contribution of epithelial derived fibroblasts to bleomycin induced lung fibrosis. Am J Respir Crit Care Med 657–665, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Teckman JH, Perlmutter DH. Retention of mutant α1-antitrypsin Z in endoplasmic reticulum is associated with an autophagic response. Am J Physiol Gastrointest Liver Physiol 279: G961–G974, 2000 [DOI] [PubMed] [Google Scholar]
- 77. Tessitore A, del PM, Sano R, Ma Y, Mann L, Ingrassia A, Laywell ED, Steindler DA, Hendershot LM, d'Azzo A. GM1-ganglioside-mediated activation of the unfolded protein response causes neuronal death in a neurodegenerative gangliosidosis. Mol Cell 15: 753–766, 2004 [DOI] [PubMed] [Google Scholar]
- 78. Thomas AQ, Lane K, Phillips J, III, Prince M, Markin C, Speer M, Schwartz DA, Gaddipati R, Marney A, Johnson J, Roberts R, Haines J, Stahlman M, Loyd JE. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med 165: 1322–1328, 2002 [DOI] [PubMed] [Google Scholar]
- 79. Torres-Gonzalez E, Bueno M, Tanaka A, Krug LT, Cheng DS, Polosukhin VV, Sorescu D, Lawson WE, Blackwell TS, Rojas M, Mora AL. Role of endoplasmic reticulum stress in age-related susceptibility to lung fibrosis. Am J Respir Cell Mol Biol 2012. January 12 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ulianich L, Garbi C, Treglia AS, Punzi D, Miele C, Raciti GA, Beguinot F, Consiglio E, Di Jeso B. ER stress is associated with dedifferentiation and an epithelial-to-mesenchymal transition-like phenotype in PC Cl3 thyroid cells. J Cell Sci 121: 477–486, 2008 [DOI] [PubMed] [Google Scholar]
- 81. Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287: 664–666, 2000 [DOI] [PubMed] [Google Scholar]
- 82. van Moorsel CH, van Oosterhout MF, Barlo NP, de Jong PA, van d V, Ruven HJ, van Es HW, van den Bosch JM, Grutters JC. Surfactant protein C mutations are the basis of a significant portion of adult familial pulmonary fibrosis in a dutch cohort. Am J Respir Crit Care Med 182: 1419–1425, 2010 [DOI] [PubMed] [Google Scholar]
- 83. Wang Y, Shen J, Arenzana N, Tirasophon W, Kaufman RJ, Prywes R. Activation of ATF6 and an ATF6 DNA binding site by the endoplasmic reticulum stress response. J Biol Chem 275: 27013–27020, 2000 [DOI] [PubMed] [Google Scholar]
- 84. Wang Y, Kuan PJ, Xing C, Cronkhite JT, Torres F, Rosenblatt RL, DiMaio JM, Kinch LN, Grishin NV, Garcia CK. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet 84: 52–59, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Watterson TL, Hamilton B, Martin R, Coulombe RA., Jr Urban particulate matter causes ER stress and the unfolded protein response in human lung cells. Toxicol Sci 112: 111–122, 2009 [DOI] [PubMed] [Google Scholar]
- 86. Weaver TE. Synthesis, processing and secretion of surfactant proteins B and C. Biochim Biophys Acta 1408: 173–179, 1998 [DOI] [PubMed] [Google Scholar]
- 87. Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol 166: 1321–1332, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, Brown MS, Goldstein JL. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell 6: 1355–1364, 2000 [DOI] [PubMed] [Google Scholar]
- 89. Yonemaru M, Kasuga I, Kusumoto H, Kunisawa A, Kiyokawa H, Kuwabara S, Ichinose Y, Toyama K. Elevation of antibodies to cytomegalovirus and other herpes viruses in pulmonary fibrosis. Eur Respir J 10: 2040–2045, 1997 [DOI] [PubMed] [Google Scholar]
- 90. Yoshida H. ER stress and diseases. FEBS J 274: 630–658, 2007 [DOI] [PubMed] [Google Scholar]
- 91. Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107: 881–891, 2001 [DOI] [PubMed] [Google Scholar]
- 92. Zhang K, Kaufman RJ. Signaling the unfolded protein response from the endoplasmic reticulum. J Biol Chem 279: 25935–25938, 2004 [DOI] [PubMed] [Google Scholar]
- 93. Zhong Q, Zhou B, Ann DK, Minoo P, Liu Y, Banfalvi A, Krishnaveni MS, Dubourd M, Demaio L, Willis BC, Kim KJ, duBois RM, Crandall ED, Beers MF, Borok Z. Role of endoplasmic reticulum stress in epithelial-mesenchymal transition of alveolar epithelial cells: effects of misfolded surfactant protein. Am J Respir Cell Mol Biol 45: 498–509, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]