Abstract
In this work, cardio-ventilatory coupling (CVC) refers to the statistical relationship between the onset of either inspiration (I) or expiration (E) and the timing of heartbeats (R-waves) before and after these respiratory events. CVC was assessed in healthy, young (<45 yr), resting, supine subjects (n = 19). Four intervals were analyzed: time from I-onset to both the prior R-wave (R-to-I) and the following R-wave (I-to-R), as well as time from E-onset to both the prior R-wave (R-to-E) and following R-wave (E-to-R). The degree of coupling was quantified in terms of transformed relative Shannon entropy (tRSE), and χ2 tests based on histograms of interval times from 200 breaths. Subjects were studied twice, from 5 to 27 days apart, and the test-retest reliability of CVC measures was computed. Several factors pointed to the relative importance of the R-to-I interval compared with other intervals. Coupling was significantly stronger for the R-to-I interval, coupling reliability was largest for the R-to-I interval, and only tRSE for the R-to-I interval was correlated with height, weight, and body surface area. The high test-retest reliability for CVC in the R-to-I interval provides support for the hypothesis that CVC strength is a subject trait. Across subjects, a peak ∼138 ms prior to I-onset was characteristic of CVC in the R-to-I interval, although individual subjects also had earlier peaks (longer R-to-I intervals). CVC for the R-to-I interval was unrelated to two separate measures of respiratory sinus arrhythmia (RSA), suggesting that these two forms of coupling (CVC and RSA) are independent.
Keywords: Shannon entropy, respiratory sinus arrhythmia, heart rate variability
cardio-ventilatory coupling (CVC) can refer to several concepts. Specifically, CVC can refer to an increase in heart rate during inspiration (5, 10, 20, 21, 26, 40), as well as to a tendency for heartbeats to occur at particular times relative to the onsets of inspiration (I) or expiration (E). In this report, we use the term CVC in this latter sense and use the term respiratory sinus arrhythmia (RSA) to refer to CVC in the former sense.
Coleman (6) noted that animals tend to have a stable number of heartbeats in each breath, i.e., a whole number ratio. Galli (14) noted the same thing in patients with heart failure. Weiss and Salzano (41) also reported whole number ratios between heartbeats and the respiratory cycle in anesthetized dogs. More recently, this basic relationship between heartbeats and breaths has been observed in healthy athletes (32) and anesthetized patients undergoing elective surgery (25).
The presence of whole number ratios between heartbeats and breaths implies CVC in the context of heartbeats occurring at specific times in the respiratory cycle. Indeed, as early as the late 1960s, several German researchers identified CVC in that a heartbeat occurred preferentially before I-onset in anesthetized as well as awake, healthy subjects (11, 18, 19). These observations were confirmed and extended in the 1990s and 2000s by Galletly, Larsen, and coworkers (12, 38). Typically, these researchers defined the time of a heartbeat from the R-wave peak in the electrocardiogram (ECG) and found that heartbeats have a tendency to occur ∼500 ms before I-onset (12, 38).
Prior reports have not compared CVC for all four of the following intervals: R-wave occurrence to I-onset (R-to-I), R-wave to E-onset (R-to-E), I-onset to R-wave (I-to-R), and E-onset to R-wave (E-to-R). We assessed and quantified CVC with respect to I-onset and E-onset, determining the strength of CVC for intervals before and after respiratory phase transitions. These comparisons may provide further insight into the nature, interpretation, and implications of CVC.
We note that there is no universally agreed on definition of coupling. In the present manuscript we use the term coupling as it has been used by Galletly, Larsen, and coworkers. In this sense, evidence for coupling emerges out of an analysis of histograms for various intervals between heartbeats and I- and E-onsets. Evidence of a statistically significant departure from chance in these histograms is evidence of coupling by this definition. As such, this definition makes no claims of causality. Causality, i.e., does the heartbeat facilitate respiratory phase transition, would be addressed in a study with a different design.
We hypothesized that CVC is a phenotypic trait in normal, healthy human subjects. The concept is that the presence and strength of CVC will vary from individual to individual but will be consistent within an individual. The test-retest reliability of CVC intensity will provide insight into the extent to which CVC is a subject trait or is influenced by occasion variance.
Finally, we were also interested in relating the intensity of CVC to the strength of RSA. Although others have indicated no correlation (22, 23, 32, 38), this is a fundamental issue because both RSA and CVC have been proposed to support a common goal of efficient gas exchange.
Thus, in the present report, we describe analytical tools for quantifying CVC, determine the test-retest reliability and the strength of CVC for all four types of intervals (R-to-I, R-to-E, I-to-R, E-to-R), and compare the relationship between CVC intensity and RSA strength in young, healthy subjects at rest.
METHODS
Subjects
Young, adult (<45 yr), healthy subjects were recruited from laboratory personnel, medical staff, and/or their relatives and associates (n = 26). Of these, only 19 subjects were included because one did not have 200 breaths in the recording period (minimum 200 breaths required for our analysis); one had premature ventricular contractions during the recording period; one had poor recording quality; and four did not return for a second study (see below). The final group of 19 subjects (8 men, 11 women) had a mean age of 28.5 yr (range: 17–43 yr), no history of major medical illness, and no current prescription medications. Subjects were studied on two occasions (from 5 to 27 days apart, median: 7 days). Each subject's visit was considered a study. The median height and weight of the subjects was 170.2 cm (range: 154.9–185.4 cm) and 72.6 kg (range: 45.4–97.5 kg). The median body mass index (BMI) of the subjects was 23.7 (range: 18.8–35.8). Three of the 19 subjects had BMI values over 30 (30.8, 32.2, and 35.8). Body surface area (BSA) was calculated according to the Mosteller formula (28).
The study was approved by the Institutional Review Board of the Louis Stokes Veterans Affairs Medical Center and all subjects gave written informed consent.
Data Acquisition
Studies were conducted during the day in a quiet room at the Cleveland Veterans Affairs Medical Center at Wade Park, Cleveland, OH. Each study lasted ∼20 min (median, range: 9.6 to 24.9 min). To measure respiratory flow, a pneumotachograph (Validyne) attached to a conventional plastic mouthpiece plus filter was suspended on a swing arm over the hospital bed and the subject adjusted the bed and swing arm so that the fit of the breathing device was comfortable in a semi-reclining position. The output of the Validyne demodulator was filtered (low-pass cutoff frequency of 300 Hz) and amplified (model USBPGF-S1, Alligator Technologies, Costa Mesa, CA). The ECG was recorded using a modified V5 lead placement, with band-pass filtering (0.3 to 300 Hz) and amplification (Grass model QP-511, West Warwick, RI). The respiratory flow and ECG signals were sampled at 2,000 Hz using LabView software and a National Instruments A/D board (Austin, TX). The signals were stored for offline analysis.
Digital Signal Preprocessing
Both the flow and ECG signals were low-pass filtered with a zero-phase (forward-reverse) digital filter (using the MATLAB “filtfilt” function, MathWorks, Natick, MA) with a 3-dB cutoff at 34 Hz. The signals were then decimated to 1 kHz. Careful visual inspection of the signals before and after digital filtering revealed that the essential components of each signal were preserved after digital filtering without phase shifts. The amplitude of the ECG signal at the R-wave peak was slightly reduced by the digital filter, but the time of the peak was preserved within 1 sample (1 ms). Furthermore, in cases with 60-Hz noise, the filtered signal was an improvement over the raw signal as a basis for detection of the peak of the R-wave.
Event Detection
Custom algorithms were created in MATLAB for event detection. For the detection of I- and E-onsets, the flow signal obtained from the pneumotachograph was integrated to obtain volume. The troughs and peaks of the volume curve were then used as indicators of the onsets of inspiration and expiration, respectively. However, each mark for the I- and E-onsets was inspected visually. This labor-intensive step was especially important because the minimum volume point may or may not be the start of I and the maximum volume point may or may not be the start of E. Both the volume and flow signals were inspected for every breath to ensure that the chosen point was either the start of inspiration or the start of expiration. The placement of each could be edited manually based on this assessment. The detection of the peak of each R-wave was based on both velocity (1st derivative) and amplitude criteria. We employed software for evaluating the accuracy of R-wave detection, with user-editable event times and examination of short or long R-to-R intervals.
Assessment of CVC
As a result of the abovementioned event detection steps, the times, in seconds (ms resolution) for each I-onset, E-onset, and R-wave peak were used as inputs to the coupling analysis. To achieve uniformity of statistical power for each subject and each visit, exactly 200 consecutive breaths were selected for analysis. These breaths were either the set that had the lowest variability for the I-to-I time intervals or the set that had the highest variability for the I-to-I time intervals. For each subject (n = 19), for each visit (n = 2), the following four intervals were evaluated: 1) R-to-I: the time from I-onset to the previous R-wave; 2) I-to-R: the time from I-onset to the next R-wave; 3) R-to-E: the time from E-onset to the previous R-wave; and 4) E-to-R: the time from each E-onset to the next R-wave.
Assessment of Temporal Autocorrelation of Event Intervals
For each subject (n = 19), for each visit (n = 2), there were 200 each of R-to-I, I-to-R, R-to-E, and E-to-R intervals. Interval length was regressed onto interval number (1…200), and the residuals from this regression were tested for temporal autocorrelation using the Durbin-Watson (DW) test. A DW statistic of 2.0 indicates the absence of temporal autocorrelation. In the present case, a DW statistic <1.758 indicated positive temporal autocorrelation, and >2.242 indicated negative temporal autocorrelation.
Histograms
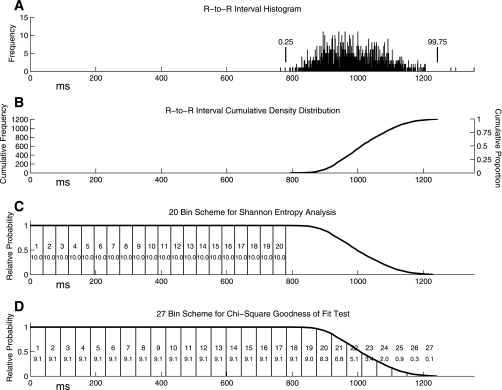
Histograms of the four interval types were constructed. The assumption of serial independence of observations was met because temporal autocorrelation was absent in these intervals. We employed two binning schemes (Fig. 1), one for each of the two coupling measures: 1) transformed relative Shannon entropy (tRSE); (2) −log10(P) from a χ2 test. We computed the histograms expected by chance. For tRSE, Shannon entropy was computed for each histogram for only the portion of the chance distribution that was flat (up to the lower 0.25% of the R-R distribution; Fig. 1A). This portion was divided into 20 bins (Fig. 1C). Shannon entropy was converted to relative Shannon entropy (RSE) by dividing by the maximum Shannon entropy [−log(1/k), where k = number of bins]. Because this measure is censored at 1.0, has values clustered very close to 1.0, and decreases as coupling strength increases, RSE was transformed to tRSE as tRSE = (−100*RSE)+100; tRSE ranges from 0 to 100, with 0 indicating no coupling and 100 indicating extreme coupling.
Fig. 1.
Illustration of the steps involved in setting up the binning schemes. The first step is to create an R-to-R interval histogram (A). Next, a cumulative density distribution is created from 0 to the 99.75 percentile of the R-to-R interval histogram (B). Then this distribution was inverted and normalized to a maximum of 1.0 (C). This final distribution is the expected distribution of R-to-I intervals (or any interval between an R wave and the immediately following or preceding I- or E-onset) assuming no coupling (chance). For transformed Relative Shannon Entropy (tRSE), the distribution from 0 to the 0.25 percentile of the R-R distribution (the flat part of the chance distribution) was divided into 20 bins (C). For the −log10(P) analysis, almost the entire distribution (up to 99.75 percentile of the R-R distribution) was divided into 27 equal bins (D). Because the sum of the expected counts in bins 23–27 was often quite small, these bins were combined into a single 23rd bin.
An RSE of 0.95 would yield a tRSE of 5 and an RSE of 0.9 would yield a tRSE of 10. This measure had a gamma distribution that was amenable to parametric statistical analysis using a generalized linear model. This measure was based on a subset of the entire potential chance distribution (the flat part), so only a subset of the available 200 intervals was used in the analysis (n ≤ 200 intervals). The number of intervals varied slightly from subject to subject.
The second coupling measure was based on a χ2 goodness-of-fit test, using all the data (up to 99.75% of the R-R distribution). This full data range was divided into 27 bins (Fig. 1D). However, to keep the expected counts >5, bins 23–27 were combined into a single 23rd bin for χ2 analysis. The negative log (base 10) of the P value from the χ2 goodness-of-fit test was computed and will be referred to as −log10(P). A P value of 0.01 would yield a −log10(P) of 2, and a P value of 0.0001 would yield a −log10(P) value of 4. Thus, a large value for −log10(P) is strong evidence for coupling. This analysis used all of the 200 intervals.
Assessment of Respiratory Sinus Arrhythmia
Direct respiratory sinus arrhythmia.
For each I-onset, we measured the two preceding R-to-R intervals (RR-2, RR-1), the two following R-to-R intervals (RR+1, RR+2), and the R-to-R interval that spanned I-onset (RR_Insp). The means for each of these intervals were calculated and used to create an index of the magnitude of respiratory sinus arrhythmia (RSA) consisting of the average of RR+1 and RR+2 divided by the average of RR-1 and RR_Insp.
Fourier-based RSA.
To compute a Fourier measure of RSA from the R-to-R intervals, we followed the general guidelines from the Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology (36) and the specific approach outlined by Singh et al. (34). We took exactly 8 min of ECG data. The R-to-R time series was interpolated to 4-Hz resolution. Next, we subtracted the mean of the time series and divided the time series by the mean. The data were then windowed using a Hann Window and then calibrated so that total power in the spectrum would equal the total variance in the time series. Our measure of RSA was the total power in the high frequency (HF-RSA) bin that ranged from 0.15 to 0.40 Hz (9 to 24 breaths/min).
Statistical Analysis
The distributions of tRSE values were non-normal and were best approximated by a gamma distribution. Similarly, the distributions of −log10(P) values were generally skewed and were best approximated by a negative binomial distribution. All ANOVA models involving these dependent variables were analyzed with generalized linear mixed model ANOVAs (Proc GLIMMIX, SAS, Cary, NC) using the base distributions noted above. These ANOVA models produce mean estimates for each coupling measure and tests that these mean estimates were non-zero. These tests will be referred to as “tests for non-zero mean estimates”. Post hoc tests were based on the “analysis of means” concept and employed a “Nelson-Hsu” adjustment for multiple comparisons (SAS). All correlation analyses involved either Pearson coefficients or Spearman rank order correlation coefficients. Autocorrelation analysis involved detrending the time series and computing the DW statistic on the residuals. Test-retest reliability was assessed with intraclass correlation coefficients (ICC) that were estimated directly from an analysis of variance table (33). The ICC is a number that ranges from 0.0 to 1.0, and Shrout and Fleiss (33) define six types. We employed type ICC(1,1) for an assessment of test-retest reliability in the absence of visit effects and type ICC(3,1) in the presence of visit effects. Cicchetti and Sparrow (3, 4) presented guidelines for interpretation of ICCs as follows: poor (below 0.40), fair (0.41–0.59), good (0.60–0.74), and excellent (above 0.75). R-to-R intervals for the RSA analysis were evaluated using a mixed-model repeated-measures ANCOVA (SAS Proc Mixed, SAS) with subjects treated as random and interval type and visit treated as fixed effects. Median heart rate was a covariate in this analysis.
RESULTS
Comparison of the Least to Most Variable 200 I-to-I Intervals
Coupling measures for the set of 200 consecutive breaths with the least variable respiratory rate were compared with the measures with the most variable respiratory rate. For our data set, respiratory rate variability did not affect the tRSE and −log10(P) coupling measures [tRSE: F = 0.04, df = 1,18, P = 0.8360; −log10(P): F = 0.17, df = 1, 18, P = 0.68]. Henceforth, all results will be reported for the least variable 200 consecutive breaths.
Temporal Autocorrelation of Intervals
The proportions of significant values for positive and negative autocorrelation (DW statistics) were calculated and compared with the proportion expected by chance. This was done for each interval type separately as well as all intervals taken together. In no case did the proportion of significant DW statistics differ significantly from that which would be expected by chance (0.05). Therefore, temporal autocorrelation was not present in these intervals.
Comparison of the Two Coupling Measures
tRSE and −log10(P) were significantly correlated to a modest degree (Pearson r = 0.66, n = 152, P < 0.001; Spearman r = 0.60, n = 152, P < 0.001). Although these two coupling measures were correlated, one measure could not replace the other because at best one accounted for only ∼45% of the other's variance.
Sample Interval Histograms
To illustrate our analysis, histograms for two subjects (#12 and #20) with strong CVC are presented in Fig. 2. (Results from the analysis of the full dataset are given below.) Table 1 contains the tRSE values for the data in Fig. 2. Table 2 contains the −log10(P) measures, and Table 3, the median heart rate and respiratory rate for the data presented in Fig. 2. The heart and respiratory rates were within normal limits. However, respiratory rates appeared to have increased slightly on visit 2. This was found in the complete data set as well and is discussed below.
Fig. 2.
The 27-bin interval histograms for 2 subjects (#12 and #20) showing strong CVC (1 subject per row). The first 2 columns present the data from the first visit and the second 2 columns present the data from the second visit. The histograms are centered on either onset of inspiration (I-onset; columns 1 and 3) or expiration (E-onset; columns 2 and 4). I-onset and E-onset occur at time 0, and 27 bins are shown before and after time 0. The number below each histogram is the bin width in ms. The alphabet marks and associated arrows (e.g., a, f) are discussed in Sample Interval Histograms.
Table 1.
tRSE and subject ranks across interval types for the histograms displayed in Fig. 2
|
Visit 1 |
Visit 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Subject # | R-to-I | I-to-R | R-to-E | E-to-R | R-to-I | I-to-R | R-to-E | E-to-R |
| 12 | 9.24 (19) | 3.83 (14) | 2.55 (15) | 1.92 (7) | 8.43 (19) | 4.58 (18) | 2.32 (12) | 2.55 (14) |
| 20 | 4.40 (15) | 4.09 (15) | 3.03 (17) | 2.08 (9) | 6.62 (18) | 3.48 (14) | 2.71 (14) | 2.55 (7) |
Rank within the set of 19 subjects per visit shown in parentheses. A high rank means a high transformed relative Shannon entropy (tRSE).
Table 2.
−log10(P) and subject ranks across interval types for the histograms displayed in Fig. 2
|
Visit 1 |
Visit 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Subject # | R-to-I | I-to-R | R-to-E | E-to-R | R-to-I | I-to-R | R-to-E | E-to-R |
| 12 | 13.45 (18)‡ | 3.42 (17)‡ | 2.16 (12)† | 1.30 (12)* | 10.89 (18)‡ | 2.77 (18)‡ | 0.40 (5) | 0.35 (6) |
| 20 | 1.64 (13)* | 1.99 (9)* | 1.69 (11)† | 0.56 (7) | 6.60 (17)‡ | 2.96 (19)‡ | 0.43 (6) | 1.23 (13)† |
Rank within the set of 19 subjects per visit shown in parentheses. A high rank means a high −log10(P), which is an index of strong cardio-ventilatory coupling (CVC).
P < 0.05;
P < 0.010;
P < 0.001.
Table 3.
Median heart rate and respiratory rate for the data presented in Fig. 2
|
Visit 1 |
Visit 2 |
|||
|---|---|---|---|---|
| Subject # | Median heart rate | Median respiration rate | Median heart rate | Median respiration rate |
| 12 | 78.7 | 13.2 | 75.1 | 15.4 |
| 20 | 55.7 | 15.3 | 60.8 | 15.5 |
Values are in cycles/min.
For subject #12, visit 1, the R-to-I histogram had a maximal value at the bin centered at 116 ms (Fig. 2, arrow a) and a secondary peak at 347 ms (Fig. 2, arrow b). The tRSE and the −log10(P) for this histogram were robust (Tables 1 and 2, respectively). The tRSE was greatest for the R-to-I interval compared with the other intervals, although the −log10(P) indicated significant coupling at several other intervals.
On visit 2, coupling for the R-to-I interval was again strong for both measures [tRSE and −log10(P)]. Furthermore, the primary peak in the R-to-I histogram also occurred in the bin centered at 116 ms (Fig. 2, arrow c). There was a second peak at 429 ms (not marked) and another at 611 ms (Fig. 2, arrow d). Thus, for subject 12, the R-to-I interval showed robust coupling on both test days.
For subject 20, for the R-to-I interval, the coupling measures were modest on visit 1 but were very strong on visit 2. Nonetheless, the R-to-I interval histograms had similar peaks on visits 1 and 2. On visit 1, peaks occurred at 118 ms (Fig. 2, arrow e) and ∼588 ms (Fig. 2, arrow f) before inspiration. On visit 2, the primary peak occurred at 118 ms again (Fig. 2, arrow g), but the later peak occurred around 878 ms (Fig. 2, arrow h).
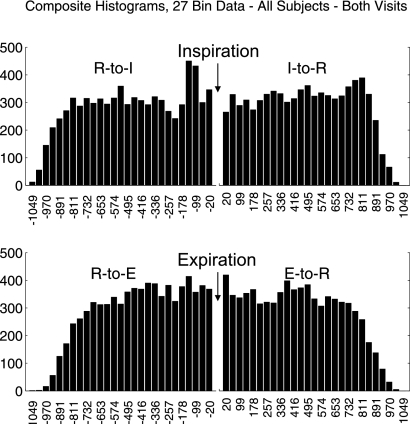
Composite 27-Bin Histograms
Composite (or cumulative) 27-bin histograms for the four intervals were based on a sum across all subjects and visits (Fig. 3). All histograms, regardless of statistical significance, contributed to this composite. The most striking features were a peak at 138.5 ms and a trough at 217.7 ms before I-onset (Fig. 3, top). For the I-to-R histogram, a peak appeared at ∼811 ms. The time from the main peak in the R-to-I histogram to the late peak in the I-to-R histogram (811+138.5 = 949 ms) was almost exactly the same as the mean R-R interval spanning inspiration (945 ms, see below). Both of the expiration-related histograms (R-to-E and E-to-R) began to roll off toward zero more quickly than the inspiration-related intervals. This indicates that long R-to-E and E-to-R intervals occurred less frequently than for R-to-I or I-to-R intervals. There does seem to be a small increased likelihood of a heartbeat immediately following the phase transition to expiration as well.
Fig. 3.
Composite 27-bin histograms for all four intervals based on a sum across subjects and visits. All histograms, regardless of statistical significance, are included in these composites. The numbers below each bar represent the average bin center, in ms, across subjects and visits. The average bin width was 39.58 ms.
Visit Effects
There were no statistically significant differences between visits for heart rate (F = 1.48, df = 1,18, P = 0.24, 2-tailed). However, respiratory rate increased slightly from visit 1 to 2 (mean: from 15.9 to 17.3 breaths/min) (F = 6.6, df= 1,18, P = 0.02). For the coupling measures [tRSE and −log10(P)], visit effects were tested as part of larger models including interval type and the interaction between visit and interval type. There were no statistically significant visit effects or visit-by-interval type interactions for the two coupling measures (smallest P value was 0.15).
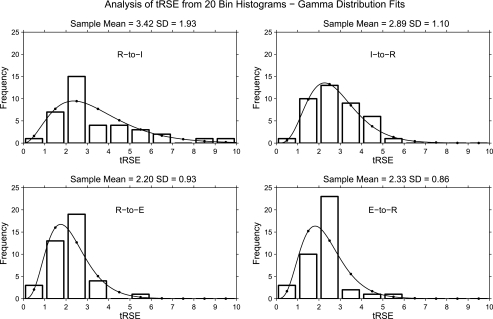
tRSE by Interval Type
Histograms of tRSE values and gamma distribution fits are displayed by interval type in Fig. 4. The highest mean tRSE was for the R-to-I interval followed by the I-to-R interval. The mean tRSE for the E-onset-related intervals was lower than the mean tRSE for the I-onset-related intervals. The F-test for the interval effect from the generalized linear mixed model ANOVA was statistically significant (F = 7.84, df=3, 54, P = 0.0002). Table 4 provides the tests for non-zero mean estimates, as well as effect size estimates for each test. Only the mean estimate for the R-to-I interval was significantly different from zero, and this effect size was large. Post hoc analysis of means indicated that the R-to-I interval was statistically larger (more coupled) than the overall mean across intervals (P value, adjusted for multiple comparisons = 0.0019) and that the R-to-E interval was significantly smaller (less coupled, P value, adjusted for multiple comparisons = 0.0094) than the overall mean.
Fig. 4.
Histograms of tRSE values by interval type along with gamma function fits.
Table 4.
Tests for non-zero mean estimates
| Measure | Interval | Mean Estimate | SE | DF | T Value | P Value | Cohen's d Effect Size | Desc. |
|---|---|---|---|---|---|---|---|---|
| tRSE | R-to-I | 1.99 | 0.21 | 54 | 3.34 | 0.0015 | 0.91 | large |
| tRSE | I-to-R | 1.41 | 0.22 | 54 | 1.57 | 0.1223 | 0.43 | moderate |
| tRSE | R-to-E | 1.19 | 0.22 | 54 | 0.79 | 0.4352 | 0.22 | small |
| tRSE | E-to-R | 1.04 | 0.23 | 54 | 0.18 | 0.8568 | 0.05 | — |
| −log10(P) | R-to-I | 3.09 | 0.08 | 54 | 13.87 | <.0001 | 3.77 | very large |
| −log10 (P) | I-to-R | 2.75 | 0.08 | 54 | 12.43 | <.0001 | 3.38 | very large |
| −log10(P) | R-to-E | 2.10 | 0.08 | 54 | 9.13 | <.0001 | 2.48 | very large |
| −log10(P) | E-to-R | 2.22 | 0.08 | 54 | 9.81 | <.0001 | 2.67 | very large |
−log10(P) by Interval Type
Histograms of −log10(P) values and negative binomial fits are displayed by interval type in Fig. 5. The highest mean −log10(P) was for the R-to-I interval followed by the I-to-R interval. The F-test for the interval effect from the generalized linear mixed model ANOVA was statistically significant (F = 3.01, df = 3,54, P < 0.04). Table 4 provides the tests for non-zero mean estimates, as well as effect size estimates for each test. All of the mean estimates were significantly larger than 0, indicating significant coupling for all intervals. The largest mean, with the largest effect size, was for the R-to-I interval. Post hoc analysis of means indicated that the R-to-I interval was statistically larger (more coupled) than the overall mean across intervals (P value, adjusted for multiple comparisons <0.03). At the P = 0.05 level [−log10(0.05)>1.301], for the R-to-I, I-to-R, R-to-E, and E-to-R intervals, respectively, the number of statistically significant χ2 were 18, 20, 17, and 13 (of a total of 38). At the P = 0.001 level, the numbers of significant coupling studies was 9, 5, 3, and 4, respectively.
Fig. 5.
Histograms of −log10(P) values by interval type along with negative binomial fits.
Test-Retest Reliability of tRSE
The intra-class correlation coefficients (ICCs) were determined for each of the four intervals for both coupling measures. The ICC for tRSE for the R-to-I interval was in the “good” range (0.69) and was significantly greater than zero (95% confidence limits do not include 0). The ICC for tRSE for the I-to-R interval was in the “fair” range (0.49) and was also significantly greater than 0. The ICCs for the expiration-related intervals were not significantly different from 0.
For −log10(P) measures, the ICC for the R-to-I interval was in the “excellent” (0.83) range and significantly greater than zero and significantly larger than the other interval types. The ICCs for the remaining intervals were not statistically significantly different from 0.
Test-Retest Reliability of Heart Rate and Respiration Rate
The ICC for median heart rate was 0.65 (good). For the respiration rate, we used ICC(3,1) rather than ICC(1,1) (see above) due to the presence of a visit effect for this measure. This ICC was 0.73 (top of the good range).
Correlations Between Coupling Measures and Heart Rate or Respiratory Rate
Spearman correlation coefficients were calculated, relating the mean tRSE and mean −log10(P) (across visits) for interval types and either mean heart rate or mean respiratory rate (2*4*2 = 16 correlation coefficients). Two correlations were statistically significant before but not after correction for multiple comparisons. These are mean tRSE for the E-to-R interval was positively correlated with the median respiratory rate (0.46, P < 0.04) and mean −log10(P) measure for the E-to-R interval was positively correlated with the median heart rate (0.49, P < 0.04). Thus, the coupling of R-to-I intervals was not dependent on heart or respiratory rates.
Relationship Between Coupling and Demographic Measures
Mean tRSE for the R-to-I interval was highly correlated with height, weight, and especially BSA (Table 5; Fig. 6). Coupling was stronger for larger subjects (taller, heavier, and larger BSA). Coupling strength was not correlated with BMI. Body size was confounded with sex: of 9 subjects in the small group (Fig. 6, bottom left), 8 were women. Given differences in height and weight, sex differences would have to be compared after controlling for height or weight, but this analysis was not conducted given the modest sample size (and low statistical power) to detect an effect. Other correlations with the other intervals were not statistically significant (Table 5). There was no correlation between age and either coupling measure for this age-restricted (<45 yr) sample.
Table 5.
Spearman correlations between coupling measures and height, weight, BSA, and BMI vs. tRSE
| Interval Type: | R-to-I | I-to-R | R-to-E | E-to-R |
|---|---|---|---|---|
| Height | 0.66 | 0.24 | 0.29 | 0.24 |
| P = 0.0022 | ns | ns | ns | |
| Weight | 0.67 | 0.18 | 0.45 | 0.36 |
| P = 0.0017 | ns | ns | ns | |
| BSA | 0.70 | 0.25 | 0.45 | 0.33 |
| P = 0.0008 | ns | ns | ns | |
| BMI | 0.40 | −0.01 | 0.32 | 0.28 |
| ns | ns | ns | ns | |
| −log10(P) | ||||
| Interval type | R-to-I | I-to-R | R-to-E | E-to-R |
| Height | 0.25 | 0.21 | 0.02 | −0.02 |
| ns | ns | ns | ns | |
| Weight | 0.45 | 0.23 | 0.02 | 0.05 |
| ns | ns | ns | ns | |
| BSA | 0.44 | 0.25 | 0.04 | 0.01 |
| ns | ns | ns | ns | |
| BMI | 0.37 | 0.09 | −0.12 | −0.01 |
| ns | ns | ns | ns | |
BSA, body surface area; BMI, body mass index; ns, not statistically significant.
Fig. 6.
Scatter plot relating rank body surface area (BSA) to rank tRSE for the R-to-I interval. ♀Female subjects.
Direct RSA Analysis
The means and standard errors for the five R-to-R interval types (RR-2, RR-1, RR_Insp, RR+1, and RR+2) were calculated across subjects and visits (Fig. 7). These R-to-R intervals were significantly different (F = 49.8, df = 4, 144, P < 0.0001). RSA consisted of shorter RR intervals in inspiration (RR+1 and RR+2) relative to the RR intervals beginning during expiration (RR-1 and RR_Insp). The difference between the means of these two sets of intervals was 149.5 ms and was statistically significant (t = 12.9, df = 144, P < 0.0001). (RR_Insp was 170 ms and RR-1 was 130 ms longer than the mean of RR+1 and RR+2.) The mean of our direct measure of RSA intensity across subjects and visits was 0.92 (± 0.05 SD) and was significantly lower than 1.0 (t = −7.26, df = 18, P < 0.0001). Our measure was also very reliable (ICC = 0.90) across visits.
Fig. 7.
R-to-R intervals before, after and spanning I-onset. Values are means (±SE) for all subjects and visits, after controlling for differences in median heart rate.
Relationship Between Direct RSA and HF Power
To validate our direct measure of RSA, we compared the rank order of direct RSA with the rank order of high-frequency power from a traditional fast Fourier transform analysis of heart rate variability. As expected, these RSA measures were very highly and positively correlated (Spearman r = 0.84, P < 0.0001).
Relationship Between RSA and Coupling
The relationship between both measures of RSA (direct RSA and HF power) and both measures of coupling [tRSE and −log10(P)] was assessed for the R-to-I interval. The rank of each measure of coupling was treated as a dependent variable and regressed onto the rank of each measure of RSA, with visit effects also in the model. The F-values for the regression were very low (0.0, 0.01, 0.15, 0.36) and do not support the hypothesis that RSA and CVC [tRSE and −log10(P)] for the R-to-I interval are correlated.
Relationship Between RSA and Demographic Measures
Mean direct RSA and mean HF power were not correlated with height, weight, or BMI (largest magnitude Spearman r = 0.12, 0.23, respectively).
DISCUSSION
CVC in awake, resting, supine, healthy subjects is strongest and most consistent for the interval between I-onset and the previous R-wave (R-to-I interval). For both coupling measures [tRSE, −log10(P)], the R-to-I interval had the largest mean and was the only interval that was significantly larger than the mean across intervals. For tRSE, the mean estimate was significantly larger than 0 only for the R-to-I interval. Although the pattern of CVC could vary across subjects, the R-wave occurred preferentially at 138 ms before I-onset. The test-retest reliability of both CVC measures was significantly greater than zero for the R-to-I interval, whereas the coupling measures for the expiratory-related intervals were unreliable. These data support the hypothesis that CVC strength for the R-to-I interval is a subject trait under these conditions. Furthermore, CVC for the R-to-I interval only (as indexed by tRSE) was strongly related to the size (height, weight, and BSA) of the subjects. Finally, CVC was unrelated to the two measures of RSA (direct RSA and HF power).
CVC has been observed in intact awake (6) and anesthetized animals (24), in clinical populations (14, 22), and in healthy awake and anesthetized subjects (11, 12, 18, 19, 38). These studies identified CVC for the R-to-I interval, whereas our study compared each interval (R-to-I, I-to-R, R-to-E, and E-to-R) systematically and statistically. We verified that the R-to-I interval is the key interval. As noted above, the R-to-I interval was the only interval significantly larger than the mean interval for both CVC measures. Our findings are consistent with Hildebrandt (17) who reported much stronger CVC in R-to-I than R-to-E intervals and with Larsen et al. (25) who identified stronger coupling in the R-to-I than I-to-R intervals.
The time between the R-wave and I-onset varies from that reported recently but is consistent with that reported in the 1960s. We found an increased probability of R-waves occurring ∼138 ms prior to I-onset. Larsen and coworkers (12, 38) reported this interval as 510 ms in anesthetized subjects (12) and 670 ms in healthy awake supine subjects (38). Although individual subjects had peaks in this time range (e.g., Fig. 2), the peaks were not sufficiently synchronous at these earlier time points to be apparent in the group average (Fig. 3). In contrast, the peak at ∼150 ms is consistent with reports by German researchers in the 1960s (11, 17, 18, 19, 31).
Differences in the method of assessing respiration and determining I-onset may account for these differences in R-to-I interval duration. Previous methodologies include the opening and closing of a Ruben nonreturn (one-way) valve (12), nasal pressure (38), a “thorakometer” (an instantaneous measure of chest circumference; 19), a pneumatic inflatable cuff around the chest (18), and a thermistor (11). All found CVC of R-I intervals in most subjects. Whether one or another provides the best or most consistent result remains to be determined and comparison of methods might be useful.
This is the first report that has evaluated the test-retest reliability CVC [tRSE: 0.69, −log10(P): 0.83]. The ICCs, in the good and excellent range, respectively, suggest that these measures are reasonably similar when assessed in the same subject 5–27 days apart. This is particularly noteworthy in light of the evidence for waxing and waning of CVC during a single recording session (38). Our findings suggest that a single estimate of CVC based on 200 breaths is a reliable characteristic of an individual, when tested under comparable conditions.
In our resting, awake, supine subjects, RSA consisted of a relative decrease in R-to-R intervals following I-onset (the RR+1 and RR+2). The average of these two intervals was ∼150 ms shorter than the two R-to-R intervals at the end of expiration. Our measure of RSA (average of RR+1 and RR+2 divided by the average of RR-1 and RR_Insp) was significantly less than 1.0 (0.92 ± 0.05 SD) and was the most reliable (test-retest ICC = 0.90) subject characteristic in this study. Furthermore, our measure of RSA was not related to either CVC measure for the R-to-I interval. This is consistent with several previous reports (13, 23, 24, 32).
The strong relationship noted between the strength of CVC (tRSE) and height, weight, and BSA has not been reported previously. However, cardiovascular measures have been correlated to height, weight, and BSA. Chirinos et al. (2) measured 11 aspects of arterial load and ventricular-arterial coupling and reported that all these measures were positively correlated with height, weight, and body size. The largest correlations were between height or weight or BSA, and stroke volume, total arterial compliance, arterial load, and end-systolic left ventricular elastance. In other studies, height was positively correlated with skin microvascular resistance in adult women (15) and with blood pressure in children, including 17 year olds (29). Two papers have reported that small vessel elasticity was positively correlated with height and/or weight (9, 43). Perhaps these correlations, including our correlation between CVC and height, weight, and BSA, represent adaptations to the extra load demands of large people. In this light, our correlations might argue for a role of CVC in enhancing the efficiency of oxygen delivery. It is interesting to note, in this context, that neither measure of RSA was correlated with body size measures.
Significance
We used the R-wave peak of the ECG signal because this is the largest and most salient component and the R-wave occurred ∼138 ms before inspiratory flow. In future studies that examine cause and effect, determining the cardio-ventilatory variables will impact the latency measure. The R-wave occurs just prior to ventricular contraction. However, other cardiac-related events occur earlier; the P-wave, which indicates atrial depolarization, occurs between 120 and 200 ms before the R-wave, and sino-atrial node depolarization occurs slightly earlier. Regarding the I-onset, diaphragmatic activity begins before air volume changes at the mouth (37), and upper airway muscles contract well before diaphragmatic activity (1, 30, 35, 42). Thus, determining CVC based on onset of EMG activity in upper-airway muscles, the diaphragm and air flow at the mouth and nose may provide substantial insight into the mechanisms and effects of CVC.
As noted above, our definition of coupling does not include the concept of causality. With the methodology we presented we cannot determine if the presence of a heartbeat just before onset of I facilitates I-onset. We can say that our data are consistent with this interpretation but we can go no further. Proof of causality requires an experiment in which one of the variables (R-wave timing or arterial pulse pressure) is manipulated and the effect on I-onset observed. This is hard to imagine doing in human subjects but could be attempted in animal studies. However, several analytical methods examine causality (for review, see 16). Perhaps the application of such methods in the future can provide additional clues to the causal relationships.
Although neural control of cardio-ventilatory functions appears to be integrated at the brain stem, integration at a systems level (typically focused on the efficiency of gas exchange) needs to be defined. At a brain stem level, although expiratory neural activity can be primarily pulse modulated (7, 8), baroreceptor input only weakly resets the respiratory rhythm (27). Both the magnitude and the direction of resetting vary, modulated by the afferent radiation of inputs from the lungs and chest wall and interdependence of medullary and pontine networks. However, in humans, chest wall activation patterns controlling tidal volume are primarily inspiratory with passive expiration during quiet wakefulness. Furthermore, the activation of upper airway, or flow modulating muscles, may occur before the diaphragm. Hence, CVC as we describe it from cardiac activation and inspiratory flow is an example where systems are modulated at a level beyond that explained currently by conceptual models based on reduced preparations. Speculation as to whether CVC is a primary or secondary controlled variable is beyond the scope of this paper yet this begs further investigation. Where and how this develops may be an important element to determine, in addition to its physiological importance.
GRANTS
This research was supported by grants from the National Institutes of Health (HL-087377 and HL-087340) and by Award Number I01BX000873 from the Biomedical Laboratory Research and Development Service of the VA Office of Research and Development.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: L.F., T.E.D., K.A.L., and K.P.S. conception and design of research; L.F. performed experiments; L.F. analyzed data; L.F., T.E.D., F.J.J., K.A.L., A.Y., M.F., C.G.W., and K.P.S. interpreted results of experiments; L.F. prepared figures; L.F. drafted manuscript; L.F., T.E.D., F.J.J., K.A.L., A.Y., M.F., C.G.W., and K.P.S. edited and revised manuscript; L.F., T.E.D., F.J.J., K.A.L., A.Y., M.F., C.G.W., and K.P.S. approved final version of manuscript.
REFERENCES
- 1. Brancatisano TP, Dodd DS, Engel LA. Respiratory activity of posterior cricoarytenoid muscle and vocal cords in humans. J Appl Physiol 57: 1143–1149, 1984 [DOI] [PubMed] [Google Scholar]
- 2. Chirinos JA, Rietzschel ER, De Buyzere ML, De Bacquer D, Gillebert TC, Gupta AK, Segers P. Arterial load and ventricular-arterial coupling: physiologic relations with body size and effect of obesity. Hypertension 54: 558–566, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cicchetti DV. The precision of reliability and validity estimates re-visited: distinguishing between clinical and statistical significance of sample size requirements. J Clin Exp Neuropsychol 23: 695–700, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Cicchetti DV, Sparrow SA. Developing criteria for establishing interrater reliability of specific items: applications to assessment of adaptive behavior. Am J Ment Defic 86: 127–137, 1981 [PubMed] [Google Scholar]
- 5. Cohen MA, Taylor JA. Short-term cardiovascular oscillations in man: measuring and modelling the physiologies. J Physiol 542: 669–683, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coleman WM. The psychological significance of bodily rhythms. J Comp Psychol 1: 213–220, 1921 [Google Scholar]
- 7. Dick TE, Morris KF. Quantitative analysis of cardiovascular modulation in respiratory neural activity. J Physiol 556: 959–970, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dick TE, Shannon R, Lindsey BG, Nuding SC, Segers LS, Baekey DM, Morris KF. Arterial pulse modulated activity is expressed in respiratory neural output. J Appl Physiol 99: 691–698, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Duprez DA, Kaiser DR, Whitwam W, Finkelstein S, Belalcazar A, Patterson R, Glasser S, Cohn JN. Determinants of radial artery pulse wave analysis in asymptomatic individuals. Am J Hypertens 17: 647–653, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Eckberg DL. Point:Counterpoint: respiratory sinus arrhythmia is due to a central mechanism vs. respiratory sinus arrhythmia is due to the baroreflex mechanism. J Appl Physiol 106: 1740–1742; discussion 1744, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Engel P, Hildebrandt G. Uber den einfluss der vigilanz auf die phasenkoppelung zwischen herzschlag und atmung. Psychologische Beitrage 15: 77–86, 1973 [Google Scholar]
- 12. Galletly DC, Larsen PD. Cardioventilatory coupling during anaesthesia. Br J Anaesth 79: 35–40, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Galletly DC, Larsen PD. Relationship between cardioventilatory coupling and respiratory sinus arrhythmia. Br J Anaesth 80: 164–168, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Galli G. Deuxieme contribution a l'étude des synchronisms cardio-respiratories. Arch Mal Coeur Vaiss 17: 208–221, 1924 [Google Scholar]
- 15. Gerhardt U, Hillebrand U, Mehrens T, Hohage H. Impact of estradiol blood concentrations on skin capillary laser Doppler flow in premenopausal women. Int J Cardiol 75: 59–64, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Hlavackova-Schindler K, Palus M, Vejmelka M, Bhattacharya J. Causality detection based on information-theoretic approaches in time series analysis. Physics Reports 441: 1–46, 2007 [Google Scholar]
- 17. Hildebrandt G. [The coordination of rhythmic functions in man]. Verh Dtsch Ges Inn Med 73: 921–941, 1967 [PubMed] [Google Scholar]
- 18. Hildebrandt G, Daumann FJ. [Coordination of pulse and respiration rhythm during work]. Int Z Angew Physiol 21: 27–48, 1965 [PubMed] [Google Scholar]
- 19. Hinderling P, Bucher K. [Synchronism between circulation and respiration in humans]. Helv Physiol Pharmacol Acta 23: 374–381, 1965 [PubMed] [Google Scholar]
- 20. Karemaker JM. Counterpoint: respiratory sinus arrhythmia is due to the baroreflex mechanism. J Appl Physiol 106: 1742–1743; discussion 1744, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Karemaker JM. Last Word on Point:Counterpoint: respiratory sinus arrhythmia is due to a central mechanism vs. respiratory sinus arrhythmia is due to the baroreflex mechanism. J Appl Physiol 106: 1750, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Larsen PD, Booth P, Galletly DC. Cardioventilatory coupling in atrial fibrillation. Br J Anaesth 82: 685–690, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Larsen PD, Galletly DC. Cardioventilatory coupling in heart rate variability: the value of standard analytical techniques. Br J Anaesth 87: 819–826, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Larsen PD, Galletly DC. Cardioventilatory coupling in the anaesthetised rabbit, rat and guinea-pig. Pflügers Arch 437: 910–916, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Larsen PD, Trent EL, Galletly DC. Cardioventilatory coupling: effects of IPPV. Br J Anaesth 82: 546–550, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Larsen PD, Tzeng YC, Sin PY, Galletly DC. Respiratory sinus arrhythmia in conscious humans during spontaneous respiration. Respir Physiol Neurobiol 174: 111–118, 2010 [DOI] [PubMed] [Google Scholar]
- 27. McMullan S, Dick TE, Farnham MM, Pilowsky PM. Effects of baroreceptor activation on respiratory variability in rat. Respir Physiol Neurobiol 166: 80–86, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mosteller RD. Simplified calculation of body-surface area. N Engl J Med 317: 1098, 1987 [DOI] [PubMed] [Google Scholar]
- 29.National Heart, Lung, and Blood Institute Blood pressure tables for children and adolescents, 2004. http://www.nhlbi.nih.gov/guidelines/hypertension/child_tbl.htm
- 30. Onal E, Lopata M, O'Connor TD. Diaphragmatic and genioglossal electromyogram responses to CO2 rebreathing in humans. J Appl Physiol 50: 1052–1055, 1981 [DOI] [PubMed] [Google Scholar]
- 31. Rascke F. The hierarchical order of cardiovascular- respiratory coupling. In: Cardiorespiratory and Cardiosomatic Psychophysiology, edited by Grossman P, Janssen KHL, Vailt D. London: Plenum, 1986, p. 207–218 [Google Scholar]
- 32. Schäfer C, Rosenblum MG, Kurths J, Abel HH. Heartbeat synchronized with ventilation. Nature 392: 239–240, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86: 420–428, 1979 [DOI] [PubMed] [Google Scholar]
- 34. Singh D, Vinod K, Saxena SC. Sampling frequency of the RR interval time series for spectral analysis of heart rate variability. J Med Eng Technol 28: 263–272, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Strohl KP, Hensley MJ, Hallett M, Saunders NA, Ingram RH., Jr Activation of upper airway muscles before onset of inspiration in normal humans. J Appl Physiol 49: 638–642, 1980 [DOI] [PubMed] [Google Scholar]
- 36.Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation 93: 1043–1065, 1996 [PubMed] [Google Scholar]
- 37. Taylor A. The contribution of the intercostal muscles to the effort of respiration in man. J Physiol 151: 390–402, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tzeng YC, Larsen PD, Galletly DC. Cardioventilatory coupling in resting human subjects. Exp Physiol 88: 775–782, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Tzeng YC, Larsen PD, Galletly DC. Mechanism of cardioventilatory coupling: insights from cardiac pacing, vagotomy, and sinoaortic denervation in the anesthetized rat. Am J Physiol Heart Circ Physiol 292: H1967–H1977, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Tzeng YC, Sin PY, Galletly DC. Human sinus arrhythmia: inconsistencies of a teleological hypothesis. Am J Physiol Heart Circ Physiol 296: H65–H70, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Weiss HR, Salzano J. Formation of whole number ratios of heart rate and breathing frequency. J Appl Physiol 29: 350–354, 1970 [DOI] [PubMed] [Google Scholar]
- 42. Wheatley JR, Brancatisano A, Engel LA. Respiratory-related activity of cricothyroid muscle in awake normal humans. J Appl Physiol 70: 2226–2232, 1991 [DOI] [PubMed] [Google Scholar]
- 43. Winer N, Sowers JR, Weber MA. Gender differences in vascular compliance in young, healthy subjects assessed by pulse contour analysis. J Clin Hypertens (Greenwich) 3: 145–152, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]