Abstract
Researchers from various disciplines, including cell and developmental biology, genetics and molecular medicine have revealed an exceptional diversity of cellular functions that are mediated by cilia-dependent mechanisms. Recent studies have directed our attention to proteins that localize to the ciliary transition zone (TZ), a small evolutionarily conserved subcompartment that is situated between the basal body and the more distal ciliary axoneme. These reports shed light on the roles of TZ proteins in ciliogenesis, ciliary protein homeostasis and specification of ciliary signaling, and pave the way for understanding their contribution to human ciliopathies. In the present review, we describe the interplay of multimeric protein complexes at the TZ, integrating morphological, genetic and proteomic data towards an account of TZ function in ciliary physiology.
Cilia: From architecture to function
From their discovery at the end of the nineteenth century, cilia (and flagella) have been recognized to be present in many branches of the eukaryotic tree and on virtually all mammalian cells [1]. The earliest studies focused on the role of cilia in motility as seen in many single-celled organisms and specialized vertebrate tissues (e.g. respiratory epithelia or cells of the reproductive tract). In the past ten years, interest in cilia has experienced a renaissance. Driven by modern developments in genetics, a rapidly growing body of experimental evidence has established the role of cilia-dependent mechanisms in cellular signaling processes, tissue homeostasis, development and disease pathogenesis.
A significant part of our existing knowledge of ciliary structure emerged with the progress in electron microscopy techniques from the 1950’s to the 1970’s (reviewed in [2]). At that time, careful and systematic ultrastructural analysis of cilia and flagella in many organisms provided a detailed and astonishingly conserved morphological map. From this map, the cilium can be dissected into distinct substructural zones with consensus anatomic features: the basal body (BB), the transition zone (TZ), the doublet zone and the singlet zone with the ciliary tip complex (Box 1). Of these zones, the TZ houses a set of remarkable protein assemblies that are thought to mediate linkage to the ciliary membrane, establishment of a barrier to membrane diffusion and a gate for proteins destined for the ciliary compartment. Moreover, in human diseases of ciliary dysfunction, the so-called “ciliopathies”, a significant number of implicated proteins localize to the TZ. Presented with this complex architecture and myriad protein constituents, it has become the task of the research community to connect the disease genes to the architecture and the architecture to function. In the present review, we will discuss TZ anatomy and function in the context of novel insights from genetic, proteomic and systems biology approaches.
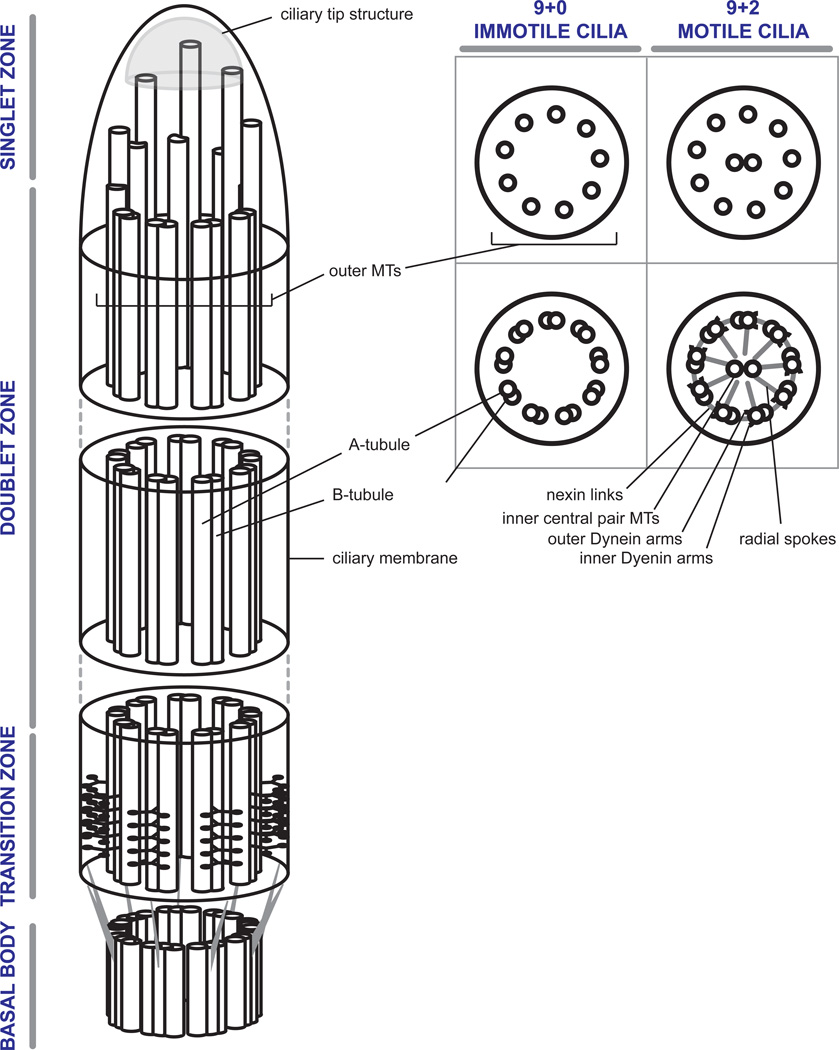
Box 1: General Aspects of Ciliary Structure.
The ciliary transition zone and axoneme arise from the basal body. The structural core of the BB consists of a nonamer of microtubule (MT) triplets, in which the A-tubule is defined as the complete MT, associated with incomplete B- and C- tubules (Box I). The BB terminates with the distal end of the C-tubule. From there on, the ciliary axoneme is composed of a scaffold of nine outer A–B-microtubule doublets. In vertebrates, variations of the common ciliary theme are limited to immotile primary cilia and motile cilia, with a few exceptions. Immotile cilia share a common 9+0 (outer doublets, no central MTs) microtubular axonemal architecture and can be found in the form of primary cilia of epithelial and mesenchymal cells, or as highly specialized sensory cilia in olfactory or retinal photoreceptor cells. Motile cilia are characterized by a 9+2 constellation (outer doublets + central MTs) and are found on multi-ciliated cells of the respiratory tract or fallopian tube, as well as in the form of sperm flagella. One notable exception are the motile 9+0 cilia found at the embryonic node which harbor dynein arms but no central pair. Toward the ciliary tip, B-tubules terminate in various organism- and cell-specific patterns, leaving behind distal A-tubule singlets. Those ultimately terminate in a ciliary tip substructure that also carries a variety of cell type specific characteristics. Not shown here are the molecules that support the dynamic process of intraflagellar transport (IFT) (reviewed in [43]). IFT is the primary mechanism by which proteins are transported within the ciliary compartment and as such plays a central role in cilium assembly and maintenance.
The ciliary transition zone and the ciliary necklace
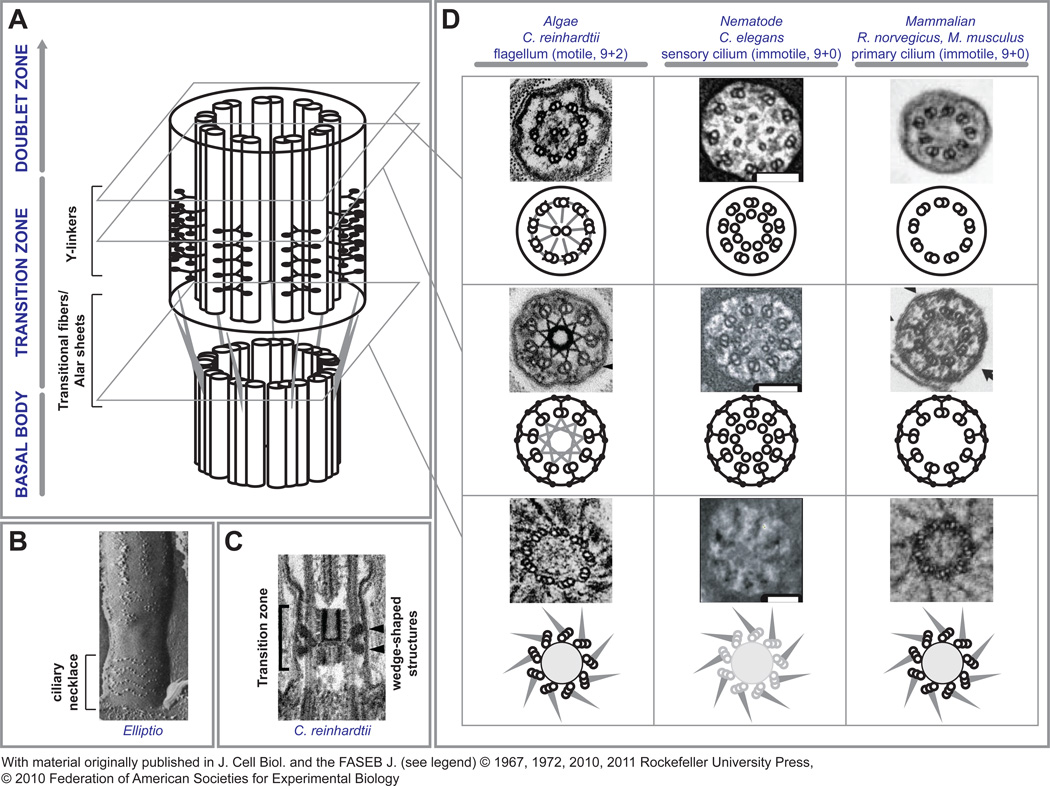
The ciliary transition zone constitutes the proximal portion of the cilium with the following defining features (Figure 1): 1. The TZ originates at the distal end of the BB. It encompasses a propeller-like array of so-called transitional fibers, which project from the B-tubule at the level of termination of the C-tubule and insert into the periciliary plasma membrane. As a result, the TZ backbone is composed of nine outer microtubule doublets with a nine-bladed propeller structure at its base [3]. 2. The main body of the TZ is characterized by the presence of multiple rows of Y-shaped linkers projecting out from the outer doublets and attaching to the ciliary membrane [4]. These linkers coincide with a characteristic circumferential arrangement of membrane particle insertions that can be observed by freeze fracture electron microscopy preparations, referred to as the ciliary necklace (Figure 1B) [5]. 3. The TZ terminates distally with the last row of Y-linkers. In 9+2 cilia, this coincides with the level of the basal plate and the proximal end of the central microtubule pair (Figure 1C).
Figure 1. The Ciliary Transition Zone.
A – A schematic representation of the ciliary transition zone shows its location between the basal body compartment and the doublet zone of the axoneme. At the distal end of the basal body, terminal triplet MT architecture and transitional fibers are seen. The A–B tubules of the triplet MTs are continuous with the doublets (not shown here for clarity) within the transition zone proper where characteristic Y-linkers span from the doublets to the surrounding membrane. It is thought that these interactions produce characteristic ciliary membrane imprints referred to as the ciliary necklace (B, reproduced with permission from [5]). In the transition zone of Chlamydomonas flagella, there is an additional central density with a barrel-shaped body within the space defined by the MT doublets and a wedge shaped density projecting towards the membrane from the outer MTs (C, reproduced with permission from [10]). D - Although remarkably conserved, subtle structural differences exist between model organisms. At the start of the transition zone, transitional fibers project from the B-tubule to the ciliary membrane at its junction with the apical cell membrane. The appearance of transitional fibers indicate the distal end of the basal body, since at that point, C-tubules terminate and the transition zone and axoneme consist only of outer MT doublets. Transitional fibers, also called alar sheets, form a propeller-like assembly leaving spaces of about 60 nm between neighboring sheets. The BB structures (grayed MT triplets) are only poorly discerned in Caenorhabditis sensory cilia, but transitional fibers can be observed. Within the transition zone, all cilia have the characteristic Y-linkers connecting the outer doublet MTs to the ciliary membrane. In addition, Chlamydomonas has a stellate fiber array within the outer doublet array connected to all A-tubules that appears near the basal plate. Within the axoneme of motile cilia, such as in Chlamydomonas, an arrangement of radial spoke proteins connect the inner central pair of MTs to the outer doublets, and nexin fibers and dynein arms connect the outer doublets to each other. The sensory cilia of Caenorhabditis and the primary cilium of mammalian cells both lack the inner central pair and the basal plate from which they originate. In addition, they lack radial spokes, nexin fibers and dynein arms (reproduced with permission from [10, 11, 30, 41, 42]). Scale bars, 100 nm.
There exist a number of key differences in transition zone features between organisms (Figure 1D). For example, motile flagella have the hallmark 9+2 configuration not seen in 9+0 primary cilia, along with the characteristic dynein arms and radial spokes. In addition, the internal cylinders within the TZ lumen are unique to the motile cilium, as seen in Chlamydomonas (Figure 1D, left). The basal body in Caenorhabditis is poorly discerned by electron microscopy but these sensory cilia do have robust transitional fibres and this arrangement may represent a minimal structure for nucleation of the transition zone [6] (Figure 1D, middle). Caenorhabditis sensory cilia also have singlet microtubules present in the lumen of the axoneme and transition zone that are unrelated to the central pair in motile cilia. Even with these differences, the high degree of morphological similarity between TZs from diverse cell types implies a set of conserved constituents and common pathways for assembly.
Many human ciliopathy syndrome proteins localize to the transition zone
Ciliopathies are defined as a group of genetic disorders whose gene products localize to the cilium. Many ciliopathies are complex syndromes that include renal cysts as one of many organ manifestations. Moreover, a large number of ciliopathy genes have been identified by genetic analyses of cystic kidney disease syndrome families. These findings have led to the ciliary hypothesis of polycystic kidney disease, which suggests that protein products of polycystic kidney disease genes localize to and exert their function at or near the primary cilium [7]. For example, PKD1 and PKD2, the gene products responsible for >95% of all cases of autosomal dominant polycystic kidney disease (ADPKD) are both found in the primary cilia of kidney epithelial cells [8].
A clinical continuum of autosomal recessive cystic kidney syndromes begins with nephronophthisis (NPHP), a form of congenital cystic renal dysplasia with progressive loss of kidney function (Box 2). As an isolated entity, NPHP is the most common cause of end-stage renal disease in the first three decades of life [9]. However, it may also be found as a component of more complex disorders. In Senior-Løken-syndrome (SLSN), NPHP is associated with retinitis pigmentosa, resulting in degeneration of retinal photoreceptor cells and consequent vision loss. In Joubert syndrome (JBTS), NPHP is accompanied by retinal degeneration, as well as central nervous system (CNS) patterning defects primarily affecting the cerebellum. In Meckel-Gruber syndrome (MKS), the most severe disease in the continuum, NPHP-like renal pathology is associated with fibrocystic changes of liver and biliary system, retinal defects, polydactyly, severe CNS malformations, such as occipital encephalocele, and perinatal lethality.
Box 2: Clinical and Pathological Features of Human Ciliopathies.
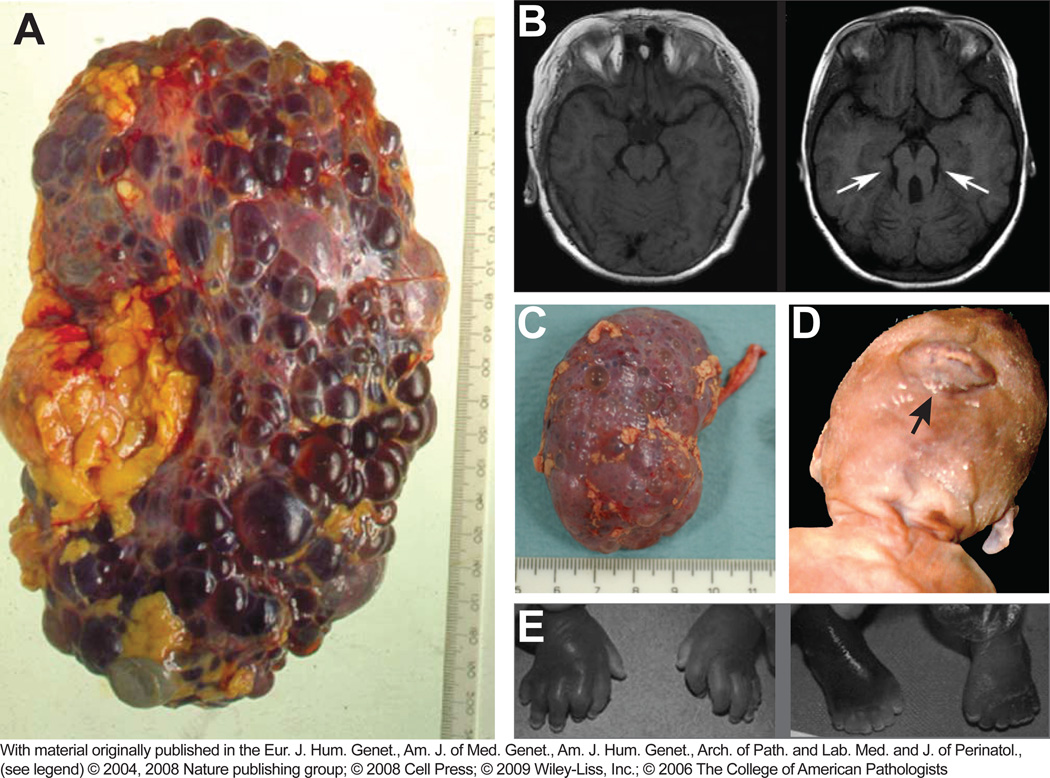
Human ciliopathies entail a large number of genetic defects that give rise to a wide spectrum of clinico-pathologic entities [9]. Disorders of the motile cilium, like Kartagener’s syndrome were the first discovered [44]. The large group of disorders of the primary cilium includes cystic malformation of the kidneys as one of the most consistent findings. In autosomal dominant polycystic kidney disease (ADPKD), cysts grow throughout the renal parenchyma, resulting in massively enlarged kidneys (Figure IIA, reprinted with permission from [45], mm ruler).
The Nephronophthisis (NPHP) spectrum of syndromes follows an autosomal recessive pattern of inheritance. In Nephronophthisis, cysts emerge in one distinct zone of the kidney parenchyma, the cortico-medullary junction, and lead to tubular atrophy and parenchymal fibrosis. Unlike ADPKD, however, kidneys are not significantly enlarged (Figure IIC, reprinted with permission from [46], cm ruler).
Senior-Løken-syndrome (SLSN) represents an association of NPHP with retinitis pigmentosa, resulting in degeneration of the retinal pigment epithelium and consecutive vision loss.
In Joubert syndrome (JBTS), variable degrees of NPHP are accompanied by retinal degeneration and CNS malformations. The characteristic cerebellar vermis hypoplasia manifests clinically in hypotonia, ataxia, abnormal eye and tongue movements, breathing pattern disturbances and psychomotor retardation. The structural equivalent is the “molar tooth sign” observed on brain imaging (Figure IIB; normal MRI on the left, molar tooth sign on the right, reprinted with permission from [47]). The phenotypic spectrum of JBTS may include further distinctive organ manifestations with variable penetrance, leading to subclasses within the cluster of Joubert syndrome related disorders (JSRD): Syndromes with predominant cerebellar, ocular and renal pathology are sometimes referred to as CORS. Syndromes including congenital liver fibrosis are also known as COACH syndrome (coloboma, oligophrenia, ataxia, cerebellar anomalies, hepatic fibrosis).
Meckel-Gruber syndrome (MKS) is characterized by renal cystic dysplasia, biliary cysts and hepatic fibrosis. CNS malformations are very severe and often involve occipital encephalocele with posterior extrusion of the brain outside the skull (Figure IID, reprinted with permission from [48]). Associated defects include Leber congenital amaurosis (LCA) and postaxial polydactyly (Figure IIE, reprinted with permission from [49]). MKS leads to fetal or perinatal death.
LCA is an isolated developmental defect of the retinal photoreceptor cell. In contrast to retinitis pigmentosa, there is no pattern of progressive vision loss, but severe vision loss from birth on. Other ciliopathies such as Bardet-Biedl syndrome (BBS), Alstrom syndrome, orofacial digital syndrome and Jeune syndrome exhibit symptoms clinically distinct from the NPHP spectrum and are reviewed elsewhere [9].
Although phenotypic overlap between human NPHP-associated ciliopathies is common, it is still remarkable that mutations in some 30 different genes manifest in a small set of well-defined clinical syndromes (Table 1 and Box 3). This is in large part due to the localization of ciliopathy genes to a common structure, the TZ of the primary cilium. This places the TZ at the center of the ciliary hypothesis; linking ultrastructural assemblies to a pleiotropy of defects in ciliated tissues. How these gene products interact to assemble the TZ and function to specify the ciliary compartment has been a central focus of recent studies. The emerging model postulates an assembly hierarchy of NPHP/JBTS/MKS gene products, acting as multimeric protein complexes to form the TZ that in turn determines the signaling repertoire of the cilium.
Table 1.
An overview of ciliopathy genes and their products
| Protein Complex |
Gene Name |
Alternate name by syndrome |
Localizationa | Protein domainsb | Selected References |
||
|---|---|---|---|---|---|---|---|
| NPHP | MKS | JBTS | |||||
| NPHP 1-4-8 | RPGRIP1L | NPHP8 | MKS5 | JBTS7 | TZ, BB | C2, CC | [53, 54] |
| NPHP4 | NPHP4 | TZ | [13, 55, 56] | ||||
| NPHP1 | NPHP1 | JBTS4 | TZ | SH3, CC | [13, 57, 58] | ||
| MKS | B9D1 | MKS9 | TZ, BB | B9 | [59, 60] | ||
| B9D2 | MKS10 | TZ | B9 | [60, 61] | |||
| TCTN1 | JBTS13 | TZ | [11, 62] | ||||
| TCTN2 | MKS8 | TZ, AX | TM | [11, 15, 63] | |||
| TCTN3 | TZ, AX | TM | [62] | ||||
| CC2D2A | MKS6 | JBTS9 | TZ | C2, CC | [11, 12, 64] | ||
| TMEM216 | MKS2 | JBTS2 | TZ, BB | TM | [65] | ||
| TMEM67 | NPHP11 | MKS3 | JBTS6 | TZ, AX | TM | [20, 66, 67] | |
| MKS1 | MKS1 | TZ | B9 | [20, 21, 60, 67, 68] | |||
| TMEM237 | JBTS14 | TZ | TM | [22] | |||
| NPHP 5–6 | Cep290 | NPHP6 | MKS4 | JBTS5 | TZ, BB | CC | [23, 25, 38, 69] |
| IQCB1 | NPHP5 | BB | IQ, CC | [23, 70] | |||
| Inversin compartment | Inversin | NPHP2 | BB, TZ, AX | ANK, IQ, CC | [26, 71–73] | ||
| NPHP3 | NPHP3 | MKS7 | AX | TPR, CC | [46, 74, 75] | ||
| Nek8 | NPHP9 | AX | Serine/Threonine kinase, RCC1 | [75–78] | |||
| Other | Glis2 | NPHP7 | C2H2 | [79, 80] | |||
| SDCCAG8 | NPHP10 | CC | [28] | ||||
| TTC21B/IFT139 | NPHP12 | JBTS11 | AX | TPR | [81, 82] | ||
| INPP5E | JBTS1 | AX | Inositol phosphatase | [83, 84] | |||
| AHI1/Jouberin | JBTS3 | SH3, WD40 | [25, 85] | ||||
| OFD1 | JBTS10 | BB | CC | [86, 87] | |||
| KIF7 | JBTS12 | AX | Kinesin motor, CC | [88, 89] | |||
| ARL13b | JBTS8 | AX | GTPase, CC | [90, 91] | |||
| XPNPEP3 | Mitochondria | Aminopeptidase | [92] | ||||
| CEP41 | JBTS15 | BB, AX | Rhod | [93] | |||
BB – basal body, TZ – transition zone, AX – axonemal compartment
TM – transmembrane, SH3 – Src homology 3, ANK – ankryin repeat, TPR – tetratricopeptide repeat, WD40 - WD40- or β-transducin domain, RCC1 – regulator of chromosome condensation 1-like, C2, B9 – membrane association domains, C2H2 – zinc finger-like domain, IQ – IQ calmodulin-binding domain.
Box 3 : Complex Genetics of Human Ciliopathies.
NPHP syndromes are characterized by oligogenicity and significant phenotypic pleiotropy, and a lack of predictable genotype-phenotype correlations [50, 51] (Table 1). For example, in MKS, the constellation of pathologic manifestations is quite complex and the penetrance of individual single organ phenotypes is variable from patient to patient. Despite the characterization of many mutations, it is difficult to predict whether an affected individual carries homozygous mutations in MKS1, in TMEM67, or in another one of the ten identified MKS genes, or in a yet unidentified gene. Moreover, certain single genes can produce the full spectrum of NPHP-related disorders, depending on the nature of the mutation. More than 100 unique human mutations were identified in the CEP290 ciliopathy gene (also referred to as NPHP6, JBTS5 or MKS4), giving rise to a range of phenotypes including isolated NPHP, LCA, characteristic SLSN, JBTS, Bardet-Biedl syndrome (BBS) or the lethal MKS [52]. We also find that some genes are frequently associated with the less severe manifestations of the NPHP continuum, whereas others are found in the context of more severe ciliopathies. Ideally, systematic genetic screening would be a routine element in the clinico-pathologic diagnosis of ciliopathy cases, but complete sequencing data is often unavailable. As our understanding evolves, we may indeed find a linkage of specific genes to more or less severe disease phenotypes. Alternatively, we may find that most ciliopathy genes, like CEP290, produce the full pathologic spectrum of ciliopathy phenotypes.
Assembling the transition zone from protein complexes
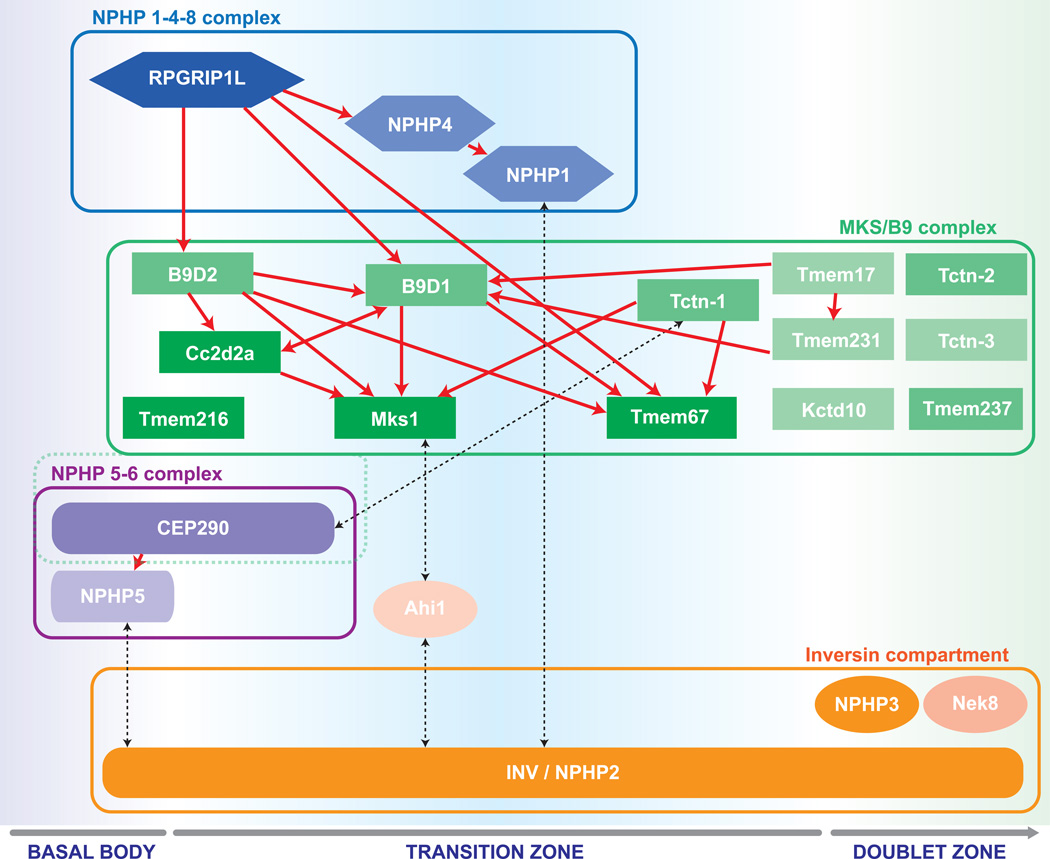
How is the intricate structure of the transition zone built and maintained? Genetic studies of TZ proteins employing mutations, knockout and RNAi knockdown strategies have revealed dependency patterns of TZ localization and, in some cases, ultrastructural correlations with specific TZ assemblies [10–14]. These studies have been complemented by proteomic analyses of affinity purified protein complexes, using various TZ proteins as “bait” [11, 15, 16] or via the direct enrichment of ciliary material [17–19]. Systematic analysis and correlation of localization data, genetic analyses and proteomic interaction patterns has revealed a preliminary map of the TZ protein network. This map involves many known and a few novel ciliopathy proteins and reveals an organization into at least four communicating multi-protein complexes: NPHP1-4-8, MKS/B9, NPHP5–6 and the Inversin compartment (Figure 2).
Figure 2. Organizational chart of transition zone functional modules.
Rpgrip1L/NPHP8 localizes independently and influences proper TZ localization of the NPHP1-4 and MKS/B9 complexes. The localization may be direct or through a cascade of consecutive localization and complex organization events. Within the MKS/B9 module, Mks1, Tmem216 and Tmem67, which are consistently associated with severe pathologies in humans, appear at the bottom of the localization hierarchy within the module. Cep290 was considered the core of its own NPHP5-6 network in one study [15], but part of an MKS-like complex in another [11]. The inversin compartment is highly dynamic and spans a long stretch of ciliary axoneme from the basal body into the distal cilium proper. Through its localization and its interactions with members of all protein modules, it may serve as a bridge and contribute to multiple distinct signaling processes.
Proteins within one module are highlighted by the same shape and color. Brighter color indicates more frequent association with severe disease. Higher position within one group and red arrows indicate intra-complex localization hierarchy. Black dotted arrows indicate physical interactions across modules.
Note that not all depicted proteins are exclusively located in the TZ, but may also be found at the basal body (Rpgrip1L, B9D1, Cc2d2a, Tmem216, Cep290, NPHP5, inversin), or in the proximal doublet zone of the cilium proper (inversin, Nphp3, Nek8, Tmem67, Tctn-2, Tctn-3).
The Rpgrip1L/NPHP8 protein organizes both the 1-4-8 module and the MKS module at the TZ. Evidence for this complex emerges from two lines of work. In cultured cells, the systematic proetomic analysis using tandem affinity purification followed by mass spectrometry-based protein identification (TAP/MS) showed clear reciprocal interactions between Rpgrip1L, Nphp1 and Nphp4 [15]. The complex was well-conserved in both epithelial (IMCD) and mesenchymal (3T3) cells demonstrating a basic core consensus of the complex across cell types. The second line of evidence came from a systematic genetic approach in Caenorhabditis, where RPGRIP1L was shown to be at the top of an assembly hierarchy controlling the localization of Nphp1 and Nphp4 and the entire nematode MKS complex [14]. Mutation of NPHP1 or NPHP4 had no effect on the MKS complex members. Interestingly, when mutated together (i.e. one mutation from 1-4-8 and one from MKS), the transition zone showed complete loss of Y-linkers and membrane attachment, defective ciliogenesis and deranged cilium protein trafficking. The multitude of associations of Rpgrip1L and its role in supporting large domains of the TZ may explain its involvement in ciliopathies across the entire severity spectrum (Table 1) and conversely, why NPHP1 and NPHP4 may be associated with less severe phenotypes.
In contrast to Rpgrip1L or NPHP1/4, nearly all of the proteins in the MKS/B9 module have been implicated in the most severe disease of the NPHP continuum, Meckel-Gruber Syndrome (MKS). This module was found through proteomic methods in multiple independent studies [11, 15, 16]. Both studies used TAP/MS methods but different baits, strengthening the evidence for a distinct MKS module. Genetic evidence in mice and Caenorhabditis, along with interactomic analyses reveal the MKS module as a multiprotein complex rich in membrane-targeting B9 and C2 domains, as well as a number of membrane proteins (Table 1). As described above any combination of MKS and NPHP1-4-8 mutations produces significant disruptions of TZ ultrastructure in nematodes [14]. Unlike mutations in Caenorhabditis, correlative ultrastructural data on cilia and TZ architecture of human MKS cases or mouse models is lacking. A recent publication describes the identification of a novel Joubert syndrome related protein, TMEM237/JBTS15, that has been localized to the TZ. Its deletion exacerbates ciliary membrane defects in an NPHP4 negative background, as described for other MKS module proteins. Although interactomic data is not yet available, it will likely be identified as an MKS complex component [22].
Comparing MKS components of Caenorhabditis with interactomic data from mammalian cells, obvious homologues for the tectonic (Tctn) family of proteins are absent from the nematode genome. Another difference between nematode and mammalian MKS proteins is their effect on ciliogenesis. While there were only few defects in isolated MKS mutations in Caenorhabditis, MKS proteins had more significant effects on ciliogenesis in mammalian cells. For example, RNAi-mediated depletion of the Mks1 protein in cell culture was reported to result in reduced ciliogenesis and defective basal body migration [20]. Genetic deletion of MKS1 in a mouse model of MKS showed tissue specific loss of cilia – with reduced ciliation in the developing neural tube but largely normal cilia in the renal tubule [21]. Similar tissue specific defects were seen in mouse models of genetic deletions of the MKS module proteins Tctn1, Tctn2 and Cc2d2a [11], but were not reported in TMEM231 and B9D1 deleted mice. One important aspect of development in worms is that all cilia reside in neurons of the sensory system and may follow a common ciliogenic program [6]. In mammals, cilia from cells of epithelial versus mesenchymal origin can have different morphological appearances and signaling roles, perhaps requiring different ciliogenic proteins. Such tissue-specific effects are likely to be reflected in varied composition or differential requirement of TZ protein modules for both, ciliogenesis and signaling.
The “NPHP5-6” or Cep290 module emerges primarily from proteomic studies in mammalian cell lines. Tandem affinity approaches show a strong interaction of Cep290 with the MKS [11], while others postulate a distinct module grouped around a Cep290-NPHP5 cluster [15], highlighting a previously described functional interaction [23]. In Chlamydomonas, immunogold electron microscopy maps Cep290 to the Y-linkers of the TZ, and its mutation results in a substantial, but not complete, loss of Y-linkers [10], indicating Cep290 contributes to Y-linker formation. Interestingly, an obvious CEP290 orthologue is lacking from the Caenorhabditis genome, where morphologically intact Y-linkers are present underscoring the role of other proteins (or a functional worm homologue of Cep290) in defining these substructures. With such a striking phenotype in Chlamydomonas and the involvement of CEP290 throughout the entire severity spectrum of human ciliopathies, it is surprising that a spontaneous in-frame deletion mutant in mice displays only retinal degeneration (rd16 mutation, [24]). Moreover, a genetic deletion has a mild JBTS-like phenotype (retinal degeneration and cerebellar patterning defects) [25] but no cystic kidney disease or defects of early development, such as we might expect. How the many recessive mutations in CEP290 result in defects more severe than the genetic deletion will require more detailed investigation.
A special role is attributed to Inversin, which interacts and colocalizes with Nphp3 and Nek8, but is not restricted to the BB or TZ. This broad localization may belie a bridging function between multiple modules within the network. Indeed, interactions were independently established with NPHP1, NPHP5 and the MKS module via the JBTS protein Ahi1 [15]. From all available evidence to date, the Inversin compartment appears dynamic, including the basal body and a variable distal extension along the ciliary shaft. Mutations in Inversin, NPHP3 or Nek8 have not been correlated with disruptions of the TZ structure itself, as yet [26].
Where can we go with such a map? Sang and colleagues were able to use the proteins in their complexes to identify the new ciliopathy genes Tectonic2 and Ataxin-10 which cause JBTS and NPHP, respectively [15]. With the possibility of identifying new ciliopathy genes a variety of proteomic, bioinformatic [27] and next generation sequencing [28] approaches, even in non-mammalian organisms, are likely to add significantly to the list of transition zone proteins and their interactions. Even more than a set of experimental approaches, these studies highlight the necessity of systematic studies. The cilium is an organelle of systems biology. Like any machine that has many moving parts, it is the assembly and coordinated effort of these parts that is necessary for proper functioning. Loss of one protein may give a dramatic phenotype, but to even hypothesize a mechanism we will need to know how that loss has affected the other components.
With the exception of Cep290, none of the described TZ proteins has been mapped to a defined TZ substructure. The next step in the characterization of NPHP and MKS protein complexes will be to localize individual proteins to specific TZ constituents. This will add a morphological dimension to the TZ protein map that is otherwise only based on interactomic and genetic data. Moreover, detailed protein-protein interactions and ultrastructural analysis will be necessary not only in the wild-type setting, but also under genetic perturbation. While immunofluorescence and GFP-tagging is possible in almost all the model ciliary systems, light microscopy does not yield the resolution to define TZ architecture. There is potential for new technologies that promise super-resolution beyond the conventional ~200 nm limit to illuminate our understanding of the TZ [29]. However, even with such technologies, the high resolution ultrastructural methods of visualizing the TZ available to models such as Chlamydomonas and Caenorhabditis must find their way to vertebrate systems (e.g [30]) to observe the more intricate elements of the TZ and their dependency on the TZ modules.
Assembling signaling pathways through the TZ trafficking gate
How are proteins trafficking through the complex assembly of structures at the ciliary base, and what role does the TZ play? It has long been postulated that a selective barrier at the ciliary base acts to recognize proteins and membrane vesicles that are intended to translocate into the ciliary compartment, while prohibiting access to others. The presence of transitional fibers, Y-linkers and necklace structures constitutes an obvious physical boundary and is suggestive of a gating mechanism. Furthermore, a variety of cytoskeletal substructures occupy a large amount of space within and around the ciliary lumen at the ciliary base. In the region of the transitional fibers, the space between two neighboring sheets is estimated not to be larger than 60 nm, thus making vesicular transport to the ciliary membrane unlikely through an axial route [31]. These considerations are further complicated by findings demonstrating that a distinct ciliary membrane domain extends into an about 1.8 µm diameter apical membrane patch around the ciliary base [32, 33]. This extraciliary region is characterized by a specific lipid and protein composition that is distinct from that of the surrounding apical membrane. This region has been proposed to be a docking site for vesicles with ciliary cargo components that would then travel laterally across the transition zone into the cilium [32, 33]. There is also evidence that entry into the cilium can be prevented by proteins tethering them to the surrounding cytoskeleton [32]. This larger region may serve as a docking site for cilium-destined cargos, but is itself far from the TZ.
Structures more proximal to the TZ have been proposed to carry out the local gating function. A barrier function has been ascribed to a septin network (Sept2) at the base of the cilium that is required for ciliogenesis and enrichment of proteins within the compartment [34]. Recent evidence suggest that Sept2 exerts this barrier fucntion through the localization of MKS/B9 protein complex members Tmem231, B9D1 and Cc2d2a [16]. This barrier appears to be passive, preventing mixing between the apical and ciliary membranes, but not “smart” enough to distinguish between ciliary and non-ciliary cargos. Disruption of TZ Y-linkers, as in Chlamydomonas CEP290 mutants or in Caenorhabditis NPHP/MKS module double mutants, resulted in the inappropriate localization of non-ciliary proteins into the cilium [10, 14]. In Chlamydomonas, flagellar proteomes from wild-type and CEP290 mutants were profiled. In addition to the relative loss of otherwise abundant flagellar proteins, some proteins were identified that are usually not found in flagella [10]. Similarly, in Caenorhabditis mutants, GFP-tagged ciliary and non-ciliary proteins were assessed for cilium localization [14]. The membrane-associated X-linked retinitis pigmentosa gene product RP2, was absent from cilia in wildtype, MKS1, TMEM67 and NPHP1 mutant worms, but present in the cilium of MKS1/TMEM67 double mutants and single mutants of B9D1, B9D2, RPGRIP1L, CC2D2A and NPHP4. The transmembrane protein TRAM-1a showed almost identical behavior. Importantly, in these genetic backgrounds, axoneme-membrane interactions and Y-linkers were ultrastructurally intact, indicating that a subtle molecular selectivity determinant has been lost. Altogether the data supports a smart barrier model of TZ function, with ciliary proteins permitted access by determinants within the TZ and non-ciliary proteins excluded. When the TZ is disrupted, ciliary proteins are either unable to enter or accumulate and non-ciliary proteins enter inappropriately. Alternatively, it has been proposed that gating may occur in a bidirectional manner, based on live observations of flagellar trafficking in Chalmydomonas [35]. As such, the inappropriate ciliary accumulation of proteins may imply an active removal pathway that is defective when the TZ is disrupted. Such a bidirectional gating is reminiscent of the nuclear pore complex, whose trafficking determinants, the Ran-Importin pathway, have been implicated in some aspects of ciliary trafficking [36].
Restrictive signals can keep proteins out of the ciliary compartment, but gatekeeping into the cilium is also dependent on permissive signal recognition [31]. The somatostatin receptor SSTR3 localizes to cilia in many genetic backgrounds. However, the adenylylate cyclase III transmembrane (ACIII) enzyme was totally absent from cilia in TCTN1, TCTN2, CC2D2A or TMEM67 null MEF cells [11]. One of the ADPKD gene products, Pkd2, was also absent from cilia in TCTN1, TCTN2 or CC2D2A null MEFs, but showed unimpaired ciliary localization in TMEM67 null cells. The Hh signaling pathway has been intimately linked to cilia through a number of studies of developmental defects in mice [37]. Activation of the hedgehog signaling pathway involves the ciliary translocation of Smoothened, a process that was shown to be dependent on Tctn1, Tctn2 and Cc2d2a, but independent of Tmem67. Similarly, Arl13b, a lipid modified and membrane associated ciliary protein, was markedly reduced in cilia of TCTN1, TCTN2 or CC2D2A null cells, while the reduction in TMEM67 null cells was modest. TCTN1/2 and CC2D2A null mice all exhibited dramatic developmental defects with situs inversus, tissue-specific ciliogenesis defects and embryonic lethality, whereas TMEM67 null mice survive until birth, have kidney cysts during embryogenesis and reduced cilia in kidney tubules. Together, this indicates that simply making a cilium is not sufficient; equipping it with the right components is just as crucial. Understanding the relationship between disruption in the TZ assembly hierarchy and the change in cilium protein localization could provide rules for protein trafficking to the cilium. Moreover, we may be able to identify those TZ proteins that recognize specific permissive signals on a cargo and the nature of these signals themselves.
From a signaling perspective, complete pathways must be assembled component by component within the cilium. The inability to target a component of a signaling pathway would result in the disruption of the cascade itself. For example, in the CEP290 mutant mouse (rd16), odorant receptors, a type of G-protein coupled receptor (GPCR), and the corresponding adenylate cyclase isoform (ACIII) were properly localized to olfactory cilia, however, the heterotrimeric G protein (Golf) that acts between the GPCR and ACIII did not [38]. As a result, these mice and patients with CEP290 mutations have poor olfaction. Ciliary signaling pathways such as G-protein coupled receptors (e.g. somatostatin or melanocortin [39]), receptor tyrosine kinases (e.g. PDGF [40]) and calcium channels [8] are all localized to and transduced through the primary cilium. Each protein in the cascade could be trafficked to the cilium independently or pre-formed signaling centers could be transported en masse. Testing how entire signaling pathways are transported and assembled within the primary cilium will require the integrative and systems biology approaches described here. Understanding these mechanisms would help to elucidate the mechanisms by which cell-specific specification of the ciliary compartment is achieved through TZ-mediated ciliary protein gating. Ultimately, we would be able to assess the types of cellular defects that translate into different severity states of ciliopathy phenotypes: the partial loss of a signaling pathway would perhaps underlie a mild phenotype, while a complete loss of cilium specification could result in more dramatic tissue dysfunction as seen in the most severely affected patients.
In summary, the complex phenotypes seen in TZ dysfunction all point to the concept of a smart gate that helps regulate ciliary protein homeostasis. Proteins that arrive at the TZ, presumably through a set of trafficking signals, are asked for their “documents” to permit them entry into the ciliary compartment. Moreover, those proteins may be queried within the ciliary compartment repeatedly and trafficked out of the cilium when no longer required. How such a gate is built from the unique ultrastructural features and protein modules reviewed here will be the central area of investigation for the future. Alongside how the transition zone itself is assembled, we will need to understand how the TZ assembles signaling pathways by documenting ciliary proteins under normal and perturbative conditions. By integrating our knowledge of molecular complexes, ultrastructural assemblies, cellular functions and organismal phenotypes, we can begin to elucidate how this small organelle exerts its control in development and homeostasis, and how its disruptions produce human disease phenotypes.
Box 1.
Box 2.
Acknowledgements
The authors thank the members of the Shah lab for fruitful discussions. Work on primary cilia in the Shah lab is supported by grants from the National Institutes of Health (P50DK074030 supporting the Harvard PKD Center) and Genzyme Corporation (Renal Innovations Program) to JVS.
Glossary
- ADPKD
autosomal dominant polycystic kidney disease
- ANK
ankyrin repeat
- BB
basal body
- BBS
Bardet-Biedl syndrome
- CC
coiled coil domain
- IFT
intraflagellar transport
- JBTS
Joubert syndrome
- LCA
Leber congenital amaurosis
- MEF
murine embryonic fibroblasts
- MKS
Meckel-Gruber syndrome
- MT
Microtubule
- NPHP
Nephronophthisis
- RCC1
“regulator of chromosome condensation-1” domain
- RPGRIP1L
X-linked Retinitis pigmentosa GTPase regulator interacting protein-1-like
- SH3
Src-homology-3 domain
- SLSN
Senior-Løken syndrome
- TZ
ciliary transition zone
Gene names are written in capital letters (TMEM67); proteins in capital and small letters (Tmem67)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carvalho-Santos Z, et al. Evolution: Tracing the origins of centrioles, cilia, and flagella. The Journal of Cell Biology. 2011;194:165–175. doi: 10.1083/jcb.201011152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisch C, Dupuis-Williams P. Ultrastructure of cilia and flagella - back to the future! Biology of the cell / under the auspices of the European Cell Biology Organization. 2011;103:249–270. doi: 10.1042/BC20100139. [DOI] [PubMed] [Google Scholar]
- 3.Anderson RG. The three-dimensional structure of the basal body from the rhesus monkey oviduct. The Journal of Cell Biology. 1972;54:246–265. doi: 10.1083/jcb.54.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibbons IR, Grimstone AV. On flagellar structure in certain flagellates. The Journal of biophysical and biochemical cytology. 1960;7:697–716. doi: 10.1083/jcb.7.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilula NB, Satir P. The ciliary necklace. A ciliary membrane specialization. The Journal of Cell Biology. 1972;53:494–509. doi: 10.1083/jcb.53.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perkins LA, et al. Mutant sensory cilia in the nematode Caenorhabditis elegans. Developmental Biology. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 7.Pazour GJ, et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoder BK, et al. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. Journal of the American Society of Nephrology : JASN. 2002;13:2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 9.Hildebrandt F, et al. Ciliopathies. The New England journal of medicine. 2011;364:1533–1543. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craige B, et al. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. The Journal of Cell Biology. 2010;190:927–940. doi: 10.1083/jcb.201006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Gonzalo FR, et al. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nature genetics. 2011;43:776–784. doi: 10.1038/ng.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorden NT, et al. CC2D2A is mutated in Joubert syndrome and interacts with the ciliopathy-associated basal body protein CEP290. American journal of human genetics. 2008;83:559–571. doi: 10.1016/j.ajhg.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jauregui AR, et al. The Caenorhabditis elegans nephrocystins act as global modifiers of cilium structure. The Journal of Cell Biology. 2008;180:973–988. doi: 10.1083/jcb.200707090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams CL, et al. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. The Journal of Cell Biology. 2011;192:1023–1041. doi: 10.1083/jcb.201012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sang L, et al. Mapping the NPHP-JBTS-MKS Protein Network Reveals Ciliopathy Disease Genes and Pathways. Cell. 2011;145:513–528. doi: 10.1016/j.cell.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chih B, et al. A ciliopathy complex at the transition zone protects the cilia as a privileged membrane domain. Nature Cell Biology. 2011;14:61–72. doi: 10.1038/ncb2410. [DOI] [PubMed] [Google Scholar]
- 17.Mayer U, et al. The proteome of rat olfactory sensory cilia. PROTEOMICS. 2009;9:322–334. doi: 10.1002/pmic.200800149. [DOI] [PubMed] [Google Scholar]
- 18.Ostrowski LE, et al. A proteomic analysis of human cilia: identification of novel components. Molecular and cellular proteomics. 2002;1:451–465. doi: 10.1074/mcp.m200037-mcp200. [DOI] [PubMed] [Google Scholar]
- 19.Pazour GJ. Proteomic analysis of a eukaryotic cilium. The Journal of Cell Biology. 2005;170:103–113. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dawe HR, et al. The Meckel-Gruber Syndrome proteins MKS1 and meckelin interact and are required for primary cilium formation. Human Molecular Genetics. 2007;16:173–186. doi: 10.1093/hmg/ddl459. [DOI] [PubMed] [Google Scholar]
- 21.Weatherbee SD, et al. A mouse model for Meckel syndrome reveals Mks1 is required for ciliogenesis and Hedgehog signaling. Human Molecular Genetics. 2009;18:4565–4575. doi: 10.1093/hmg/ddp422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang L, et al. TMEM237 Is Mutated in Individuals with a Joubert Syndrome Related Disorder and Expands the Role of the TMEM Family at the Ciliary Transition Zone. American journal of human genetics. 2011;89:713–730. doi: 10.1016/j.ajhg.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schäfer T, et al. Genetic and physical interaction between the NPHP5 and NPHP6 gene products. Human Molecular Genetics. 2008;17:3655–3662. doi: 10.1093/hmg/ddn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang B, et al. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Human Molecular Genetics. 2006;15:1847–1857. doi: 10.1093/hmg/ddl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lancaster MA, et al. Defective Wnt-dependent cerebellar midline fusion in a mouse model of Joubert syndrome. Nature Medicine. 2011;17:726–731. doi: 10.1038/nm.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiba D, et al. Localization of Inv in a distinctive intraciliary compartment requires the C-terminal ninein-homolog-containing region. Journal of Cell Science. 2009;122:44–54. doi: 10.1242/jcs.037408. [DOI] [PubMed] [Google Scholar]
- 27.Li JB, et al. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell. 2004;117:541–552. doi: 10.1016/s0092-8674(04)00450-7. [DOI] [PubMed] [Google Scholar]
- 28.Otto EA, et al. Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nature genetics. 2010;42:840–850. doi: 10.1038/ng.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toomre D, Bewersdorf J. A new wave of cellular imaging. Annual Review of Cell and Developmental Biology. 2010;26:285–314. doi: 10.1146/annurev-cellbio-100109-104048. [DOI] [PubMed] [Google Scholar]
- 30.Gluenz E, et al. Beyond 9+0: noncanonical axoneme structures characterize sensory cilia from protists to humans. The FASEB Journal. 2010;24:3117–3121. doi: 10.1096/fj.09-151381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nachury MV, et al. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annual Review of Cell and Developmental Biology. 2010;26:59–87. doi: 10.1146/annurev.cellbio.042308.113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Francis SS, et al. A hierarchy of signals regulates entry of membrane proteins into the ciliary membrane domain in epithelial cells. The Journal of Cell Biology. 2011;193:219–233. doi: 10.1083/jcb.201009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vieira OV, et al. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:18556–18561. doi: 10.1073/pnas.0608291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu Q, et al. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science. 2010;329:436–439. doi: 10.1126/science.1191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lechtreck K-F, et al. The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. The Journal of Cell Biology. 2009;187:1117–1132. doi: 10.1083/jcb.200909183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dishinger JF, et al. Ciliary entry of the kinesin-2 motor KIF17 is regulated by importin-beta2 and RanGTP. Nature Cell Biology. 2010 doi: 10.1038/ncb2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nature Reviews Genetics. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McEwen DP, et al. Hypomorphic CEP290/NPHP6 mutations result in anosmia caused by the selective loss of G proteins in cilia of olfactory sensory neurons. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15917–15922. doi: 10.1073/pnas.0704140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berbari NF, et al. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci U S A. 2008;105:4242–4246. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider L, et al. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Current biology : CB. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Horst CJ, et al. Cytoskeletal-membrane interactions: a stable interaction between cell surface glycoconjugates and doublet microtubules of the photoreceptor connecting cilium. The Journal of Cell Biology. 1987;105:2973–2987. doi: 10.1083/jcb.105.6.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ringo DL. Flagellar motion and fine structure of the flagellar apparatus in Chlamydomonas. The Journal of Cell Biology. 1967;33:543–571. doi: 10.1083/jcb.33.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishikawa H, Marshall WF. Ciliogenesis: building the cell's antenna. Nature Reviews Molecular Cell Biology. 2011;12:222–234. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- 44.Afzelius BA. A human syndrome caused by immotile cilia. Science (New York, NY) 1976;193:317–319. doi: 10.1126/science.1084576. [DOI] [PubMed] [Google Scholar]
- 45.Boucher C, Sandford R. Autosomal dominant polycystic kidney disease (ADPKD, MIM 173900, PKD1 and PKD2 genes, protein products known as polycystin-1 and polycystin-2) European Journal of Human Genetics. 2004;12:347–354. doi: 10.1038/sj.ejhg.5201162. [DOI] [PubMed] [Google Scholar]
- 46.Bergmann C, et al. Loss of nephrocystin-3 function can cause embryonic lethality, Meckel-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am J Hum Genet. 2008;82:959–970. doi: 10.1016/j.ajhg.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parisi MA. Clinical and molecular features of Joubert syndrome and related disorders. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. 2009;151C:326–340. doi: 10.1002/ajmg.c.30229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alexiev BA, et al. Meckel-Gruber syndrome: pathologic manifestations, minimal diagnostic criteria, and differential diagnosis. Archives of pathology & laboratory medicine. 2006;130:1236–1238. doi: 10.5858/2006-130-1236-MS. [DOI] [PubMed] [Google Scholar]
- 49.Kumari N, et al. Post-mortem examination of prenatally diagnosed fatal renal malformation. Journal of Perinatology. 2008;28:736–742. doi: 10.1038/jp.2008.93. [DOI] [PubMed] [Google Scholar]
- 50.Chaki M, et al. Genotype-phenotype correlation in 440 patients with NPHP-related ciliopathies. Kidney International. 2011 doi: 10.1038/ki.2011.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoefele J, et al. Evidence of oligogenic inheritance in nephronophthisis. Journal of the American Society of Nephrology. 2007;18:2789–2795. doi: 10.1681/ASN.2007020243. [DOI] [PubMed] [Google Scholar]
- 52.Coppieters F, et al. CEP290, a gene with many faces: mutation overview and presentation of CEP290base. Human Mutation. 2010;31:1097–1108. doi: 10.1002/humu.21337. [DOI] [PubMed] [Google Scholar]
- 53.Arts HH, et al. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nature genetics. 2007;39:882–888. doi: 10.1038/ng2069. [DOI] [PubMed] [Google Scholar]
- 54.Delous M, et al. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nature genetics. 2007;39:875–881. doi: 10.1038/ng2039. [DOI] [PubMed] [Google Scholar]
- 55.Habbig S, et al. NPHP4, a cilia-associated protein, negatively regulates the Hippo pathway. The Journal of Cell Biology. 2011;193:633–642. doi: 10.1083/jcb.201009069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mollet G, et al. Characterization of the nephrocystin/nephrocystin-4 complex and subcellular localization of nephrocystin-4 to primary cilia and centrosomes. Hum Mol Genet. 2005;14:645–656. doi: 10.1093/hmg/ddi061. [DOI] [PubMed] [Google Scholar]
- 57.Fliegauf M, et al. Nephrocystin specifically localizes to the transition zone of renal and respiratory cilia and photoreceptor connecting cilia. Journal of the American Society of Nephrology : JASN. 2006;17:2424–2433. doi: 10.1681/ASN.2005121351. [DOI] [PubMed] [Google Scholar]
- 58.Hildebrandt F, et al. A novel gene encoding an SH3 domain protein is mutated in nephronophthisis type 1. Nature genetics. 1997;17:149–153. doi: 10.1038/ng1097-149. [DOI] [PubMed] [Google Scholar]
- 59.Hopp K, et al. B9D1 is revealed as a novel Meckel syndrome (MKS) gene by targeted exon-enriched next-generation sequencing and deletion analysis. Human Molecular Genetics. 2011;20:2524–2534. doi: 10.1093/hmg/ddr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams CL, et al. Functional redundancy of the B9 proteins and nephrocystins in Caenorhabditis elegans ciliogenesis. Molecular Biology of the Cell. 2008;19:2154–2168. doi: 10.1091/mbc.E07-10-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dowdle WE, et al. Disruption of a ciliary B9 protein complex causes Meckel syndrome. American journal of human genetics. 2011;89:94–110. doi: 10.1016/j.ajhg.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reiter JF, Skarnes WC. Tectonic, a novel regulator of the Hedgehog pathway required for both activation and inhibition. Genes and Development. 2006;20:22–27. doi: 10.1101/gad.1363606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shaheen R, et al. A TCTN2 mutation defines a novel Meckel Gruber syndrome locus. Human Mutation. 2011;32:573–578. doi: 10.1002/humu.21507. [DOI] [PubMed] [Google Scholar]
- 64.Tallila J, et al. Identification of CC2D2A as a Meckel syndrome gene adds an important piece to the ciliopathy puzzle. American journal of human genetics. 2008;82:1361–1367. doi: 10.1016/j.ajhg.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valente EM, et al. Mutations in TMEM216 perturb ciliogenesis and cause Joubert, Meckel and related syndromes. Nature genetics. 2010;42:619–625. doi: 10.1038/ng.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith UM, et al. The transmembrane protein meckelin (MKS3) is mutated in Meckel-Gruber syndrome and the wpk rat. Nature genetics. 2006;38:191–196. doi: 10.1038/ng1713. [DOI] [PubMed] [Google Scholar]
- 67.Tammachote R, et al. Ciliary and centrosomal defects associated with mutation and depletion of the Meckel syndrome genes MKS1 and MKS3. Human Molecular Genetics. 2009;18:3311–3323. doi: 10.1093/hmg/ddp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kyttälä M, et al. MKS1, encoding a component of the flagellar apparatus basal body proteome, is mutated in Meckel syndrome. Nature genetics. 2006;38:155–157. doi: 10.1038/ng1714. [DOI] [PubMed] [Google Scholar]
- 69.Sayer JA, et al. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nature genetics. 2006;38:674–681. doi: 10.1038/ng1786. [DOI] [PubMed] [Google Scholar]
- 70.Otto EA, et al. Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior-Loken syndrome and interacts with RPGR and calmodulin. Nature genetics. 2005;37:282–288. doi: 10.1038/ng1520. [DOI] [PubMed] [Google Scholar]
- 71.Otto EA, et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nature genetics. 2003;34:413–420. doi: 10.1038/ng1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simons M, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nature genetics. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yokoyama T, et al. Reversal of left-right asymmetry: a situs inversus mutation. Science. 1993;260:679–682. doi: 10.1126/science.8480178. [DOI] [PubMed] [Google Scholar]
- 74.Olbrich H, et al. Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapeto-retinal degeneration and hepatic fibrosis. Nature genetics. 2003;34:455–459. doi: 10.1038/ng1216. [DOI] [PubMed] [Google Scholar]
- 75.Shiba D, et al. Inv acts as a molecular anchor for Nphp3 and Nek8 in the proximal segment of primary cilia. Cytoskeleton. 2010;67:112–119. doi: 10.1002/cm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu S, et al. A defect in a novel Nek-family kinase causes cystic kidney disease in the mouse and in zebrafish. Development. 2002;129:5839–5846. doi: 10.1242/dev.00173. [DOI] [PubMed] [Google Scholar]
- 77.Otto EA, et al. NEK8 mutations affect ciliary and centrosomal localization and may cause nephronophthisis. J Am Soc Nephrol. 2008;19:587–592. doi: 10.1681/ASN.2007040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sohara E, et al. Nek8 regulates the expression and localization of polycystin-1 and polycystin-2. J Am Soc Nephrol. 2008;19:469–476. doi: 10.1681/ASN.2006090985. Epub 2008 Jan 2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Attanasio M, et al. Loss of GLIS2 causes nephronophthisis in humans and mice by increased apoptosis and fibrosis. Nature genetics. 2007;39:1018–1024. doi: 10.1038/ng2072. [DOI] [PubMed] [Google Scholar]
- 80.Zhang F, et al. Characterization of Glis2, a novel gene encoding a Gli-related, Krüppel-like transcription factor with transactivation and repressor functions. Roles in kidney development and neurogenesis. The Journal of biological chemistry. 2002;277:10139–10149. doi: 10.1074/jbc.M108062200. [DOI] [PubMed] [Google Scholar]
- 81.Davis EE, et al. TTC21B contributes both causal and modifying alleles across the ciliopathy spectrum. Nature genetics. 2011;43:189–196. doi: 10.1038/ng.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tran PV, et al. THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nature genetics. 2008;40:403–410. doi: 10.1038/ng.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bielas SL, et al. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nature genetics. 2009;41:1032–1036. doi: 10.1038/ng.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jacoby M, et al. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nature genetics. 2009;41:1027–1031. doi: 10.1038/ng.427. [DOI] [PubMed] [Google Scholar]
- 85.Ferland RJ, et al. Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nature genetics. 2004;36:1008–1013. doi: 10.1038/ng1419. [DOI] [PubMed] [Google Scholar]
- 86.Ferrante MI, et al. Identification of the gene for oral-facial-digital type I syndrome. American journal of human genetics. 2001;68:569–576. doi: 10.1086/318802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Singla V, et al. Ofd1, a Human Disease Gene, Regulates the Length and Distal Structure of Centrioles. Developmental Cell. 2010;18:410–424. doi: 10.1016/j.devcel.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liem KF, et al. Mouse Kif7/Costal2 is a cilia-associated protein that regulates Sonic hedgehog signaling. Proc Natl Acad Sci U S A. 2009;106:13377–13382. doi: 10.1073/pnas.0906944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Putoux A, et al. KIF7 mutations cause fetal hydrolethalus and acrocallosal syndromes. Nature genetics. 2011;43:601–606. doi: 10.1038/ng.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cantagrel V, et al. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. American journal of human genetics. 2008;83:170–179. doi: 10.1016/j.ajhg.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Caspary T, et al. The graded response to Sonic Hedgehog depends on cilia architecture. Developmental Cell. 2007;12:767–778. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 92.O'Toole JF, et al. Individuals with mutations in XPNPEP3, which encodes a mitochondrial protein, develop a nephronophthisis-like nephropathy. The Journal of clinical investigation. 2010;120:791–802. doi: 10.1172/JCI40076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee JE, et al. CEP41 is mutated in Joubert syndrome and is required for tubulin glutamylation at the cilium. Nature genetics. 2012 doi: 10.1038/ng.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]