Abstract
Irregular vestibular afferents exhibit significant phase leads with respect to angular velocity of the head in space. This characteristic and their connectivity with vestibulospinal neurons suggest a functionally important role for these afferents in producing the vestibulo-collic reflex (VCR). A goal of these experiments was to test this hypothesis with the use of weak galvanic stimulation of the vestibular periphery (GVS) to selectively activate or suppress irregular afferents during passive whole body rotation of guinea pigs that could freely move their heads. Both inhibitory and excitatory GVS had significant effects on compensatory head movements during sinusoidal and transient whole body rotations. Unexpectedly, GVS also strongly affected the vestibulo-ocular reflex (VOR) during passive whole body rotation. The effect of GVS on the VOR was comparable in light and darkness and whether the head was restrained or unrestrained. Significantly, there was no effect of GVS on compensatory eye and head movements during volitional head motion, a confirmation of our previous study that demonstrated the extravestibular nature of anticipatory eye movements that compensate for voluntary head movements.
Keywords: vestibulo-ocular reflex, vestibulo-collic reflex, eye movements, galvanic vestibular stimulation
application of galvanic currents to the vestibular periphery has been shown to affect a subpopulation of afferent fibers innervating vestibular hair cells in all of the vestibular sense organs (Goldberg 2000; Kim and Curthoys 2004). Specifically, irregularly firing axons show a high degree of sensitivity to weak galvanic currents, whereas regular afferents are relatively unaffected (Baird et al. 1988; Goldberg et al. 1984). An afferent's sensitivity to electrical stimulation is one of several properties used to distinguish between the two types (for review see Eatock and Songer 2011; Goldberg 2000). Afferents vary in their anatomy, response dynamics, firing regularity, and response gains (Baird et al. 1988). Irregular afferents are characterized by calyceal endings (but may be dimorphic), are associated with more centrally located hair cells, and have spontaneous irregular firing rates. With respect to their dynamic responses, irregular units fall into two categories: those with relatively low gains at higher frequencies and others, which are part of a continuum of regular, intermediate, and irregular units with linearly increasing gains as a function of discharge regularity. The low-gain units have calyx endings, while the high-gain units tend to be dimorphs (Baird et al. 1988; Goldberg 2000).
Previous experiments have shown that galvanic vestibular stimulation (GVS) does not affect the vestibulo-ocular reflex (VOR) during sinusoidal motion or brief velocity steps in primates (Angelaki and Perachio 1993; Chen-Huang et al. 1997; Minor and Goldberg 1991). Angelaki and Perachio (1993) did find that anodal GVS reduced eye velocity during prolonged velocity steps lasting several seconds and during off-axis angular rotation (Angelaki et al. 1992). On the basis of these results, they concluded that irregular otolith afferents might be essential for the generation of steady-state nystagmus during off-vertical axis rotations (OVAR) and for velocity storage and the VOR at sinusoidal frequencies <0.1 Hz. The limited effects of irregular afferent “functional” ablation are surprising, as experimenters have also shown that there is no preferential innervation of second-order vestibular neurons by the different afferent types (Boyle et al. 1992; Chen-Huang et al. 1997; Highstein et al. 1987). One possible explanation for this apparent paradox is the influence of extravestibular factors such as target distance or attention (Chen-Huang et al. 1997; Chen-Huang and McCrea 1998) on the responses of neurons within the vestibular nucleus. Alternatively, irregular afferents might predominantly influence vestibular control of head stability (Angelaki and Perachio 1993; Bilotto et al. 1982) rather than the VOR, although this idea has never been directly tested in head-unrestrained animals.
To directly test the latter hypothesis, we performed galvanic stimulation in head-unrestrained guinea pigs. The present study presents evidence for effects of GVS on vestibular compensatory head movements during passive whole body rotations. Furthermore, here we show that in the guinea pig GVS has a significant effect on the VOR during both sinusoidal and transient head motion over a broad range of movement frequencies and velocities. In contrast, there is no effect of GVS on the anticipatory compensatory eye movements that occur during self-generated (active) head movements of the same animals (Shanidze et al. 2010b).
The approach of the present study differs in two critical ways from previous work. First, our experiments were done in the guinea pig, unlike the seminal primate studies of Minor and Goldberg (1991). Second, both eye and head responses to horizontal vestibular stimulation were measured in animals whose heads were unrestrained, a more natural preparation. Previous experiments have described the effects of GVS on vestibular nerve activity in the guinea pig. Kim and Curthoys (2004) replicated, in anesthetized guinea pigs, the preferential effect of GVS on irregular afferents found in chinchilla (e.g., Baird et al. 1988) and monkey (e.g., Goldberg et al. 1984). To control for any possible influence of head restraint on the effectiveness of GVS, we also report data from both head-restrained and head-unrestrained guinea pigs.
MATERIALS AND METHODS
Experimental and surgical procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Michigan's University Committee on Use and Care of Animals (UCUCA).
Surgical Procedures and Testing
For eye coil, head post, and electrode implantation, the animals were anesthetized with an intramuscular cocktail of ketamine (0.40 ml/kg) and xylazine (0.50 ml/kg) and were administered saline solution (20 ml) and atropine (0.125 ml/kg) subcutaneously for each surgical procedure. A heating pad was used to maintain the animals' body temperature. Vital signs were monitored until the animal became mobile and could stand upright. Three guinea pigs were prepared for chronic eye and head movement recording by implantation of a two-dimensional search coil in the right eye and a head post on the skull with dental acrylic (C&B Metabond, Parkell, Edgewood, NY). A second search coil was affixed to the head post to record head movements. The guinea pigs' bodies were restrained, but their heads were free to move during vestibular stimulation. Passive whole body angular rotation about an Earth vertical axis centered between the animals' ears was used to evoke reflex responses. Animals were rotated sinusoidally at frequencies ranging from 0.2 to 10 Hz, all tested at velocities ranging from 20 to 90°/s (20, 30, 40, 60, 80, 90°/s; accelerations of 300–3,000°·s−1·s−1). To mimic voluntary head movements, abrupt transient “velocity steps” between 20 and 90°/s with peak accelerations up to 2,000°·s−1·s−1 were used (for details see Shanidze et al. 2010a).
Galvanic Stimulation
Stimulating electrodes were implanted bilaterally in the middle ear. The electrodes and leads were assembled prior to the surgery and gas sterilized. Each electrode consisted of two Teflon-coated, 32-gauge, platinum-iridium wires (A-M Systems, Sequim, WA) soldered to a set of stainless steel connectors. Each electrode had a ball, 0.05 mm in diameter, on the implanted end. A retro-auricular incision was made and the dorsal bulla exposed. The bulla was drilled to provide access to the middle ear. With a surgical microscope, the ossicles, cochlea, and round window were observed through the opening as landmarks for placement of the stimulating electrodes. One electrode was placed near the round window, wedged in place, and fixed with Vetbond (3M, St. Paul, MN). The second electrode, serving as a ground, was implanted in the bulla distal to the first electrode and fixed in place with Vetbond. Metabond was used to seal the bulla opening. The leads from both electrodes were led subcutaneously to the skull and attached to a previously constructed acrylic pad. Currents ranging between 20 and 80 μA and comparable to those used previously in guinea pig (Kim and Curthoys 2004) were applied either cathodally or anodally (to achieve either excitation or inhibition of the vestibular periphery, respectively) and were timed to occur in relation to the vestibular stimulus. Currents were applied bilaterally and in temporal synchrony with separate constant-current isolated pulse stimulators (model 2100, A-M Systems) for each ear. For each experiment, currents were balanced between the two ears. Current was applied to each ear to determine thresholds at which vestibular nystagmus was elicited. Currents were then applied bilaterally for 1–2 s and adjusted so that no eye movements were invoked in the absence of rotational stimuli with either cathodal or anodal bilateral stimulation.
During the experiment, fully awake animals were restrained and placed on a servo-controlled turntable (Neurokinetics, Pittsburgh, PA; see Shanidze et al. 2010a). Video camera recordings using infrared illumination were used to ensure that the animals remained alert and to confirm that head position remained relatively upright and aligned with the body axis during stimulation. Eye and head movements were recorded with the electromagnetic search coil technique (e.g., Robinson 1963 in human; Fuchs and Robinson 1966; Judge et al. 1980 in monkey; Shanidze et al. 2010a in guinea pig). A Primelec search coil system (D. Florin, Ostring, Switzerland; model CS681) generated three orthogonal electromagnetic fields around the guinea pig. The Primelec field coils were stationary relative to the world, and the animals were rotated within the generated fields. In this configuration, measured eye and head movement signals were eye-in-space and head-in-space relative to the Earth fixed coordinate frame established by the field coils. Eye position, head position, and body velocity data were each sampled at 1,000 Hz by a dedicated data acquisition system (CED Power 1401, Cambridge Electronic Design, Cambridge, UK). Data were analyzed off-line with custom software written in Spike 2 (Cambridge Electronic Design) and MATLAB (The MathWorks, Natick, MA) environments. A smoothing filter with a 0.005- or 0.01-s time constant (time constants were applied uniformly across all data traces on a given date but could differ across dates) was applied to all acquired position channels, and eye and head velocity were computed by differentiating the position data.
Head Restraint Experiments
To control for a possible effect of head restraint on GVS responses, animals were placed in a modified body restraint with an attached mold of the head. To create the custom mold, a guinea pig was anesthetized and tightly wrapped in protective covering. The area around its head was then filled with dental impression material (AlgiNot, Kerr, Sybron Dental Specialties, Washington, DC). Once hardened, the mold could be attached to the body restraint and comfortably tightened around the animal's head. The animal was then placed in the recording setup, and vestibular and galvanic stimulation were applied as described above.
Data Analysis
Sinusoidal stimulation.
For sinusoidal rotations, rapid eye movements were removed with a computer algorithm tuned to each frequency. The algorithm used both velocity and acceleration threshold criteria to detect the onset and offset of rapid eye movements. The thresholds were adjusted by an experienced user to optimize the removal of rapid eye movements. Desaccaded cycles of eye-in-space, eye-in-head, head-in-space, and head-on-body were averaged and fit with a sinusoid (of the corresponding frequency) using a least squares algorithm and checked by the experimenter. The sinusoid fit to the data was used to compute the amplitude and phase of the response relative to the stimulus. To quantify VOR gain, we computed the ratio of eye-in-head velocity to head-in-space velocity amplitudes; analogously, for compensatory head movement (CHV), we divided the head-on-body velocity by that of the body-in-space. VOR and compensatory head movement phase shifts were computed by subtracting phase values from the same pairs of fits as the gains. These computations were done in the time domain.
Sinusoidal stimuli were typically applied in blocks of 10 (low frequencies) or 20 (high frequencies) cycles of the same amplitude and frequency. Stimulation frequencies were separated into two groups: “low frequency” (0.1, 0.2, 0.5, 1, 2, 5 Hz) and “high frequency” (8, 10 Hz). The distinction was made based on previously published data, which showed a biomechanical resonance for stimulation frequencies of 8 or 10 Hz (e.g., Fig. 4, Shanidze et al. 2010a). Peak-to-peak stimulus amplitudes ranged from 20°/s to 90°/s. Galvanic stimulation was applied during the first 5 or 10 cycles of a block; the remaining cycles were used as control data (Fig. 1A, top). Cycles with and without GVS were fit separately; the cycle immediately preceding the onset and the cycle following the offset of stimulation were omitted to avoid possible GVS onset/offset transients. Since galvanic stimulation was applied for half of the sinusoidal stimulus block, each set of cycles with GVS was paired with a subsequent set of control cycles within the same block. This procedure yielded a pair of gain and phase values for both compensatory eye and head movements that could be directly compared with one another (Figs. 2–4). Unless noted otherwise, the Wilcoxon signed-rank test was used for the comparison statistics because the data were not normally distributed.
Fig. 4.
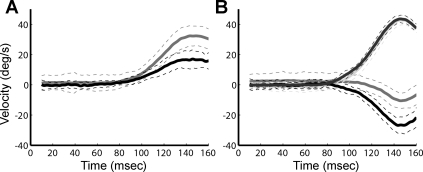
Anodal GVS suppression of compensatory eye movements. Each panel shows averaged responses to 30°/s velocity steps during a representative experiment. A: averaged eye-in-space velocity. Black, control; gray, anodal GVS. B: averaged head-in-space velocity. Dark gray, anodal GVS; pale gray, control (note that the pale gray control trace is nearly overlaid by the darker gray anodal GVS trace); gray, eye-in-head anodal GVS; black, control. Dashed lines represent 1 standard deviation.
Fig. 1.
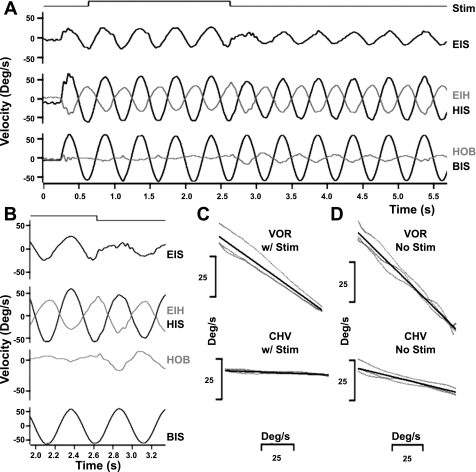
Anodal galvanic vestibular stimulation (GVS) suppression of compensatory eye and head movements. A: 2-Hz sinusoidal rotation at 60°/s with bilateral anodal galvanic stimulation (cycles 2–5) and without GVS (cycles 7–11). During stimulation (Stim, top trace) eye-in-head velocity (EIH, light gray, 3rd trace from top) and head-on-body velocity (HOB, light gray, 4th trace from top) are decreased. EIS, eye-in space; HIS, head-in space; BIS, body-in-space. B: higher-temporal resolution panel of cycles 5 and 6 showing EIS, EIH, HIS, HOB, and BIS velocities. C: responses during anodal GVS. Eye vs. head velocity {top; for cycles 2–5, regression slope [vestibulo-ocular (VOR) gain] = −0.58} and head vs. body velocity {bottom; regression slope [compensatory head movement (CHV) gain] = −0.03}. D: responses with no GVS. Eye vs. head velocity for cycles 7–11 without GVS [regression slope (VOR gain) = −0.77] and head vs. body velocity [regression slope (CHV gain) = −0.19].
Fig. 2.
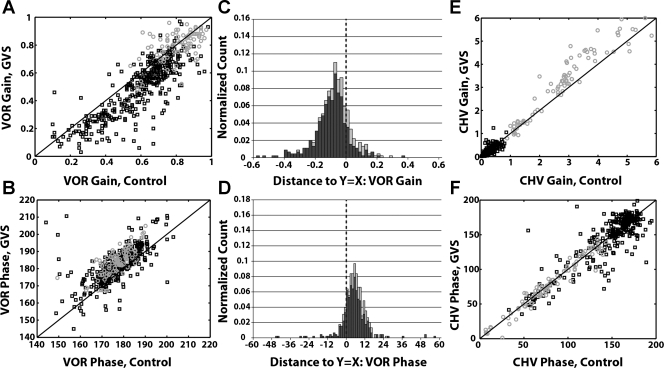
Comparison of eye and head movement responses with and without anodal GVS. A: VOR gain for control vs. paired anodal GVS cycles. B: VOR phase for control vs. paired anodal GVS cycles. C: VOR gain is reduced during anodal GVS: distribution of distances of points in A from the unity slope line (called residuals in text). D: VOR phase is increased during anodal GVS: distribution of distances of points in B from the unity slope line. E: head movement response gain (CHV) for control vs. paired anodal GVS cycles. F: head movement phase shifts for control vs. paired anodal GVS cycles. For all panels: black squares or dark bars, low frequencies (<8 Hz); gray circles or light bars, high frequencies (8 and 10 Hz). Solid black lines in A, B, E, and F represent unity slope lines where the plotted responses would be equal. Distributions are normalized by the total number of points across all frequencies in each distribution.
Fig. 3.
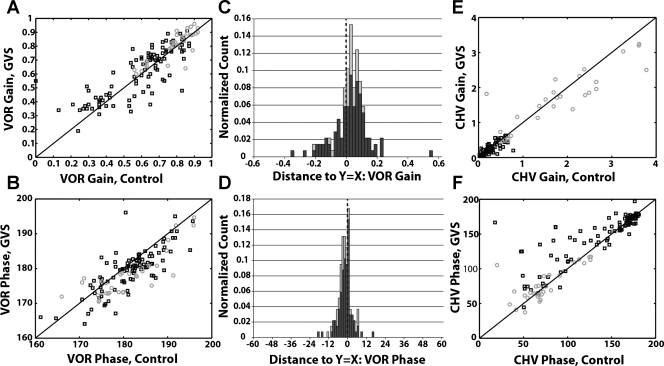
Comparison of eye and head movement responses with and without cathodal GVS. A: VOR gain for control vs. paired cathodal GVS cycles. B: VOR phase for control vs. paired cathodal GVS cycles. C: VOR gain is increased during cathodal GVS: distribution of distances of points in A from the unity slope line (called residuals in text). D: VOR phase is decreased during cathodal GVS: distribution of distances of points in B from the unity slope line. E: head movement response gain (CHV) for control vs. paired cathodal GVS cycles. F: head movement phase shifts for control vs. paired cathodal GVS cycles. For all panels: black squares or dark bars, low frequencies (<8 Hz); gray circles or light bars, high frequencies (8 and 10 Hz). Solid black lines in A, B, E, and F represent unity slope lines where the plotted responses would be equal. Distributions are normalized by the total number of points across all frequencies in each distribution.
Transient stimulation.
For transient step trials, our approach differed from the sinusoidal motion experiments in several key aspects. Unlike sinusoidal trials, where GVS was applied over consecutive cycles of motion, each step was treated as a single trial. On GVS trials, constant current was applied for 800 ms, beginning 400 ms before and persisting 400 ms after the onset of body acceleration. Thus any onset transients of GVS occurred well before the onset of body acceleration. GVS was applied for ∼50% of all transient trials, selected randomly by the computer. The values of eye and head velocity were measured at 90% of the peak body velocity (Shanidze et al. 2010a); control and GVS step values were grouped separately. We also examined response values at the time of peak body acceleration and during the steady-state portion of each step and found that the results were qualitatively similar regardless of the time at which the measurement was made. To quantify eye movements, the eye-in-head velocity value was plotted against the head-in-space velocity value for each step, and an overall slope was computed for all control points with robust regression (rightward and leftward steps were analyzed separately). Slopes were similarly computed for all GVS points for each animal's test date. These slopes were used as an estimate of the gain of the VOR for each animal, for trials with and without GVS. This analysis yielded a set of VOR gain value pairs whose differences represented the effects of GVS. Head movement responses were analyzed in a similar fashion.
Active head movements.
To evaluate effects of GVS on compensatory eye movement responses to self-generated head movement, animals were placed in the experimental setup and allowed to move their heads freely without external vestibular stimulation. Eye and head movements were recorded as described above. GVS was applied randomly during epochs of active head movement. Control and intervening GVS blocks of active head movements with similar speeds and accelerations were analyzed to calculate compensatory eye movement gain and eye movement latency with respect to an active head movement. The gains were computed using data samples taken over the entirety of the control or GVS block and performing a robust regression of eye-in-head versus head-in-space velocities. Time points where both head-in-space and eye-in-space velocities were in the same direction were excluded, as they represented anticompensatory behavior. To calculate latency we first divided each control or GVS block into 25-ms segments. For each segment, a cross-correlation of head-in-space and eye-in-head velocities was then performed, and only latencies with negative correlation coefficients were considered as per Cullen et al. (1996). For the GVS vs. control comparison, the gains and latencies of consecutive control and GVS blocks were subtracted, yielding a difference value for each pair of control and GVS blocks.
Statistical analysis.
To ascertain the significance of gain changes associated with GVS during transient steps or active head movements, a nonparametric permutation test was performed. Following the general procedure described by Nichols and Holmes (2002), we examined differences between each set of gains (e.g., control VOR gain minus GVS VOR gain) across all dates and amplitudes of head movement. From each distribution, we calculated the mean of the differences as the observed statistic. Using this mean, we compared it against a null distribution representing the null hypothesis that the test condition (GVS or control) has no effect on behavior. The null distribution was generated by relabeling the test condition of the values in each pair difference and calculating the mean of the differences after relabeling. If computationally feasible, the values of the null distribution were composed of the means from a complete permutation of all label orders. To make the required computational state feasible, if the number of permutations exceeded 2∧14, a randomized permutation test was performed instead with 10,000 permutations, each with randomly chosen labels. The P value was calculated as the proportion of statistic values in the null distribution equal to, or more extreme than, the observed mean (Nichols and Holmes 2002).
RESULTS
Sinusoidal Rotation: Inhibitory GVS
Inhibitory galvanic vestibular stimulation (anodal GVS) had a significant effect on compensatory head and eye movements as shown in Fig. 1. Figure 1A shows an animal's responses to 2-Hz sinusoidal rotations at 60°/s with bilateral anodal galvanic stimulation (cycles 2–5) and without GVS (cycles 6–11). During GVS, eye-in-space (EIS, 2nd trace from top) velocity was greater and head-on-body velocity (gray trace, HOB) was less. Greater eye-in-space velocity (e.g., gaze velocity) during stimulation indicates reduced retinal image stability during an externally driven rotation. The stimulus-evoked suppression of compensatory eye velocity is evident in the higher-temporal resolution panel (Fig. 1B) and in the plots of eye vs. head velocity during GVS (compare Fig. 1C, top, with Fig. 1D, top). The VOR gain was 0.58 ± 0.01 with anodal stimulation (Fig. 1C) and 0.77 ± 0.01 without stimulation (Fig. 1D). Similarly, compensatory head relative to body velocity (CHV) was reduced during anodal GVS stimulation (compare Fig. 1, C, bottom, and D, bottom). Since inertial forces and volitional movements, as well as the vestibulo-collic reflex (VCR), may produce compensatory head movements, we characterize head movement responses with the general acronym CHV, rather than VCR. CHV gain was 0.03 ± 0.00 with anodal stimulation (Fig. 1C) and 0.19 ± 0.01 without stimulation (Fig. 1D).
GVS Effects on Compensatory Eye Movements
To quantify the effect of anodal GVS on the VOR, we compared VOR gain with GVS versus VOR gain in the absence of GVS for paired cycles within each block of sinusoidal rotations (see materials and methods). Figure 2A shows a scatterplot of these data for all of the animals tested in the study. If the gain during GVS equaled that of paired control cycles, then the plotted data points should be evenly distributed about the unity slope line (Fig. 2A). Instead, it is evident that the majority of the data points fall below this line, indicating that VOR gain in the presence of GVS was less than VOR gain in a paired control cycle. To quantify this observation, we measured the distance of each data point from the unity slope line and plotted the distribution of these residuals (Fig. 2C). These residual values are equivalent to the paired differences between responses with and without GVS within a sinusoidal trial. If there were no effect of GVS on gain, the distribution would be centered about zero. For frequencies of rotation <8 Hz, the distribution is clearly shifted negatively (median = −0.09, P < 0.0001).
In general, VOR gain increases with frequency of stimulation, accounting for much of the spread in gain values shown in Fig. 2 (Shanidze et al. 2010a). For the highest frequencies of rotation where head velocity may exceed body velocity (8–10 Hz), the gain of the VOR was much higher on average than during lower-frequency oscillations (Shanidze et al. 2010a). Anodal GVS did not suppress the elevated VOR gains seen at these frequencies (Fig. 2A, median = −0.02, P = 0.18).
Figure 2B shows VOR phase shifts for the same paired cycles used to assess changes in VOR gain. Over the entire tested frequency range the majority of the plotted phase shifts fall above the unity slope line, indicating that anodal GVS increased the phase lag of the VOR. This observation is quantified in Fig. 2D, which shows the distribution of the GVS-induced changes in phase; for frequencies <8 Hz the median change in phase was 4.00° (P < 0.0001), and for frequencies ≥8 Hz the median phase change was 6.65° (P < 0.0001).
Anodal GVS also influenced compensatory head movements (CHV) made in response to body rotation. Figure 2E shows CHV gain for the same set of paired GVS and control cycles used to illustrate changes in VOR gain. During low-frequency rotations (<8 Hz), where inertial forces were less dominant, anodal GVS was associated with reduced CHV gain (median = −0.02, P < 0.0001; Fig. 2E) but not with a significant difference in phase (−0.05°, P = 0.65; Fig. 2F).
In a previous report (Shanidze et al. 2010a), we showed that compensatory head velocity relative to the body increased dramatically and often exceeded body velocity for rotational frequencies ≥8 Hz. This effect is evident in Fig. 2E, where CHV gain during 8- to 10-Hz rotations is up to six times greater than what would be compensatory (gain = 1) and where many of the plotted data points actually fall above the unity slope line (Fig. 2E), implying, paradoxically, a further increase in head movement during anodal GVS. This change was statistically significant for gain (median = +0.33, P < 0.0001) but not for phase shift (−0.30°, P = 0.34; Fig. 2F). Greater head velocity during GVS implies that vestibular inputs to the neck exerted a suppressive effect on head movement during high-frequency head oscillations. Although seemingly paradoxical, the effect is consistent with the VCR goal of stabilizing the head in space.
At frequencies >8 Hz, the acceleration of the body is quite large (∼1,500–5,600°·s−1·s−1) and suggests a dominant influence of biomechanical factors such as inertial acceleration (Shanidze et al. 2010b). Consistent with this hypothesis, Fig. 2F shows that head-on-body velocity tended to be in phase with body acceleration regardless of the presence or absence of GVS. In comparison, phase shifts during lower-frequency oscillations tended toward 180° (Fig. 2F).
In summary, the overall effect of anodal GVS reflected a reduction in vestibular control. However, the specifics of this effect differed according to stimulus frequency range. In particular, at low frequencies where vestibular influences were more dominant, anodal GVS caused decreases in ocular stability, which were reflected by parallel increases in eye movement relative to the world (control 15.40 ± 8.94°/s vs. anodal GVS 20.04 ± 10.40°/s, P < 0.0001) and head movement relative to the world (control 37.92 ± 18.78°/s vs. anodal GVS 39.27 ± 18.74°/s, P < 0.0001).
Effects of Excitatory GVS
Excitatory GVS (cathodal GVS) caused a consistent improvement in ocular stability. Figure 3A shows VOR gains during sinusoidal cycles with cathodal GVS plotted against paired control cycles without GVS. For cathodal GVS, the majority of the data points fall above the unity slope line, indicating an increase in VOR gain with GVS. The VOR was enhanced at low and high frequencies (median = +0.03; P < 0.05; Fig. 3A). Concurrently, there was a small increase in VOR phase lead during cathodal GVS for both low and high frequencies of rotation (low: median = −1.75, high: median = −3.7; Fig. 3B, P < 0.001). Although smaller in magnitude, the effects of cathodal GVS on VOR gain and phase mirrored those of anodal GVS on VOR gain and phase.
Cathodal GVS also had limited influence on CHV (Fig. 3, E and F). CHV gain was not changed with GVS versus without GVS for low-frequency rotation (Fig. 3E; median = 0.00, P > 0.62). However, head movements during high-frequency stimulation, when paired with cathodal GVS, displayed a decrease in head velocity (functionally an improvement in head stability relative to space; Fig. 3E; median = −0.11, P < 0.05). At low frequencies, CHV phase was significantly different (median = 1.75°, P < 0.001). In contrast, no differences in phase shift were observed at high frequencies of rotation with cathodal GVS (P > 0.77).
On the whole, these results mirror those for anodal GVS. Figures 2C and 3C show distributions of residuals for VOR gain with anodal (Fig. 2C) and cathodal (Fig. 3C) GVS. A comparison of these residual distributions shows that VOR gains during anodal GVS were less than their paired controls, whereas VOR gains during cathodal GVS were greater than their paired controls. Figures 2D and 3D demonstrate a similar pattern of effects of GVS on the phase.
Stimulation in the light.
For some test dates we performed the experiments in light as well as darkness to determine whether the response to stimulation was dependent on vision. Consistent with previous findings (Shanidze et al. 2010a), we found that in the light there was an improvement on both ocular and head stability (Kolmogorov-Smirnov 2-sample test, P < 0.05) for both anodal GVS and control conditions. In both control and GVS conditions, eye and head velocities were decreased in light versus darkness (eye: control: dark 11.56 ± 6.63°/s, light 5.71 ± 3.42°/s; anodal GVS: dark 14.88 ± 7.15°/s, light 6.60 ± 3.52°/s; head: control: dark 34.98 ± 15.43°/s, light 32.45 ± 20.41°/s; anodal GVS: dark 36.31 ± 16.27°/s, light 33.50 ± 21.20°/s). This decrement in eye velocity resulted in comparable changes in gain. For example, at low frequencies (where light was likely to play a role; Shanidze et al. 2010a) the median difference for VOR gain was 0.045, which was significant (P < 0.0005), and the median difference in CHV gain was 0.01, also significant (P < 0.05). We examined whether the influence of vision was quantitatively different in control versus GVS conditions and found no effect for either eye or head responses (all P values >0.05, unbalanced 2-way ANOVA). Because the effects of light on performance did not differ between control and GVS conditions, we can conclude that there was no interaction between the presence or absence of a visual stimulus and GVS.
Transient Velocity Steps
During brief transient rotations GVS had a significant effect on the magnitude of compensatory eye movements (Table 1) but a smaller effect on compensatory head movements. Figure 4 shows averaged transient responses of one animal at 30°/s (W3, Table 1). Figure 4A shows reduced gaze stability on trials with anodal GVS compared with control trials (compare eye-in-space with GVS to eye-in-space without GVS). An increase in eye velocity would be expected if head-in-space velocity also increased as a result of GVS suppression of compensatory head movement. However, as shown in Fig. 4B, head-in-space velocity on control trials essentially overlays head-in-space velocity on GVS trials. Figure 4B illustrates the suppression of eye-in-head velocity on GVS trials compared with the control trials (same data set as Fig. 4A).
Table 1.
VOR gain differences between control and anodal GVS during transient velocity steps
| Animal/Date | Left Control − Anodal GVS | Right Control − Anodal GVS |
|---|---|---|
| D1 | −0.19 | −0.02 |
| D2 | 0.03 | −0.08 |
| V1 | −0.07 | −0.26 |
| V2 | −0.02 | −0.26 |
| V3 | −0.02 | −0.11 |
| V4 | 0.12 | −0.24 |
| W1 | −0.21 | −0.11 |
| W2 | −0.12 | −0.29 |
| W3 | 0.01 | −0.30 |
| W4 | −0.32 | −0.19 |
| HF1 | −0.01 | −0.23 |
VOR, vestibulo-ocular reflex; GVS, galvanic vestibular stimulation.
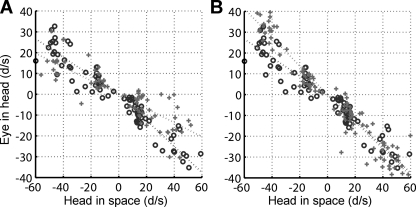
Figure 5 is a scatterplot of eye-in-head versus head-in-space velocities for a representative experiment. Each data point represents a single transient. Anodal GVS reduced the mean VOR gain for either leftward steps (EIH vs. HIS slope, left control: 0.44, left GVS: 0.33) or rightward steps (right control: 0.63, right GVS: 0.34) for the experiment shown in Fig. 5A. Across all animals and test dates anodal GVS caused a reduction in mean gain; the mean difference was −0.08 ± 0.13 for leftward steps and −0.19 ± 0.10 for rightward steps. The effect is greater for rightward steps but is significant in both directions (right: P < 0.001; left: P < 0.05). The apparent asymmetry is related to an asymmetry in the animals' control responses since control gains were higher for rightward steps (during GVS and control experiments). Figure 5B shows the results of cathodal GVS, which produced a small increase in VOR gain in either step direction for the experiment illustrated in Fig. 5B; however, across all animals and test dates there was no significant change in gain (rightward, +0.14, P > 0.99; leftward, +0.07, P > 0.99).
Fig. 5.
VOR suppression with anodal GVS (A) or VOR enhancement with cathodal GVS (B). Data from a representative experiment (W2, Table 1). Circles represent control trials, and plus symbols represent GVS trials.
No significant effect of either anodal or cathodal GVS was detected on compensatory head movements in either direction [mean difference of BIS vs. HOB slopes: −0.01 left (P > 0.32) and +0.01 right (P > 0.77)]. Similarly, cathodal GVS failed to have a significant effect on CHV [left mean = +0.2 (P > 0.99), right mean = −0.01 (P > 0.99)].
Weak galvanic currents suppress irregular afferents. On the basis of their responses to angular acceleration, one might expect to detect a change in VOR latency if these afferents were selectively ablated by GVS. However, there were no significant latency changes (as measured by waveform correlation; see Methods, Shanidze et al. 2010a, 2010b for details) for either eye or head responses with either cathodal or anodal stimulation (e.g., anodal GVS: head P = 0.48 and P = 0.23, eye P = 0.06 and P = 0.68, right and left rotations, respectively).
Stimulation in the light.
As with periodic rotation, experiments were performed in light and darkness during some test dates. To determine whether there was an effect of light on an animal's performance we compared values obtained in the light with those obtained in the dark on the same test date. We found no effect of light in the control condition on eye-in-space or head-in-space movement (Kolmogorov-Smirnov 2-sample test, P < 0.05; Shanidze et al. 2010a). In agreement with the results of periodic stimulation, there was no interaction between light and galvanic stimulation for eye or head responses (all P values > 0.05, unbalanced 2-way ANOVA).
Effects of GVS on VOR in head-fixed guinea pig.
Minor and Goldberg (1991), among others, reported no suppression of the VOR during anodal GVS. Their experiments were performed in head-restrained primates. To test whether the differences in our results were due to the ability of the animals' heads to move freely, we repeated the experiments (both sines and transient steps) in an animal with a restrained head. For both sinusoidal and transient rotations we found anodal GVS to suppress the VOR. For sinusoidal rotations, eye-in-head velocity decreased across all frequencies (low control: 13.54 ± 4.92°/s, anodal GVS: 11.25 ± 4.23°/s; high control: 27.96 ± 8.75°/s, anodal GVS: 21.83 ± 6.85°/s), causing a decrement in the animal's ability to maintain gaze (eye-in-space velocity: low control 36.81 ± 9.31°/s, anodal GVS 38.96 ± 10.47°/s; high control 47.59 ± 7.86°/s, anodal GVS 55.05 ± 8.43°/s; P < 0.001). For transient steps, the decrease in performance closely mimicked those reported for head-unrestrained animals. The overall decrease in VOR gain was significant (ANOVA, P < 0.05; mean VOR gain difference: right −0.24, left −0.02).
Active Head Movements
GVS did not influence compensatory eye movements associated with voluntary head movements. As previously reported (Shanidze et al. 2010b), for compensatory eye movement mean gain (eye/head) across all animals during active head movements was higher than during passive stimulation (active: gain = 0.91 ± 0.11; passive periodic rotation: low-frequency VOR gain = 0.59 ± 0.18, high-frequency VOR gain = 0.81 ± 0.10; passive transient steps: right VOR gain = 0.45 ± 0.21, left VOR gain = 0.31 ± 0.16). Furthermore, the mean latency of compensatory ocular responses in relation to active head movement was effectively zero (mean = −0.06 ± 0.18 ms), consistent with the anticipatory nature of the movement (Shanidze et al. 2010b). In our previous study, we reported that anticipatory responses occurred even in the absence of a functional vestibular system, suggesting that GVS should have no effect on anticipatory responses. Consistent with this hypothesis, there was no measurable difference between compensatory eye movements that occurred with and those that occurred without GVS during self-generated head movements (anodal GVS: P = 0.08; cathodal GVS: P = 0.19).
To determine whether GVS influenced self-generated head velocity we compared distributions of head-in-space velocity during active movement epochs that occurred in the absence of GVS with those that occurred during GVS. No statistical difference was found (anodal GVS: P = 0.17, cathodal GVS: P = 0.26) suggesting that changes in vestibular afference, as modulated by GVS, do not significantly alter the trajectories of planned voluntary head movements.
During epochs of active head movement the animals occasionally generated eye and head movements in the same direction, presumably to shift their line of sight. To compare whether there was relatively more gaze change occurring under either of the GVS or control conditions, we examined data samples where eye-in-head and head-in-space velocities had the same sign (because the eye and head are moving in the same direction, the instances are anticompensatory). We analyzed eye velocity amplitude during these epochs of anticompensatory movement to determine whether there was any influence of GVS on the generated quick phases. During cathodal GVS, the proportion of eye movements in the anticompensatory direction was greater than control (P = 0.02), although these epochs corresponded to significantly lower mean eye-in-space velocities than control (Kolmogorov-Smirnov 2-sample test, P < 0.05; means: right: control = 38.98°/s, cathodal GVS = 26.00°/s; left: control = −41.45°/s, cathodal GVS = −34.36°/s). For anodal GVS, there was a trend for a higher number of anticompensatory eye movements than control (P = 0.05). The distribution of eye-in-space velocities corresponding to these movements was also different between control and GVS (Kolmogorov-Smirnov 2-sample test, P < 0.05), although no clear trend in mean velocity changes could be ascertained (right: control = 60.39°/s, anodal GVS = −57.47°/s; left: control = −56.46°/s, anodal GVS = −72.19°/s).
DISCUSSION
GVS Effects on CHV and Their Relationship to the VCR
Irregular vestibular afferents exhibit more phasic responses to angular rotation and innervate central vestibular neurons that project to the cervical spinal cord (Boyle et al. 1992). It has been hypothesized that their dynamic characteristics could help compensate for the inertial and biomechanical properties of the head and neck; thus the phase-advanced signal encoded by these afferents might significantly influence the VCR and vestibular control of head stability (Bilotto et al. 1982; Boyle et al. 1992; Fernandez and Goldberg 1971; Peterson et al. 1988; Schor et al. 1998). A goal of this study was to directly test this hypothesis. Previous studies have established that weak anodal currents selectively suppress the discharge of irregular afferents in primates and guinea pigs (Kim and Curthoys 2004; Minor and Goldberg 1991). If irregular afferent activity is functionally significant for the VCR, then anodal galvanic stimulation should reduce VCR gain and potentially destabilize the head in space during passive whole body rotation in animals whose heads are unrestrained. Our experimental results support this hypothesis because there were statistically significant decrements in compensatory head velocity (CHV) relative to body velocity when anodal GVS was applied during sinusoidal rotation (Figs. 1 and 2). In some instances, the suppression of CHV was substantial. For example, nearly a 70% reduction in gain is illustrated in Fig. 1, C and D, during 2-Hz sinusoidal rotations. Overall, however, the measurable effect of GVS on CHV was small for periodic stimuli and not significant for brief transient rotations, from which one might conclude that irregular afferents contribute minimally to CHV and, more specifically, to the VCR. Two factors are likely to contribute to these weak effects of GVS. First, we used currents sufficient to suppress irregular afferents (Kim and Curthoys 2004) but not regular afferents, which would continue to provide significant inputs to the neck. Second, the VCR is only one source of head movement drive; proprioception and inertial forces may also produce compensatory movement. Consistent with this interpretation, GVS had minimal effects on compensatory head velocity during transient steps (Fig. 4) where head velocity was measured at a point in time when there was concurrent high acceleration (up to 1,600°·s−1·s−1).
During high-frequency sinusoidal oscillations (≥8 Hz), head speeds exceeded imposed stimulus speeds and were likely driven by a both a biomechanical resonance and inertial forces (Shanidze et al. 2010a). At these frequencies, anodal GVS further destabilized the head in space (Fig. 2E), resulting in significantly greater head-in-space velocity compared with control cycles. Thus, despite the presence of large inertial forces and in contrast to aperiodic, transient steps, irregular inputs to central vestibular neurons with descending axons appeared to exert a stabilizing influence on the head at high rotational frequencies near resonance consistent with their dynamic properties and the functional role of the VCR. An interesting aspect of the high-frequency sinusoidal data was that anodal GVS had no significant influence on CHV phase shift (Fig. 2F), despite the phase-advanced dynamics of the irregular afferents. Instead, phase shifts clustered near 90°, apparently reflecting the inertially driven character of the head movements.
GVS Effects on Direct VOR Central Pathways
Unlike vestibular control of the head, it is widely assumed that signals encoded by regular vestibular afferents dominate activity in the direct VOR pathway (Brontë-Stewart and Lisberger 1994). In what is now a classical study, Minor and Goldberg (1991) showed that anodal GVS had no effect on the VOR of monkeys during passive sinusoidal rotations above 0.2 Hz or dynamic segments of transient rotations, and this finding was subsequently confirmed by two other laboratories (Angelaki and Perachio 1993; Chen-Huang et al. 1997). In contrast to the findings in monkeys, we observed significant effects of GVS on the guinea pig's VOR during sinusoidal rotations at frequencies >0.2 Hz (Figs. 1–3) and during brief transient rotations (Fig. 4 and Table 1). Our results are not due to nonselective suppression (or activation) of regular afferents. Kim and Curthoys (2004) showed that weak galvanic currents, similar to those employed in this study, selectively suppressed irregular afferents in guinea pigs just as they did in nonhuman primates. Furthermore, our results are consistent with evidence that irregular afferents provide synaptic inputs to central neurons in VOR pathways as well as to neurons in VCR pathways (Highstein et al. 1987).
A possible conclusion is that GVS suppression of the VOR in guinea pigs is a species-based difference in the synaptic weight of irregular afferents on neurons in the central VOR pathways. Guinea pigs are lateral-eyed, afoveate, terrestrial animals. Unlike primates, they do not produce smooth pursuit eye movements (Marlinsky and Kröller 2000), which, in monkeys, share the central pathways that process signals related to the VOR (Roy and Cullen 2002). Furthermore, in the mouse, another afoveate species, Beraneck and Cullen (2007) showed that putative secondary vestibular neurons with eye position sensitivity (“ES neurons”) encode a signal correlated with eye velocity during optokinetic nystagmus (OKN). However, in contrast to previous findings in primates (Blazquez and Highstein 2007; Waespe and Henn 1979), mouse vestibular-only cells (“VO neurons”) do not exhibit activity related to either the visual motion stimulus or eye movement during OKN. The guinea pig has been previously shown to have neural circuitry intermediate to that of primates compared with mice or rabbits for OKN (Lui et al. 1994), and so further electrophysiological studies are needed to determine whether the effects of GVS on VOR show species-specific differences for this animal.
The presence or absence of head restraint is another potentially significant factor, since any head movement (active or passive) that is concurrent with the rotational stimulus will directly modify the afferent vestibular signal itself as well as produce proprioceptive inflow to vestibular neurons (Roy and Cullen 2002). Moreover, head movement has been shown to alter experimental outcomes in a number of behaviors, for example, sound localization (Populin 2006). In the case of the vestibular system, electrophysiological recordings from the primate vestibular nucleus have shown that the activity of VO neurons changes when the head is allowed to move (for review, see Cullen and Roy 2004). Previously (Shanidze et al. 2010a), we reported VOR and head movement responses in head-unrestrained guinea pigs to a wide range of passive whole body rotational speeds and frequencies. These results were comparable to responses previously shown in guinea pigs tested with restrained heads (Escudero et al. 1993). In the two studies of guinea pigs, VOR performance (gain) was quite similar despite the difference in head restraint. This finding indicates that signal processing in the VOR pathways is not substantially modified when the head is free to move compared with head-restrained experiments. Our results confirm that the presence or absence of head restraint does not influence the effect of GVS on the guinea pig's VOR, despite the potentially large differences in central signals within the vestibular nuclei.
GVS Effects on Indirect VOR Central Pathways
Angelaki and colleagues reported a 30–50% reduction in eye velocity during the steady-state portion of prolonged constant-velocity stimuli and during the steady state of OVAR in monkeys (Angelaki et al. 1992; Angelaki and Perachio 1993). A recent review by Laurens and Angelaki (2011) makes a strong case that the function of velocity storage is not solely to improve the low-frequency responses of canal dynamics but instead to act as a multisensory integrator to improve overall canal and otolith function in a midrange of frequencies (0.2–0.5 Hz). The data of Angelaki and Perachio imply that suppression of irregular afferents reduces the time constant of this integrator by some undefined mechanism. We cannot exclude the possibility of a similar effect in the guinea pig as the cause of the changes in VOR gain that we observed. However, the frequencies of our sinusoidal stimuli were greater (up to a factor of 10) than the midrange described by the Laurens and Angelaki primate model and the duration of the velocity steps employed in our experiments were much shorter (400 ms) than the 1–3 s range predicted by the model of Laurens and Angelaki. Moreover, for the velocity steps, the gain change we report was apparent within 100 ms of the onset of the step (Fig. 4). If an effect of GVS on velocity storage is a viable explanation for our findings, then we must assume either that the integrator in the guinea pig's velocity storage network is more dependent on irregular afferent activity than the primate's or that the time constant of the network is much shorter, an unlikely assumption in our opinion since canal dynamics are quite accurate over the frequency range employed in our experiments.
Functional Role of Retinal Stability in the Guinea Pig
Although detailed evidence for the central connectivity of vestibular afferents is not available in the guinea pig, it is likely that the basic VOR pathways in the brain stem are fundamentally similar to those of primates (Babalian 1997; Burian et al. 1990; Ris et al. 1995). To account for our experimental findings, we hypothesize that polysynaptic, possibly extravestibular, inputs sculpt vestibular afference in accord with species-specific behavior. In particular, there are fundamental differences in the relative importance of vestibular and visual sensation in afoveate guinea pigs compared with primates with well-developed visual systems. Guinea pigs live in burrows and feed at dawn and dusk in their natural habitat (Finlay and Sengelaub 1981). They do not have foveas; their retinas are relatively homogeneous and have a low density of photoreceptors, primarily rods (Choudhury 1978; Hughes 1977); and they have poor visual acuity (estimated maximally at 2.7 c/°; Buttery et al. 1991). Consistent with this morphology and lifestyle, guinea pigs make few spontaneous saccades and do not produce smooth pursuit eye movements (Escudero et al. 1993).
The VOR stabilizes retinal images during head movements and, in primates, maximizes visual acuity. However, guinea pigs have poor acuity, so reduction of blur due to image motion is unlikely to be a primary function of the guinea pig's VOR. Land (1999) and Walls (1962) argued that detection of motion in the external environment is also dependent on retinal stability; if the eye moves in space, then the brain must determine the amount of retinal image motion induced by the animal's self-generated motion versus imposed movement by the environment. For example, if one spins and then abruptly stops, the world may be perceived as spinning for a brief time. The misperception occurs because vestibular sensation persists after one stops spinning because of central velocity storage that effectively extends the cupula time constant. Detection of relative motion between an object and its environment is also enhanced by retinal image stability (Nakayama 1981). In accord with Land, we suggest that the function of the guinea pig's VOR is to stabilize retinal images so as to distinguish movements of other animals (e.g., a predator) in the environment from self-motion. This functional distinction may represent a fundamental difference between foveate and afoveate species in how vestibular nuclei neurons integrate vestibular inputs with inputs related to visual sensation.
For example, in the primate, a powerful set of visual mechanisms assist or even supplant vestibular control of compensatory eye movements. First, foveal-based smooth pursuit stabilizes retinal images on the fovea regardless of the source of image motion (self-generated or external). During passive rotation, the gain of the VOR is significantly improved during visual fixation of a target (Baloh and Halmagyi 1996; Schweigart et al. 1999) because smooth pursuit and the VOR act synergistically. In the clinic, vestibular nystagmus is readily suppressed by real or even imagined fixation targets (reviewed in Baloh and Halmagyi 1996), a clear demonstration that visual inputs or cognition can dominate vestibular signals in VOR pathways. Second, fusional vergence stabilizes retinal images because it maintains binocular correspondence of foveated targets (reviewed in Baloh and Halmagyi 1996).
In the guinea pig, foveal visual mechanisms are not available to assist the VOR in stabilizing the retinal image. Instead, we hypothesize that irregular vestibular afferents, with greater sensitivity to angular acceleration (Goldberg 2000), enhance VOR responses to movement and thus play a greater role in image stabilization in the guinea pig than they do in the primate. In monkeys, the influence of irregular afferents on secondary vestibular neurons may be regulated or suppressed to allow more powerful visual mechanisms to dominate or modulate vestibular signals. This idea is consistent with previous findings, which showed that polysynaptic inhibitory pathways could be responsible for the central cancellation of irregular afferent inputs in primates (Chen-Huang et al. 1997). Although anodal GVS had no effect on the averaged activity of secondary vestibular neurons (type I PVP), the activity of individual cells was modulated by GVS (Chen-Huang et al. 1997), confirming the presence of irregular inputs on those cells. This result and our data suggest that recordings from central vestibular neurons in guinea pigs will be needed to determine the influence of irregular inputs to specific classes of vestibular neurons, e.g., those likely to project into the VCR-related pathways (VO neurons) and those that project into VOR pathways (cells with eye movement sensitivity). For example, in the mouse, Beraneck and Cullen (2007) found that the firing rates of neurons with eye movement sensitivity, “ES” neurons, were modulated by optokinetic signals. In primates, eye movement and vestibular-only (VO cells) exhibit firing rate modulation correlated with optokinetic stimulation (Waespe and Henn 1977).
Independence of Optokinetic and GVS Influence on the VOR
Although the VOR effectively stabilizes retinal images for rapid movements, it performs poorly for low-frequency stimuli (Baird et al. 1988; Highstein et al. 2005; for review, see Goldberg 2000). However, in the light, full-field, slowly moving images induce an optokinetic reflex in guinea pigs (and other species) that reduces retinal slip over a frequency range where the VOR is deficient (Azzena et al. 1974; Lui et al. 1999; Marlinsky and Kröller 2000). Consistent with these reports, we found that in the light the guinea pig's compensatory eye movements were enhanced during low-velocity periodic motion (Shanidze et al. 2010a). However, anodal GVS had no effect on low-frequency visual enhancement of the VOR. Visual enhancement and anodal GVS suppression both occurred, but there was no interaction suggesting either linear addition of signals in a common circuit or that optokinetic and GVS influences were exerted in parallel pathways.
Ocular Compensation During Self-Generated Head Movements
During self-generated head movements we were unable to detect any influence of GVS on head or eye speed. This result extends our previous finding that anticipatory compensatory eye movements associated with active head movements occur independently of vestibular afference (Shanidze et al. 2010b). Whatever the source of the anticipatory motor command (proprioceptive or efference copy), the anticipatory response is more effective than the passive VOR in stabilizing the eye in space (King and Shanidze 2011; Shanidze et al. 2010b). Retinal stability during rapid self-generated head turns would be especially important for an animal to distinguish object motion in the environment from self-motion (Land 1999). Thus an anticipatory movement in association with a self-generated head or body movement not only reduces retinal slip but also could provide perceptual suppression of any remaining slip associated with the animal's voluntary movement.
The failure of GVS to influence compensation for volitional head movements is evidence that the anticipatory response produces all or most of the ocular compensation. If there were a significant and synergistic passive VOR component, then that component would have been suppressed by GVS and we should have detected a difference in the compensatory eye movements that occurred during GVS and control epochs of self-generated head movement. Thus this finding supports the idea that an efference copy of the motor command to move the head is used to cancel the sensory consequence (reafference) of the planned head movement (Cullen et al. 2011; Sadeghi et al. 2010; Shanidze et al. 2010b; von Holst and Mittelstaedt 1950). If the active head movement occurs as planned, then the vestibular nerve signal is effectively nullified centrally by the efference copy and would produce no VOR-related eye movement. Our GVS data are consistent with this model of vestibular processing and the principle of reafference (von Holst and Mittelstaedt 1950).
GRANTS
This research was supported by National Institutes of Health Grants P30 NDC-005188-07, R21 DC-008607-01, F31 DC-010947, and T32 DC-000011-30.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.S. and W.M.K. conception and design of research; N.S., J.D., and W.M.K. performed experiments; N.S., K.L., J.D., and W.M.K. analyzed data; N.S. and W.M.K. interpreted results of experiments; N.S., K.L., and W.M.K. prepared figures; N.S. and W.M.K. drafted manuscript; N.S., J.D., and W.M.K. edited and revised manuscript; N.S. and W.M.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the contribution of Deborah Colesa for her advice and expertise in helping us develop our middle ear surgical approach and electrode design and Joonkoo Park for his advice on our statistical approaches. Dwayne Valliencourt designed and built the specialized animal restraints, and Chris Ellinger kept our electronics running.
REFERENCES
- Angelaki DE, Perachio AA. Contribution of irregular semicircular canal afferents to the horizontal vestibuloocular response during constant velocity rotation. J Neurophysiol 69: 996–999, 1993 [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Perachio AA, Mustari MJ, Strunk CL. Role of irregular otolith afferents in the steady-state nystagmus during off-vertical axis rotation. J Neurophysiol 68: 1895–1900, 1992 [DOI] [PubMed] [Google Scholar]
- Azzena G, Azzena M, Marini R. Optokinetic nystagmus and the vestibular nuclei. Exp Neurol 42: 158–168, 1974 [DOI] [PubMed] [Google Scholar]
- Babalian A, Vibert N, Assie G, Serafin M, Mühlethaler M, Vidal P. Central vestibular networks in the guinea-pig: functional characterization in the isolated whole brain in vitro. J Neurosci 81: 405–426, 1997 [DOI] [PubMed] [Google Scholar]
- Baird R, Desmadryl G, Fernández C, Goldberg J. The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J Neurophysiol 60: 182–203, 1988 [DOI] [PubMed] [Google Scholar]
- Baloh WR, Halmagyi MG. Disorders of the Vestibular System. New York: Oxford Univ. Press, 1996 [Google Scholar]
- Beraneck M, Cullen K. Activity of vestibular nuclei neurons during vestibular and optokinetic stimulation in the alert mouse. J Neurophysiol 98: 1549–1565, 2007 [DOI] [PubMed] [Google Scholar]
- Bilotto G, Goldberg J, Peterson B, Wilson V. Dynamic properties of vestibular reflexes in the decerebrate cat. Exp Brain Res 47: 343–352, 1982 [DOI] [PubMed] [Google Scholar]
- Blazquez PM, Highstein SM. Visual-vestibular interaction in vertical vestibular only neurons. Neuroreport 18: 1403–1406, 2007 [DOI] [PubMed] [Google Scholar]
- Boyle R, Goldberg J, Highstein S. Inputs from regularly and irregularly discharging vestibular nerve afferents to secondary neurons in squirrel monkey vestibular nuclei. III. Correlation with vestibulospinal and vestibuloocular output pathways. J Neurophysiol 68: 471–484, 1992 [DOI] [PubMed] [Google Scholar]
- Brontë-Stewart H, Lisberger S. Physiological properties of vestibular primary afferents that mediate motor learning and normal performance of the vestibulo-ocular reflex in monkeys. J Neurosci 14: 1290–1308, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burian M, Gstoettner W, Mayr R. Brainstem projection of the vestibular nerve in the guinea pig: an HRP (horseradish peroxidase) and WGA-HRP (wheat germ agglutinin-HRP) study. J Comp Neurol 293: 165–177, 1990 [DOI] [PubMed] [Google Scholar]
- Buttery R, Hinrichsen C, Weller W, Haight J. How thick should a retina be? A comparative study of mammalian species with and without intraretinal vasculature. Vision Res 31: 169–187, 1991 [DOI] [PubMed] [Google Scholar]
- Chen-Huang C, McCrea R. Contribution of vestibular nerve irregular afferents to viewing distance-related changes in the vestibulo-ocular reflex. Exp Brain Res 119: 116–130, 1998 [DOI] [PubMed] [Google Scholar]
- Chen-Huang C, McCrea R, Goldberg J. Contributions of regularly and irregularly discharging vestibular-nerve inputs to the discharge of central vestibular neurons in the alert squirrel monkey. Exp Brain Res 114: 405–422, 1997 [DOI] [PubMed] [Google Scholar]
- Choudhury B. Retinotopic organization of the guinea pig's visual cortex. Brain Res 144: 19–29, 1978 [DOI] [PubMed] [Google Scholar]
- Cullen K, Brooks JX, Jamali M, Carriot J, Massot C. Internal models of self-motion: computations that suppress vestibular reafference in early vestibular processing. Exp Brain Res 210: 377–388, 2011 [DOI] [PubMed] [Google Scholar]
- Cullen K, Roy J. Signal processing in the vestibular system during active versus passive head movements. J Neurophysiol 91: 1919–1933, 2004 [DOI] [PubMed] [Google Scholar]
- Cullen K, Rey C, Guitton D, Galiana H. The use of system identification techniques in the analysis of oculomotor burst neuron spike train dynamics. J Comput Neurosci 3: 347–368, 1996 [DOI] [PubMed] [Google Scholar]
- Eatock R, Songer J. Vestibular hair cells and afferents: two channels for head motion signals. Annu Rev Neurosci 34: 501–534, 2011 [DOI] [PubMed] [Google Scholar]
- Escudero M, de Waele C, Vibert N, Berthoz A, Vidal P. Saccadic eye movements and the horizontal vestibulo-ocular and vestibulo-collic reflexes in the intact guinea-pig. Exp Brain Res 97: 254–262, 1993 [DOI] [PubMed] [Google Scholar]
- Fernandez C, Goldberg J. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol 34: 661–675, 1971 [DOI] [PubMed] [Google Scholar]
- Finlay B, Sengelaub D. Toward a neuroethology of mammalian vision: ecology and anatomy of rodent visuomotor behavior. Behav Brain Res 3: 133–149, 1981 [DOI] [PubMed] [Google Scholar]
- Fuchs A, Robinson D. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol 21: 1068–1070, 1966 [DOI] [PubMed] [Google Scholar]
- Goldberg J. Afferent diversity and the organization of central vestibular pathways. Exp Brain Res 130: 277–297, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J, Smith C, Fernández C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol 51: 1236–1256, 1984 [DOI] [PubMed] [Google Scholar]
- Highstein S, Goldberg J, Moschovakis A, Fernández C. Inputs from regularly and irregularly discharging vestibular nerve afferents to secondary neurons in the vestibular nuclei of the squirrel monkey. II. Correlation with output pathways of secondary neurons. J Neurophysiol 58: 719–738, 1987 [DOI] [PubMed] [Google Scholar]
- Highstein S, Rabbitt R, Holstein G, Boyle R. Determinants of spatial and temporal coding by semicircular canal afferents. J Neurophysiol 93: 2359–2370, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. The Handbook of Sensory Physiology, Vol. VII/5, Part A, edited by Crescetelli F. New York: Springer, 1977 [Google Scholar]
- Judge S, Richmond B, Chu F. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res 20: 535–538, 1980 [DOI] [PubMed] [Google Scholar]
- Kim J, Curthoys I. Responses of primary vestibular neurons to galvanic vestibular stimulation (GVS) in the anaesthetised guinea pig. Brain Res Bull 64: 265–271, 2004 [DOI] [PubMed] [Google Scholar]
- King WM, Shanidze N. Anticipatory eye movements stabilize gaze during self-generated head movements. Ann NY Acad Sci 1233: 219–225, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land M. Motion and vision: why animals move their eyes. J Comp Physiol A 185: 341–352, 1999 [DOI] [PubMed] [Google Scholar]
- Laurens J, Angelaki DE. The functional significance of velocity storage and its dependence on gravity. Exp Brain Res 210: 407–422, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui F, Benassi C, Biral G, Corazza R. Olivofloccular circuit in oculomotor control: binocular optokinetic stimulation. Exp Brain Res 125: 211–216, 1999 [DOI] [PubMed] [Google Scholar]
- Lui F, Giolli RA, Blanks RH, Tom EM. Pattern of striate cortical projections to the pretectal complex in the guinea pig. J Comp Neurol 344: 598–609, 1994 [DOI] [PubMed] [Google Scholar]
- Marlinsky V, Kröller J. Optokinetic eye movements elicited by an apparently moving visual pattern in guinea pigs. Exp Brain Res 131: 350–358, 2000 [DOI] [PubMed] [Google Scholar]
- Minor L, Goldberg J. Vestibular-nerve inputs to the vestibulo-ocular reflex: a functional-ablation study in the squirrel monkey. J Neurosci 11: 1636–1648, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K. Differential motion hyperacuity under conditions of common image motion. Vision Res 21: 1475–1482, 1981 [DOI] [PubMed] [Google Scholar]
- Nichols T, Holmes A. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15: 1–25, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson B, Baker J, Goldberg J, Banovetz J. Dynamic and kinematic properties of the vestibulocollic and cervicocollic reflexes in the cat. Prog Brain Res 76: 163–172, 1988 [DOI] [PubMed] [Google Scholar]
- Populin L. Monkey sound localization: head-restrained versus head-unrestrained orienting. J Neurosci 26: 9820–9832, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ris L, de Waele C, Serafin M, Vidal P, Godaux E. Neuronal activity in the ipsilateral vestibular nucleus following unilateral labyrinthectomy in the alert guinea pig. J Neurophysiol 74: 2087–2099, 1995 [DOI] [PubMed] [Google Scholar]
- Robinson D. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng 10: 137–145, 1963 [DOI] [PubMed] [Google Scholar]
- Roy J, Cullen K. Vestibuloocular reflex signal modulation during voluntary and passive head movements. J Neurophysiol 87: 2337–2357, 2002 [DOI] [PubMed] [Google Scholar]
- Sadeghi SG, Minor LB, Cullen KE. Neural correlates of motor learning in the vestibulo-ocular reflex: dynamic regulation of multimodal integration in the macaque vestibular system. J Neurosci 30: 10158–10168, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor R, Steinbacher B, Yates B. Horizontal linear and angular responses of neurons in the medial vestibular nucleus of the decerebrate cat. J Vestib Res 8: 107–116, 1998 [PubMed] [Google Scholar]
- Schweigart G, Mergner T, Barnes G. Eye movements during combined pursuit, optokinetic and vestibular stimulation in macaque monkey. Exp Brain Res 127: 54–66, 1999 [DOI] [PubMed] [Google Scholar]
- Shanidze N, Kim A, Raphael Y, King W. Eye-head coordination in the guinea pig. I. Responses to passive whole-body rotations. Exp Brain Res 205: 395–404, 2010a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanidze N, Kim A, Loewenstein S, Raphael Y, King W. Eye-head coordination in the guinea pig. II. Responses to self-generated (voluntary) head movements. Exp Brain Res 205: 445–454, 2010b [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Holst E, Mittelstaedt H. Das Reafferenzprinzip. Naturwissenschaften 20: 464–476, 1950 [Google Scholar]
- Waespe W, Henn V. Neuronal activity in the vestibular nuclei of the alert monkey during vestibular and optokinetic stimulation. Exp Brain Res 27: 523–538, 1977 [DOI] [PubMed] [Google Scholar]
- Waespe W, Henn V. The velocity response of vestibular nucleus neurons during vestibular, visual, and combined angular acceleration. Exp Brain Res 37: 337–347, 1979 [DOI] [PubMed] [Google Scholar]
- Walls G. The evolutionary history of eye movements. Vision Res 2: 69–80, 1962 [Google Scholar]