Abstract
Bilateral voluntary contractions involve functional changes in both primary motor cortices. We investigated whether a voluntary contraction controlled by one hemisphere can influence oscillatory processes contralaterally. Corticomuscular coherence was calculated between EEG recorded over the motor cortex hand representation and electromyogram from the first dorsal interosseous muscle when the nondominant hand performed a precision grip task. The dominant arm remained at rest or performed a finger abduction or an elbow flexion task at 10, 40, and 70% of maximal isometric voluntary contraction (MVC). Mean coherence in the 15- to 30-Hz range in the hand performing a precision grip increased during 40% (by 72%) and 70% (by 73%) but not during 10% of MVC in the finger abduction task. Similarly, in the elbow flexion task, mean coherence increased during 40% (by 40%) and 70% (by 48%) but not during 10% of MVC. No differences were observed between the increments in coherence between the finger abduction and elbow flexion tasks at a given force level. We speculate that these results reflect the increased complexity of controlling a fine motor task with one hand while performing a strong contraction with the contralateral hand and suggest that increased oscillatory corticomuscular coupling may contribute to successful task performance.
Keywords: primary motor cortex, oscillations, corticospinal drive, β-band, sensorimotor
bilateral voluntary contractions of arm muscles involve activity-dependent adaptations in both primary motor cortices (M1s; Ashe 1997; Cardoso de Oliveira et al. 2001; Carson 2005; Donchin et al. 1998). In humans, previous electrophysiological studies using transcranial magnetic stimulation have demonstrated that a voluntary contraction with an arm muscle changes corticospinal output measured in the contralateral voluntarily active arm (Netz et al. 1995; Soteropoulos and Perez 2011; Stinear and Byblow 2004; Yedimenko and Perez 2010). It has been proposed that these changes take place, at least in part, at the cortical level (Diedrichsen et al. 2003; Giovannelli et al. 2009; Soteropoulos and Perez 2011; Yedimenko and Perez 2010).
It is possible to obtain information about the synaptic drive to spinal motoneurons during a voluntary contraction by coherence analysis (Baker 2007; Witham et al. 2010, 2011). Coherence measures the strength of correlations between two signals in the frequency domain (Rosenberg et al. 1989). Corticomuscular coherence between EEG and electromyography (EMG) around 15–30 Hz (the “β-band”) is likely to be mediated, at least in part, by synchronous oscillatory discharge in fast corticospinal axons, propagated to spinal motoneurons via their monosynaptic connections (Baker et al. 1997, 2003; Conway et al. 1995; Kilner et al. 1999). However, a growing body of evidence suggests that afferent feedback pathways also contribute to this phenomenon (Baker et al. 2006; Riddle and Baker 2005; Tsujimoto et al. 2009; Witham et al. 2010, 2011).
During unimanual tasks, corticomuscular coherence in the 15- to 30-Hz frequency band is modulated in a task-dependent manner (Baker et al. 1999; Feige et al. 2000; Salenius et al. 1997). Coherence might reflect functional aspects of the task, such as compliance (Kilner et al. 2000), displacement (Riddle and Baker 2006), attention (Kristeva-Feige et al. 2002), or the level of force (Brown et al. 1998; Mima et al. 1999; Omlor et al. 2011; Witte et al. 2007). During bimanual tasks, comparatively little is known about the modulation of corticomuscular coherence. In monkeys, ∼25-Hz oscillatory activity is present in M1 on both sides, and these oscillations are strongly synchronized during bimanual manipulations (Murthy and Fetz 1996). In humans, corticomuscular coherence measured in one hand is modulated by the state (Kilner et al. 2003) and direction (Gross et al. 2005) of the movement of the contralateral hand, suggesting a degree of dependency between cortical oscillators. Therefore, we hypothesized that corticomuscular coherence during a voluntary contraction in one hand will be influenced by the level of voluntary contraction exerted by the contralateral arm. Based on previous studies (Gross et al. 2005; Kilner et al. 2003; Murthy and Fetz 1996), we predicted that corticomuscular coherence in a hand performing a steady voluntary contraction will increase to a larger extent during increasing levels of contralateral force.
METHODS
Subjects.
Ten healthy volunteers (8 female, age 26.6 ± 5.1 yr, mean ± SD; 8 right-handed) participated in the study. All subjects gave their informed written consent to the experimental procedure, which was approved by the Ethical Committee of the Medical Faculty, Newcastle University. Subjects were preselected out of a total of 20 subject that were screened to ensure that they showed significant corticomuscular coherence with the 1st dorsal interosseous (1DI) muscle during an auxotonic contraction; this allowed us to measure changes in coherence magnitude during our different experimental conditions.
Motor task.
Subjects were seated comfortably in an armchair. In all experiments, the nondominant hand performed a precision grip by squeezing a short length of plastic tubing (8-mm inside diameter, 11-mm outside diameter; PVC, order code 800/010/455/800; Portex) between finger and thumb with the arm flexed at the elbow by 90°. Subjects were instructed to perform the same movement with the precision grip hand regardless of what the opposite hand was doing. No visual feedback was presented for the hand performing the precision grip task. The precision grip was performed while the dominant arm remained at rest or performed a finger abduction or an elbow flexion task. In the finger abduction task, the dominant hand was placed in the posture illustrated in Fig. 1A. The arm was flexed at the elbow by 90° with the forearm pronated and the shoulder at 0° of abduction. The index finger was attached to a strain gauge, which measured force in the abduction-adduction direction. In different blocks of trials, subjects were required to exert forces of 10, 40, or 70% of the maximal voluntary contraction (MVC), determined during two to three brief (3–5 s) contractions performed at the start of the testing session (30-s rest between contractions). During these maximal contractions, subjects were verbally encouraged throughout the contractions to perform maximally, and visual feedback was provided (Gandevia 2001). Subjects were asked to move the black cursor, corresponding to force exerted, into the gray target by using the dominant arm (Fig. 1C). Contraction onset was signaled by the appearance of the black cursor; subjects were instructed to perform the precision grip simultaneously with the production of the force under visual feedback by this cue. Three seconds later, the cursor disappeared, cuing the end of the contraction. There was a 2-s delay before the start of the next trial. For brevity, we refer to these task conditions as Finger10, Finger40, and Finger70.
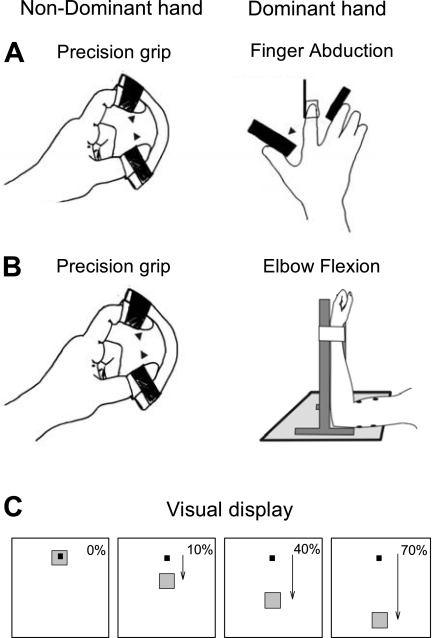
Fig. 1.
Experimental setup. A and B: schematic of the experimental setup showing the posture of both hands and 1 arm during testing. Note that the nondominant hand always performed a precision grip while the dominant arm performed a finger abduction (A) or elbow flexion (B) task. C: diagram showing the visual display presented to all subjects for the dominant arm. Subjects were instructed by the monitor to perform 10, 40, and 70% of the maximal voluntary index finger abduction or elbow flexion by activating the 1st dorsal interosseous (1DI) and biceps brachii muscles, respectively. The condition in which the dominant arm remained at rest was used as baseline. The black square is the cursor that subjects were instructed to move by performing isometric voluntary contractions with either the 1DI or biceps brachii. The gray square was the target to which the black cursor had to be moved; its position corresponded to the different maximal voluntary contraction (MVC) levels.
In the elbow flexion task, the dominant arm was held in the posture illustrated in Fig. 1B, with shoulder flexed by 90°, the elbow flexed at 90°, and the forearm supinated. The wrist was attached to the device by straps; a strain gauge measured force in the elbow flexion-extension direction. Task performance proceeded as for the finger abduction task, with subjects exerting elbow flexion forces at 10, 40, or 70% of MVC controlled by the visual display. Subjects completed 75 repetitions of the finger abduction and elbow flexion tasks at each contraction strength; the order of performance of each block was randomized. Additional verbal feedback was provided to the subjects to ensure that they reached the target with the dominant hand without making corrections. We refer to these task conditions as Elbow10, Elbow40, and Elbow70.
We assessed whether the consistency of motor performance of the precision grip hand was affected when the contralateral arm was being used. For each subject, the mean level of rectified 1DI EMG activity (in the precision grip hand) was calculated separately for each of the 75 repetitions on each task condition (across the same epoch that was used for the coherence analysis). After that, the variance from all trials for each bilateral condition (10, 40, and 70% of MVC) was compared with the variance during the unimanual condition (0% of MVC) across all subjects.
Recordings.
EMG was recorded bilaterally from the 1DI and biceps brachii muscles using adhesive surface electrodes (2-cm interelectrode distance; Biotrace 0713C; MSB, Marlbrough, United Kingdom). For measurements of corticomuscular coherence, EEG activity was recorded from sensorimotor cortex bilaterally, using pairs of adhesive electrodes (AMBU Neuroline 720, Wet Gel Snap Electrode) positioned 3 cm lateral and 2 cm anterior or posterior to the vertex. EEG from each side was derived from a differential recording between the electrode pair on that side; the anterior electrode was connected to the noninverting input of the amplifier. This is the same montage as used in our previous publications on corticomuscular coherence. Signals were amplified (EMG: gain, 500–2,000, band pass, 30 Hz to 2 kHz; EEG: gain, 50,000, band pass, 3 Hz to 2 kHz) and sampled to disk at 5 kHz together with force signals and task markers [Spike2 software; Cambridge Electronic Design (CED)].
Corticomuscular coherence.
The procedures for calculation of corticomuscular coherence between EEG and EMG signals have been described in detail in previous publications (Baker et al. 1997; Soteropoulos and Baker 2006). As corticomuscular coherence is known to be present during steady contractions, we confined the analysis to the last 1.6 s of the hold phase of the task (see shaded area in Fig. 2A).
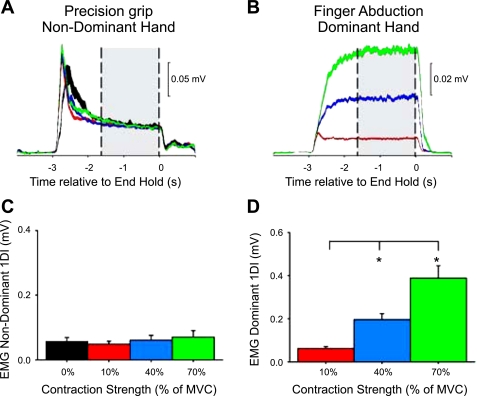
Fig. 2.
Electromyography (EMG) recordings during the finger abduction task. In a single representative subject, mean rectified EMG activity in the nondominant hand performing a precision grip (A) while the contralateral hand remained at rest (black line) or performed a finger abduction task (B) consisting of 10% (red line), 40% (blue line), and 70% (green line) of MVC is shown. The gray bars mark the region over which coherence analysis was performed. Shading indicates ±1 SE around the mean. C and D: group data (n = 10) showing mean rectified EMG activity (%MVC) in the hand performing the precision grip (C) and the finger abduction task (D). The abscissa shows the force level produced in finger abduction, and the ordinate shows the mean rectified EMG activity during the period used for measurement of corticomuscular coherence. Error bars indicate SE; *P < 0.05.
Denoting the fast Fourier transform (FFT) of the ith segment of EEG by Xi(f) and of the ith segment of rectified EMG data by Yi(f), the coherence (Coh) at frequency (f) was estimated as:
| (1) |
where L is the number of data sections available for analysis, and * denotes complex conjugate. The use of 2 4,096-data-point segments per trial (corresponding to a time period of 1.64 s extending back from the end of trial) with a sampling rate of 5 kHz provided a 0.92-Hz frequency resolution in the coherence spectra. The significance level for the coherence was calculated as (Rosenberg et al. 1989):
| (2) |
where coherence larger than Z was considered significant at P < α (here, α = 0.05).
Repeated-measures ANOVA was used to determine the effect of activation level (0, 10, 40, and 70%) with 1DI and biceps brachii on mean 15- to 30-Hz corticomuscular coherence, mean rectified EMG activity, and mean normalized 15- to 30-Hz EMG and EEG power. EEG power was normalized to the total power (summed over all frequencies) obtained in the baseline condition. This allowed an estimate of the proportion of power contributed by a given frequency band; fixing the reference as the baseline condition made changes in power easier to interpret. EMG power was normalized relative to the power at the first frequency bin (F = 0 Hz), which corresponds to the direct current level in the EMG. The mean level of EMG has previously been shown to relate to force output (Lawrence and De Luca 1983; Milner-Brown and Stein 1975), particularly at low to moderate contractions. A two-way ANOVA was used to examine the effect of task (finger abduction vs. elbow flexion) and activation level on mean corticomuscular coherence, mean rectified EMG activity, and mean normalized EMG and EEG power. An additional two-way ANOVA was used to examine the effect of task (precision grip vs. finger abduction) and activation level (10, 40, and 70%) on mean rectified EMG activity. Bonferroni post hoc test was performed on significant comparisons. Significance was set at P < 0.05. Group data are presented as means ± SD. Pearson correlation analysis was used to test correlations as needed.
RESULTS
Finger abduction task.
Figure 2A illustrates data from a single representative subject during unilateral and bilateral voluntary contractions of the 1DI muscle. In this subject, the mean rectified EMG activity in the hand performing the precision grip was maintained constant (Fig. 2A) while the contralateral hand performed Finger10, Finger40, or Finger70 (Fig. 2B) activations. In all subjects, mean rectified EMG activity in the precision grip hand remained similar across conditions (F = 1.69, P = 0.19, n = 10; ∼10% of MVC; Fig. 2C). The mean rectified EMG activity was increased in the hand performing the finger task (F = 36.9, P < 0.001, n = 10; Fig. 2D). Post hoc testing showed a significant increase in EMG activity during Finger40 (P < 0.01) and Finger70 (P < 0.001) compared with Finger10. A comparison between mean rectified EMG activity in both hands revealed no differences during Finger10 (P = 0.69) and an increase in EMG activity in the hand performing the finger abduction task compared with the hand performing the precision grip during Finger40 (P < 0.01) and Finger70 (P < 0.001). Variability in motor performance by the precision grip hand (see methods for details) remained similar across conditions (F = 1.47, P = 0.2, n = 10).
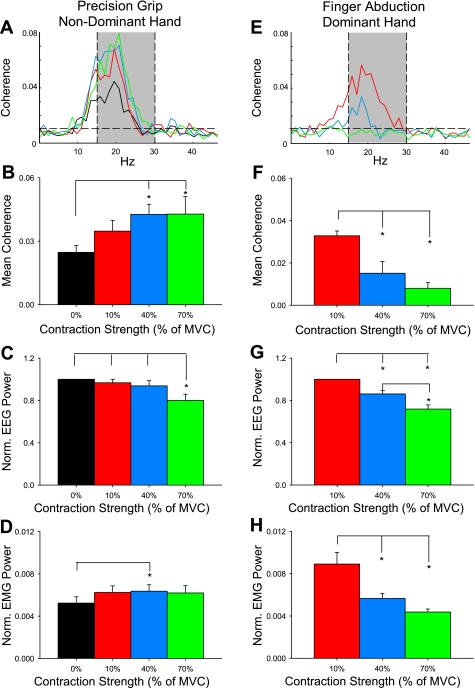
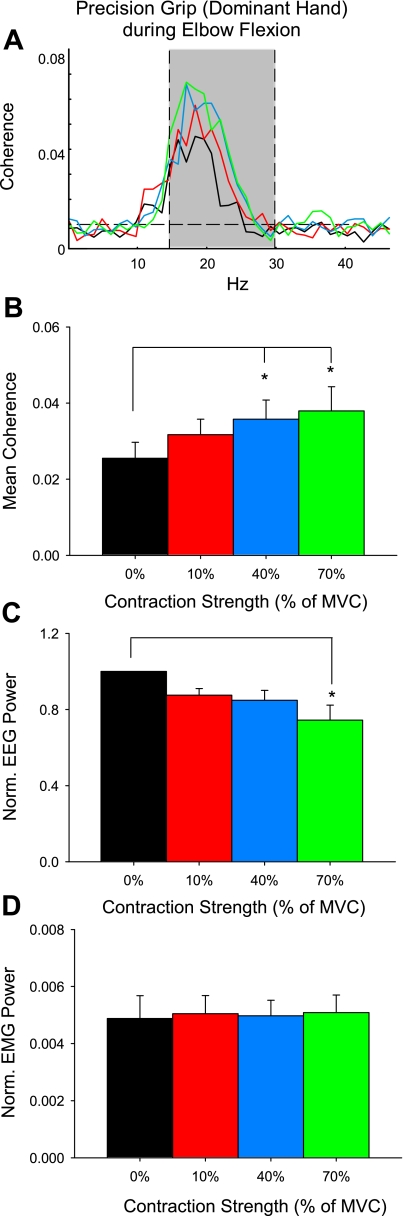
The population mean corticomuscular coherence in the hand performing the precision grip is shown in Fig. 3A (mean across subjects in the 15- to 30-Hz range: 0.024 ± 0.01, range across subjects: 0.0097–0.043, see shaded area). We found an increase in mean 15- to 30-Hz coherence in the precision grip hand at increasing levels of finger abduction force performed by the contralateral hand (color code is similar to previous plots; F = 6.4, P < 0.01, n = 10; Fig. 3B). Post hoc testing showed a significant increase during Finger40 (by 72.6 ± 45.1%; P < 0.01) and Finger70 (by 73.8 ± 78.1%; P < 0.01) compared with baseline. Mean coherence remained unchanged during Finger10 compared with baseline (by 40.5 ± 48.1%; P = 0.2), and it was similar between the higher force levels (P = 0.9). In the same frequency band, normalized EEG power was decreased during Finger70 compared with Finger40 (P < 0.01), Finger10 (P < 0.01), and baseline (P < 0.01; Fig. 3C). Changes in normalized EMG power were only seen during Finger40 compared with the baseline (P = 0.02; Fig. 3D).
Fig. 3.
Corticomuscular coherence during the finger abduction task. Traces show the corticomuscular coherence averaged across 10 subjects in the nondominant hand performing a precision grip (A) and the dominant hand performing the finger abduction task (E) during rest (black line) and 10% (red line), 40% (blue line), and 70% (green line) of MVC. Averaged measures over the 15- to 30-Hz frequency band: B and F, mean coherence; C and G, normalized (Norm.) EEG power; D and H, EMG power. Left column indicates measures made relating to nondominant hand; right column indicates measures made relating to dominant hand. Error bars indicate SE; *P < 0.05.
We also measured the population mean corticomuscular coherence in the 15- to 30-Hz frequency band in the hand that performed the finger abduction task (mean across subjects, 0.019 ± 0.007; range across subjects, 0.008–0.032; see shaded area in Fig. 3E). Coherence was decreased during Finger70 (by 45.9 ± 26%; P < 0.001) and Finger40 (by 24.4 ± 8.2%; P < 0.001) compared with Finger10 (Fig. 3F). No differences were found between the higher force levels (P = 0.3). In the same frequency band, mean normalized EEG power was decreased during Finger70 compared with Finger40 (P < 0.01) and Finger10 (P < 0.001; Fig. 3G). Similarly, the normalized EMG power was decreased during Finger70 (P < 0.001) and Finger40 (P < 0.01) compared with Finger10 (Fig. 3H).
When we compared the corticomuscular coherence in the 15- to 30-Hz band between the hand performing the precision grip and the hand performing the finger abduction task, we found an effect of side (F = 17.8, P < 0.01), MVC level (F = 4.3, P = 0.02), and in their interaction (F = 11.1, P < 0.001). Note that coherence values were similar on both sides during Finger10 (P = 0.7; see red bars in Fig. 3, B and F). The level of coherence was decreased during Finger40 (P < 0.001) and Finger70 (P < 0.01) in the hand performing the finger abduction task compared with the hand performing the precision grip.
Elbow flexion task.
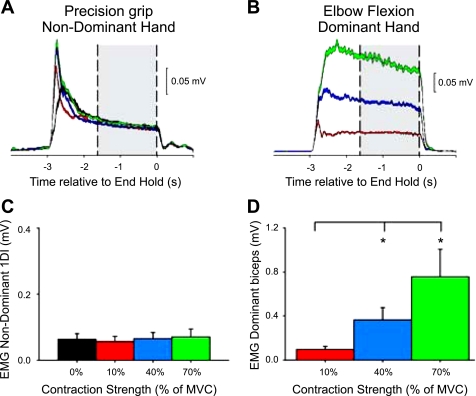
To examine whether changes in the magnitude of corticomuscular coherence were specific to the voluntary contraction of the contralateral 1DI, we also asked our subjects to perform an Elbow flexion task. Figure 4, A and B, illustrates data from a single representative subject. One subject was excluded from further analysis because of lack of constant EMG level in 1DI during different levels of activation of the contralateral biceps. In the remaining nine subjects, mean rectified 1DI EMG activity in the hand performing the precision grip was maintained constant across conditions (F = 2.0, P = 0.13, ∼10% of MVC; Fig. 4C). In the arm performing the elbow flexion task, mean rectified biceps EMG activity was increased during Elbow40 (P = 0.01) and Elbow70 (P = 0.01) compared with Elbow10 (Fig. 4D).
Fig. 4.
EMG recordings during the elbow flexion task. Averaged EMG activity in a single representative subject in the nondominant hand performing the precision grip (A) while the contralateral arm remained at rest (black line) or performed the elbow flexion task (B) consisting of contractions at 10% (red line), 40% (blue line), and 70% (green line) of MVC with the biceps brachii. The gray bars show the region over which the coherence analysis was performed. Shading indicates ±1 SE around the mean. C and D: group data (n = 9) showing mean rectified EMG activity (%MVC) in the precision grip hand (C) and in the arm performing the elbow flexion task (D). Error bars indicate SE; *P < 0.05.
Figure 5A shows the population mean corticomuscular coherence in the 15- to 30-Hz band in the hand that performed the precision grip during contralateral elbow flexion (mean across subjects: 0.025 ± 0.01, range across subjects: 0.007–0.039). Mean coherence was increased with increasing levels of biceps activation (F = 4.1, P < 0.01; Fig. 5B). Post hoc testing showed a significant increase during Elbow40 (by 40.1 ± 52.4%; P < 0.01) and Elbow70 (by 48.6 ± 66.4%; P < 0.01) compared with baseline but not between Elbow10 (24.1 ± 42.8%) and baseline (P = 0.1). Mean normalized EEG power was decreased during Elbow70 compared with Elbow40 (P = 0.02), Elbow10 (P < 0.01), and baseline (P < 0.001; Fig. 5C), whereas the normalized EMG power remained similar across all conditions (F = 1.3, P = 0.29; Fig. 5D).
Fig. 5.
Corticomuscular coherence in the hand performing a precision grip during the elbow flexion task. A: corticomuscular coherence averaged over 9 subjects measured in the nondominant 1DI during a precision grip task while the dominant arm remained at rest (black line) or performed 10% (red line), 40% (blue line), and 70% (green line) of MVC on the elbow flexion task. Shown are mean coherence (B), normalized EEG power (C), and EMG power (D) in the 15- to 30-Hz band. Error bars indicate SE; *P < 0.05.
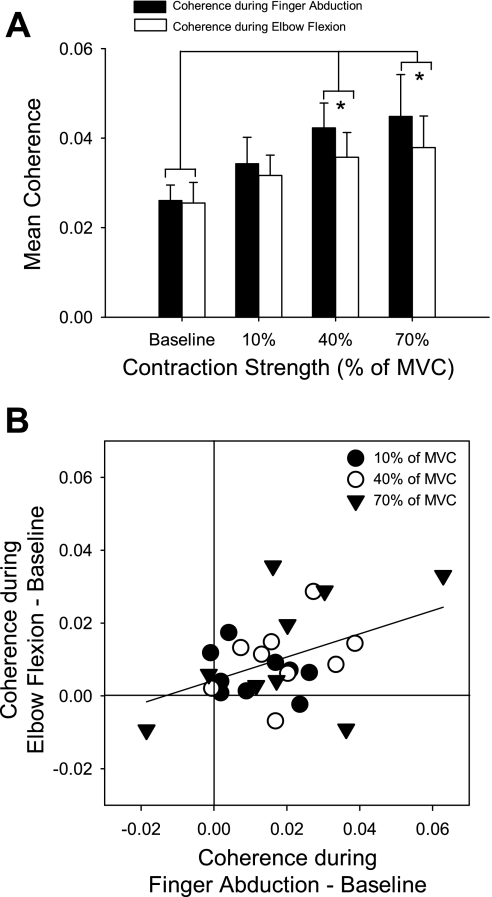
Finally, we compared the magnitude of corticomuscular coherence in the 15- to 30-Hz band in the hand performing the precision grip while the contralateral hand performed the finger abduction or elbow flexion task. We found an effect of activation level (F = 5.5, P < 0.01) but not muscle (F = 1.1, P = 0.3) nor their interaction (F = 0.9, P = 0.4; Fig. 6A). Corticomuscular coherence was significantly increased from the baseline condition during 40% (P < 0.01) and 70% (P < 0.001) but not during 10% of MVC (P > 0.05) regardless of whether subjects activated the contralateral 1DI or biceps brachii. Figure 6B shows the relation in single subjects between the changes in mean coherence relative to baseline for a contralateral biceps activation vs. a contralateral 1DI activation; the different symbols mark different levels of effort. There was a positive correlation (r2 = 0.21, P = 0.01) indicating that also at a single subject level similar changes occurred for a given strength of activation on either motor task.
Fig. 6.
Comparison of corticomuscular coherence in the hand performing a precision grip during contralateral finger abduction and elbow flexion tasks. A: coherence averaged across 9 subjects in the 15- to 30-Hz band as a function of contraction strength (0, 10, 40, and 70% of MVC) and task type (finger abduction, black bars; elbow flexion, white bars). Error bars show SE, computed across subjects. *P < 0.05. B: correlation between the change from baseline (contralateral limb resting) of coherence in nondominant 1DI during the finger abduction and elbow flexion tasks. Different symbols mark different contraction strengths. Line indicates best fit.
DISCUSSION
The present study investigated whether a voluntary contraction controlled by one hemisphere can influence oscillatory processes and oscillatory corticomuscular coupling in the other. We showed that strong isometric activations (≥40% of MVC) increased corticomuscular coherence in the 15- to 30-Hz frequency range in the contralateral hand. This occurred regardless of whether a distal intrinsic hand muscle or a proximal elbow flexor was activated. By contrast, weaker effort (10% of MVC) did not affect contralateral oscillatory coupling.
Effects of changing contraction strength on corticomuscular coherence measured from the muscle for which contraction varies.
Previous reports have described the changes in corticomuscular coherence when the muscle in which coherence is measured is voluntarily activated by different amounts. However, in common with most studies on corticomuscular coherence, the range of force levels has generally been limited to low levels of force and unilateral contractions (Chakarov et al. 2009; Kilner et al. 1999; Witte et al. 2007). In our study, we used stronger forces and bilateral contractions that limit a direct comparison with these previous studies. Brown et al. (1998) studied stronger forces and found that at MVC 15- to 30-Hz coherence was reduced, to be replaced by a new band of significant coherence ∼40 Hz (the Piper rhythm). This rhythm can also be seen at intermediate force levels around 60–80% of MVC (Brown et al. 1998). Interestingly, the present data showed a progressive reduction in the 15- to 30-Hz coherence with increasing force level; however, it was not replaced by a ∼40-Hz peak, even at the highest force tested of 70% of MVC (Fig. 3E). This suggests that the reduction in 15- to 30-Hz and appearance of ∼40-Hz corticomuscular coherence with increasing activation strength are two distinct phenomena, which can under some conditions be separated. The fact that activation of the contralateral limb prevents ∼40-Hz coherence may provide a useful technique for studying the origin of this activity further.
We can only speculate as to why 15- to 30-Hz corticomuscular coherence might be reduced with increasing force levels; however, the concomitant reduction in both EEG and EMG power at these frequencies suggests that there is a decreased reliance on oscillatory drive to muscle during stronger contractions. It has previously been shown that the firing rate of motor cortical cells (including identified corticomotoneuronal cells) saturates at higher forces (Evarts et al. 1983; Maier et al. 1993) as does the BOLD signal localized to M1 (Dettmers et al. 1995, 1996). It is likely that whereas the corticospinal tract (and its monosynaptic corticomotoneuronal connections) contributes a substantial part of the input required to recruit motoneurons during the fine control of the hand associated with weak forces, other pathways begin to contribute at higher force levels. One example of such an alternative pathway might be the reticulospinal tract (see Baker 2011), which is known to provide input to motoneurons involved in controlling the hand (Riddle et al. 2009) and for which outputs may be easier to detect at high force levels in humans (Ziemann et al. 1999). An alternative explanation for the decrease in 15- to 30-Hz corticomuscular coherence is that during strong voluntary contractions, the cortical motor network becomes less synchronized as reflected by the decreased EEG spectral power (Kristeva et al. 2007; Mendez-Balbuena et al. 2012).
Effects of changing contraction strength on corticomuscular coherence measured from a contralateral muscle.
In addition to changes in corticomuscular coherence in the dominant hand seen when that hand changed its activation strength, we also saw changes in corticomuscular coherence with the nondominant hand, even though it maintained a constant contraction. This is in agreement with previous studies demonstrating that aspects of a voluntary contraction with one hand can modulate coherence in the other (Gross et al. 2005; Kilner et al. 2003). In contrast to the changes in the dominant hand, coherence in the nondominant hand was increased with higher levels of contralateral effort. This is unlikely to reflect simply an increase in oscillatory control, since paradoxically there was a tendency for β-band power in the EEG to reduce and in the EMG to increase with increasing force. It has been previously demonstrated that modulations in coherence and EEG or EMG power can be dissociated in some circumstances (Baker and Baker 2003; Riddle et al. 2004; Kristeva et al. 2007; Omlor et al. 2007).
Previous studies have shown that there is an increased tendency to generate unwanted contralateral activity in the supposedly inactive limb during increasing levels of unilateral voluntary contraction (“mirroring”; Giovannelli et al. 2009; Sehm et al. 2009; Zijdewind et al. 2006). Some mirroring may result from recruitment of brain stem output pathways, such as the reticulospinal tract, for which axons have diffuse patterns of termination across multiple segmental levels, and bilaterally (Baker 2011; Davidson and Buford 2006; Peterson et al. 1975). Additionally, changes in corticospinal output from M1 ipsilateral to the contracting hand are produced by strong, not weak, levels of force (Hortobagyi et al. 2003; Muellbacher et al. 2000; Perez and Cohen 2008).
Performing a fine, low-force task (such as a precision grip) requires careful control of small groups of muscles to achieve the required patterns of digit fractionation (Schieber 1995). This is achieved not only by facilitation of some motoneuron pools, but also inhibition of others (Jankowska and Tanaka 1974; Kasser and Cheney 1985). Inhibition prevents inappropriate activation of muscles, which could be generated given the divergent patterns of excitatory connections made by corticomotoneuronal cells (Buys et al. 1986). Generating the correct pattern of activity across motoneuron pools that are initially at rest may be relatively straightforward: inappropriate activation of a given muscle will not occur so long as any facilitation of motoneurons remains below threshold. By contrast, if a strong contralateral contraction induces diffuse mirrored activation of many motoneuron pools, a far better focused motor command will be required, with stronger inhibition of muscles for which contraction must be prevented. Previous evidence has associated an increase in corticomuscular coherence with good motor performance (Kristeva et al. 2007; Perez et al. 2006; Witte et al. 2007) and speculated that oscillatory reafference may be important in interpretation of sensory feedback from the periphery (Baker 2007; Riddle and Baker 2006; Witham et al. 2010, 2011). The increased coherence seen with strong contralateral activation may thus reflect the increased difficulty in generating a fine fractionated finger movement in this situation and also possibly in monitoring feedback relating to successful performance.
Increased coherence with the 1DI muscle occurred to a similar extent during increasing contralateral activation strengths of both the 1DI and biceps muscles. This finding may support the above interpretation. Corticospinal drive in a finger muscle can be modulated by contralateral activation of either a distal or a proximal arm muscle (Sohn et al. 2003; Soteropoulos and Perez 2011). The level of mirrored muscle activation generated via less selective, noncorticospinal output pathways would also be unlikely to vary greatly with the identity of the prime muscle activated. In each case, we speculate that the increased potential for mirror activity in the hand that needs to perform a fine precision grip results in a more demanding motor task.
GRANTS
This work was funded by the Wellcome Trust, the Biotechnology and Biological Sciences Research Council (United Kingdom), and the National Institute of Neurological Disorders and Stroke, National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.A.P., D.S.S., and S.N.B. conception and design of research; M.A.P., D.S.S., and S.N.B. performed experiments; M.A.P., D.S.S., and S.N.B. analyzed data; M.A.P., D.S.S., and S.N.B. interpreted results of experiments; M.A.P., D.S.S., and S.N.B. prepared figures; M.A.P., D.S.S., and S.N.B. drafted manuscript; M.A.P., D.S.S., and S.N.B. edited and revised manuscript; M.A.P., D.S.S., and S.N.B. approved final version of manuscript.
REFERENCES
- Ashe J. Force and the motor cortex. Behav Brain Res 87: 255–269, 1997 [DOI] [PubMed] [Google Scholar]
- Baker MR, Baker SN. The effect of diazepam on motor cortical oscillations and corticomuscular coherence studied in man. J Physiol 546: 931–942, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN. Oscillatory interactions between sensorimotor cortex and the periphery. Curr Opin Neurobiol 6: 649–655, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN. The primate reticulospinal tract, hand function and functional recovery. J Physiol 589: 5603–5612, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN, Chiu M, Fetz EE. Afferent encoding of central oscillations in the monkey arm. J Neurophysiol 95: 3904–3910, 2006 [DOI] [PubMed] [Google Scholar]
- Baker SN, Kilner JM, Pinches EM, Lemon RN. The role of synchrony and oscillations in the motor output. Exp Brain Res 128: 109–117, 1999 [DOI] [PubMed] [Google Scholar]
- Baker SN, Olivier E, Lemon RN. Coherent oscillations in monkey motor cortex and hand muscle EMG show task-dependent modulation. J Physiol 501: 225–241, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN, Pinches EM, Lemon RN. Synchronization in monkey motor cortex during a precision grip task. II. Effect of oscillatory activity on corticospinal output. J Neurophysiol 89: 1941–1953, 2003 [DOI] [PubMed] [Google Scholar]
- Brown P, Salenius S, Rothwell JC, Hari R. Cortical correlate of the Piper rhythm in humans. J Neurophysiol 80: 2911–2917, 1998 [DOI] [PubMed] [Google Scholar]
- Buys EJ, Lemon RN, Mantel GW, Muir RB. Selective facilitation of different hand muscles by single corticospinal neurones in the conscious monkey. J Physiol 381: 529–549, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso de Oliveira S, Gribova A, Donchin O, Bergman H, Vaadia E. Neural interactions between motor cortical hemispheres during bimanual and unimanual arm movements. Eur J Neurosci 14: 1881–1896, 2001 [DOI] [PubMed] [Google Scholar]
- Carson RG. Neural pathways mediating bilateral interactions between the upper limbs. Brain Res Brain Res Rev 49: 641–662, 2005 [DOI] [PubMed] [Google Scholar]
- Chakarov V, Naranjo JR, Schulte-Mönting J, Omlor W, Huethe F, Kristeva R. β-Range EEG-EMG coherence with isometric compensation for increasing modulated low-level forces. J Neurophysiol 102: 1115–1120, 2009 [DOI] [PubMed] [Google Scholar]
- Conway BA, Halliday DM, Farmer SF, Shahani U, Maas P, Weir AI, Rosenberg JR. Synchronization between motor cortex and spinal motoneuronal pool during the performance of a maintained motor task in man. J Physiol 489: 917–924, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Bilateral actions of the reticulospinal tract on arm and shoulder muscles in the monkey: stimulus triggered averaging. Exp Brain Res 173: 25–39, 2006 [DOI] [PubMed] [Google Scholar]
- Dettmers C, Connelly A, Stephan KM, Turner R, Friston KJ, Frackowiak RS, Gadian DG. Quantitative comparison of functional magnetic resonance imaging with positron emission tomography using a force-related paradigm. Neuroimage 4: 201–209, 1996 [DOI] [PubMed] [Google Scholar]
- Dettmers C, Fink GR, Lemon RN, Stephan KM, Passingham RE, Silbersweig D, Holmes A, Ridding MC, Brooks DJ, Frackowiak RS. Relation between cerebral activity and force in the motor areas of the human brain. J Neurophysiol 74: 802–815, 1995 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Hazeltine E, Nurss WK, Ivry RB. The role of the corpus callosum in the coupling of bimanual isometric force pulses. J Neurophysiol 90: 2409–2418, 2003 [DOI] [PubMed] [Google Scholar]
- Donchin O, Gribova A, Steinberg O, Bergman H, Vaadia E. Primary motor cortex is involved in bimanual coordination. Nature 395: 274–278, 1998 [DOI] [PubMed] [Google Scholar]
- Evarts EV, Fromm C, Kröller J, Jennings VA. Motor cortex control of finely graded forces. J Neurophysiol 49: 1199–1215, 1983 [DOI] [PubMed] [Google Scholar]
- Feige B, Aertsen A, Kristeva-Feige R. Dynamic synchronization between multiple cortical motor areas and muscle activity in phasic voluntary movements. J Neurophysiol 84: 2622–2629, 2000 [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789, 2001 [DOI] [PubMed] [Google Scholar]
- Giovannelli F, Borgheresi A, Balestrieri F, Zaccara G, Viggiano MP, Cincotta M, Ziemann U. Modulation of interhemispheric inhibition by volitional motor activity: an ipsilateral silent period study. J Physiol 587: 5393–5410, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Pollok B, Dirks M, Timmermann L, Butz M, Schnitzler A. Task-dependent oscillations during unimanual and bimanual movements in the human primary motor cortex and SMA studied with magnetoencephalography. Neuroimage 26: 91–98, 2005 [DOI] [PubMed] [Google Scholar]
- Hortobagyi T, Taylor JL, Petersen NT, Russell G, Gandevia SC. Changes in segmental and motor cortical output with contralateral muscle contractions and altered sensory inputs in humans. J Neurophysiol 90: 2451–2459, 2003 [DOI] [PubMed] [Google Scholar]
- Jankowska E, Tanaka R. Neuronal mechanism of the disynaptic inhibition evoked in primate spinal motoneurones from the corticospinal tract. Brain Res 75: 163–166, 1974 [DOI] [PubMed] [Google Scholar]
- Kasser RJ, Cheney PD. Characteristics of corticomotoneuronal post-spike facilitation and reciprocal suppression of EMG activity in the monkey. J Neurophysiol 53: 959–978, 1985 [DOI] [PubMed] [Google Scholar]
- Kilner JM, Baker SN, Salenius S, Hari R, Lemon RN. Human cortical muscle coherence is directly related to specific motor parameters. J Neurosci 20: 8838–8845, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Baker SN, Salenius S, Jousmäki V, Hari R, Lemon RN. Task-dependent modulation of 15–30 Hz coherence between rectified EMGs from human hand and forearm muscles. J Physiol 516: 559–570, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Salenius S, Baker SN, Jackson A, Hari R, Lemon RN. Task-dependent modulations of cortical oscillatory activity in human subjects during a bimanual precision grip task. Neuroimage 18: 67–73, 2003 [DOI] [PubMed] [Google Scholar]
- Kristeva R, Patino L, Omlor W. Beta-range cortical motor spectral power and corticomuscular coherence as a mechanism for effective corticospinal interaction during steady-state motor output. Neuroimage 36: 785–792, 2007 [DOI] [PubMed] [Google Scholar]
- Kristeva-Feige R, Fritsch C, Timmer J, Lücking CH. Effects of attention and precision of exerted force on beta range EEG-EMG synchronization during a maintained motor contraction task. Clin Neurophysiol 113: 124–131, 2002 [DOI] [PubMed] [Google Scholar]
- Lawrence JH, De Luca CJ. Myoelectric signal versus force relationship in different human muscles. J Appl Physiol 54: 1653–1659, 1983 [DOI] [PubMed] [Google Scholar]
- Maier M, Bennett KM, Hepp-Reymond MC, Lemon RN. Contribution of the monkey cortico-motoneuronal system to the control of force in precision grip. J Neurophysiol 69: 772–785, 1993 [DOI] [PubMed] [Google Scholar]
- Mendez-Balbuena I, Huethe F, Schulte-Mönting J, Leonhart R, Manjarrez E, Kristeva R. Corticomuscular coherence reflects interindividual differences in the state of the corticomuscular network during low-level static and dynamic forces. Cereb Cortex 22: 628–638, 2012 [DOI] [PubMed] [Google Scholar]
- Milner-Brown HS, Stein RB. The relation between the surface electromyogram and muscular force. J Physiol 246: 549–569, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima T, Simpkins N, Oluwatimilehin T, Hallett M. Force level modulates human cortical oscillatory activities. Neurosci Lett 275: 77–80, 1999 [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Facchini S, Boroojerdi B, Hallett M. Changes in motor cortex excitability during ipsilateral hand muscle activation in humans. Clin Neurophysiol 111: 344–349, 2000 [DOI] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Oscillatory activity in sensorimotor cortex of awake monkeys: synchronization of local field potentials and relation to behavior. J Neurophysiol 76: 3949–3967, 1996 [DOI] [PubMed] [Google Scholar]
- Netz J, Ziemann U, Hömberg V. Hemispheric asymmetry of transcallosal inhibition in man. Exp Brain Res 104: 527–533, 1995 [DOI] [PubMed] [Google Scholar]
- Omlor W, Patino L, Hepp-Reymond MC, Kristeva R. Gamma-range corticomuscular coherence during dynamic force output. Neuroimage 34: 1191–1198, 2007 [DOI] [PubMed] [Google Scholar]
- Omlor W, Patino L, Mendez-Balbuena I, Schulte-Mönting J, Kristeva R. Corticospinal beta-range coherence is highly dependent on the pre-stationary motor state. J Neurosci 31: 8037–8045, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Cohen LG. Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. J Neurosci 28: 5631–5640, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Lundbye-Jensen J, Nielsen JB. Changes in corticospinal drive to spinal motoneurones following visuo-motor skill learning in humans. J Physiol 573: 843–855, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BW, Maunz RA, Pitts NG, Mackel RG. Patterns of projection and braching of reticulospinal neurons. Exp Brain Res 23: 333–351, 1975 [DOI] [PubMed] [Google Scholar]
- Riddle CN, Baker SN. Digit displacement, not object compliance, underlies task dependent modulations in human corticomuscular coherence. Neuroimage 33: 618–627, 2006 [DOI] [PubMed] [Google Scholar]
- Riddle CN, Baker SN. Manipulation of peripheral neural feedback loops alters human corticomuscular coherence. J Physiol 566: 625–639, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle CN, Baker MR, Baker SN. The effect of carbamazepine on human corticomuscular coherence. Neuroimage 22: 333–340, 2004 [DOI] [PubMed] [Google Scholar]
- Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci 29: 4993–4999, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg JR, Amjad AM, Breeze P, Brillinger DR, Halliday DM. The Fourier approach to the identification of functional coupling between neuronal spike trains. Prog Biophys Mol Biol 53: 1–31, 1989 [DOI] [PubMed] [Google Scholar]
- Salenius S, Portin K, Kajola M, Salmelin R, Hari R. Cortical control of human motoneuron firing during isometric contraction. J Neurophysiol 77: 3401–3405, 1997 [DOI] [PubMed] [Google Scholar]
- Schieber MH. Muscular production of individuated finger movements: the roles of extrinsic finger muscles. J Neurosci 15: 284–297, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehm B, Perez MA, Xu B, Hidler J, Cohen LG. Functional neuroanatomy of mirroring during a unimanual force generation task. Cereb Cortex 20: 34–45, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn YH, Jung HY, Kaelin-Lang A, Hallett M. Excitability of the ipsilateral motor cortex during phasic voluntary hand movement. Exp Brain Res 148: 176–185, 2003 [DOI] [PubMed] [Google Scholar]
- Soteropoulos DS, Baker SN. Cortico-cerebellar coherence during a precision grip task in the monkey. J Neurophysiol 95: 1194–1206, 2006 [DOI] [PubMed] [Google Scholar]
- Soteropoulos DS, Perez MA. Physiological changes underlying bilateral isometric arm voluntary contractions in healthy humans. J Neurophysiol 105: 1594–602, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear JW, Byblow WD. Modulation of human cervical premotoneurons during bilateral voluntary contraction of upper-limb muscles. Muscle Nerve 29: 506–514, 2004 [DOI] [PubMed] [Google Scholar]
- Tsujimoto T, Mima T, Shimazu H, Isomura Y. Directional organization of sensorimotor oscillatory activity related to the electromyogram in the monkey. Clin Neurophysiol 120: 1168–1173, 2009 [DOI] [PubMed] [Google Scholar]
- Witham CL, Riddle CN, Baker MR, Baker SN. Contributions of descending and ascending pathways to corticomuscular coherence in humans. J Physiol 589: 3789–3800, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witham CL, Wang M, Baker SN. Corticomuscular coherence between motor cortex, somatosensory areas and forearm muscles in the monkey. Front Syst Neurosci 4: 38, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte M, Patino L, Andrykiewicz A, Hepp-Reymond MC, Kristeva R. Modulation of human corticomuscular beta-range coherence with low-level static forces. Eur J Neurosci 26: 3564–3570, 2007 [DOI] [PubMed] [Google Scholar]
- Yedimenko JA, Perez MA. The effect of bilateral isometric forces in different directions on motor cortical function in humans. J Neurophysiol 104: 2922–2931, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Ishii K, Borgheresi A, Yaseen Z, Battaglia F, Hallett M, Cincotta M, Wassermann EM. Dissociation of the pathways mediating ipsilateral and contralateral motor-evoked potentials in human hand and arm muscles. J Physiol 518: 895–906, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijdewind I, Butler JE, Gandevia SC, Taylor JL. The origin of activity in the biceps brachii muscle during voluntary contractions of the contralateral elbow flexor muscles. Exp Brain Res 175: 526–535, 2006 [DOI] [PubMed] [Google Scholar]