Abstract
The relatively simple clock neuron network of Drosophila is a valuable model system for the neuronal basis of circadian timekeeping. Unfortunately, many key neuronal classes of this network are inaccessible to electrophysiological analysis. We have therefore adopted the use of genetically encoded sensors to address the physiology of the fly's circadian clock network. Using genetically encoded Ca2+ and cAMP sensors, we have investigated the physiological responses of two specific classes of clock neuron, the large and small ventrolateral neurons (l- and s-LNvs), to two neurotransmitters implicated in their modulation: acetylcholine (ACh) and γ-aminobutyric acid (GABA). Live imaging of l-LNv cAMP and Ca2+ dynamics in response to cholinergic agonist and GABA application were well aligned with published electrophysiological data, indicating that our sensors were capable of faithfully reporting acute physiological responses to these transmitters within single adult clock neuron soma. We extended these live imaging methods to s-LNvs, critical neuronal pacemakers whose physiological properties in the adult brain are largely unknown. Our s-LNv experiments revealed the predicted excitatory responses to bath-applied cholinergic agonists and the predicted inhibitory effects of GABA and established that the antagonism of ACh and GABA extends to their effects on cAMP signaling. These data support recently published but physiologically untested models of s-LNv modulation and lead to the prediction that cholinergic and GABAergic inputs to s-LNvs will have opposing effects on the phase and/or period of the molecular clock within these critical pacemaker neurons.
Keywords: live imaging; GCaMP; Epac1-camps; adenosine 3′,5′-cyclic monophosphate
the circadian clock is an innate temporal program that rhythmically orchestrates most aspects of animal behavior and physiology (Aschoff 1981). Circadian clocks are a ubiquitous adaptation to the predictable and often severe daily and seasonal fluctuations that characterize much of the Earth's surface. The proper entrainment of these endogenous clocks to local time is a prerequisite for the optimal orchestration of clock-controlled behavioral and physiological outputs with an ever-changing environment. In animals, the master circadian clock comprises a network of central brain neurons, each of which expresses a highly conserved molecular clockwork (Herzog 2007), and the proper entrainment of this network to the 24-h rhythm of the environment is critical for human health and psychological well-being (Foster 2010; Schwartz and Roth 2006). Clock neuron networks are closely associated with input from the visual system in animals, and environmental light-dark cycles are thought to be the network's most salient entrainment cue (Golombek and Rosenstein 2009; Helfrich-Förster 2004). However, the clock neuron network is sensitive to many environmental, physiological, and social cues and likely responds to a diverse set of modulatory inputs to maintain entrainment to the external world (Challet 2007). The disruption of the normal timing of these inputs is thought to be the cause of circadian misalignment in humans (Arble et al. 2010). Understanding how the brain's clock integrates various modulatory inputs is therefore critical for understanding circadian timekeeping and its misalignment.
Compared to the mammalian clock center in the hypothalamic suprachiasmatic nuclei, the circadian clock neuron network of the fly Drosophila melanogaster is simple, consisting of fewer than 200 neurons (Kaneko and Hall 2000; Shafer et al. 2006). Subsets of these neurons, the large and small ventrolateral neurons (l-LNvs and s-LNvs), are critical for the control of sleep and arousal and for several aspects of circadian timekeeping (Chung et al. 2009; Parisky et al. 2008; Renn et al. 1999; Shang et al. 2008; Sheeba et al. 2008a; Yoshii et al. 2009). The s-LNvs are thought to be the dominant neuronal pacemaker of the circadian clock neuron network under light-dark cycles and under constant darkness and temperature (Grima et al. 2004; Rieger et al. 2006; Stoleru et al. 2004, 2005). Given the important roles that these neurons serve in timekeeping, an understanding of the physiological basis of their circadian function is critical to our understanding of the clock network in Drosophila. The l-LNvs—probably because of their large cell body diameters and superficial locations—have been the focus of detailed electrophysiological analysis within the intact adult clock neuron network (Fogle et al. 2011; McCarthy et al. 2011; Park and Griffith 2006; Sheeba et al. 2008b). In contrast, the electrophysiological analysis of s-LNvs is challenging, and, to our knowledge, only one electrophysiological experiment, which described the resting membrane potential of s-LNvs across the diurnal cycle, has been reported for this neuron type (Cao and Nitabach 2008). Thus the physiological properties of the critical adult s-LNvs remain largely unknown. Likewise, the physiology of the majority of clock neuron classes has not been addressed through electrophysiological observations, likely owing to inaccessibility. For this reason we have sought to employ live imaging methods for the measurement of Ca2+ and cAMP signaling to extend the physiological analysis of the adult clock neuron network beyond the l-LNvs.
Acetylcholine (ACh) and γ-aminobutyric acid (GABA) have both been proposed as important neurochemical modulators of the clock neuron networks of both insects and mammals (Albus et al. 2005; Dahdal et al. 2010; Hamasaka et al. 2005; Liu and Reppert 2000). Electrophysiological studies of l-LNvs have established that these neurons are excited by ACh through nicotinic acetylcholine receptors (McCarthy et al. 2011) and are inhibited by GABA, likely via ionotropic GABAA receptors (Chung et al. 2009; McCarthy et al. 2011; Parisky et al. 2008). A growing body of work indicates that the l-LNvs are important GABA-modulated regulators of sleep and arousal in the adult fly (Chung et al. 2009; Parisky et al. 2008; Shang et al. 2008; Sheeba et al. 2008a). Several lines of evidence suggest that, like l-LNvs, s-LNvs are reciprocally modulated by ACh and GABA (Chung et al. 2009; Dahdal et al. 2010; Hamasaka et al. 2005; Parisky et al. 2008; Wegener et al. 2004). Nevertheless, the predicted physiological effects of these transmitters on adult s-LNvs have not yet been directly tested in the adult brain.
Although live cAMP imaging has been previously conducted on adult s-LNvs (Shafer et al. 2008; Shang et al. 2011), previous attempts to address the acute Ca2+ responses of the s-LNvs to neurotransmitters have focused on dissociated and cultured LNvs of the larval brain (Dahdal et al. 2010; Hamasaka et al. 2005; Wegener et al. 2004). These neurons persist through metamorphosis to become the adult s-LNvs (Helfrich-Förster 1997) and may maintain many aspects of their larval physiology. Nevertheless, the circadian clock network of the adult brain is radically different from the relatively simple larval clock network, and the s-LNv neurons of the adult likely acquire a large number of new synaptic inputs and neuronal targets during metamorphosis (Helfrich-Förster et al. 2007; Kaneko and Hall 2000; Kaneko et al. 1997). Thus it is likely that some aspects of s-LNv physiology will differ between the larval and adult stages of Drosophila. For this reason it is critical that models of connectivity and modulation be tested in the intact adult clock neuron network. Here we test the efficacy and limitations of genetically encoded Ca2+ and cAMP sensors for measuring the physiological responses of deeply situated neurons of the adult circadian clock neuron network and use these tools to investigate the physiological effects of ACh and GABA on the critical s-LNv pacemakers of the adult Drosophila brain.
METHODS
Fly strains.
Expression of the GCaMP3.0 and Epac1-camps sensors was achieved with the previously described; UAS-GCaMP3.0; (Tian et al. 2009) and ;UAS-Epac1-camps(50A); elements (Shafer et al. 2008). We created stable lines expressing these sensors in l- and s-LNvs, by combining each of these second chromosome UAS elements with the X-chromosome PDF driver Pdf(M)-GAL4 (Renn et al. 1999). These Pdf-GAL4; UAS-GCaMP3.0; and Pdf-GAL4;UAS-Epac1-camps flies were reared under a 12:12-h light-dark cycle at 25°C on cornmeal-yeast-sucrose media. Male flies were used for all live imaging experiments and were dissected and imaged 2–4 days after adult emergence. Only flies dissected during the day were used for our experiments.
Dissection and solutions.
Flies were anesthetized on ice, and the brains were dissected directly into ice-cold Tübingen and Düsseldorf Drosophila Ringer solution consisting of (in mM) 46 NaCl, 182 KCl, 3 CaCl2, and 10 Tris, pH 7.2 (Sullivan et al. 2000). All cuticle, compound eye tissue, and large trachea were removed from the dissected brains. Brains were mounted anterior surface up in drop of hemolymph-like saline (HL3) consisting of (in mM) 70 NaCl, 5 KCl, 1.5 CaCl2, 20 MgCl2, 10 NaHCO3, 5 trehalose, 115 sucrose, and 5 HEPES, pH 7.1 (Stewart et al. 1994) placed on the center of a 35-mm Falcon dish (Becton Dickenson Labware, Franklin Lakes, NJ). A petri dish insert for a PS-8H perfusion system (Bioscience Tools, San Diego, CA) was lowered around the brain. Brains were allowed to recover for 5–10 min before the start of imaging experiments. HL3 flow was established across the brain at the beginning of each experiment with the gravity-fed PS-8H perfusion system. Test compounds were applied by switching perfusion flow from the main HL3 line to a second line containing test compound for 30 s, followed by a return to HL3 flow. For vehicle controls, we switched to a second HL3 perfusion line for 30 s, followed by a return to the main HL3 line. All test compounds were purchased from Sigma-Aldrich (St. Louis, MO) and were dissolved in HL3.
For both GCaMP3.0 and Epac1-camps imaging experiments, single brains were treated with multiple doses of agonist and with vehicle controls unless otherwise noted. A typical brain received two to five agonist stimulations of differing concentrations and was allowed to recover for 5–10 min between stimulations with continuous washout with HL3 saline. Thus, for dose-response experiments, multiple concentrations of agonist and a control perfusion were delivered to each brain. Although this approach revealed dose-dependent effects of agonist treatments, the magnitudes of the individual responses were likely affected somewhat by previous treatments. We therefore used only single-agonist perfusions when comparing neuronal responses in the presence or absence of tetrodotoxin (TTX). We performed TTX treatments by diluting TTX in both the HL3 perfusion flow and in each solution of diluted agonist. To ensure that TTX had time to penetrate our intact brain preparation, brains were incubated in 2 μM TTX for 20 min before imaging and experienced 60–90 s of 2 μM TTX flow before being treated with agonist solutions containing 2 μM TTX.
Live imaging and analysis.
Live imaging of the GCaMP3.0 and Epac1-camps sensors was conducted on an Olympus FV1000 laser scanning confocal microscope (Olympus, Center Valley, PA). LNvs were first located based on their basal sensor expression under epifluorescent illumination using green fluorescent protein (GFP) optics. The l- and s-LNvs were differentiated based on their cell body sizes and locations. We used a 60 × 1.10 N/A W, FUMFL N objective with a dipping cone and correction collar for all live imaging experiments (Olympus). For GCaMP3.0 imaging experiments, frames were scanned with a 488-nm laser at 10 Hz for a recording duration of 5 min and GFP emission was directed to a photomultiplier tube using standard GFP optics. Epac1-camps FRET imaging was performed by scanning frames with a 440-nm laser at a frequency of 1 Hz for 5 min. Cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP) emission were separated by means of a SDM510 dichroic mirror. In the l-LNvs, some cAMP responses to GABA were observed to have relatively long latencies; Epac1-camps time courses for GABA treatments were therefore extended to a recording duration of 10 min. For both GCaMP3.0 and Epac1-camps experiments, regions of interest (ROIs) were selected over single cell bodies of l- and s-LNvs within a single focal plane. To reduce movement artifacts for GCaMP3.0 experiments, the confocal aperture was increased to a diameter of 564 μm to increase the thickness of the optical section, and focal planes were focused on center of somata of interest. The ratiometric nature of Epac1-camps made these precautions unnecessary for our cAMP imaging experiments. Mean ROI pixel intensities for GCaMP3.0 fluorescence or Epac1-camps CFP and YFP fluorescence (value range: 0–4,095) were collected for each time point with Olympus Fluoview software (v. 10).
Raw GCaMP3.0 fluorescence traces were processed with custom analysis software written in MATLAB (MathWorks, Natick MA). GCaMP3.0 traces were first filtered with a 10-point moving average to remove high-frequency noise. Each filtered intensity trace was then transformed to a percent fluorescence change (ΔF/F0) trace with the following equation: [(Fn − F0)/F0] × 100, where Fn is the intensity value at each point in time and F0 is the baseline fluorescence calculated as the average fluorescence intensity recorded during the first 10 s of imaging. Mean ± SE traces were created based on all filtered and normalized GCaMP3.0 fluorescence traces for each neuronal class and treatment. The maximum increase in GCaMP3.0 fluorescence was determined for each filtered and normalized trace based on the entire 5-min duration of the recording. These values were then used to determine the mean maximum GCaMP3.0 fluorescence change for each treatment and neuron type. Rarely, individual neurons displayed rather large monotonic increases in GCaMP3.0 fluorescence over the course of an experiment. We defined outlier plots as monotonic plots with maximal changes that were two standard deviations or more from the mean maximal change for the vehicle treatment group. These plots were removed from the data set prior to the application of statistical tests.
Time course data collected for Epac1-camps experiments consisted of raw CFP and YFP values for each time point. The YFP channel was corrected to subtract CFP spillover into the YFP channel with the following equation: YFPsoc = YFP − (CFP × 0.444), where YFPsoc is the spillover-corrected YFP intensity, YFP and CFP are the raw intensity values, and 0.444 is the proportion of CFP emission that spills over into the YFP channel on our imaging system. The inverse FRET ratio, which is proportional to changes in cAMP, was calculated by taking the ratio CFP/YFPsoc. Raw inverse FRET traces were filtered with a 10-point moving average to remove high-frequency noise, and the initial time point for each trace was normalized to “1.0.” Filtered and normalized inverse FRET ratio traces were averaged to create average traces for each treatment and neuron type and were expressed as percent changes in inverse Epac1-camps FRET. The maximum increase or decrease in inverse Epac1-camps FRET was determined for each individual trace from the entire 5- or 10-min duration of the recording. These values were used to generate the mean maximum changes in inverse Epac1-camps FRET for each treatment and neuron type. For our nicotine experiments, the maximum change in inverse Epac1-camps FRET was determined for the first 3 min of the time course to compensate for the steadily increasing baseline observed for all vehicle control plots in this experiment.
For all comparisons of mean maximum change in GCaMP3.0 fluorescence or Epac1-camps inverse FRET ratio, a Kruskal-Wallis one-way ANOVA was performed on the maximum response magnitudes for each set of experimental compounds and neuron type and a Dunn's multiple comparison test was performed to determine which treatments within the group of compounds tested produced responses significantly different from vehicle controls. Statistical significance was set at P < 0.05 for the ANOVA and multiple comparisons test. For pairwise comparisons of maximum changes in GCaMP3.0 fluorescence or inverse Epac1-camps FRET, we employed the Mann Whitney U-test. All data were statistically analyzed and plotted with Prism 5 (GraphPad, San Diego, CA). For all data, sample sizes are reported as two numbers, separated by a comma, representing the number of neurons and number of brains sampled, respectively.
RESULTS
s-LNvs retain their receptivity to cholinergic agonists in the adult brain.
We first investigated the Ca2+ responses of l-LNvs, which have been previously shown by electrophysiology to be responsive to ACh (McCarthy et al. 2011), to cholinergic agonists. For vehicle-treated l-LNvs, a gradual, steady, and relatively small decline in GCaMP3.0 fluorescence was typically observed (Fig. 1A). We found no evidence for spontaneous increases in GCaMP3.0 fluorescence in vehicle-treated or untreated l-LNvs, despite the fact that these neurons have been shown to fire spontaneous streams of action potentials, sometimes organized as low-frequency bursts (McCarthy et al. 2011; Sheeba et al. 2008b). However, given the relatively slow nature of the return to basal Ca2+ levels following bursts of action potentials in neurons (e.g., Irwin and Allen 2009) and the well-described kinetics of GCaMP3.0 responses to neuronal firing (Tian et al. 2009), we would not expect this sensor to detect such spontaneous activity.
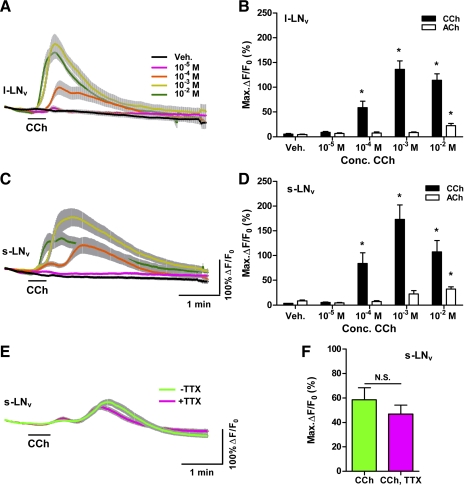
Fig. 1.
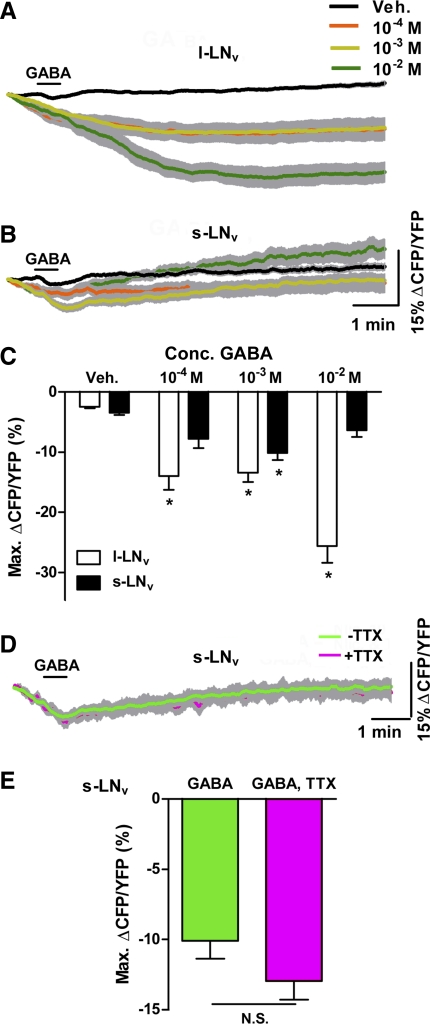
Cholinergic agonists cause Ca2+ increases in adult ventrolateral neurons (LNvs). A: mean GCaMP3.0 fluorescence traces from single large (l)-LNv somata from brains treated with 30-s (black bar) perfusions of 10−5, 10−4, 10−3, and 10−2 M carbamoylcholine (CCh) and vehicle (Veh) controls. Error bars indicate SE. For CCh treatments, nveh = 21 neurons from 10 brains (21,10), n10−5 = 17,7; n10−4 = 27,11; n10−3 = 16,7; n10−2 = 10,5. Key in A also applies to C. B: mean ± SE maximum GCaMP3.0 fluorescence increases (ΔF/F0) for l-LNv CCh data shown in A and for an identical range of acetylcholine (ACh) treatments. Asterisks indicate a mean maximum fluorescence change that was significantly different from the change evoked by Veh perfusion (P < 0.05 by Kruskal-Wallis ANOVA and Dunn's multiple comparisons test). For ACh treatments, nveh = 29,9; n10−5 = 21,7; n10−4 = 21,7; n10−3 = 24,8; n10−2 = 32,11. C: mean ± SE GCaMP3.0 fluorescence traces recorded from single small (s)-LNv somata from brains treated with 30-s perfusions of various CCh concentrations and Veh. Scale bars in C also apply to A. Sample sizes were nveh = 24,9; n10−5 = 15,5; n10−4 = 15,5; n10−3 = 15,5; n10−2 = 13,6. D: summary of mean maximum GCaMP3.0 fluorescence increases for s-LNv CCh responses shown in C and for an identical range of ACh concentrations. Sample sizes for ACh treatments were nveh = 20,7; n10−5 = 18,7; n10−4 = 18,7; n10−3 = 21,8; n10−2 = 29,11. Asterisks indicate a mean maximum fluorescent intensity change that was significantly different from the change evoked by Veh perfusion (P < 0.05 by Kruskal-Wallis ANOVA and Dunn's multiple comparisons test). E: mean traces of GCaMP3.0 fluorescence recorded from single s-LNv somata from brains treated with 30-s (black bar) perfusions of 10−4 M CCh with (magenta; n = 9,5) or without (green; n = 10,5) the presence of 2 μM tetrodotoxin (TTX). F: mean maximum GCaMP3.0 fluorescence increases for s-LNv data shown in E. There was no significant difference in mean maximum fluorescence increases between the +TTX and −TTX conditions (P = 0.2775 by Mann-Whitney U-test). N.S., not significant.
We first tested the responses of single l-LNvs to 30-s perfusions of 10−5, 10−4, 10−3, and 10−2 M ACh and vehicle. Only ACh delivered at 10−2 M resulted in a significant increase in GCaMP3.0 fluorescence compared with vehicle controls (Fig. 1B). Given the fact that electrophysiological analysis of l-LNvs has established that l-LNvs are directly receptive to ACh (McCarthy et al. 2011), we wondered whether the apparent low sensitivity of these neurons to bath-applied ACh might be due to degradation or uptake of the transmitter within our largely intact brain preparation. We therefore tested the responses of single l-LNvs to 30-s perfusions of the cholinergic agonist carbamoylcholine (CCh) (Fig. 1, A and B). Perfusion of 10−5 M CCh did not result in significant Ca2+ responses compared with vehicle controls in l-LNvs, but CCh concentrations of 10−4 M and greater produced significant increases in GCaMP3.0 fluorescence [Fig. 1, A and B, and Supplemental Movie S1; P < 0.05 by Kruskal-Wallis 1-way ANOVA with Dunn's multiple comparisons test; for CCh: nveh = 21 neurons from 10 brains (21,10), n10−5 = 17,7; n10−4 = 27,11; n10−3 = 16,7; n10−2 =10,5; for ACh: nveh = 29,9; n10−5 = 21,7; n10−4 = 21,7; n10−3 = 24,8; n10−2 = 32,11].1 Thus the l-LNvs displayed Ca2+ increases consistent with neuronal excitation in response to bath-applied cholinergic agonists, with CCh eliciting much larger responses than ACh (Fig. 1B). These results indicated that GCaMP3.0 is suitable for the measurement of excitatory Ca2+ responses within single clock neuron somata of the explanted adult brain.
We next asked whether the s-LNvs of the adult brain also respond to cholinergic agonists. Figure 1C shows the mean GCaMP3.0 traces of s-LNvs to 30-s perfusions of 10−5, 10−4, 10−3, and 10−2 M CCh and vehicle. The s-LNvs displayed significant increases in GCaMP3.0 fluorescence in response to CCh concentrations of 10−4 M and higher (Fig. 1D and Supplemental Movie S1; P < 0.05 by Kruskal-Wallis 1-way ANOVA with Dunn's multiple comparisons test; for CCh: nveh = 24,9; n10−5 = 15,5; n10−4 = 15,5; n10−3 = 15,5; n10−2 = 13,6). The response of s-LNvs to 10−4 M CCh was markedly bimodal, with a large peak preceded by a smaller peak (Fig. 1C). This bimodality was evident in individual plots and was not due to differing latencies among individual neurons (data not shown). Higher doses of CCh caused responses that were less bimodal and dominated by a single large peak of GCaMP3.0 fluorescence. Like l-LNvs, the s-LNvs displayed stronger responses to CCh than to ACh (Fig. 1D; for ACh: nveh = 20,7; n10−5 = 18,7; n10−4 = 18,7; n10−3 = 21,8; n10−2 = 29,11).
Given the largely intact nature of our explanted brain preparation and the widespread use of ACh as an excitatory neurotransmitter in the fly brain, it is possible that some or even all of the responses of the s-LNvs to cholinergic agonists were indirect responses mediated by neuronal intermediaries. A widely used approach to determining whether a neurophysiological response is due to direct excitation employs the voltage-gated Na+ channel blocker TTX (Soderlund 2005). We therefore asked whether the Ca2+ response of s-LNvs to 10−4 M CCh persisted in the presence of 2 μM TTX, which has been used previously for electrophysiological and live imaging experiments on the explanted fly brain (McCarthy et al. 2011; Shang et al. 2011; Sheeba et al. 2008b). In the presence of TTX, s-LNvs displayed bimodal GCaMP3.0 fluorescence increases in response to 10−4 M CCh that were highly similar to those of −TTX controls (Fig. 1E). In fact, there was no significant difference in the magnitudes of the CCh-induced GCaMP3.0 fluorescence increases displayed by s-LNvs in the presence and absence of TTX, although the +TTX responses trended toward lower magnitudes (Fig. 1F; P = 0.2775 by Mann-Whitney U-test; n = 10,5 for −TTX and n = 9,5 for +TTX). Our results indicate that, like l-LNvs, s-LNvs are directly excited by cholinergic agonists in the adult brain.
Nicotinic ACh receptors mediate Ca2+ responses of s-LNvs to cholinergic agonists.
Previous studies have shown that nicotinic receptors mediate the cholinergic response of adult l-LNvs (McCarthy et al. 2011). Furthermore, work in the dissociated larval central nervous system (CNS) indicates that larval s-LNvs express nicotinic ACh receptors (Dahdal et al. 2010; Wegener et al. 2004), suggesting that adult s-LNvs will likewise express nicotinic receptors. We therefore measured the effects of bath-applied nicotine on Ca2+ dynamics within single LNvs. Thirty-second perfusions of nicotine evoked clear increases in GCaMP3.0 fluorescence in both l- and s-LNvs (Fig. 2, A, B, E, and F; P < 0.05 for 10−4 M CCh in l-LNvs and for 10−5 and 10−4 M CCh in s-LNvs by Kruskal-Wallis 1-way ANOVA and Dunn's multiple comparisons test; for l-LNvs, nveh = 12,5; n10−5 = 12,5; n10−4 = 12,5; n10−3 =12,5; for s-LNvs, nveh = 12,5; n10−5 = 12,5; n10−4 = 13,5; n10−3 = 12,5). Thus both l- and s-LNvs displayed excitatory Ca2+ increases in response to nicotine.
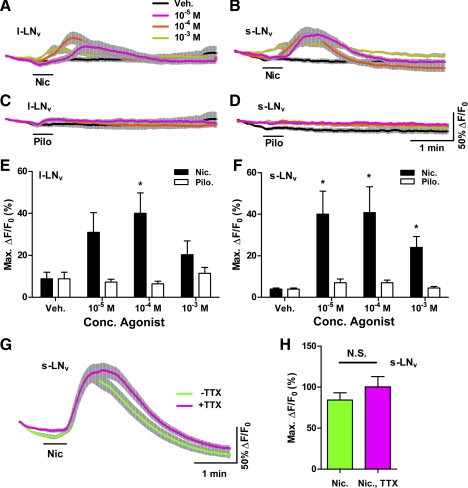
Fig. 2.
A nicotinic but not a muscarinic ACh receptor agonist causes Ca2+ increases in adult LNvs. A and B: mean ± SE GCaMP3.0 fluorescence traces recorded from single l-LNv (A) and s-LNv (B) somata from brains treated with 30-s (black bar) perfusions of 10−5, 10−4, and 10−3 M nicotine (Nic) and vehicle (Veh). For A: nveh = 12 neurons from 5 brains (12,5); n10−5 = 12,5; n10−4 = 12,5; n10−3 = 12,5. For B: nveh = 12,5; n10−5 = 12,5; n10−4 = 13,5; n10−3 = 12,5. Key in A also applies to B–D. C and D: mean ± SE GCaMP3.0 fluorescence traces recorded from single l-LNv (C) and s-LNv (D) somata in response to 30-s (black bar) perfusions of 10−5, 10−4, and 10−3 M pilocarpine (Pilo) and Veh. For C, nveh = 12; n10−5 = 12,5; n10−4 = 12,5; n10−3 = 12,5. For D, nveh= 12,5; n10−5 = 12,5; n10−4 = 12,5; n10−3 = 12,5. E: summary of mean ± SE maximum GCaMP3.0 fluorescence increases displayed by l-LNvs in response to Veh, Nic, and Pilo based on data shown in A and C. F: summary of mean ± SE maximum GCaMP3.0 fluorescence increases displayed by s-LNvs in response to Veh, Nic, and Pilo based on data in B and D. For E and F, asterisks indicate a mean maximum fluorescence increase that was significantly different from the change evoked by Veh (P < 0.05, by Kruskal-Wallis ANOVA and Dunn's multiple comparisons test). G: mean ± SE GCaMP3.0 fluorescence traces recorded from single s-LNv somata from brains treated with 30-s (black bar) perfusions of 10−4 M Nic in the presence (magenta; n = 27,10) or absence (green; n = 25,8) of 2 μM TTX. H: summary of mean maximum GCaMP3.0 fluorescence increases displayed by s-LNvs in response to 10−4 M Nic in the presence (magenta) or absence (green) of TTX based on data shown in G. There was no significant difference in mean maximum fluorescence increases between the +TTX and −TTX conditions (P = 0.3793 by Mann-Whitney U-test).
We wondered whether the activation of muscarinic ACh receptors might also have contributed to the LNv Ca2+ responses to CCh. We therefore asked whether bath application of the muscarinic agonist pilocarpine had measurable effects on Ca2+ dynamics in l- and s-LNvs. Thirty-second perfusions of 10−5 to 10−3 M pilocarpine had no significant effects on GCaMP3.0 fluorescence in either l- or s-LNvs (Fig. 2, C–F; P > 0.05 for all pairwise comparisons of pilocarpine treatments with vehicle control by Kruskal-Wallis 1-way ANOVA with Dunn's multiple comparisons test; for l-LNvs, nveh = 12,5; n10−5 = 12,5; n10−4 = 12,5; n10−3 = 12,5; for s-LNvs, nveh = 12,5; n10−5 = 12,5; n10−4 = 12,5; n10−3 = 12,5). Thus the muscarinic agonist had no measurable effects on the Ca2+ dynamics of l- and s-LNvs, consistent with previous work on dissociated larval LNvs indicating that these neurons are not sensitive to pilocarpine (Wegener et al. 2004).
We next confirmed that the effects of nicotine on Ca2+ in the s-LNvs were direct by comparing the GCaMP3.0 responses of s-LNvs treated with 10−4 M nicotine in the presence or absence of 2 μM TTX. Under both conditions, 30-s perfusions of nicotine caused clear increases in GCaMP3.0 fluorescence whose maximum changes were not significantly different (Fig. 2, G and H; for −TTX, n = 25,8; for +TTX n = 27,10; P = 0.3793 by Mann-Whitney U-test). Thus s-LNvs are directly receptive to nicotine, further supporting the conclusion that these critical pacemaker neurons express nicotinic ACh receptors in the adult brain.
Carbamoylcholine causes acute cAMP increases in ventrolateral neurons.
G protein and cAMP signaling is critical for the maintenance of normally paced circadian rhythms in animals (Dahdal et al. 2010; O'Neill et al. 2008). We therefore wondered whether cholinergic excitation of adult LNvs had acute effects on cAMP signaling. To further characterize the nature of the cholinergic responses in these neurons, we used the genetically encoded cAMP sensor Epac1-camps to measure the effects of bath-applied cholinergic agonists on cAMP signaling. Figure 3A shows the mean inverse Epac1-camps FRET traces for single l-LNvs treated with 30-s perfusions of 10−5, 10−4, 10−3, and 10−2 M CCh and vehicle. Concentrations of 10−4 and 10−3 M CCh evoked significant increases in Epac1-camps FRET ratio in l-LNvs compared with vehicle controls (Fig. 3C; P < 0.05 by Kruskal-Wallis 1-way ANOVA with Dunn's multiple comparisons test; nveh = 15,6; n10−5 = 17,7; n10−4 = 17,7; n10−3 = 17,7; n10−2 = 17,7). The Epac1-camps responses to CCh appeared to be biphasic, with a small and brief decrease in inverse FRET followed by a large increase in the inverse FRET ratio (Fig. 3A). However, this initial loss of inverse FRET may simply have been a reflection of a steady loss of inverse FRET, which was apparent in the vehicle controls, preceding the large CCh-induced cAMP increase (Fig. 3A). The 10−2 M CCh treatment did not result in significant cAMP increases compared with vehicle controls, consistent with previous work in dissociated larval neurons, which revealed decreased Ca2+ responses at very high ACh doses (Wegener et al. 2004) (Fig. 3, A and C).
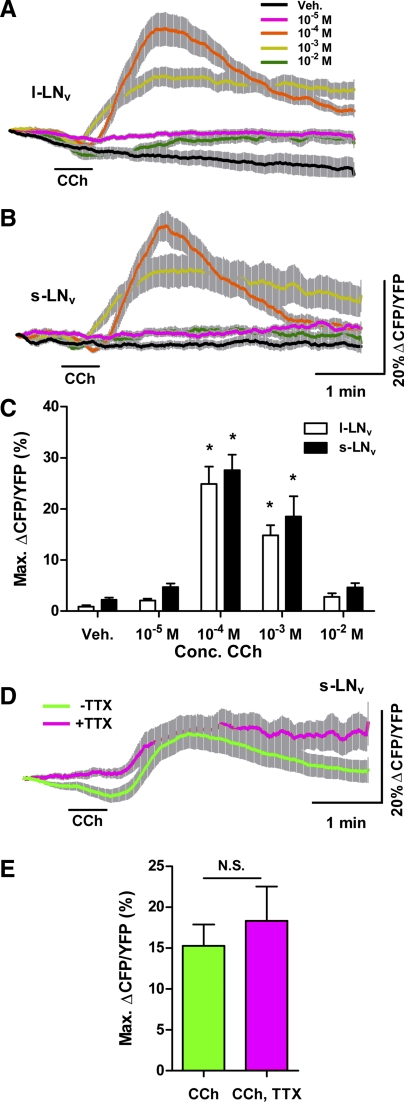
Fig. 3.
The general cholinergic agonist CCh causes cAMP increases in adult LNvs. A and B: mean ± SE inverse Epac1-camps FRET traces recorded from single l-LNv (A) and s-LNv (B) somata from brains treated with 30-s perfusions of 10−5, 10−4, 10−3, and 10−2 M CCh. Scale bars in B also apply to A. For A, nveh = 15 neurons from 6 brains (15,6); n10−5 = 17,7; n10−4 = 17,7; n10−3 = 17,7; n10−2 =17,7. For B, nveh = 12,5; n10−5 = 15,6; n10−4 = 15,6; n10−3 = 15,6; n10−2 = 15,6. Key in A also applies to B. CFP, cyan fluorescent protein; YFP, yellow fluorescent protein. C: summary of mean ± SE maximum increases in inverse Epac1-camps FRET from l- and s-LNvs data displayed in A and B. Asterisks indicate a mean maximum inverse FRET ratio change that was significantly different from the ratio change evoked by Veh (P < 0.05 by Kruskal-Wallis ANOVA and Dunn's multiple comparisons test). D: mean inverse Epac1-camps FRET traces for single s-LNv somata from brains treated with 30-s perfusions of 10−4 M CCh in the presence (magenta; n = 25,10) or absence (green; n = 34,10)of 2 μM TTX. E: summary of mean maximum increases in inverse Epac1-camps FRET based on s-LNv data displayed in D. There was no significant difference in maximum inverse FRET change between the +TTX and −TTX conditions (P = 0.4074 by Mann-Whitney U-test).
The s-LNvs displayed a highly similar pattern of Epac1-camps responses to perfusions of 10−5, 10−4, 10−3, and 10−2 M CCh (Fig. 3B). Concentrations of 10−4 and 10−3 M CCh evoked significant increases in Epac1-camps FRET ratio in s-LNvs compared with vehicle controls (Fig. 3C; P < 0.05 by Kruskal-Wallis 1-way ANOVA with Dunn's multiple comparisons test; nveh = 12,5; n10−5 = 15,6; n10−4 = 15,6; n10−3 = 15,6; n10−2 = 15,6). Like l-LNvs, the s-LNvs displayed biphasic responses to 10−4 and 10−3 M CCh and failed to display significant responses to the 10−2 M treatment relative to vehicle controls (Fig. 3B; P > 0.05 by Kruskal-Wallis with Dunn's multiple comparisons test). For s-LNvs the bimodality of the cAMP response was less equivocal than that of l-LNvs, as the vehicle controls displayed no obvious loss of inverse FRET immediately following perfusion. We do not know the cellular basis for this bimodality or how it might relate to the bimodality observed in the s-LNv Ca2+ response to 10−4 M CCh (Fig. 1C), but it might reflect the nature of the coupling of acute ionotropic excitation to cAMP increases in these neurons. Nevertheless, these results suggest that cholinergic excitation is coupled to cAMP increases in the l- and s-LNvs of the adult brain.
To confirm that the cAMP responses of the critical s-LNv pacemakers were in fact a direct response to CCh and not an indirect modulatory effect of other CCh-stimulated pathways, we asked whether CCh-induced cAMP increases in s-LNvs persisted in the presence of TTX. Indeed, the cAMP responses of s-LNvs to CCh was direct, as the increase in inverse Epac1-camps FRET caused by 10−4 M CCh persisted in the presence of 2 μM TTX (Fig. 3, D and E). There was no significant difference in the maximum increases in inverse Epac1-camps FRET between the +TTX and −TTX conditions (Fig. 3E; P = 0.4074 by Mann-Whitney U-test; n = 25,10 for +TTX and n = 34,10 for −TTX). These results indicate that direct cholinergic excitation is coupled to cAMP increases in the critical s-LNv pacemakers of the adult brain.
Nicotinic ACh receptors mediate cAMP responses of s-LNvs to cholinergic agonists.
The finding that cholinergic excitation is coupled to cAMP increases in the LNvs has important implications for how the molecular clock within these neurons might be affected by synaptic inputs, as cAMP signaling is thought to be a central component of circadian timekeeping in animals (Levine et al. 1994; O'Neill et al. 2008). We therefore sought to characterize the cholinergic stimulation of cAMP in the LNvs by pharmacologically addressing the nature of the ACh receptors that underlie it. Bath-applied 10−4 and 10−5 M nicotine, doses that caused consistent Ca2+ responses in LNvs (Fig. 2, A and B), also caused inverse Epac1-camps FRET increases in both l- and s-LNvs (Fig. 4, A and B). The l-LNvs also responded to 10−5 M nicotine (Fig. 4A). Because of constantly increasing inverse FRET levels in vehicle-treated and nonresponding neurons, our conservative statistical analysis indicated significant cAMP responses only at the 10−4 M dose for l-LNvs and at the 10−5 M dose for s-LNvs (Fig. 4, E and F; P < 0.05 for these 2 treatments vs. vehicle controls by Kruskal-Wallis 1-way ANOVA and Dunn's multiple comparisons test, P > 0.05 for all remaining doses of nicotine vs. vehicle perfusion; for l-LNvs, nveh = 18,5; n10−5 = 18,5; n10−4 = 18,5; n10−3 = 17,5; for s-LNvs, nveh = 14,5; n10−5 = 14,5; n10−4 = 14,5; n10−3 = 14,5). The steady increase in the inverse Epac1-camps FRET observed for vehicle controls was due to an uneven photobleaching of YFP and CFP, with greater bleaching for the former fluorophore, as has been reported in previous studies using Epac1-camps (Börner et al. 2011). It was not clear why this occurred in some experiments but not others. Nevertheless, these results indicate that bath-applied nicotine causes increases in cAMP in l- and s-LNvs.
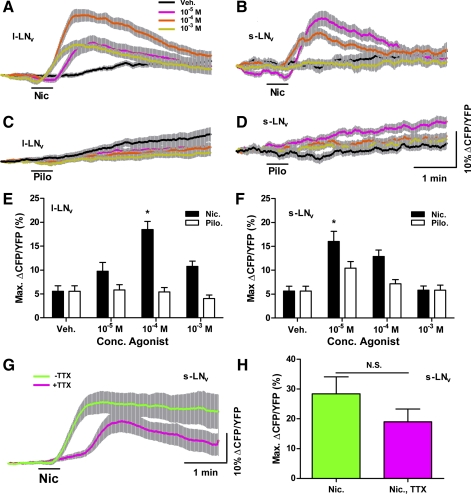
Fig. 4.
A nicotinic but not a muscarinic receptor agonist causes cAMP increases in adult LNvs. A and B: mean ± SE inverse Epac1-camps FRET traces recorded from single l-LNv (A) and s-LNv (B) somata from brains treated with 30-s perfusions (black bar) of 10−5, 10−4, and 10−3 M nicotine (Nic) and vehicle (Veh). For A, nveh = 18 neurons from 5 brains (18,5); n10−5 = 18,5; n10−4 = 18,5; n10−3 = 17,5. For B, nveh = 14,5; n10−5 = 14,5; n10−4 = 14,5; n10−3 = 14,5. Key in A also applies to B–D. C and D: mean ± SE inverse Epac1-camps FRET traces recorded from single l-LNv (C) and s-LNv (D) somata from brains treated with 30-s perfusions of 10−5, 10−4, and 10−3 M pilocarpine (Pilo) and Veh. For C, nveh = 18,5; n10−5 = 21,6; n10−4 = 18,5; n10−3 = 18,5. For D, nveh = 14,5; n10−5 = 14,5; n10−4 = 14,5; n10−3 = 14,5. Scale bars in D apply to A–D. E: summary of mean ± SE maximum increases in inverse Epac1-camps FRET for l-LNv responses to Nic, Pilo, and Veh shown in A and C. F: summary of mean maximum increases in inverse Epac1-camps FRET for s-LNv responses to Nic, Pilo, and Veh shown in B and D. For E and F, asterisks indicate a mean maximum change in inverse FRET that was significantly different from the change evoked by Veh (P < 0.05, by Kruskal-Wallis ANOVA and Dunn's multiple comparisons test). G: mean ± SE inverse Epac1-camps FRET traces recorded from single s-LNv somata from brains treated with 30-s perfusions of 10−4 M Nic in the presence (magenta; n = 14,6) or absence (green; n = 13,6) of 2 μM TTX. H: summary ± SE of mean maximum increases in inverse FRET displayed by s-LNvs based on data in G. Mean maximum increase in inverse FRET did not differ significantly between the +TTX and −TTX conditions (P = 0.2541 by Mann-Whitney U-test).
We wondered whether the activation of muscarinic ACh receptors might also have contributed to the LNv cAMP responses to CCh (Fig. 3, A–C). We therefore tested the effects of pilocarpine perfusion on inverse Epac1-camps FRET levels in l- and s-LNvs. Thirty-second perfusions of 10−5 to 10−3 M pilocarpine had no measurable effects on inverse Epac1-camps FRET in l- or s-LNvs (Fig. 4, C–F; P > 0.05 for all comparisons of pilocarpine dose and vehicle control by Kruskal-Wallis 1-way ANOVA and Dunn's multiple comparisons test; for l-LNvs, nveh = 18,5; n10−5 = 21,6; n10−4 = 18,5; n10−3 = 18,5; for s-LNvs, nveh = 14,5; n10−5 = 14,5; n10−4 = 14,5; n10−3 = 14,5). These results suggest that muscarinic pathways do not significantly affect cAMP levels in adult LNvs.
To confirm that the cAMP responses of s-LNvs to nicotine were direct responses rather than indirect modulatory effects of other nicotine-sensitive pathways, we asked whether nicotine-induced cAMP increases in s-LNvs persisted in the presence of TTX. Indeed, the cAMP responses of s-LNvs to nicotine were direct, as significant increases in inverse Epac1-camps FRET in response to 30-s perfusions of 10−4 M nicotine persisted in the presence of 2 μM TTX (Fig. 4, G and H). The s-LNvs appeared to display slightly smaller and briefer cAMP responses in the presence of toxin (Fig. 4G), but a statistical comparison of the maximum changes in inverse Epac1-camps FRET displayed in the +TTX and −TTX conditions revealed no significant differences in the mean maximum increases (Fig. 4H; P = 0.2541 by Mann-Whitney U-test; n = 14,6 for +TTX and n = 13,6 for −TTX). Taken together, our results indicate that the direct activation of nicotinic ACh receptors is coupled to cAMP increases in s-LNvs of the adult brain.
GABA antagonizes cholinergic excitation in adult LNvs.
We next tested the ability of the GCamp3.0 and Epac1-camps sensors to detect inhibitory responses in single adult LNv soma. The l-LNvs express the GABAA receptor RDL, an inhibitory ligand-gated Cl− channel (Chung et al. 2009; Parisky et al. 2008), and electrophysiological analysis has established that bath application of GABA inhibits l-LNv action potentials, likely via GABAA receptors (McCarthy et al. 2011). We therefore asked whether GCaMP3.0 imaging could detect acute GABAergic inhibition in l-LNvs. Thirty-second perfusions of GABA alone at concentrations of 10−4, 10−3, and 10−2 M had no significant effects on GCaMP3.0 fluorescence compared with vehicle controls (Fig. 5, A and C; P > 0.05 for all 3 concentrations by Kruskal-Wallis 1-way ANOVA and Dunn's multiple comparisons test; nveh = 14,5; n10−4 = 14,5; n10−3 = 14,5; n10−2 = 14,5). It is possible that GABA alone might not alter Ca2+ levels significantly, particularly if Ca2+ was already at or near baseline levels, or that GCaMP3.0 is insufficiently sensitive to detect inhibitory effects on intracellular Ca2+. We wondered whether GCaMP3.0 could detect GABAergic inhibition when GABA is coapplied with an excitatory stimulus, as has been shown previously for dissociated larval LNvs (Dahdal et al. 2010). We therefore tested whether coapplication of GABA with CCh would reduce or abolish the direct CCh response of l-LNvs. In the presence of 2 μM TTX, coapplication of 10−3 M GABA and 10−4 M CCh significantly reduced the Ca2+ response to CCh by the l-LNv (Fig. 5, D and F; P = 0.0096 by Mann-Whitney U-test; nCCh = 12,5 and nCCh&GABA = 12,5).
Fig. 5.
GABA alone has no acute effects on GCaMP3.0 fluorescence in adult LNvs but inhibits their CCh-induced Ca2+ responses. A and B: mean ± SE GCaMP3.0 fluorescence traces recorded from single l-LNv (A) and s-LNv (B) somata from brains treated with 30-s (black bar) perfusions of 10−4, 10−3, and 10−2 M GABA and vehicle (Veh). Scale bars in B apply to both A and B. For A, nveh = 14 neurons from 5 brains (14,5); n10−4 = 14,5; n10−3 = 14,5; n10−2 = 14,5. For B, nveh = 17,5; n10−4 = 17,5; n10−3 = 17,5; n10−2 = 17,5. Key in A also applies to B. C: summary of mean maximum GCaMP3.0 fluorescence changes for l-LNv and s-LNv responses to GABA. Values are based on data shown in A and B. There was no significant difference between the effects of any concentration of GABA perfusion and Veh controls (P > 0.05 by Kruskal-Wallis ANOVA and Dunn's multiple comparisons test). D and E: mean ± SE GCaMP3.0 fluorescence traces recorded from single l-LNv (D) and s-LNv (E) somata from brains treated with 30-s perfusions of 10−4 M CCh with (magenta) and without (green) coapplication of 10−3 M GABA. These experiments were conducted in the presence of 2 μM TTX. Sample sizes were nCCh = 12,5 and nCCh&GABA = 12,5 for D and nCCh = 11,5 and nCCh&GABA = 11,5 for E. F: summary of mean ± SE maximum GCaMP3.0 fluorescence intensity increases recorded from l-LNv and s-LNv in response to 10−4 M CCh with or without coapplication of 10−3 M GABA in the presence of 2 μM TTX. Values are based on data shown in D and E. Asterisks indicate a significant difference in mean maximum CCh-induced increase in GCaMP3.0 fluorescence recorded in the presence or absence of GABA (Pl-LNv = 0.0096, Ps-LNv = 0.0122 by Mann-Whitney U-test).
We next examined GABA's effects on s-LNvs, whose physiological responses to GABA have not been previously addressed in the adult brain. As for l-LNvs, perfusion of GABA alone had no significant effects on GCaMP3.0 fluorescence in s-LNvs (Fig. 5, B and C; P > 0.05 for all 3 concentrations by Kruskal-Wallis 1-way ANOVA and Dunn's multiple comparisons test; nveh = 17,5; n10−4 = 17,5; n10−3 = 17,5; n10−2 = 17,5), but coapplication of GABA and CCh revealed that GABA significantly reduced the s-LNv response to CCh in the presence of 2 μM TTX (Fig. 5, E and F: P = 0.0122 by Mann-Whitney U-test; nCCh = 11,5 and nCCh&GABA = 11,5). Thus, as predicted by studies on dissociated larval LNvs (Dahdal et al. 2010), adult s-LNvs were directly inhibited by bath-applied GABA.
GABA causes acute reduction in cAMP levels in adult LNvs.
There is evidence for the expression of both ionotropic GABAA receptors and metabotropic GABAB receptors by the PDF-positive l- and s-LNvs (Chung et al. 2009; Dahdal et al. 2010; Hamasaka et al. 2007; Parisky et al. 2008). In mammals GABAB receptors are negatively coupled to adenylate cyclase through Go signaling, and Drosophila GABAB receptors expressed in mammalian cell lines can elicit decreases in cAMP (Bettler et al. 2004; Mezler et al. 2001). If GABAB receptors signal through Go in Drosophila and are indeed present in the LNvs, we would predict that GABA application would result in a reduction in cAMP levels. Thirty-second perfusions of GABA alone at 10−4 to 10−2 M resulted in significant reductions in inverse Epac1-camps FRET in l-LNvs relative to vehicle controls, consistent with a GABA-induced loss of cAMP in these neurons (Fig. 6, A and C; P < 0.05 for these concentrations compared with vehicle by Kruskal-Wallis 1-way ANOVA and Dunn's multiple comparisons test; nveh = 30,11; n10−4 = 17,6; n10−3 = 17,6; n10−2 = 17,7). Likewise, 30-s perfusions of 10−3 M GABA resulted in significant decreases in inverse Epac1-camps FRET in s-LNvs (Fig. 6, B and C; P < 0.05 for this dose compared with vehicle by Kruskal-Wallis 1-way ANOVA and Dunn's multiple comparisons test, P > 0.05 for the remaining concentrations; nveh = 26,11; n10−4 = 26,11; n10−3 = 14,5; n10−2 = 11,5). The GABA-induced loss of inverse FRET displayed by l- and s-LNvs was due to a reciprocal increase in YFP and decreases in CFP emission (i.e., caused by bona fide FRET changes) and not due to uneven photobleaching (data not shown).
Fig. 6.
GABA causes cAMP decreases in adult LNvs. A and B: mean ± SE inverse Epac1-camps FRET traces recorded from single l-LNv (A) and s-LNv (B) somata from brains treated with 30-s perfusions (black bar) of 10−4, 10−3, and 10−2 M GABA. Scale bars in B also apply to A. For A, nveh = 30 neurons from 11 brains (30,11); n10−4 = 17,6; n10−3 = 17,6; n10−2 = 17,7. For B, nveh = 26,11; n10−4 = 26,11; n10−3 = 14,5; n10−2 = 11,5. Key in A also applies to B. C: summary of mean ± SE maximum decreases in inverse Epac1-camps FRET recorded from l- and s-LNvs in response to GABA perfusion. Values are based on data shown in A and B. Asterisks indicate a maximum decrease in inverse Epac1-camps FRET that was significantly different from the maximum decrease evoked by Veh (P < 0.05 by Kruskal-Wallis ANOVA and Dunn's multiple comparisons test). D: mean ± SE inverse Epac1-camps FRET traces recorded from single s-LNv somata from brains treated with 30-s perfusions (black bar) of 10−3 M GABA in the presence (magenta; n = 34,12) or absence (green; n = 30,11) of 2 μM TTX. E: summary of mean maximum decreases in inverse Epac1-camps FRET displayed by s-LNvs in response to 10−3 M GABA in the presence or absence of 2 μM TTX. Values are based on data displayed in D. There was no significant difference in mean maximum loss of inverse Epac1-camps FRET between the +TTX and −TTX conditions (P = 0.0503 by Mann-Whitney U-test).
Although both classes of LNv neurons responded to GABA with cAMP decreases, the character of their responses differed. The l-LNv cAMP response was relatively large and long lasting, with cAMP levels remaining low for the duration of the 10-min time course experiment (Fig. 6A). The s-LNv response to 10−3 M GABA, in contrast, was relatively small, and transient compared with the l-LNv responses (compare Fig. 6, A and B). Furthermore, in contrast to l-LNvs, s-LNvs displayed no apparent response to 10−2 M GABA, whereas l-LNvs displayed large cAMP decreases in response to this dose (compare Fig. 6, A and B). These results suggest that bath-applied GABA causes significant reductions of cAMP in both l- and s-LNvs and that these two neuronal classes may differ in their transduction of GABAergic input.
Anatomical and genetic evidence suggests that the responses of s-LNvs to GABAergic inputs are the result of the direct effects of agonist binding receptors expressed by s-LNvs (Chung et al. 2009; Dahdal et al. 2010; Hamasaka et al. 2007; Parisky et al. 2008). We therefore asked whether GABA-induced cAMP decreases persisted in s-LNvs in the presence of 2 μM TTX. Thirty-second perfusions of 10−3 M GABA produced clear decreases in inverse Epac1-camps FRET in the presence of TTX with magnitudes that were not significantly different from those displayed in the absence of toxin (Fig. 6, D and E; P = 0.0503 by Mann-Whitney U-test; n = 34,12 for +TTX and n = 30,11 for −TTX). We conclude that GABA's inhibitory effect on cAMP in the s-LNvs was direct.
GABA antagonizes cholinergic cAMP increases in s-LNvs.
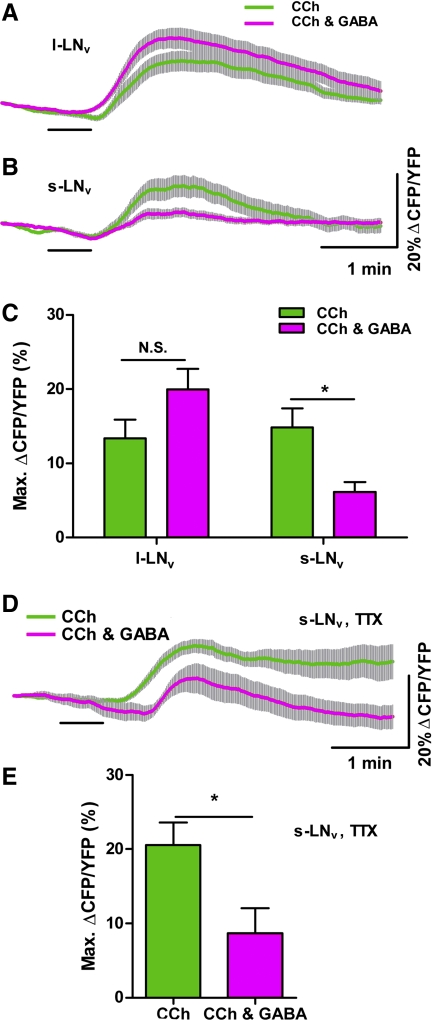
Our GCaMP3.0 imaging experiments indicated that GABA antagonizes CCh's effects on Ca2+ in l- and s-LNvs. We next asked whether GABA also inhibits the excitatory cAMP increases caused by CCh in these neurons. Coapplication of 10−3 M GABA with 10−4 M CCh did not significantly reduce the CCh-induced increase in cAMP in the l-LNv (Fig. 7, A and C; P = 0.0915 by Mann-Whitney U-test; nCCh = 17,5 and nCCh&GABA = 17,5), although in some single-neuron traces a residual decrease in cAMP that resembled an inhibitory cAMP response to GABA was observed following the excitatory CCh response (data not shown). In contrast, coapplication of 10−3 M GABA significantly reduced the cAMP response to 10−4 M CCh in s-LNvs (Fig. 7, B and C; P = 0.0048 by Mann-Whitney U-test; nCCh = 21,7 and nCCh&GABA = 21,8). The inhibitory effect of GABA on CCh-induced cAMP increases was direct, as coapplication of 10−3 M GABA significantly reduced the magnitude of the cAMP response to CCh in the presence of 2 μM TTX (Fig. 7, D and E; P = 0.0173 by Mann-Whitney U-test; nCCh,TTX = 12,5 and nCCh&GABA,TTX = 9,5). These results suggest that GABAergic input directly antagonizes the effects of cholinergic input on cAMP levels within the s-LNvs in adult brain.
Fig. 7.
GABA inhibits CCh-induced cAMP increases in s-LNvs but not in l-LNvs. A and B: mean ± SE inverse Epac1-camps FRET ratio traces recorded from single l-LNv (A) and s-LNv (B) somata from brains treated with 30-s perfusions of 10−4 M CCh applied with (magenta) or without (green) 10−3 M GABA. Scale bars in B also apply to A. For A, nCCh = 17 neurons from 5 brains (17,5); nCCh&GABA = 17,5. For B, nCCh = 21,7; nCCh&GABA = 21,8. Key in A also applies to B. C: summary of mean ± SE maximum inverse Epac1-camps FRET increases recorded in l- and s-LNvs in response to 10−4 M CCh applied alone (green) or with 10−3 M GABA (magenta). Values are based on data displayed in A and B. GABA did not significantly change the cAMP response to CCh in l-LNvs (P = 0.0915 by Mann-Whitney U-test). GABA significantly reduced the CCh-induced the cAMP response in s-LNvs (*P = 0.0048 by Mann-Whitney U-test). D: mean ± SE inverse Epac1-camps FRET traces recorded from single s-LNv somata from brains treated with 30-s perfusions of 10−4 M CCh applied with (magenta; n = 9,5) or without (green; n = 12,5) 10−3 M GABA in the presence of 2 μM TTX. E: summary of mean ± SE maximum increases in inverse Epac1-camps FRET displayed by s-LNvs in response to 10−4 M CCh applied with (magenta) or without (green) 10−3 M GABA in the presence of TTX. Values are based on data displayed in D. GABA significantly reduced the CCh-induced cAMP response of s-LNvs in the presence of toxin (*P = 0.0173 by Mann-Whitney U-test).
DISCUSSION
In Drosophila, circadian timekeeping appears to be distributed across multiple classes of clock neuron and is thought to depend on physiological interactions between them (Grima et al. 2004; Lin et al. 2004; Peng et al. 2003; Picot et al. 2007; Rieger et al. 2006; Stoleru et al. 2004, 2005). Likewise, the synchronization of the circadian clock neuron network to the environment is maintained, at least in part, by inputs from peripheral sensory receptors, which must ultimately modulate the physiology and molecular timekeeping of the clock neuron network (Helfrich-Förster 2002; Helfrich-Förster et al. 2001; Klarsfeld et al. 2004; Sehadova et al. 2009). However, the physiological basis of clock network timekeeping, its modulation by sensory inputs, and the neurotransmitters employed by these pathways are largely unknown in the adult fly brain. The electrophysiological inaccessibility of much of the fly's circadian clock neuron network is a fundamental barrier to understanding the neural basis of timekeeping in the fly. Recently developed genetically encoded sensors for Ca2+ and cAMP signaling make possible the measurement of physiological responses in electrophysiologically inaccessible neurons of the clock network. Here we have tested the performance of two of these sensors, the Ca2+ sensor GCaMP3.0 and the cAMP sensor Epac1-camps, within single deeply situated adult clock neurons and have used them to test the predicted effects of ACh and GABA on the critical s-LNv pacemakers of the adult circadian clock neuron network.
Testing the sensitivity of genetically encoded sensors in single adult clock neuron somata.
The l-LNvs are the only neurons of the adult clock neuron network that have been routinely analyzed electrophysiologically in the intact adult brain (Cao and Nitabach 2008; Fogle et al. 2011; McCarthy et al. 2011; Park and Griffith 2006; Sheeba et al. 2008b). We therefore tested the performance of GCaMP3.0 and Epac1-camps within the l-LNv in response to modulators with established excitatory and inhibitory effects on these neurons. For single l-LNvs, we find that GCaMP3.0 readily detects acute responses to excitatory agonists but fails to detect acute ionotropic inhibitory responses. Given the strong coupling of neuronal excitation with Ca2+ entry, it was not surprising that GCaMP3.0 could detect excitatory responses within single l-LNvs. In contrast, the suitability of genetically encoded Ca2+ sensors for the measurement of acute inhibitory responses seems less straightforward. Nevertheless, GCaMP sensors can be used to detect inhibitory effects in the presence of excitation, as previously reported (Dahdal et al. 2010; Ignell et al. 2009).
Previous experiments on dissociated larval LNvs found that GABA application resulted in significant decreases in Ca2+ as reported by fura-2 imaging (Hamasaka et al. 2005). We suspect that this might reflect the high sensitivity of the synthetic Ca2+ dye relative to genetically encoded sensors. Indeed, experiments using an earlier version of GCaMP in a similar dissociated larval preparation were unable to detect these inhibitory responses to GABA (Dahdal et al. 2010). We therefore suspect that the Ca2+ changes induced by GABA application are too low for detection by GCaMP3.0 and that coapplication of putative inhibitors with excitatory agonists is necessary to address inhibition when using this sensor. We wonder if newly developed Cl− sensors (Markova et al. 2008; Waseem et al. 2010) might be more suitable than GCaMP3.0 for the detection of such acute inhibitory input within deep networks of the fly brain. In contrast to our results with GCaMP3.0 and Ca2+ signaling, we found that Epac1-camps was capable of detecting inhibitory effects on cAMP signaling. However, it is important to note that despite the fact that Epac1-camps is nearly 10 times more sensitive to cAMP than to cyclic guanosine monophosphate (cGMP) in vitro (Nikolaev et al. 2004), it appears to be sensitive to physiologically relevant cGMP levels in fly neurons (Shakiryanova and Levitan 2008).
The l-LNv neurons displayed much stronger responses to CCh compared with ACh (Fig. 1). The relatively intact nature of our brain preparation may account for the relatively high efficacy of CCh in our study. ACh, a widespread excitatory neurotransmitter in the insect CNS, is tightly regulated in the intact brain via degradation by acetylcholinesterases (Treherne and Smith 1965a, 1965b). Consistent with this notion, previous studies of cholinergic responses in another dipteran CNS have reported a relatively high efficacy of CCh relative to ACh (Brotz et al. 1995).
Testing predicted physiological responses of s-LNvs to cholinergic and GABAergic stimulation.
Having characterized the sensitivity of GCaMP3.0 and Epac1-camps for imaging excitatory and inhibitory responses within the physiologically well-studied l-LNvs, we turned our attention to the s-LNvs. Unlike l-LNvs, these neurons are critical for the maintenance of circadian rhythms and are thought to play a central role in the synchronization of the various clock neuron classes (Grima et al. 2004; Rieger et al. 2006; Shafer and Taghert 2009; Stoleru et al. 2004). Understanding the physiological basis of s-LNv function is therefore critical to our understanding the clock network. The small diameters and deep brain locations of the s-LNvs have made electrophysiological analysis challenging (Cao and Nitabach 2008). We therefore sought to test the predicted effects of cholinergic and GABAergic agonists on s-LNvs using genetically encoded sensors.
Our results indicate that s-LNvs maintain their receptivity to cholinergic agonists via nicotinic ACh receptors, as predicted by previous work on the dissociated larval CNS (Dahdal et al. 2010; Wegener et al. 2004). Ca2+ and cAMP responses of s-LNvs to nicotine persisted in the presence of TTX, indicating that s-LNvs maintain direct receptivity to cholinergic input through nicotinic receptors after metamorphosis. Furthermore, we have found no evidence for sensitivity to muscarinic agonists by l- and s-LNvs. CCh- and nicotine-induced Ca2+ and cAMP responses displayed similar time courses, suggesting a strong coupling of excitation and cAMP production in these neurons. Given the important role that cAMP signaling plays in the maintenance of circadian rhythms (Levine et al. 1994; O'Neill et al. 2008), these results suggest that cholinergic modulation of s-LNvs might contribute to the entrainment of the molecular circadian rhythms of these neurons through the modulation of cAMP levels.
ACh is a widespread excitatory neurotransmitter in the fly brain and serves as the transmitter of most sensory neurons (Buchner et al. 1986; Gorczyca and Hall 1987; Nässel 1991). Thus, there are many potential sources for the cholinergic modulation of the s-LNvs. One prime candidate in this regard is input from the Hofbauer-Buchner eyelet (H-B eyelet), an extraretinal light input pathway that has been implicated in the entrainment of circadian rhythms to light-dark cycles (Helfrich-Förster et al. 2002; Malpel et al. 2002; Rieger et al. 2003). The axons of the H-B eyelet express choline acetyltransferase and project to the accessory medulla, where they terminate near s-LNv arborizations (Helfrich-Förster et al. 2002; Malpel et al. 2002; Yasuyama and Meinertzhagen 1999).
Our results also support the prediction, based on anatomical and genetic studies, that GABA inhibits adult s-LNvs (Chung et al. 2009; Dahdal et al. 2010; Hamasaka et al. 2005; Parisky et al. 2008). Furthermore, the acute GABAergic reduction of cAMP in s-LNvs is consistent with recent work suggesting that GABAB receptors expressed by these neurons are an important component of circadian timekeeping (Dahdal et al. 2010; Hamasaka et al. 2005). A recent report has shown that Go signaling is required for the GABAergic inhibition of dissociated larval LNvs and that Go signaling via phospholipase C in adult LNvs is critical for the maintenance of normally paced locomotor rhythms (Dahdal et al. 2010). GABAB receptors and Go signaling can also be negatively coupled to adenylate cyclase, as has been reported for Drosophila GABAB receptors in heterologous cell culture (Mezler et al. 2001), although some doubt has been cast upon the presence of such negative cAMP coupling in the fly brain (Dahdal et al. 2010; Ferris et al. 2006).
Like ACh, GABA is a widespread neurotransmitter in the adult fly brain (Küppers et al. 2003), and the potential sources of the GABAergic input to s-LNvs are manifold. GABA immunoreactivity is found in the accessory medulla where projections from the s-LNv are found (Hamasaka et al. 2005), and GABA is released by many neurons of the visual system (Nässel 1991). Interestingly, GABA is thought to modulate light input to the accessory medulla of the cockroach (Petri et al. 2002), suggesting that GABA might play a similar modulatory role in the fly through the modulation of the s-LNv response to input from external photoreceptors. One interesting possibility is that GABA might act to modulate the response of s-LNvs to cholinergic input coming from the H-B eyelet or other sensory inputs.
In conclusion, our results experimentally confirm the prediction—based on previously published experiments in the dissociated larval CNS and on genetic and anatomical experiments in adults—that s-LNvs are modulated antagonistically by cholinergic and GABAergic input. Importantly, our work establishes for the first time that this antagonism extends to cAMP signaling, which is known to be a central component of molecular timekeeping in animals (Levine et al. 1994; O'Neill et al. 2008). We therefore predict that acute cholinergic and GABAergic stimulation of s-LNvs have opposing effects on the phase and or period of the molecular clock within these critical neuronal pacemakers. Finally, the utility of the genetically encoded sensors used in this study within deeply situated adult clock neurons indicates that such physiological analysis can now be extended to the entire clock neuron network with these tools. These imaging methods, particularly when combined with the considerable genetic tools available in the fly, will be critical for advancing our understanding of circadian timekeeping in the brain.
GRANTS
This work was supported by a National Institutes of Health Pathway to Independence Award (National Institute of Neurological Disorders and Stroke R00NS-62953) to O. T. Shafer.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.R.L. and O.T.S. conception and design of research; K.R.L. performed experiments; K.R.L. analyzed data; K.R.L. and O.T.S. interpreted results of experiments; K.R.L. and O.T.S. prepared figures; K.R.L. and O.T.S. drafted manuscript; K.R.L. and O.T.S. edited and revised manuscript; O.T.S. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Paul Taghert and Vivek Jayaraman for kindly providing fly stocks and Robert Denver, Richard Hume, and Zepeng Yao for helpful comments on the manuscript.
Footnotes
Supplemental Material for this article is available online at the Journal website.
REFERENCES
- Albus H, Vansteensel MJ, Michel S, Block GD, Meijer JH. A GABAergic mechanism is necessary for coupling dissociable ventral and dorsal regional oscillators within the circadian clock. Curr Biol 15: 886–893, 2005 [DOI] [PubMed] [Google Scholar]
- Arble DM, Ramsey KM, Bass J, Turek FW. Circadian disruption and metabolic disease: findings from animal models. Best Pract Res Clin Endocrinol Metab 24: 785–800, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschoff J. Biological Rhythms. New York: Plenum, 1981 [Google Scholar]
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABAB receptors. Physiol Rev 84: 835–867, 2004 [DOI] [PubMed] [Google Scholar]
- Börner S, Schwede F, Schlipp A, Berisha F, Calebiro D, Lohse MJ, Nikolaev VO. FRET measurements of intracellular cAMP concentrations and cAMP analog permeability in intact cells. Nat Protoc 6: 427–438, 2011 [DOI] [PubMed] [Google Scholar]
- Brotz TM, Egelhaaf M, Borst A. A preparation of the blowfly (Calliphora erythrocephala) brain for in vitro electrophysiological and pharmacological studies. J Neurosci Methods 57: 37–46, 1995 [DOI] [PubMed] [Google Scholar]
- Buchner E, Buchner S, Crawford G, Mason WT, Salvaterra PM, Sattelle DB. Choline acetyltransferase-like immunoreactivity in the brain of Drosophila melanogaster. Cell Tissue Res 246: 57–62, 1986 [Google Scholar]
- Cao G, Nitabach MN. Circadian control of membrane excitability in Drosophila melanogaster lateral ventral clock neurons. J Neurosci 28: 6493–6501, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challet E. Minireview: entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology 148: 5648–5655, 2007 [DOI] [PubMed] [Google Scholar]
- Chung BY, Kilman VL, Keath JR, Pitman JL, Allada R. The GABAA receptor RDL acts in peptidergic PDF neurons to promote sleep in Drosophila. Curr Biol 19: 386–390, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahdal D, Reeves DC, Ruben M, Akabas MH, Blau J. Drosophila pacemaker neurons require G protein signaling and GABAergic inputs to generate twenty-four hour behavioral rhythms. Neuron 68: 964–977, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris J, Ge H, Liu L, Roman G. Go signaling is required for Drosophila associative learning. Nat Neurosci 9: 1036–1040, 2006 [DOI] [PubMed] [Google Scholar]
- Fogle KJ, Parson KG, Dahm NA, Holmes TC. CRYPTOCHROME is a blue-light sensor that regulates neuronal firing rate. Science 331: 1409–1413, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster FRG. A sense of time: body clocks, sleep and health. Dtsch Med Wochenschr 135: 2601–2608, 2010 [DOI] [PubMed] [Google Scholar]
- Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev 90: 1063–1102, 2009 [DOI] [PubMed] [Google Scholar]
- Gorczyca M, Hall J. Immunohistochemical localization of choline acetyltransferase during development and in Chats mutants of Drosophila melanogaster. J Neurosci 7: 1361–1369, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima B, Chelot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature 431: 869–873, 2004 [DOI] [PubMed] [Google Scholar]
- Hamasaka Y, Rieger D, Parmentier ML, Grau Y, Helfrich-Förster C, Nässel DR. Glutamate and its metabotropic receptor in Drosophila clock neuron circuits. J Comp Neurol 505: 32–45, 2007 [DOI] [PubMed] [Google Scholar]
- Hamasaka Y, Wegener C, Nässel DR. GABA modulates Drosophila circadian clock neurons via GABAB receptors and decreases in calcium. J Neurobiol 65: 225–240, 2005 [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C. Development of pigment-dispersing hormone-immunoreactive neurons in the nervous system of Drosophila melanogaster. J Comp Neurol 380: 335–354, 1997 [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C. The circadian clock in the brain: a structural and functional comparison between mammals and insects. J Comp Physiol A 190: 601–613, 2004 [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C. The circadian system of Drosophila melanogaster and its light input pathways. Zoology 105: 297–312, 2002 [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C, Edwards T, Yasuyama K, Wisotzki B, Schneuwly S, Stanewsky R, Meinertzhagen IA, Hofbauer A. The extraretinal eyelet of Drosophila: development, ultrastructure, and putative circadian function. J Neurosci 22: 9255–9266, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Förster C, Shafer OT, Wülbeck C, Grieshaber E, Rieger D, Taghert P. Development and morphology of the clock-gene-expressing lateral neurons of Drosophila melanogaster. J Comp Neurol 500: 47–70, 2007 [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C, Winter C, Hofbauer A, Hall JC, Stanewsky R. The circadian clock of fruit flies is blind after elimination of all known photoreceptors. Neuron 30: 249–261, 2001 [DOI] [PubMed] [Google Scholar]
- Herzog ED. Neurons and networks in daily rhythms. Nat Rev Neurosci 8: 790–802, 2007 [DOI] [PubMed] [Google Scholar]
- Ignell R, Root CM, Birse RT, Wang JW, Nässel DR, Winther ÅME. Presynaptic peptidergic modulation of olfactory receptor neurons in Drosophila. Proc Natl Acad Sci USA 106: 13070–13075, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RP, Allen CN. GABAergic signaling induces divergent neuronal Ca2+ responses in the suprachiasmatic nucleus network. Eur J Neurosci 30: 1462–1475, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Hall JC. Neuroanatomy of cells expressing clock genes in Drosophila: transgenic manipulation of the period and timeless genes to mark the perikarya of circadian pacemaker neurons and their projections. J Comp Neurol 422: 66–94, 2000 [DOI] [PubMed] [Google Scholar]
- Kaneko M, Helfrich-Förster C, Hall JC. Spatial and temporal expression of the period and timeless genes in the developing nervous system of Drosophila: newly identified pacemaker candidates and novel features of clock gene product cycling. J Neurosci 17: 6745–6760, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarsfeld A, Malpel S, Michard-Vanhée C, Picot M, Chélot E, Rouyer F. Novel features of cryptochrome-mediated photoreception in the brain circadian clock of Drosophila. J Neurosci 24: 1468–1477, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küppers B, Sánchez-Soriano N, Letzkus J, Technau GM, Prokop A. In developing Drosophila neurones the production of γ-amino butyric acid is tightly regulated downstream of glutamate decarboxylase translation and can be influenced by calcium. J Neurochem 84: 939–951, 2003 [DOI] [PubMed] [Google Scholar]
- Levine JD, Casey CI, Kalderon DD, Jackson FR. Altered circadian pacemaker functions and cyclic AMP rhythms in the Drosophila learning mutant dunce. Neuron 13: 967–974, 1994 [DOI] [PubMed] [Google Scholar]
- Lin Y, Stormo GD, Taghert PH. The neuropeptide pigment-dispersing factor coordinates pacemaker interactions in the Drosophila circadian system. J Neurosci 24: 7951–7957, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Reppert SM. GABA synchronizes clock cells within the suprachiasmatic circadian clock. Neuron 25: 123–128, 2000 [DOI] [PubMed] [Google Scholar]
- Malpel S, Klarsfeld A, Rouyer F. Larval optic nerve and adult extra-retinal photoreceptors sequentially associate with clock neurons during Drosophila brain development. Development 129: 1443–1453, 2002 [DOI] [PubMed] [Google Scholar]
- Markova O, Mukhtarov M, Real E, Jacob Y, Bregestovski P. Genetically encoded chloride indicator with improved sensitivity. J Neurosci Methods 170: 67–76, 2008 [DOI] [PubMed] [Google Scholar]
- McCarthy EV, Wu Y, deCarvalho T, Brandt C, Cao G, Nitabach MN. Synchronized bilateral synaptic inputs to Drosophila melanogaster neuropeptidergic rest/arousal neurons. J Neurosci 31: 8181–8193, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezler M, Müller T, Raming K. Cloning and functional expression of GABAB receptors from Drosophila. Eur J Neurosci 13: 477–486, 2001 [DOI] [PubMed] [Google Scholar]
- Nässel DR. Neurotransmitters and neuromodulators in the insect visual system. Prog Neurobiol 37: 179–254, 1991 [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Bünemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem 279: 37215–37218, 2004 [DOI] [PubMed] [Google Scholar]
- O'Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hastings MH. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science 320: 949–953, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJL, Kang K, Liu X, Garrity PA, Rosbash M, Griffith LC. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron 60: 672–682, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Griffith LC. Electrophysiological and anatomical characterization of PDF-positive clock neurons in the intact adult Drosophila brain. J Neurophysiol 95: 3955–3960, 2006 [DOI] [PubMed] [Google Scholar]
- Peng Y, Stoleru D, Levine JD, Hall JC, Rosbash M. Drosophila free-running rhythms require intercellular communication. PLoS Biol 1: e13, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri B, Homberg U, Loesel R, Stengl M. Evidence for a role of GABA and Mas-allatotropin in photic entrainment of the circadian clock of the cockroach Leucophaea maderae. J Exp Biol 205: 1459–1469, 2002 [DOI] [PubMed] [Google Scholar]
- Picot M, Cusumano P, Klarsfeld A, Ueda R, Rouyer F. Light activates output from evening neurons and inhibits output from morning neurons in the Drosophila circadian clock. PLoS Biol 5: e315, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn SCP, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99: 791–802, 1999 [DOI] [PubMed] [Google Scholar]
- Rieger D, Shafer OT, Tomioka K, Helfrich-Forster C. Functional analysis of circadian pacemaker neurons in Drosophila melanogaster. J Neurosci 26: 2531–2543, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger D, Stanewsky R, Helfrich-Förster C. Cryptochrome, compound eyes, Hofbauer-Buchner eyelets, and ocelli play different roles in the entrainment and masking pathway of the locomotor activity rhythm in the fruit fly Drosophila melanogaster. J Biol Rhythms 18: 377–391, 2003 [DOI] [PubMed] [Google Scholar]
- Schwartz JRL, Roth T. Shift work sleep disorder: burden of illness and approaches to management. Drugs 66: 2357–2370, 2006 [DOI] [PubMed] [Google Scholar]
- Sehadova H, Glaser FT, Gentile C, Simoni A, Giesecke A, Albert JT, Stanewsky R. Temperature entrainment of Drosophila's circadian clock involves the gene nocte and signaling from peripheral sensory tissues to the brain. Neuron 64: 251–266, 2009 [DOI] [PubMed] [Google Scholar]
- Shafer OT, Helfrich-Förster C, Renn SCP, Taghert PH. Reevaluation of Drosophila melanogaster's neuronal circadian pacemakers reveals new neuronal classes. J Comp Neurol 498: 180–193, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer OT, Kim DJ, Dunbar-Yaffe R, Nikolaev VO, Lohse MJ, Taghert PH. Widespread receptivity to neuropeptide PDF throughout the neuronal circadian clock network of Drosophila revealed by real-time cyclic AMP imaging. Neuron 58: 223–237, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer OT, Taghert PH. RNA-interference knockdown of Drosophila pigment dispersing factor in neuronal subsets: the anatomical basis of a neuropeptide's circadian functions. PLoS One 4: e8298, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakiryanova D, Levitan ES. Prolonged presynaptic posttetanic cyclic GMP signaling in Drosophila motoneurons. Proc Natl Acad Sci USA 105: 13610–13613, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Griffith L, Rosbash M. Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proc Natl Acad Sci USA 105: 19587–19594, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Haynes P, Pirez N, Harrington KI, Guo F, Pollack J, Hong P, Griffith LC, Rosbash M. Imaging analysis of clock neurons reveals light buffers the wake-promoting effect of dopamine. Nat Neurosci 14: 889–895, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeba V, Fogle KJ, Kaneko M, Rashid S, Chou YT, Sharma VK, Holmes TC. Large ventral lateral neurons modulate arousal and sleep in Drosophila. Curr Biol 18: 1537–1545, 2008a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeba V, Gu H, Sharma VK, O'Dowd DK, Holmes TC. Circadian- and light-dependent regulation of resting membrane potential and spontaneous action potential firing of Drosophila circadian pacemaker neurons. J Neurophysiol 99: 976–988, 2008b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund DM. Sodium channels. In: Comprehensive Molecular Insect Science, edited by Gilbert LI, Latrou K, Gill SS. Amsterdam: Elsevier, 2005 [Google Scholar]
- Stewart BA, Atwood HL, Renger JJ, Wang J, Wu CF. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol A 175: 179–191, 1994 [DOI] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature 431: 862–868, 2004 [DOI] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Nawathean P, Rosbash M. A resetting signal between Drosophila pacemakers synchronizes morning and evening activity. Nature 438: 238–242, 2005 [DOI] [PubMed] [Google Scholar]
- Sullivan W, Ashburner M, Hawley RS. Drosophila Protocols. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2000, p. xiv [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, Bargmann CI, Jayaraman V, Svoboda K, Looger LL. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods 6: 875–881, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treherne JE, Smith DS. The metabolism of acetylcholine in the intact central nervous system of an insect (Periplaneta americana L.). J Exp Biol 43: 441–454, 1965a [DOI] [PubMed] [Google Scholar]
- Treherne JE, Smith DS. The penetration of acetylcholine into the central nervous tissues of an insect (Periplaneta americana L.). J Exp Biol 43: 13–21, 1965b [DOI] [PubMed] [Google Scholar]
- Waseem T, Mukhtarov M, Buldakova S, Medina I, Bregestovski P. Genetically encoded Cl-Sensor as a tool for monitoring of Cl-dependent processes in small neuronal compartments. J Neurosci Methods 193: 14–23, 2010 [DOI] [PubMed] [Google Scholar]
- Wegener C, Hamasaka Y, Nässel DR. Acetylcholine increases intracellular Ca2+ via nicotinic receptors in cultured PDF-containing clock neurons of Drosophila. J Neurophysiol 91: 912–923, 2004 [DOI] [PubMed] [Google Scholar]
- Yasuyama K, Meinertzhagen IA. Extraretinal photoreceptors at the compound eye's posterior margin in Drosophila melanogaster. J Comp Neurol 412: 193–202, 1999 [DOI] [PubMed] [Google Scholar]
- Yoshii T, Wülbeck C, Sehadova H, Veleri S, Bichler D, Stanewsky R, Helfrich-Förster C. The neuropeptide pigment-dispersing factor adjusts period and phase of Drosophila's clock. J Neurosci 29: 2597–2610, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.