Abstract
Myocardial ischaemia/reperfusion (I/R)-induced remodelling generally includes cell death (necrosis and apoptosis), myocyte hypertrophy, angiogenesis, cardiac fibrosis, and myocardial dysfunction. It is becoming increasingly clear that microRNAs (miRNAs or miRs), a group of highly conserved small (∼18–24 nucleotide) non-coding RNAs, fulfil specific functions in the reperfused myocardium towards post-infarct remodelling. While miR-21, -133, -150, -195, and -214 regulate cardiomyocyte hypertrophy, miR-1/-133 and miR-208 have been elucidated to influence myocardial contractile function. In addition, miR-21, -24, -133, -210, -494, and -499 appear to protect myocytes against I/R-induced apoptosis, whereas miR-1, -29, -199a, and -320 promote apoptosis. Myocardial fibrosis can be regulated by the miR-29 family and miR-21. Moreover, miR-126 and miR-210 augment I/R-induced angiogenesis, but miR-24, -92a, and -320 suppress post-infarct neoangiogenesis. In this review, we summarize the latest advances in the identification of myocardial ischaemia-associated miRNAs and their functional significance in the modulation of I/R-triggered remodelling. Controversial effects of some miRNAs in post-infarct remodelling will be also discussed.
Keywords: MicroRNA, Ischaemia/reperfusion, Myocardial infarction, Post-infarct remodelling
1. Introduction
Myocardial infarction remains a significant and yet unsolved health problem, which seriously affects the human health. An infarct usually causes insufficient blood supply and oxidative stress, which result in necrosis of cardiac tissue, myocardial inflammation, pathological remodelling, and left ventricular dysfunction.1–3 Early reperfusion of the ischaemic region by thrombolytic treatment or surgical revascularization, albeit effective for salvaging a damaged heart, can lead to additional injury.4–6 As summarized by Kloner,7 four types of reperfusion injury have been observed in experimental animals, as follows: (i) myocyte death; (ii) microvasculature damage; (iii) the stunned myocardium, where myocytes display a prolonged period of contractile dysfunction following coronary blood flow restoration; and (iv) reperfusion arrhythmias. Although tremendous advances have been made in understanding the mechanisms of myocardial ischaemia/reperfusion (I/R) injury (see reviews elsewhere 1–5 and in this issue),1–5 the translation of these findings into the clinical setting has been largely disappointing.8 This may reflect our still incomplete knowledge of the biology of I/R. In addition, intervention using a single molecule might not be possible to protect the heart against a process involving multiple pathophysiological components.
MicroRNAs (miRNAs or miRs) are endogenous, single-stranded, non-coding RNAs ranging from ∼18 to 24 nucleotides in length. They are highly conserved and ubiquitously expressed in all species.9 Based on genomic locations of the miRNA genes, miRNAs can be divided into the following groups: (i) intergenic miRNAs, which are independently transcribed from their own genes; (ii) intronic miRNAs, which originate from introns of their host protein-coding genes; (iii) exonic miRNAs, which are located within exons of protein-coding genes; and (iv) untranslated region (UTR) miRNAs, which are generated from 5′ or 3′ UTR of protein-coding genes. Of the human miRNAs identified so far, it is estimated that ∼42% are intergenic, ∼44% intronic, ∼7% exonic, and <7% UTR miRNAs.10 In general, miRNAs act as endogenous repressors of target genes, either by inhibiting translation and/or by promoting degradation of the mRNA, or alternatively by increasing translation.9 It is widely accepted that an individual miRNA can influence hundreds of gene transcripts to co-ordinate complex programmes of gene expression and, thereby, effect global changes in the physiology of a cell.9–12 Accordingly, miRNAs have been implicated as key molecular players in virtually all cellular processes, including proliferation, differentiation, ageing, death, and the maintenance of stem cell self-renewal.10,13–15 Numerous studies have revealed that alterations in the spectrum of intra-/extracellular miRNAs are correlated with various cardiovascular conditions, such as myocardial infarction, hypertrophy, cardiomyopathy, and arrhythmias.16–22 The unique signature of miRNA expression may hold promise as a novel diagnostic tool for cardiovascular disease. Moreover, animal models with alteration of intracellular miRNA (over-expression or knockout) have demonstrated that miRNAs produce pleiotropic effects on stress-triggered cardiac remodelling.23,24 In this review, we highlight the latest advances in the identification of myocardial ischaemia-associated miRNAs and their functional significance in the modulation of I/R-induced cardiomyocyte death (necrosis/apoptosis), myocardial inflammation, fibrosis, compromised contractile function, and neoangiogenesis.
2. Expression profile of miRNAs in ischaemic/reperfused hearts
Similar to protein-coding genes, the primary transcripts of intergenic miRNAs are independently regulated by transcription factors.25 For example, we recently identified that the promoter of miR-144/451 contains GATA-binding motifs, and over-expression of GATA-4 increased the expression of miR-144/451 in cardiomyocytes.26 While intronic miRNAs may be transcribed as part of their host genes, recent studies have found that one-third of intronic miRNAs bear transcription initiation regions independently from the promoters of their host genes.27 For instance, miR-499 is an intronic miRNA within the myosin heavy chain gene Myh7b, but the intron of the Myh7b gene has been identified to contain the p53-binding site. As a result, p53 can regulate the expression of miR-499, but not of Myh7b.28 In addition, the expression of miRNA can be regulated at the post-transcriptional level by Dicer/Drosha, two major enzymes that control the generation of mature miRNAs (see reviews elsewhere).9,29 Therefore, it is reasonable that levels of miRNAs can be dynamically altered in the heart upon I/R injury, as myocardial I/R causes dysregulation of hundreds of proteins, including transcription factors and Dicer/Drosha.
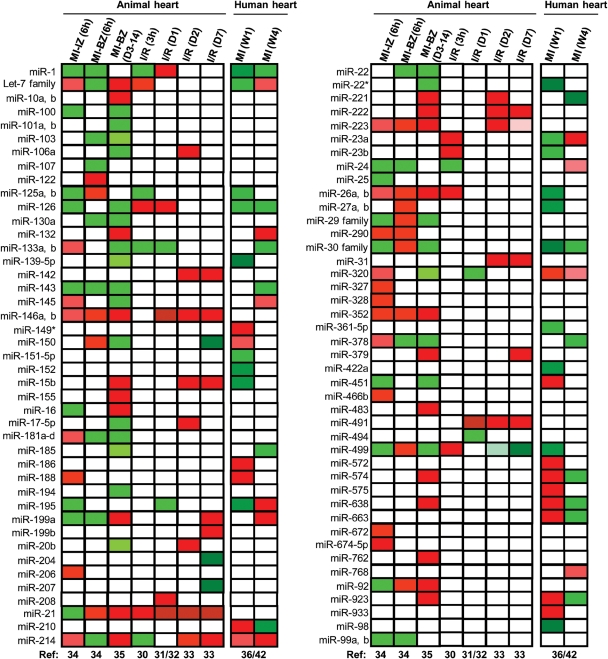
Indeed, recent work from our laboratory and others has indicated that mature miRNA levels are altered in ischaemic/reperfused hearts from animal models and human patients (Figure 1).30–36 He et al.30 examined the miRNA expression profile in Sprague–Dawley rat hearts treated with 1 h ischaemia followed by 3 h reperfusion, and observed that a total of 16 miRNAs were significantly dysregulated, of which 10 miRNAs were up-regulated and six down-regulated [Figure 1; I/R(3 h)]. However, in another rat model subjected to myocardial I/R (30 min/24 h), results of miRNA array analysis revealed that miR-1, -126, and -208 were up-regulated, whereas miR-21, 133, and -195 were down-regulated in these I/R hearts, compared with sham-operated control rats [Figure 1; I/R (D1)].31 Using the same myocardial I/R protocol (I/R, 30 min/24 h) in FVB/N mice, we observed that a total of six miRNAs were dysregulated, with down-regulation of miR-320 and up-regulation of miR-7, miR-21, miR-146b, miR-491, and miR-9651 in the I/R heart [Figure 1; I/R (D1)].32 Interestingly, Roy et al.33 examined the alterations of miRNA expression in the C57BL/6 mouse heart treated with 30 min of ischaemia, followed by 2 or 7 days of reperfusion. They reported that levels of 13 miRNAs were significantly increased on day 2 post-I/R [Figure 1; I/R (D2)], whereas on day 7 post-I/R, nine miRNAs were up-regulated, together with down-regulation of six miRNAs [Figure 1; I/R (D7)].
Figure 1.
MicroRNA (miRNA) expression profiles in animal models subjected to myocardial infarction or ischaemia/reperfusion and in human patients with myocardial infarction. The state of myocardial infarction and ischaemia/reperfusion is listed in the top row. Data summarized in the heat map are collected from eight independent studies which are represented in nine columns. Red indicates up-regulation of miRNAs and green indicates down-regulation. The let-7 family includes let-7b, c, d, e, f, g, h, i, and j; miR-29 family includes miR-29a, b, and c; and miR-30 family includes miR-30a, b, c, d, and e. Abbreviations: BZ, border zone; D3-14, on days 3 and 14 post-MI; I/R, ischaemia/reperfusion; IZ, infarction zone; MI, myocardial infarction; Ref, references; W1, 1 week post-MI; and W4, 4 weeks post-MI.
Additionally, in a rat model of permanent myocardial infarction (MI), Dong and colleagues34 found that 6 h of coronary artery occlusion resulted in differential expression of 38 miRNAs (21 up-regulated and 17 down-regulated) in the infarcted zone and 33 miRNAs dysregulated (19 up-regulated and 14 down-regulated) in the border zone compared with those in the non-infarcted area [Figure 1; MI-IZ (6 h) and MI-BZ (6 h)]. The miRNA expression signature in the late phase of MI (3 and 14 days post-infarction) was reported by van Rooij et al.35 [Figure 1; MI-BZ (D3-14)]. Recently, Bostjancic et al.36 analysed the miRNA expression profile in infarcted heart tissues from 39 patients with MI, which were divided into two groups (1 and 4 weeks post-infarction). Of 719 miRNAs analysed, 77 miRNAs were differentially expressed, of which 47 were dysregulated in hearts 1 week post-infarction, and 30 miRNAs were altered in hearts 4 weeks post-infarction [Figure 1; MI (W1) and MI (W4)].
Collectively, diverse profiles of miRNA expression in the ischaemic/reperfused or infarcted myocardium, described above and summarized in Figure 1, suggest that the alteration of miRNAs is dynamic and condition dependent. We would reasonably speculate that dysregulated miRNAs that occur in the early phase of reperfusion may be associated with cell death and oxidative stress, while those miRNAs altered in the later phase of reperfusion may contribute to post-infarct remodelling or function as compensatory mechanisms. Accordingly, those miRNAs changed in the ischaemic pre-conditioned heart should confer cardioprotection against I/R injury.34,37 However, it remains largely unclear whether these dysregulated miRNAs together or individually determine the clinical outcome of post-infarct myocardial remodelling. In addition, we should take into consideration that the myocardium contains cardiomyocytes, endothelial cells, fibroblasts, and other cell types; and the miRNA profiling described above was performed in pooled RNAs isolated from the whole heart. Moreover, some miRNAs play divergent roles in different cell types, as reviewed below. Therefore, to better understand mechanisms associated with post-infarct remodelling, it is necessary to dissect the consequence of these I/R-related miRNAs individually in various cell types within the heart in vivo and in vitro.
3. Role of miR-1/miR-133 in myocardial I/R injury
miR-1 and miR-133 are clustered on the same chromosomal locus and transcribed together as a single transcript, which becomes two independent, mature miRNAs with distinct biological functions.38 miR-1 expression is restricted to heart and skeletal muscle and is regulated by transcription factors: serum response factor and myocyte enhancer factor-2 (MEF2)/MyoD, respectively.39,40 However, among three members of the miR-133 family (miR-133a-1, miR-133a-2, and miR-133b), only miR-133b is not expressed in cardiomyocytes.41 Therefore, while three related clusters, namely miR-1-1/miR-133a-2, miR-206/miR-133b, and miR-1-2/miR-133a-1, have similar sequences and expression patterns, each miRNA is regulated by its own promoters.41 Indeed, miR-1 is one of the most strongly up-regulated miRNAs, whereas miR-133a is one of the down-regulated miRNAs in rat hearts upon acute myocardial I/R.31 Exceptionally, in human infarcted hearts, both miR-1 and miR-133a are down-regulated.36,42 Recent functional studies indicate that miR-1 and miR-133 have opposite effects in the regulation of stress-induced myocyte survival, with a pro-apoptotic role of miR-1 and anti-apoptotic role of miR-133.43 Increased levels of miR-1 in H9C2 rat embryonic ventricle cells or isolated rat cardiomyocytes provoked stress-induced apoptosis;31,43,44 in contrast, over-expression of miR-133 elicited protective effects against H2O2-triggered cell death.43 The opposite consequences of miR-1/miR-133 may be largely attributed to different targets that they modulate. Increased miR-1 causes down-regulation of multiple anti-apoptotic genes, such as Hsp60, Hsp70, IGF-1 and Bcl-2; whereas miR-133 negatively regulates a pro-apoptotic gene (i.e. Caspase-9).31,43,44 In addition, while miR-133a-over-expressing hearts displayed a similar hypertrophic response to wild-type hearts upon transverse aortic banding, cardiac fibrosis was significantly reduced, somehow implying protective effects of miR-133a on I/R-triggered cardiac remodelling.45 On the contrary, several recent studies further indicate that miR-1 promoted cardiac arrhythmogenesis by inhibiting the expression of protein phosphatase 2A regulatory subunit B56α, connexin 43 and Kir2.1.46–48 Consistently, injection of miR-1 into the infarcted myocardium exacerbated arrhythmogenesis, whereas specific knockdown of miR-1 suppressed arrhythmias.47 Collectively, these data suggest that approaches to either lower cardiac miR-1 levels or increase miR-133 levels during an ischaemic event might potentially attenuate I/R-induced myocardial injury.
4. Controversial effects of miR-21 in I/R-induced myocardial remodelling
miR-21 has been implicated in a variety of disorders and is highly up-regulated during cardiac remodelling,49,50 but the precise function of miR-21 in cardiac stress responses remains obscure. A recent study by Sabatel et al.51 indicates that over-expression of miR-21 reduced endothelial cell proliferation, migration, and tube formation by targeting RhoB, whereas knockdown of miR-21 using an locked nucleic acid (LNA)-anti-miR led to an opposite effect. They also showed that RhoB silencing impaired endothelial cell migration and tubulogenesis, thus providing a possible mechanism for miR-21 to inhibit angiogenesis.51 Furthermore, the therapeutic potential of miR-21 as an angiogenesis inhibitor was demonstrated in vivo in a mouse model of choroidal neovascularization.51 Given that coronary angiogenesis is instrumental in functional compensation and restoration of the infarcted heart, inhibition of miR-21 may offer cardioprotection against post-infarct injury.
Nonetheless, miR-21 is involved in pro-survival signalling of cardiomyocytes by targeting several pro-apoptotic genes, i.e. programmed cell death 4, phosphatase and tensin homolog (PTEN) and Fas ligand (FasL).52–54 Transgenic mice with cardiac-specific over-expression of miR-21 were tolerant to I/R-mediated myocardial injury.54 Conversely, silencing of miR-21 using antagomiR increased H2O2-triggered cardiomyocyte necrosis and apoptosis.52,53 In addition, miR-21 was up-regulated in the mouse heart after ischaemic pre-conditioning and sublethal heat shock, which are believed to offer cardioprotection.37,53,55 Likewise, adenovirus-mediated in vivo delivery of miR-21 reduced myocardial infarct size and improved left ventricular remodelling 2 weeks after infarction.34
Furthermore, miR-21 has been also shown to promote fibroblast survival, which may contribute to myocardial fibrosis.33,56 Thum et al.56 showed that knockdown of miR-21 with antagomiRs mitigated interstitial fibrosis and cardiac remodelling after aortic banding. They identified that miR-21 negatively regulated the expression of Sprouty-1 (Spry1), which suppressed mitogen-activated protein kinase signalling, especially in cardiac fibroblasts.56 Likewise, Roy and colleagues33 reported that miR-21 was up-regulated in the border zone, a fibroblast-rich region adjacent to the infarct, after myocardial I/R. Increased levels of miR-21 in the heart inhibited the PTEN expression in cardiac fibroblasts, which conversely up-regulated the matrix metalloproteinase-2 expression, leading to promotion of fibrosis in the infarcted heart.33 Together, these studies indicate that knockdown of miR-21 in the heart may be a therapeutic approach to the treatment of post-infarct fibrosis. However, these salutary effects elicited by down-regulation of miR-21 are totally contradicted by a recent study from Olson's group.57 Neither genetic knockout of miR-21 nor pharmacological inhibition of miR-21 by tiny LNAs (seed-targeting 8-mer locked nucleic acid oligonucleotides) altered the pathological responses of the heart to pressure overload or myocardial infarction.57 This study indicates that miR-21 seems not to be essential for cardiac hypertrophy, fibrosis, and cardiac dysfunction in response to myocardial infarction or other stresses in mice. As a matter of fact, in cultured myocytes, miR-21 has been reported to be both pro- and anti-hypertrophic.20,58 Reasons for the discrepancy between these studies are unclear; however, miR-21 is expressed predominantly in cardiac fibroblasts, not cardiomyocytes. It is possible that much of the miR-21 enrichment observed during cardiac disease might be ascribed primarily to an increase in the number of cardiac fibroblast cells, implying a rather limited physiological relevance of miR-21, at least in cardiomyocytes. This is supported by the fact that cardiomyocyte-specific over-expression of miR-21 in vivo does not evoke an obvious cardiac phenotype.54 In addition, miR-21 is broadly expressed in multiple tissues, including the vasculature,51 which may hamper the discrimination of primary and secondary cardiac effects in models that use global knockdown of miR-21, such as intravenous injection of antagomiRs. Furthermore, the genetic deletion model may yield compensatory effects during development, which may prevent the proper assessment of physiological alterations; and tiny LNAs (8-mer) are less effective to target miRNAs. Indeed, several studies recently confirmed that 8-mer anti-miR-21 LNAs did not affect pressure-overload-induced cardiac hypertrophy, fibrosis, and cardiac dysfunction,59 whereas highly specific, 22- and 15-nucleotide-long anti-miR-21 oligonucleotides effectively inhibited myocardial and pulmonary fibrosis.59,60
Collectively, these results indicate that miR-21 inhibits endothelial cell proliferation, migration, and tubulogenesis, whereas it promotes cardiomyocyte and fibroblast survival upon myocardial I/R. The potential role of miR-21 in cardiac fibrosis and hypertrophy is still debated. Thus, global knockdown or over-expression of miR-21 in the reperfused myocardium might yield tangled effects on post-infarct remodelling.
5. Dual roles of miR-29 and miR-24 in the myocardium towards post-infarct remodelling
Like miR-21, the expression of miR-29 is greater in cardiac fibroblasts than in cardiomyocytes.35 While the alteration of miR-29 expression in reperfused hearts has not been reported, van Rooij et al. observed that all members of the miR-29 family (miR-29a, b, and c) were down-regulated after MI, particularly in the border zone.35 Intriguingly, miR-29 controls a subset of fibrosis-related genes, including several collagens, fibrillins, laminins, integrins, and elastin.35 Down-regulation of miR-29 with antagomiRs in vivo and in vitro caused the increased expression of collagens. Supportively, over-expression of miR-29 in fibroblasts diminished the expression of collagen transcripts.35 Given that cardiac fibrosis is a common sequela of MI, reduced expression of miR-29 observed in the infarcted myocardium may contribute to post-infarct remodelling, including the stiffening of ventricular walls and compromised contractility.
In addition to modulation of pro-fibrotic genes, miR-29 has been shown to negatively regulate several anti-apoptotic genes, including Tcl-1 (oncogene, T-cell leukaemia/lymphoma 1), Mcl-1 (a member of the antiapoptotic Bcl-2 family), YY1 (Yin Yang 1), p85α (the regulatory subunit of phosphoinositide 3-kinase (PI3K)), CDC42 (cell division cycle 42), and DNMT3 (DNA methyltransferase 3).61–64 For instance, Ye et al.65 showed that knockdown of miR-29 by tail-vein injection of antagomiRs increased the expression of Mcl-1 in the mouse heart, consequently inhibiting I/R-induced myocardial apoptosis/necrosis, and leading to mitigation of cardiac remodelling. This study indicates that systemically reduced miR-29 appears to be beneficial to the myocardium in response to I/R. Notably, Ye et al.65 observed that treatment of rats with pioglitazone, a peroxisome proliferator-activated receptor (PPAR)-γ agonist, significantly reduced miR-29 levels in the heart, which may contribute to its reduction of myocardial infarct size. However, systematic inhibition of miR-29 by the aforementioned approaches also influences fibroblasts, which may jeopardize its therapeutic potential in anti-post-infarct remodelling. Therefore, its double-edged function should be taken into consideration when we decide to down-regulate miR-29 levels in the heart. The time window and cell type in which miR-29 inhibition occurs may affect its consequence on cardiac remodelling upon I/R. Immediate down-regulation of miR-29 in cardiomyocytes upon infarction should be protective, whereas chronic inhibition of miR-29 in fibroblasts may be detrimental in the reperfused myocardium.
miR-24 is enriched in cardiac endothelial cells and considerably up-regulated after MI.66 Fiedler et al.66 demonstrated that blocking endothelial miR-24 limited the myocardial infarct size of mice via prevention of endothelial apoptosis and enhancement of vascularity, which led to preserved cardiac function and survival. However, miR-24 expression is down-regulated in the ischaemic border and infarct zone of the animal left ventricle after MI and I/R.35,67 Qian et al.67 showed that in vivo local delivery of miR-24 into the infarcted hearts of mice inhibited cardiomyocyte apoptosis, attenuated infarct size, and reduced cardiac dysfunction. This anti-apoptotic effect on cardiomyocytes in vivo is partly mediated by the Bcl-2 homology domain 3 (BH3)-only domain-containing protein, Bim.67 Hence, the dual roles of miR-24, acting on cardiac endothelial cells and myocytes, suggest that the time point of intervention on miR-24 may be important for the post-infarct remodelling. Transient over-expression of miR-24 in the infarcted myocardium would inhibit I/R-induced myocyte death (necrosis and apoptosis), leading to attenuation of the later remodelling. However, during the post-infarct remodelling phase, endogenous miR-24 should be down-regulated in the heart to promote myocardial angiogenesis. Moreover, given that miR-24 is also expressed in fibroblasts,67 it would be interesting to know whether increased miR-24 enhances post-infarct cardiac fibrosis or functions in cardiac fibroblasts to promote cardiomyocyte survival through the activation of paracrine secretory pathways. Therefore, future studies will be needed to examine the consequence of fibroblast-specific over-expression of miR-24 in the heart.
6. Protective effects of miR-126, miR-210, miR-494, and miR-499 in the heart against post-I/R remodelling
As summarized in Figure 1, miRNAs are regulated in the reperfused myocardium towards post-infarct remodelling. Numerous studies have indicated that a group of miRNAs (i.e. miR-126, miR-210, miR-494, and miR-499) positively interfere with the process of post-myocardial infarct remodelling at multiple levels, i.e. inhibition of myocyte apoptosis, mitigation of myocardial inflammation, and promotion of neoangiogenesis.68–82
miR-126 has been implicated in the maintenance of vascular integrity and promotion of vessel growth as a pro-angiogenic factor, both in vitro and in vivo.68–75 Genetic deletion of miR-126 resulted in profound vascular defects, phenotypes that were previously ascribed to the host gene Egfl7, because of inadvertent deletion of miR-126 in the Egfl7-null mouse.69–72 Although a significant fraction of miR-126-null mice died embryonically because of vessel leakage, those mice that escaped neonatal lethality were particularly susceptible to vascular rupture after MI as a result of a deficit in neovascularization of the infarcted tissue.70 Consistently, antagomiR-mediated silencing of miR-216 impaired ischaemia-induced angiogenesis.74 The pro-angiogenic effect of miR-126 was attributed, at least in part, to the repression of Spred-1, an intracellular inhibitor of vascular endothelial growth factor (VEGF)/fibroblast growth factor (FGF)-mediated angiogenesis and phosphatidylinositol-3-kinase regulatory subunit PIK3R2 (p85β).70,71 Additionally, miR-126 is enriched in endothelial cell-derived apoptotic bodies and acts to down-regulate the expression of RGS16 (a member of the regulator of G protein signalling family).73 Reduced RGS16 enables CXCR4 (CXC chemokine CXCL12 receptor) to stimulate an autoregulatory feedback loop that enhances the phosphorylation of extracellular-signal-regulated kinase 1/2(ERK1/2) and induces the production of more CXCL12, which functions as a signal to mobilize progenitor cells and as an anti-apoptotic factor.73 Interestingly, miR-126 is also expressed in endothelial progenitor cells, a stem cell population that might contribute to post-MI cardiac regeneration.75 As a result, we would speculate that miR-126 may influence cardiac repair by affecting the homing ability of circulating progenitor cells. In addition to a direct role in angiogenesis, miR-126 influences the process of leucocyte infiltration and vascular inflammation.68,69 It is reported that the expression of vascular cell adhesion molecules is modulated by miR-126, thus affecting tumour necrosis factor-α-stimulated leucocyte adherence to endothelial cells and vessel inflammation.68,69 Collectively, these results indicate that systematic up-regulation of cardiac miR-126 may be instrumental in the reperfused myocardium to subdue post-infarct remodelling. Like miR-126, miR-210 has been shown to possess pro-angiogenic properties in vivo and in vitro. Fasanaro et al.76 provided evidence that up-regulation of miR-210 in normoxic conditions increased endothelial cell tubulogenesis and migration, whereas blockade of miR-210 in the presence of hypoxia decreased capillary-like formation, endothelial cell migration, and cell survival. They also validated ephrin-A3 as a nodal target of miR-210, the inhibition of which was a major contributor to miR-210-mediated cell survival, migration, and tube formation in response to hypoxia.76 Furthermore, recent studies also indicate that levels of miR-210 were increased in cardiomyocytes during hypoxic conditions through both hypoxia-inducible factor-dependent and –independent pathways.77 Over-expression of miR-210 in cardiomyocytes dampened the generation of reactive oxygen species, thereby suppressing apoptosis in response to oxidative stress.77 Likewise, a recent study suggests that cytoprotection afforded by ischaemic pre-conditioning may be party mediated by the induction of miR-210, which promoted mesenchymal stem cell survival by blocking caspase-8-associated protein-2.78 Importantly, the therapeutic potential of miR-210 was recently investigated in vivo by Hu et al.79 They directly injected a minicircle vector carrying miR-210 precursor into the infarcted mouse heart, and observed a significant improvement of left ventricular fractional shortening, increased myocyte survival and neovascularization, compared with the miR-scramble controls.79 Taken together, these studies consistently suggest that miR-210 plays a cardioprotective role at multiple levels and would be a potential therapeutic agent for the treatment of ischaemic heart disease.
Unlike miR-126 and miR-210, miR-494 is down-regulated in the murine heart upon in vivo I/R, whereas it is up-regulated in ex vivo ischaemic/reperfused mouse hearts.80 Interestingly, predicted targets of miR-494 include both pro-apoptotic (i.e. suppressor of cytokine signaling 6 (SOCS6), phosphatase and tensin homolog (PTEN), Rho-associated, coiled-coil containing protein kinase 1 (ROCK1), and Ca2+/calmodulin-dependent protein kinase II delta (CAMKII-δ) and anti-apototic genes (i.e. fibroblast growth factor receptor 2 (FGFR2), leukemia inhibitory factor (LIF), apoptosis inhibitor 5 (Api5), insulin-like growth factor-1 receptor (IGF1R)), and fibroblast growth factor 7 (FGF7)). Using transgenic mouse models with cardiac-specific over-expression of miR-494, we observed that elevation of miR-494 improved the post-I/R recovery of cardiac function, and suppressed I/R-triggered cardiomyocyte apotosis and necrosis.80 Although we did verify that three pro-apoptotic genes (PTEN, ROCK1, and CAMKIIδ) and two anti-apoptotic genes (FGFR2 and LIF) were bona fide targets of miR-494, the individual contribution of these targets to the downstream Akt pathway appeared unequal. In miR-494-over-expressing cardiomyocytes, the impact of PTEN, ROCK1, and CAMKIIδ (either individually or in combination) on Akt signalling seems more significant than the impact of FGFR2, LIF, or other anti-apoptotic targets, thereby tilting the balance of the Akt pathway towards activation. These interesting findings suggest that divergent targets of an miRNA may work unequally to balance a common signalling pathway and eventually determine its phenotype. Future studies will be needed to examine the effects of miR-494 on non-cardiomyocytes in the reperfused myocardium towards post-infarct remodelling.
miR-499 is among the top cardiac-enriched microRNAs, along with the well-studied microRNAs, miR-1 and miR-133. miR-499 is distinct from miR-1/miR-133 in that it is located in an intron of Myh7b.27,82 Mice expressing lower levels of miR-499 (12-fold) displayed normal phenotype in cardiac pathophysiology, whereas transgenic mice expressing higher levels of miR-499 (28-fold) developed cardiac hypertrophy, as demonstrated by gross pathology at 5 weeks of life.82 Recently, a seminal study by Ozsolak et al.27 demonstrated that miR-499 was down-regulated in the heart in ischaemic conditions. Interestingly, they showed that miR-499 transgenic mice (seven-fold) are resistant to I/R-induced left ventricular remodelling through regulation of mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Knockdown of endogenous miR-499 with its specific antagomiR aggravated deleterious cardiac remodelling after I/R, as revealed by increased collagen deposition, cardiomyocyte hypertrophy, and compromised contractile function. These results suggest that suitably increased miR-499 in the heart may provide favourable effects in retarding the process of post-infarct remodelling.
7. Detrimental role of miR-92a, miR-199a, and miR-320 in myocardial I/R injury
Members of the miR-17-92 cluster (encoding miR-17, -18a, -19a/b, -20a, and miR-92a) have been demonstrated to influence angiogenesis either positively or negatively, depending on the cellular context.83 In contrast to the pro-angiogenic effects of miR-18a and miR-19a in tumour angiogenesis, miR-92a was found to block angiogenesis and vessel formation in vitro and in vivo by targeting pro-angiogenic genes, such as the integrin subunits α5, the histone deacetylase Sirtuin 1, the sphingosine-1-phosphate receptor 1, and the mitogen-activated kinase kinase 4.83,84 Accordingly, using a mouse hindlimb ischaemia model, Bonauer et al.84 observed that inhibition of miR-92a by antagomiR significantly promoted neovascularization in ischaemic limbs. Likewise, treatment of mouse acute myocardial infarction with antagomiR-92a improved left ventricular function, reduced the infarct size/myocardial apoptosis, and significantly augmented the number of blood vessels, particularly in the infarct border zone. Together, these results suggest that miR-92a as an endogenous repressor of cardiac angiogenesis may play a detrimental role in post-infarct remodelling.
The miR-199 family contains three miRNAs: miR-199a-1, miR-199a-2, and miR-199b. They are all encoded by the antisense strand of an intron of a dynamin gene (Dnm2, Dnm3, and Dnm1, respectively).85,86 Furthermore, miR-199a-2 is co-transcribed with miR-214.85,86 The transcriptional regulation of miR-199 in the heart is unknown, but Rane et al.87 reported recently that miR-199a was rapidly down-regulated in cardiomyocytes during hypoxic conditions, most probably via a post-transcriptional mechanism, because the expression level of the miR-199a precursor was unaffected. Down-regulation of miR-199a increased the expression of hypoxia-inducible factor-1α and Sirtuin 1. Interestingly, Rane et al.87 demonstrated that knockdown of miR-199a during hypoxia induced apoptosis, whereas knockdown of miR-199a before hypoxia surprisingly imitated pre-conditioning and protected cardiomyocytes against hypoxic damage. Murakami et al.88 recently showed that expression levels of miR-199a positively correlated to the progressed liver fibrosis of animal models and human samples. Thus, future investigation will be needed to determine whether knockdown of miR-199a also reduces fibrosis in the infarcted heart.
Similar to miR-199a, the expression of miR-320 was decreased in the ischaemic/reperfused hearts.32 We demonstrated that transgenic mice with cardiac-specific over-expression of miR-320 exhibited increased apoptosis and infarct size after I/R injury. Conversely, administration of miR-320 antagomiRs reduced infarct size, probably at least partly by derepression of the cardioprotective heat-shock protein 20.32 In addition, up-regulation of miRNA-320 was observed in myocardial microvascular endothelial cells from diabetic rats.89 Transfection of miR-320 into myocardial microvascular endothelial cells impaired angiogenesis by repressing the insulin-like growth factor 1 (IGF-1) expression.89 Conversely, the miR-320 inhibitor significantly enhanced the proliferation and migration of diabetic myocardial microvascular endothelial cells.89 Hence, we would conjecture that targeting miR-320 with antagomiRs after MI could be a new treatment option to decrease cardiomyocyte loss and increase neovascularization.
8. Conclusions and future directions
The clinical outcome of myocardial I/R is determined by numerous mediators and signalling pathways that are involved in myocyte death (necrosis, apotosis, and autophagy), myocyte hypertrophy, cardiac fibrosis, impaired angiogenesis, and compromised contractile function. Identification of I/R-associated miRNAs is important not only for the treatment of post-infarct remodelling, but also for use as diagnostic or prognostic biomarkers in ischaemic heart disease.21,22,90 In addition to the miRNAs reviewed above, those miRNAs which have been shown to correlate with tumour cell death/survival, if they are dysexpressed in the ischaemic/reperfused heart, should interfere in post myocardial-infarct remodelling. For example, members of the miR-15 family (including miR-15a, miR-15b, miR-16-1, miR-16-2, miR-195, miR-424, and miR-497) have been consistently found to be strongly up-regulated in ischaemic hearts and failing hearts.16,17 While the exact role of the miR-15 family in myocardial I/R injury is not clear, studies in other cell types suggest that the miR-15 family might induce apoptosis by down-regulation of the anti-apoptotic factor Bcl-2.91 In this respect, antagomiR-induced knockdown of the miR-15 family might be a means to prevent I/R-induced cardiomyocyte apoptosis. Furthermore, those miRNAs associated with regulation of the inflammatory pathway (i.e. miR-146, -155, and -125b),92–94 oxidative stress (i.e. miR-451),95 cardiac autophagy (i.e. miR-204),96 and endoplasmic reticum stress (i.e. miR-221/222)97 may also represent potential avenues to the therapeutic modulation of post-infarct remodelling. Generally, over-expression of protective miRNAs by virus-vector or direct injection of miRNA mimics, whereas specific down-regulation of those detrimental miRNAs by antisense strategies (see reviews elsewhere),23,98,99 into the reperfused myocardium would be instrumental to retard/halt post-infarct remodelling. In addition, some pharmacological agents may regulate miRNA expression and confer cardioprotection against I/R injury. For instance, propranolol (a non-selective β-blocker) down-regulates miR-1 expression and provides ischaemic cardioprotection,48 whereas pioglitazone (a peroxisome proliferator-activated receptor-γ agonist) significantly inhibits the expression of miR-29, leading to the attenuation of myocardial remodelling upon I/R.65 While modulation of miRNA levels directly or indirectly by the aforementioned approaches offers a therapeutic advantage to interfere with myocardial I/R injury, the complexity of miRNA targets might yield different consequences in various cell types within the heart. Hence, to acquire better therapeutic effects of a specific miRNA on the reperfused myocardium, we should consider the best time window for altering certain miRNA levels and the possibility of regulating miRNA expression in specific cell types.
Conflict of interest: none declared.
Funding
Research in the Fan lab was supported by NIH grant HL-087861 and University of Cincinnati Center for Environmental Genetics (CEG) grant NIEHS P30-ES006096.
References
- 1.Hori M, Nishida K. Oxidative stress and left ventricular remodeling after myocardial infarction. Cardiovasc Res. 2009;81:457–464. doi: 10.1093/cvr/cvn335. doi:10.1093/cvr/cvn335. [DOI] [PubMed] [Google Scholar]
- 2.Landmesser U, Wollert KC, Drexler H. Potential novel pharmacological therapies for myocardial remodelling. Cardiovasc Res. 2009;81:519–527. doi: 10.1093/cvr/cvn317. doi:10.1093/cvr/cvn317. [DOI] [PubMed] [Google Scholar]
- 3.Sun Y. Myocardial repair/remodelling following infarction: roles of local factors. Cardiovasc Res. 2009;81:482–490. doi: 10.1093/cvr/cvn333. doi:10.1093/cvr/cvn333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takemura G, Nakagawa M, Kanamori H, Minatoguchi S, Fujiwara H. Benefits of reperfusion beyond infarct size limitation. Cardiovasc Res. 2009;83:269–276. doi: 10.1093/cvr/cvp032. doi:10.1093/cvr/cvp032. [DOI] [PubMed] [Google Scholar]
- 5.Eefting F, Rensing B, Wigman J, Pannekoek WJ, Liu WM, Cramer MJ, et al. Role of apoptosis in reperfusion injury. Cardiovasc Res. 2004;61:414–426. doi: 10.1016/j.cardiores.2003.12.023. doi:10.1016/j.cardiores.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 6.Piper HM, García-Dorado D, Ovize M. A fresh look at reperfusion injury. Cardiovasc Res. 1998;38:291–300. doi: 10.1016/s0008-6363(98)00033-9. doi:10.1016/S0008-6363(98)00033-9. [DOI] [PubMed] [Google Scholar]
- 7.Kloner RA. Does reperfusion injury exist in humans? J Am Coll Cardiol. 1993;21:537–545. doi: 10.1016/0735-1097(93)90700-b. doi:10.1016/0735-1097(93)90700-B. [DOI] [PubMed] [Google Scholar]
- 8.Downey JM, Cohen MV. Why do we still not have cardioprotective drugs? Circ J. 2009;73:1171–1177. doi: 10.1253/circj.cj-09-0338. doi:10.1253/circj.CJ-09-0338. [DOI] [PubMed] [Google Scholar]
- 9.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. doi:10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z. MicroRNA: a matter of life or death. World J Biol Chem. 2010;1:41–54. doi: 10.4331/wjbc.v1.i4.41. doi:10.4331/wjbc.v1.i4.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. doi:10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 12.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. doi:10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/s0092-8674(01)00616-x. doi:10.1016/S0092-8674(01)00616-X. [DOI] [PubMed] [Google Scholar]
- 14.Liu N, Olson EN. MicroRNA regulatory networks in cardiovascular development. Dev Cell. 2010;18:510–525. doi: 10.1016/j.devcel.2010.03.010. doi:10.1016/j.devcel.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fazi F, Nervi C. MicroRNA: basic mechanisms and transcriptional regulatory networks for cell fate determination. Cardiovasc Res. 2008;79:553–561. doi: 10.1093/cvr/cvn151. doi:10.1093/cvr/cvn151. [DOI] [PubMed] [Google Scholar]
- 16.van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA. 2006;103:18255–18260. doi: 10.1073/pnas.0608791103. doi:10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikeda S, Kong SW, Lu J, Bisping E, Zhang H, Allen PD, et al. Altered microRNA expression in human heart disease. Physiol Genomics. 2007;31:367–373. doi: 10.1152/physiolgenomics.00144.2007. doi:10.1152/physiolgenomics.00144.2007. [DOI] [PubMed] [Google Scholar]
- 18.Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, et al. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation. 2007;116:258–267. doi: 10.1161/CIRCULATIONAHA.107.687947. doi:10.1161/CIRCULATIONAHA.107.687947. [DOI] [PubMed] [Google Scholar]
- 19.Sayed D, Hong C, Chen IY, Lypowy J, Abdellatif M. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ Res. 2007;100:416–424. doi: 10.1161/01.RES.0000257913.42552.23. doi:10.1161/01.RES.0000257913.42552.23. [DOI] [PubMed] [Google Scholar]
- 20.Cheng Y, Ji R, Yue J, Yang J, Liu X, Chen H, et al. MicroRNAs are aberrantly expressed in hypertrophic heart: do they play a role in cardiac hypertrophy? Am J Pathol. 2007;170:1831–1840. doi: 10.2353/ajpath.2007.061170. doi:10.2353/ajpath.2007.061170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta SK, Bang C, Thum T. Circulating microRNAs as biomarkers and potential paracrine mediators of cardiovascular disease. Circ Cardiovasc Genet. 2010;3:484–488. doi: 10.1161/CIRCGENETICS.110.958363. doi:10.1161/CIRCGENETICS.110.958363. [DOI] [PubMed] [Google Scholar]
- 22.Zhu H, Fan GC. Extracellular/circulating microRNAs and their potential role in cardiovascular disease. Am J Cardiovasc Dis. 2011;1:138–149. [PMC free article] [PubMed] [Google Scholar]
- 23.van Rooij E, Olson EN. MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest. 2007;117:2369–2376. doi: 10.1172/JCI33099. doi:10.1172/JCI33099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thum T, Catalucci D, Bauersachs J. MicroRNAs: novel regulators in cardiac development and disease. Cardiovasc Res. 2008;79:562–570. doi: 10.1093/cvr/cvn137. doi:10.1093/cvr/cvn137. [DOI] [PubMed] [Google Scholar]
- 25.Saini HK, Griffiths-Jones S, Enright AJ. Genomic analysis of human microRNA transcripts. Proc Natl Acad Sci USA. 2007;104:17719–17724. doi: 10.1073/pnas.0703890104. doi:10.1073/pnas.0703890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Wang X, Zhu H, Zhu C, Wang Y, Pu WT, et al. Synergistic effects of the GATA-4-mediated miR-144/451 cluster in protection against simulated ischemia/reperfusion-induced cardiomyocyte death. J Mol Cell Cardiol. 2010;49:841–850. doi: 10.1016/j.yjmcc.2010.08.007. doi:10.1016/j.yjmcc.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozsolak F, Poling LL, Wang Z, Liu H, Liu XS, Roeder RG, et al. Chromatin structure analyses identify miRNA promoters. Genes Dev. 2008;22:3172–3183. doi: 10.1101/gad.1706508. doi:10.1101/gad.1706508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang JX, Jiao JQ, Li Q, Long B, Wang K, Liu JP, et al. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med. 2011;17:71–78. doi: 10.1038/nm.2282. doi:10.1038/nm.2282. [DOI] [PubMed] [Google Scholar]
- 29.Bauersachs J, Thum T. Biogenesis and regulation of cardiovascular microRNAs. Circ Res. 2011;109:334–347. doi: 10.1161/CIRCRESAHA.110.228676. doi:10.1161/CIRCRESAHA.110.228676. [DOI] [PubMed] [Google Scholar]
- 30.He B, Xiao J, Ren AJ, Zhang YF, Zhang H, Chen M, et al. Role of miR-1 and miR-133a in myocardial ischemic postconditioning. J Biomed Sci. 2011;18:22–31. doi: 10.1186/1423-0127-18-22. doi:10.1186/1423-0127-18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang Y, Zheng J, Sun Y, Wu Z, Liu Z, Huang G. MicroRNA-1 regulates cardiomyocyte apoptosis by targeting Bcl-2. Int Heart J. 2009;50:377–387. doi: 10.1536/ihj.50.377. doi:10.1536/ihj.50.377. [DOI] [PubMed] [Google Scholar]
- 32.Ren XP, Wu J, Wang X, Sartor MA, Qian J, Jones K, et al. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation. 2009;119:2357–2366. doi: 10.1161/CIRCULATIONAHA.108.814145. doi:10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, et al. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res. 2009;82:21–29. doi: 10.1093/cvr/cvp015. doi:10.1093/cvr/cvp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, et al. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J Biol Chem. 2009;284:29514–29525. doi: 10.1074/jbc.M109.027896. doi:10.1074/jbc.M109.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. doi:10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bostjancic E, Zidar N, Glavac D. MicroRNA microarray expression profiling in human myocardial infarction. Dis Markers. 2009;27:255–268. doi: 10.3233/DMA-2009-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin C, Salloum FN, Kukreja RC. A novel role of microRNA in late preconditioning: upregulation of endothelial nitric oxide synthase and heat shock protein 70. Circ Res. 2009;104:572–575. doi: 10.1161/CIRCRESAHA.108.193250. doi:10.1161/CIRCRESAHA.108.193250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–233. doi: 10.1038/ng1725. doi:10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436:214–220. doi: 10.1038/nature03817. doi:10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 40.Sweetman D, Goljanek K, Rathjen T, Oustanina S, Braun T, Dalmay T, et al. Specific requirements of MRFs for the expression of muscle specific microRNAs, miR-1, miR-206 and miR-133. Dev Biol. 2008;321:491–499. doi: 10.1016/j.ydbio.2008.06.019. doi:10.1016/j.ydbio.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 41.Townley-Tilson WH, Callis TE, Wang D. MicroRNAs 1, 133, and 206: critical factors of skeletal and cardiac muscle development, function, and disease. Int J Biochem Cell Biol. 2010;42:1252–1255. doi: 10.1016/j.biocel.2009.03.002. doi:10.1016/j.biocel.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bostjancic E, Zidar N, Stajer D, Glavac D. MicroRNAs miR-1, miR-133a, miR-133b and miR-208 are dysregulated in human myocardial infarction. Cardiology. 2010;115:163–169. doi: 10.1159/000268088. doi:10.1159/000268088. [DOI] [PubMed] [Google Scholar]
- 43.Xu C, Lu Y, Pan Z, Chu W, Luo X, Lin H, et al. The muscle-specific microRNAs miR-1 and miR-133 produce opposing effects on apoptosis by targeting HSP60, HSP70 and caspase-9 in cardiomyocytes. J Cell Sci. 2007;120:3045–3052. doi: 10.1242/jcs.010728. doi:10.1242/jcs.010728. [DOI] [PubMed] [Google Scholar]
- 44.Yu XY, Song YH, Geng YJ, Lin QX, Shan ZX, Lin SG, et al. Glucose induces apoptosis of cardiomyocytes via microRNA-1 and IGF-1. Biochem Biophys Res Commun. 2008;376:548–552. doi: 10.1016/j.bbrc.2008.09.025. doi:10.1016/j.bbrc.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 45.Matkovich SJ, Wang W, Tu Y, Eschenbacher WH, Dorn LE, Condorelli G, et al. MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circ Res. 2010;106:166–175. doi: 10.1161/CIRCRESAHA.109.202176. doi:10.1161/CIRCRESAHA.109.202176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terentyev D, Belevych AE, Terentyeva R, Martin MM, Malana GE, Kuhn DE, et al. miR-1 overexpression enhances Ca2+ release and promotes cardiac arrhythmogenesis by targeting PP2A regulatory subunit B56α and causing CaMKII-dependent hyperphosphorylation of RyR2. Circ Res. 2009;104:514–521. doi: 10.1161/CIRCRESAHA.108.181651. doi:10.1161/CIRCRESAHA.108.181651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13:486–491. doi: 10.1038/nm1569. doi:10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 48.Lu Y, Zhang Y, Shan H, Pan Z, Li X, Li B, et al. MicroRNA-1 downregulation by propranolol in a rat model of myocardial infarction: a new mechanism for ischaemic cardioprotection. Cardiovasc Res. 2009;84:434–441. doi: 10.1093/cvr/cvp232. doi:10.1093/cvr/cvp232. [DOI] [PubMed] [Google Scholar]
- 49.da Costa Martins PA, De Windt LJ. miR-21: a miRaculous Socratic paradox. Cardiovasc Res. 2010;87:397–400. doi: 10.1093/cvr/cvq196. doi:10.1093/cvr/cvq196. [DOI] [PubMed] [Google Scholar]
- 50.Cheng Y, Zhang C. MicroRNA-21 in cardiovascular disease. J Cardiovasc Transl Res. 2010;3:251–255. doi: 10.1007/s12265-010-9169-7. doi:10.1007/s12265-010-9169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sabatel C, Malvaux L, Bovy N, Deroanne C, Lambert V, Gonzalez ML, et al. MicroRNA-21 exhibits antiangiogenic function by targeting RhoB expression in endothelial cells. PLoS One. 2011;6:e16979. doi: 10.1371/journal.pone.0016979. doi:10.1371/journal.pone.0016979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng Y, Liu X, Zhang S, Lin Y, Yang J, Zhang C. MicroRNA-21 protects against the H2O2-induced injury on cardiac myocytes via its target gene PDCD4. J Mol Cell Cardiol. 2009;47:5–14. doi: 10.1016/j.yjmcc.2009.01.008. doi:10.1016/j.yjmcc.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng Y, Zhu P, Yang J, Liu X, Dong S, Wang X, et al. Ischaemic preconditioning-regulated miR-21 protects heart against ischaemia/reperfusion injury via anti-apoptosis through its target PDCD4. Cardiovasc Res. 2010;87:431–439. doi: 10.1093/cvr/cvq082. doi:10.1093/cvr/cvq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sayed D, He M, Hong C, Gao S, Rane S, Yang Z, et al. MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. J Biol Chem. 2010;285:20281–20290. doi: 10.1074/jbc.M110.109207. doi:10.1074/jbc.M110.109207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yin C, Wang X, Kukreja RC. Endogenous microRNAs induced by heat-shock reduce myocardial infarction following ischemia–reperfusion in mice. FEBS Lett. 2008;582:4137–4142. doi: 10.1016/j.febslet.2008.11.014. doi:10.1016/j.febslet.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. doi:10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 57.Patrick DM, Montgomery RL, Qi X, Obad S, Kauppinen S, Hill JA, et al. Stress-dependent cardiac remodeling occurs in the absence of microRNA-21 in mice. J Clin Invest. 2010;120:3912–3916. doi: 10.1172/JCI43604. doi:10.1172/JCI43604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tatsuguchi M, Seok HY, Callis TE, Thomson JM, Chen JF, Newman M, et al. Expression of microRNAs is dynamically regulated during cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2007;42:1137–1141. doi: 10.1016/j.yjmcc.2007.04.004. doi:10.1016/j.yjmcc.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thum T, Chau N, Bhat B, Gupta SK, Linsley PS, Bauersachs J, et al. Comparison of different miR-21 inhibitor chemistries in a cardiac disease model. J Clin Invest. 2011;121:461–462. doi: 10.1172/JCI45938. doi:10.1172/JCI45938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Obad S, dos Santos CO, Petri A, Heidenblad M, Broom O, Ruse C, et al. Silencing of microRNA families by seed-targeting tiny LNAs. Nat Genet. 2011;43:371–378. doi: 10.1038/ng.786. doi:10.1038/ng.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pekarsky Y, Santanam U, Cimmino A, Palamarchuk A, Efanov A, Maximov V, et al. Tcl1 expression in chronic lymphocytic leukemia is regulated by miR-29 and miR-181. Cancer Res. 2006;66:11590–11593. doi: 10.1158/0008-5472.CAN-06-3613. doi:10.1158/0008-5472.CAN-06-3613. [DOI] [PubMed] [Google Scholar]
- 62.Mott JL, Kobayashi S, Bronk SF, Gores GJ. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene. 2007;26:6133–6140. doi: 10.1038/sj.onc.1210436. doi:10.1038/sj.onc.1210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang H, Garzon R, Sun H, Ladner KJ, Singh R, Dahlman J, et al. NF-κB–YY1–miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma. Cancer Cell. 2008;14:369–381. doi: 10.1016/j.ccr.2008.10.006. doi:10.1016/j.ccr.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park SY, Lee JH, Ha M, Nam JW, Kim VN. miR-29 miRNAs activate p53 by targeting p85α and CDC42. Nat Struct Mol Biol. 2009;16:23–29. doi: 10.1038/nsmb.1533. doi:10.1038/nsmb.1533. [DOI] [PubMed] [Google Scholar]
- 65.Ye Y, Hu Z, Lin Y, Zhang C, Perez-Polo JR. Downregulation of microRNA-29 by antisense inhibitors and a PPAR-γ agonist protects against myocardial ischaemia–reperfusion injury. Cardiovasc Res. 2010;87:535–544. doi: 10.1093/cvr/cvq053. doi:10.1093/cvr/cvq053. [DOI] [PubMed] [Google Scholar]
- 66.Fiedler J, Jazbutyte V, Kirchmaier BC, Gupta SK, Lorenzen J, Hartmann D, et al. MicroRNA-24 regulates vascularity after myocardial infarction. Circulation. 2011;124:720–730. doi: 10.1161/CIRCULATIONAHA.111.039008. doi:10.1161/CIRCULATIONAHA.111.039008. [DOI] [PubMed] [Google Scholar]
- 67.Qian L, Van Laake LW, Huang Y, Liu S, Wendland MF, Srivastava D. miR-24 inhibits apoptosis and represses Bim in mouse cardiomyocytes. J Exp Med. 2011;208:549–560. doi: 10.1084/jem.20101547. doi:10.1084/jem.20101547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008;79:581–588. doi: 10.1093/cvr/cvn156. doi:10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- 69.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA. 2008;105:1516–1521. doi: 10.1073/pnas.0707493105. doi:10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. doi:10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, et al. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. doi:10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuhnert F, Mancuso MR, Hampton J, Stankunas K, Asano T, Chen CZ, et al. Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development. 2008;135:3989–3993. doi: 10.1242/dev.029736. doi:10.1242/dev.029736. [DOI] [PubMed] [Google Scholar]
- 73.Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2:ra81. doi: 10.1126/scisignal.2000610. doi:10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 74.van Solingen C, Seghers L, Bijkerk R, Duijs JM, Roeten MK, van Oeveren-Rietdijk A, et al. Antagomir-mediated silencing of endothelial cell specific microRNA-126 impairs ischemia-induced angiogenesis. J Cell Mol Med. 2009;13:1577–1585. doi: 10.1111/j.1582-4934.2008.00613.x. doi:10.1111/j.1582-4934.2008.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Q, Kandic I, Kutryk MJ. Dysregulation of angiogenesis-related microRNAs in endothelial progenitor cells from patients with coronary artery disease. Biochem Biophys Res Commun. 2011;405:42–46. doi: 10.1016/j.bbrc.2010.12.119. doi:10.1016/j.bbrc.2010.12.119. [DOI] [PubMed] [Google Scholar]
- 76.Fasanaro P, D'Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, et al. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008;283:15878–15883. doi: 10.1074/jbc.M800731200. doi:10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mutharasan RK, Nagpal V, Ichikawa Y, Ardehali H. MicroRNA-210 is upregulated in hypoxic cardiomyocytes through Akt- and p53-dependent pathways and exerts cytoprotective effects. Am J Physiol Heart Circ Physiol. 2011;301:H1519–H1530. doi: 10.1152/ajpheart.01080.2010. doi:10.1152/ajpheart.01080.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim HW, Haider HK, Jiang S, Ashraf M. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem. 2009;284:33161–33168. doi: 10.1074/jbc.M109.020925. doi:10.1074/jbc.M109.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu S, Huang M, Li Z, Jia F, Ghosh Z, Lijkwan MA, et al. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation. 2010;122:S124–S131. doi: 10.1161/CIRCULATIONAHA.109.928424. doi:10.1161/CIRCULATIONAHA.109.928424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang X, Zhang X, Ren XP, Chen J, Liu H, Yang J, et al. MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardiac injury. Circulation. 2010;122:1308–1318. doi: 10.1161/CIRCULATIONAHA.110.964684. doi:10.1161/CIRCULATIONAHA.110.964684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu L, Jiang Y, Zhang H, Greenlee AR, Han Z. Overexpressed miR-494 down-regulates PTEN gene expression in cells transformed by anti-benzo(a)pyrene-trans-7,8-dihydrodiol-9,10-epoxide. Life Sci. 2010;86:192–198. doi: 10.1016/j.lfs.2009.12.002. doi:10.1016/j.lfs.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 82.Shieh JT, Huang Y, Gilmore J, Srivastava D. Elevated miR-499 levels blunt the cardiac stress response. PLoS One. 2011;6:e19481. doi: 10.1371/journal.pone.0019481. doi:10.1371/journal.pone.0019481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Doebele C, Bonauer A, Fischer A, Scholz A, Reiss Y, Urbich C, et al. Members of the microRNA-17-92 cluster exhibit a cell-intrinsic antiangiogenic function in endothelial cells. Blood. 2010;115:4944–4950. doi: 10.1182/blood-2010-01-264812. doi:10.1182/blood-2010-01-264812. [DOI] [PubMed] [Google Scholar]
- 84.Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. doi: 10.1126/science.1174381. doi:10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 85.Watanabe T, Sato T, Amano T, Kawamura Y, Kawamura N, Kawaguchi H, et al. Dnm3os, a non-coding RNA, is required for normal growth and skeletal development in mice. Dev Dyn. 2008;237:3738–3748. doi: 10.1002/dvdy.21787. doi:10.1002/dvdy.21787. [DOI] [PubMed] [Google Scholar]
- 86.Lee YB, Bantounas I, Lee DY, Phylactou L, Caldwell MA, Uney JB. Twist-1 regulates the miR-199a/214 cluster during development. Nucleic Acids Res. 2009;37:123–128. doi: 10.1093/nar/gkn920. doi:10.1093/nar/gkn920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rane S, He M, Sayed D, Vashistha H, Malhotra A, Sadoshima J, et al. Downregulation of miR-199a derepresses hypoxia-inducible factor-1α and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res. 2009;104:879–886. doi: 10.1161/CIRCRESAHA.108.193102. doi:10.1161/CIRCRESAHA.108.193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murakami Y, Toyoda H, Tanaka M, Kuroda M, Harada Y, Matsuda F, et al. The progression of liver fibrosis is related with overexpression of the miR-199 and 200 families. PLoS One. 2011;6:e16081. doi: 10.1371/journal.pone.0016081. doi:10.1371/journal.pone.0016081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang XH, Qian RZ, Zhang W, Chen SF, Jin HM, Hu RM. MicroRNA-320 expression in myocardial microvascular endothelial cells and its relationship with insulin-like growth factor-1 in type 2 diabetic rats. Clin Exp Pharmacol Physiol. 2009;36:181–188. doi: 10.1111/j.1440-1681.2008.05057.x. doi:10.1111/j.1440-1681.2008.05057.x. [DOI] [PubMed] [Google Scholar]
- 90.Widera C, Gupta SK, Lorenzen JM, Bang C, Bauersachs J, Bethmann K, et al. Diagnostic and prognostic impact of six circulating microRNAs in acute coronary syndrome. J Mol Cell Cardiol. 2011;51:872–875. doi: 10.1016/j.yjmcc.2011.07.011. doi:10.1016/j.yjmcc.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 91.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. doi:10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. doi:10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ma X, Becker Buscaglia LE, Barker JR, Li Y. MicroRNAs in NF-κB signaling. J Mol Cell Biol. 2011;3:159–166. doi: 10.1093/jmcb/mjr007. doi:10.1093/jmcb/mjr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hulsmans M, De Keyzer D, Holvoet P. MicroRNAs regulating oxidative stress and inflammation in relation to obesity and atherosclerosis. FASEB J. 2011;25:2515–2527. doi: 10.1096/fj.11-181149. doi:10.1096/fj.11-181149. [DOI] [PubMed] [Google Scholar]
- 95.Yu D, dos Santos CO, Zhao G, Jiang J, Amigo JD, Khandros E, et al. miR-451 protects against erythroid oxidant stress by repressing 14-3-3ζ. Genes Dev. 2010;24:1620–1633. doi: 10.1101/gad.1942110. doi:10.1101/gad.1942110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xiao J, Zhu X, He B, Zhang Y, Kang B, Wang Z, et al. MiR-204 regulates cardiomyocyte autophagy induced by ischemia-reperfusion through LC3-II. J Biomed Sci. 2011;18:35. doi: 10.1186/1423-0127-18-35. doi:10.1186/1423-0127-18-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dai R, Li J, Liu Y, Yan D, Chen S, Duan C, et al. miR-221/222 suppression protects against endoplasmic reticulum stress-induced apoptosis via p27(Kip1)- and MEK/ERK-mediated cell cycle regulation. Biol Chem. 2010;391:791–801. doi: 10.1515/BC.2010.072. doi:10.1515/BC.2010.072. [DOI] [PubMed] [Google Scholar]
- 98.Frost RJ, van Rooij E. miRNAs as therapeutic targets in ischemic heart disease. J Cardiovasc Transl Res. 2010;3:280–289. doi: 10.1007/s12265-010-9173-y. doi:10.1007/s12265-010-9173-y. [DOI] [PubMed] [Google Scholar]
- 99.Poller W, Hajjar R, Schultheiss HP, Fechner H. Cardiac-targeted delivery of regulatory RNA molecules and genes for the treatment of heart failure. Cardiovasc Res. 2010;86:353–364. doi: 10.1093/cvr/cvq056. doi:10.1093/cvr/cvq056. [DOI] [PMC free article] [PubMed] [Google Scholar]