Abstract
Mitochondria play an important role in regulating the life and death of cells. They provide the cell with energy via oxidative phosphorylation but can quickly turn into death-promoting organelles in response to stress by disrupting adenosine triphosphate synthesis, releasing pro-death proteins, and producing reactive oxygen species. Due to their high-energy requirement, cardiac myocytes are abundant in mitochondria and as a result, particularly vulnerable to mitochondrial defects. Myocardial ischaemia and reperfusion are associated with mitochondrial dysfunction and cell death. Therefore, future therapies will focus on preserving mitochondrial integrity and function in hopes of minimizing the impact of ischaemia/reperfusion (I/R) injury. It is well established that myocardial I/R activates both necrosis and apoptosis, and that blocking either process reduces the levels of injury. However, recent studies have demonstrated that alterations in mitochondrial dynamics or clearance of mitochondria via autophagy also can contribute to cell death in the myocardium. In this review, we will discuss these new developments and their impact on the role of cardiac mitochondria in cell death following reperfusion in the heart.
Keywords: Mitochondria, Fission, Fusion, Autophagy, Myocytes
1. Introduction
Mitochondria are important gate-keepers of life and death in the cell. In cardiac myocytes, the mitochondria occupy up to 30% of the total volume and provide energy to the contracting cell in the form of adenosine triphosphate (ATP) via oxidative phosphorylation. The mitochondria are also very sensitive to alterations in the cellular environment and can quickly switch from being a supporter of life to a promoter of cell death. Therefore, it is not surprising that mitochondrial dysfunction is associated with the loss of myocytes and subsequent development of heart failure. It is well known that myocardial ischaemia and reperfusion results in mitochondrial dysfunction and cell death via both necrosis and apoptosis. Necrosis is induced via opening of the mitochondrial permeability transition pore (mPTP) which results in swelling and subsequent rupture of mitochondria.1 Apoptosis is activated by permeabilization of the outer mitochondrial membrane and release of pro-death proteins, such as cytochrome c by the pro-apoptotic Bcl-2 members Bax and Bak.2,3 Recently, it has become clear that alterations in mitochondrial dynamics and autophagy also contribute to loss of myocytes during ischaemia/reperfusion (I/R).
Studies have shown that mitochondria are highly dynamic organelles that can undergo fission or fusion in response to changes in the cellular environment. In fact, mitochondrial fission has been linked to increased mitochondrial production of reactive oxygen species (ROS),4,5 impaired function,6 and activation of cell death.7–9 In addition, mitochondria are cleared in the cell by autophagy (also called mitophagy) and both defective and excessive mitophagy have been linked to cell death.10–14 Mitochondrial fission and mitophagy are activated in response to ischaemia and reperfusion in the myocardium. In this review, we discuss the roles of mitochondrial dynamics and mitophagy in myocardial cell death following the reperfusion phase.
2. Ischaemia–reperfusion injury and opening of the mPTP
Myocardial infarction occurs when the blood flow to the myocardium is disrupted due to a sudden and sustained thrombotic occlusion of a coronary artery. As a consequence, anaerobic glycolysis is initiated, lactate is produced, and the physiological pH is reduced in the myocytes.15 The influx of hydrogen ions into the mitochondria dissipates the mitochondrial membrane potential (Δψm) and activates the Na+–H+ exchanger (NHE) causing an elevation in sodium levels. To reduce the levels of Na+ in the mitochondria, the sodium–calcium exchanger (Na+–Ca2+ exchanger) is activated, thus introducing Ca2+ into the mitochondria. Upon reperfusion, the Na+–Ca2+ exchanger is reversed and starts extruding Ca2+ from mitochondria.16 The endoplasmic reticulum (ER) is now known to be tethered to mitochondria which facilitates rapid uptake of Ca2+ into mitochondria released from the ER.17,18 It was recently reported that Ca2+ release from the ER was initiated upon reperfusion with subsequent uptake by the mitochondria and loss of Δψm.19 During reperfusion, Ca2+ uptake into mitochondria occurs via the mitochondrial calcium uniporter.20,21
The re-introduction of blood flow to the heart in a timely manner is of paramount importance to salvage the tissue. Paradoxically, this reperfusion phase is also the stage where most of the cells in the heart die. During ischaemia, the mPTP in the inner mitochondrial membrane, which is responsible for causing primarily necrotic cell death, remains closed as the high levels of hydrogen ions inhibits the opening.15 Instead, excess uptake of Ca2+, increased pH, and elevated production of ROS at the onset of reperfusion promote opening of the mPTP. ROS are normal by-products of oxidative phosphorylation. However, the sudden return of oxygen during reperfusion and restoration of mitochondrial respiration will increase mitochondrial ROS formation at levels that exceed the cells antioxidant capacity.22–24 Opening of the mPTP leads to an influx of osmolytes, depolarization of the Δψm, and hydrolysis of ATP. Further insult will cause the inner membrane in the mitochondria to swell with subsequent rupture of the outer membrane, which disrupts its function and releases pro-death proteins into the cytosol. Necrotic cells undergo extensive organelle and cell swelling with subsequent plasma membrane rupture. Also, the release of cellular components into the extracellular space induces inflammation in the tissue. Studies have demonstrated that either inhibiting the mPTP directly25–27 or targeting the upstream regulators of mPTP, e.g. the NHE, lead to cardioprotection.28 Also, mice lacking cyclophilin D (CypD), an essential component of the mPTP, are resistant to I/R injury.29,30 Nevertheless, transient opening of the mPTP has also been demonstrated to play a physiological role in regulating mitochondrial calcium levels for proper metabolic function.31
3. Activation of pro-apoptotic Bax/Bak in the reperfused myocardium
I/R also activates apoptosis in the heart.2,3,32 Apoptosis is an energy-dependent process that triggers cell death through programmed self-destruction. Cells undergoing apoptosis are characterized by cell shrinkage, plasma membrane blebbing, condensation of the nucleus, fragmentation of the DNA, and nucleus. In contrast to necrosis, apoptosis does not trigger an inflammatory response as cellular contents are not released into the extracellular space but are instead engulfed by macrophages. The Bcl-2 proteins are important regulators of mitochondrial integrity in cells. These proteins are categorized into three groups based on their domain architecture: (1) anti-apoptotic Bcl-2, Bcl-XL and Mcl-1, which contain all four Bcl-2 homology (BH) regions and maintain mitochondrial integrity, (2) pro-apoptotic proteins containing only the BH1–3 regions and function to permeabilize the outer mitochondrial membrane (Bax and Bak), and (3) BH3-only proteins, such as Bad, Bnip3, Nix, Bid, and Puma. The BH3-only proteins are initially activated by various cellular stressors, such as oxidative stress, DNA damage, and hypoxia, and they activate downstream effectors Bax and/or Bak. The resulting permeabilization of the outer mitochondrial membrane causes the release of pro-apoptotic proteins, such as cytochrome c, apoptosis-inducing factor, and Smac/DIABLO (Second Mitochondria-derived Activator of Caspases/Direct IAP Binding Protein with Low PI).33 The Bcl-2 proteins have been found to play major roles in I/R. For instance, transgenic mice overexpressing Bcl-2 in the heart had reduced levels of apoptosis, smaller infarcts, and improved cardiac function after I/R compared with wild-type mice.34–36 Similarly, Bax-deficient mice had reduced mitochondrial damage and infarct size after I/R.37
4. Cell death by necrosis, apoptosis, and autophagy in the infarcted myocardium
Necrosis, apoptosis, and autophagy all contribute to cell death in the reperfused heart. During ischaemia, most myocytes are lost via necrosis due to extended oxygen deprivation and ATP depletion. Upon reperfusion, both apoptosis and necrosis are rapidly activated in cells that are still viable in the risk zone. As discussed earlier, mitochondrial Ca2+ overload upon reperfusion and increased oxidative stress results in opening of the mPTP pore, a major contributor of necrotic cell death.16,22,24,29,38 Similarly, increased production of ROS activates the mitochondrial cell death pathway in myocytes.39 Although both necrosis and apoptosis are activated upon reperfusion, the acute loss of cells during reperfusion is primarily due to necrosis. Instead, apoptotic cell death occurs at a lower incidence than necrosis but takes places for an extended period of time after I/R. Studies have demonstrated the presence of apoptotic myocytes as early as 30 min40–42 and as late as 3 days after initiation of reperfusion.43,44 This continuous activation of apoptosis in the myocardium after I/R can result in substantial loss of myocytes overtime which contributes to development of heart failure.
The autophagic–lysosomal pathway is responsible for the degradation and recycling of cytoplasmic components and organelles.45 Autophagy is rapidly enhanced in response to nutrient deprivation to provide the cells with amino acids and fatty acids. It is also important in cellular quality control by degrading cytotoxic protein aggregates and damaged organelles.45 Autophagy is also rapidly enhanced during the ischaemic phase and upon reperfusion.11,14,46 Most studies suggest that a moderate level of autophagy in response to stress is beneficial,11,47,48 whereas excessive autophagy is detrimental to the cell.11,12 A recent study found that autophagy was enhanced as early as 30 min after coronary ligation in both the ischaemic and non-ischaemic regions and that inhibition of autophagy resulted in increased injury.49 This study also found that prolonged ischaemia resulted in impaired autophagy. Other studies have found that inhibition of autophagy genetically or pharmacologically results in increased susceptibility to I/R injury.10,11,49 Similarly, enhancing autophagy in the heart can reduce acute I/R injury.11,47,49 In contrast, some studies have found that enhanced autophagy can be detrimental during reperfusion.11,12 It is very possible that the duration and levels of autophagy plays an important role in determining whether autophagy will be protective or detrimental to myocytes during reperfusion. Excessive autophagy can result in removal of too many essential organelles, such as mitochondria, and therefore contribute to the development of heart failure.
5. Mitochondrial dynamics
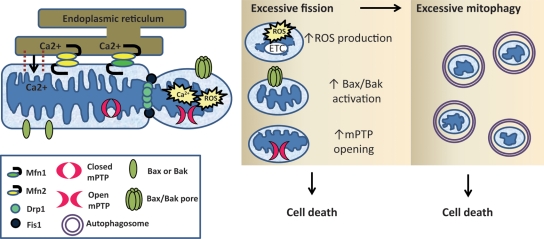
It has become clear that mitochondrial fission and fusion play important roles in regulating life and death of cells (Figure 1). Mitochondria are highly dynamic organelles that are constantly undergoing fission and fusion to adapt to changes in the cellular environment. These processes are regulated by several different GTPases. Fusion of the outer mitochondrial membrane is regulated by mitofusins 1 and 2 (Mfn1 and Mfn2), whereas fusion of the inner membrane is regulated by optic atrophy protein 1 (Opa1).50–53 During mitochondrial fission, dynamin-related protein 1 (Drp1) translocates to the outer mitochondrial membrane, where it interacts with fission protein 1 (Fis1) to promote division of mitochondria.54,55 All of these proteins are expressed in the mammalian heart56–60 and recent studies suggest that they play important roles in regulating cell survival and death in the myocardium.
Figure 1.
Recently elucidated pathways leading to mitochondrial-dependent cell death include excessive mitochondrial fission and mitophagy. Mfn2 on the mitochondria will form homo- or heterodimeric complexes with Mfn2 or Mfn1 on the ER for calcium transfer into the mitochondria. Overloading of mitochondrial calcium leads to opening of the mPTP at the onset of reperfusion. Excessive mitochondrial fission by Drp1 and Fis1 produces dysfunctional mitochondrial fragments that have increased production of ROS. These abnormally small mitochondrial fragments are also more sensitive to mPTP opening and Bax/Bak activation. Moreover, smaller dysfunctional mitochondria are more likely to be removed by autophagy and excessive mitophagy following mitochondrial fission will lead to energetic failure and cell death.
6. Functional role of mitochondrial dynamics in the myocardium
Studies have been initiated to investigate the functional role of mitochondrial dynamics in the myocardium and it is clear that mitochondrial fission and fusion are important for normal heart function. For instance, Wakabayashi et al.61 discovered that deletion of Drp1 was embryonic lethal and that Drp1-deficient embryonic myocytes had reduced contractility. Dorn and colleges found that knockdown of fusion proteins mitochondrial assembly regulatory factor or Opa1 led to development of cardiomyopathy in Drosophila.57 Another study found that a mutation in Drp1 resulted in cardiomyopathy in mice.62 Although these studies suggest that proper fission is important for normal function of mitochondria, alterations in mitochondrial dynamics have also been implicated in I/R injury. Drp1-dependent mitochondrial fission occurs following myocardial ischaemia and pharmacological inhibition of fission reduced I/R injury.56 This study discovered that inhibition of fission during I/R reduced mPTP opening and cell death in cardiac myocytes.56 In addition, Chen et al.63 demonstrated that coronary ligation in adult rat caused a reduction in Opa1 levels which correlated with increased mitochondrial fragmentation. Furthermore, ischaemia induced loss of Opa1 and subsequent mitochondrial fission in H9c2 cells, and overexpression of Opa1 preserved mitochondrial morphology during ischaemia.63
Many studies have reported that excessive Drp1-mediated mitochondrial fission contributes to apoptotic cell death.7–9,64,65 Mitochondrial fission occurs within the same time frame as activation of the proapoptotic Bcl-2 family member Bax and permeabilization of the mitochondrial outer membrane. Drp-1 also co-localizes with Bax at defined foci on the mitochondrial membrane at the onset of apoptosis.8,66 However, fission is not required for Bax/Bak-dependent apoptosis and inhibiting Drp1 only delays cell death.64,65 Parone et al.64 discovered that inhibiting the fission machinery partially prevented cytochrome c release from mitochondria but had no effect on the release of Smac/DIABLO. Similarly, Sugioka et al.65 found that cells with fragmented mitochondria were more sensitive to apoptotic stimuli. Thus, these studies suggest that Drp1-mediated fission is not a prerequisite for apoptosis but rather increases sensitivity to apoptotic stimuli perhaps by enhancing cytochrome c release.
There is evidence that aberrant mitochondrial fission impairs mitochondrial bioenergetic function. Excessive fission results in abnormally small mitochondria with fragmented cristae67 and smaller mitochondria are less efficient in ATP production.6 Other studies have found that fragmented mitochondria have enhanced mitochondrial ROS production.4,5 Since ROS are a major by-product of oxidative phosphorylation, it is possible that fission alters the spatial orientation of the enzymes in the electron transport chain. This in turn may lead to uncoupling of respiration and increased ROS. Alternatively, it is possible that fission of mitochondria results in uneven distribution of mitochondrial components, such as antioxidant enzymes.
The role of mitochondrial fusion proteins in the myocardium is also under investigation. Papanicolaou et al.58 discovered that conditional deletion of Mfn2 in cardiac myocytes resulted in development of mild cardiac hypertrophy and systolic dysfunction in mouse hearts. Surprisingly, the same study found that Mfn2−/− hearts were more resistant to I/R injury and exhibited less cell death after I/R.58 Mfn2-deficient myocytes were more resistant to oxidative stress-induced cell death and Ca2+-mediated mPTP opening,58 suggesting that Mfn2 plays a role in promoting mPTP opening. Apart from mediating mitochondrial fusion, Mfn2 on the mitochondria also functions to tether the mitochondria to the ER. The resulting close proximity of these sub-cellular organelles allow the mitochondria to absorb excess cytotoxic Ca2+ that is released from the ER.17 It is therefore possible that Mfn2-mediated tethering of ER to mitochondria may affect mPTP opening at reperfusion via uptake of excessive levels of Ca2+. Nevertheless, it should be noted that Mfn2 has pleiotropic effects and there are conflicting data with respect to whether Mfn2 promotes cellular survival or death. Several studies have found that Mfn2 can also inhibit apoptosis in cells. For instance, overexpression of a dominant-active form of Mfn2 protected against Bax-mediated cytochrome c release,68 downregulation of Mfn2 exacerbated ceramide-induced mitochondrial dysfunction and cytochrome c release,58 and Mfn2 overexpression protected against IR injury in HL-1 cells.56 In contrast, elevated Mfn2 levels were detected in myocytes undergoing apoptosis following H2O2 treatment, myocardial infarction, or I/R injury.69 Clearly, further studies are needed to understand the functional role of Mfn2 in cardiac cells.
7. Mitochondrial autophagy
Autophagy is a process involved in the elimination of unnecessary or dysfunctional organelles in the cell. The removal of dysfunctional mitochondria by autophagy is also referred to as mitophagy. The importance of regular turnover of mitochondria by autophagy in the heart was demonstrated in the study by Nakai et al.10 who found that genetic disruption of autophagy led to rapid accumulation of dysfunctional mitochondria in myocytes and development of cardiac dysfunction. Autophagy and mitophagy are also induced in the heart in response to I/R.14,47 Properly targeted mitophagy leads to decreased cell death in the ischaemic myocardium.47 Conversely, a disrupted or over-activated autophagic mechanism is implicated in pathological cell death during heart failure,70 hypertrophy,71 and during reperfusion.11,47
It is clear that removal of dysfunctional mitochondria is important for cellular survival. Damaged mitochondria can serve as a source of ROS which can cause further damage to adjacent mitochondria. It was demonstrated that in the rigid and well-defined spatial arrangement of mitochondria in the adult cardiac myocyte, damage to a few mitochondria via laser quickly propagated the deleterious signals to the neighbouring mitochondria via a ROS-dependent process.72 Damaged mitochondria can also leak pro-apoptotic proteins such as cytochrome c which results in activation of caspases and cell death.33,73 Mitophagy has also been shown to play an important role in ischaemic preconditioning presumably by removing weak mitochondria that might be more prone to mPTP opening during reperfusion.74 Thus, mitophagy protects by preventing activation of unnecessary cell death which is important in a post-mitotic cell such as the myocyte. However, it is not surprising that excessive mitophagy is detrimental to myocytes. The contracting myocyte is a high energy requiring cell and removal of too many mitochondria by autophagosomes will create an energy deficiency and result in cell death. In summary, further studies are required to determine exactly what the threshold for mitophagy is in myocytes and why only certain conditions induces excess mitophagy.
8. Regulation of mitophagy by Bnip3 and Nix
Although the Bcl-2 proteins are important regulators of the mitochondrial cell death pathway, they are also important regulators of autophagy. It has been demonstrated that binding of Bcl-2 and Bcl-XL to Beclin-1 at the BH3 region prevents formation of the omegasome, an ER-associated platform for the initial formation of pre-autophagosomal vesicles.75 The omegasome is created when Beclin-1 associates with vesicles containing Vps34, a class III phosphatidylinositol 3-kinases (PI3K).76 The Bcl-2/Beclin-1 complex is also regulated by BH3-only proteins. A BH3-like domain was detected in Beclin-1 and disruption of this domain interfered with the ability to bind to Bcl-XL.75,77 The usage of the mimetic drug ABT-737 for Bad as well as the Bad protein itself disrupted the Beclin-1-Bcl-2/Bcl-XL complex and restored autophagy.75 Expression of Bad also impaired the interaction of Beclin-1 and Bcl-XL at the ER interface.75 Similarly, phosphorylation of Bcl-2 by c-Jun N-terminal kinase 1 (JNK1) inhibited this binding.78 In contrast, binding of Bcl-2 to Beclin-1 is enhanced by the presence of the recently characterized nutrient-deprivation autophagy factor-1.79
Bcl-2/adenovirus E1B nineteen-kilodalton interacting protein (Bnip3) and its homologue Nix (also called Bnip3L) are pro-apoptotic BH3-only proteins which play key roles in the pathogenesis of heart failure.80,81 Nix has been implicated in cardiac hypertrophy and development of cardiomyopathy,81 whereas Bnip3 plays a role in I/R injury and post-infarct remodelling.82–84 Both Bnip3 and Nix have been reported to mediate mitochondrial dysfunction via opening of the mPTP80,85,86 and via activation of Bax/Bak.87,88 Recent studies have demonstrated that Bnip3 and Nix are potent inducers of mitophagy. We found that mitophagy was specifically induced in myocytes overexpressing Bnip3 and that inhibiting this process resulted in increased cell death.13,14,89 Interestingly, Bnip3 induced mitochondrial autophagy in cells lacking a functional mPTP90 and in Bax/Bak-deficient fibroblasts,89 suggesting that Bnip3 activates this process even in the absence of mitochondrial permeabilization and apoptosis. Moreover, Nix has been found to be essential for mitochondrial clearance via autophagy during reticulocyte maturation.91 Similar to our findings with Bnip3, Nix-mediated mitochondrial clearance was normal in Bax/Bak-null reticulocytes and unaffected by inhibitors of the mPTP in wild-type reticulocytes.91 Thus, these data suggest that the induction of Bnip3- and Nix-dependent mitophagy is a separately activated process that is independent of Bax/Bak and the mPTP.
It is still unclear exactly how Bnip3 and Nix mark mitochondria for removal by autophagosomes. It has been suggested that the Nix-dependent loss of Δψm plays an important role in targeting the mitochondria to autophagosomes for clearance during erythroid maturation.92 Sandoval et al.92 found that treatment with compounds that caused mitochondrial depolarization restored mitophagy in Nix−/− cells. We found that Bnip3 selectively induced degradation of proteins involved in oxidative phosphorylation.89 Thus, it is possible that the impairment in the electron transport chain and reduced ATP synthesis serve to target the mitochondria for autophagy. We also recently found that Bnip3-mediated mitophagy involves recruitment of Parkin to mitochondria prior to their autophagy.93 Parkin is an E3 ubiquitin ligase that is selectively recruited to dysfunctional mitochondria to promote their removal by autophagy.94 In addition, Nix and Bnip3 can directly interact with autophagy proteins microtubule-associated protein 1 light chain 3 (LC3) and gamma-aminobutyric acid receptor-associated protein (GABARAP) on the autophagosome,89,95 which might also serve to tether the mitochondrion to the autophagosome.
9. Role of the mPTP in mitophagy
The opening of the mPTP at the onset of reperfusion is synonymous with cardiac cell death. However, opening of the mPTP has also been implicated in the selective removal of damaged mitochondria.96–98 In the heart, starvation-induced mitophagy was found to be reduced in CypD-deficient hearts compared with wild-type mice.99 Cyclosporine A, a known inhibitor of the mPTP, has also been demonstrated to inhibit upregulation of autophagy following reperfusion.29 Although Bnip3 induced opening of the mPTP,86 we found that the presence of CsA had no effect on Bnip3-mediated mitophagy in cardiac myocytes.90 Also, Bnip3 was still a potent inducer of autophagy in mouse embryonic fibroblasts (MEFs) isolated from the CypD−/− mice. CypD−/− MEFs lack a functional mPTP and are resistant to opening induced by hydrogen peroxide. These studies suggest that the role of mPTP in mitophagy is context dependent.
10. Mitochondrial dynamics and mitophagy
It has become clear that mitochondrial dynamics play an important role in regulating mitophagy. The link between mitochondrial morphology and mitophagy was initially established when Twig et al.100 demonstrated that mitochondrial fission produced fragments with different membrane potential. The mitochondrial fragment with higher membrane potential had a higher chance of re-fusion, while the fragment with lower membrane potential was subjected to autophagy.100 Gomes et al.6 subsequently demonstrated that fused mitochondria were spared from starvation-induced autophagy. Since then, many studies have reported that inhibition of mitochondrial fission or induction of fusion abolished mitophagy in cells.67,93 We recently reported that Drp1-mediated mitochondrial fission is required for mitophagy by Bnip3 in adult cardiac myocytes and inhibition of fission or induction of fusion inhibited mitophagy.93 It is still unknown why mitochondria must undergo fission before removal by autophagosomes. However, it is possible that mitochondria are too large to be engulfed by autophagosomes and fission will produce smaller fragments that can more easily be engulfed by the autophagosomes or fission is required to segregate dysfunctional mitochondria prior to removal by autophagy. Interestingly, since fission is a prerequisite for mitophagy, it is possible that too much fission will also lead to excessive mitophagy and subsequent cell death.
11. Conclusion
It is clear that mitochondrial dysfunction is a major contributor to loss of myocytes during myocardial ischaemia and subsequent reperfusion. With a reduced number of myocytes, the heart is no longer able to sustain contractility and heart failure develops. This is a major clinical problem and development of novel therapeutic interventions to reduce myocardial cell death following infarction is ongoing. Since mitochondria play key roles in regulating life and death of the cardiac myocytes, future therapies should be aimed at preserving or restoring mitochondrial function in the myocytes. Mitochondrial activations of necrosis and apoptosis are both known contributors to cell death in the reperfused myocardium. Mitochondrial dynamics and mitophagy are also important processes involved in regulating both cell death and survival in the myocardium. Thus, these pathways might represent novel therapeutic targets to preserve mitochondrial function in the reperfused myocardium. However, many important questions regarding the effect of modulating these processes in the heart remain to be answered. A better understanding of the complex mechanisms associated with mitochondrial dysfunction is necessary to identify potential novel targets and therapeutic strategies to preserve mitochondrial function and cell viability in the reperfused myocardium.
Conflict of interest: none declared.
Funding
This manuscript was supported by NIH grants R01HL087023, R01HL101217, and R01HL092136.
References
- 1.Schaper J, Elsasser A, Kostin S. The role of cell death in heart failure. Circ Res. 1999;85:867–869. doi: 10.1161/01.res.85.9.867. [DOI] [PubMed] [Google Scholar]
- 2.Anversa P, Cheng W, Liu Y, Leri A, Redaelli G, Kajstura J. Apoptosis and myocardial infarction. Basic Res Cardiol. 1998;93(Suppl. 3):8–12. doi: 10.1007/s003950050195. [DOI] [PubMed] [Google Scholar]
- 3.Buja LM, Entman ML. Modes of myocardial cell injury and cell death in ischemic heart disease. Circulation. 1998;98:1355–1357. doi: 10.1161/01.cir.98.14.1355. [DOI] [PubMed] [Google Scholar]
- 4.Shenouda SM, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE, et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation. 2011;124:444–453. doi: 10.1161/CIRCULATIONAHA.110.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci USA. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomes LC, Di Benedetto G, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol. 2011;13:589–598. doi: 10.1038/ncb2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breckenridge DG, Stojanovic M, Marcellus RC, Shore GC. Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J Cell Biol. 2003;160:1115–1127. doi: 10.1083/jcb.200212059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 9.Germain M, Mathai JP, McBride HM, Shore GC. Endoplasmic reticulum BIK initiates DRP1-regulated remodelling of mitochondrial cristae during apoptosis. EMBO J. 2005;24:1546–1556. doi: 10.1038/sj.emboj.7600592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 11.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 12.Zhai P, Sciarretta S, Galeotti J, Volpe M, Sadoshima J. Differential roles of GSK-3{beta} during myocardial ischemia and ischemia/reperfusion. Circ Res. 2011;109:502–511. doi: 10.1161/CIRCRESAHA.111.249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamacher-Brady A, Brady NR, Gottlieb RA, Gustafsson AB. Autophagy as a protective response to Bnip3-mediated apoptotic signaling in the heart. Autophagy. 2006;2:307–309. doi: 10.4161/auto.2947. [DOI] [PubMed] [Google Scholar]
- 14.Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, et al. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007;14:146–157. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- 15.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths EJ, Ocampo CJ, Savage JS, Rutter GA, Hansford RG, Stern MD, et al. Mitochondrial calcium transporting pathways during hypoxia and reoxygenation in single rat cardiomyocytes. Cardiovasc Res. 1998;39:423–433. doi: 10.1016/s0008-6363(98)00104-7. [DOI] [PubMed] [Google Scholar]
- 17.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 18.Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu FH, Tian Z, Zhang WH, Zhao YJ, Li HL, Ren H, et al. Calcium-sensing receptors regulate cardiomyocyte Ca2+ signaling via the sarcoplasmic reticulum-mitochondrion interface during hypoxia/reoxygenation. J Biomed Sci. 2010;17:50. doi: 10.1186/1423-0127-17-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 23.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nazareth W, Yafei N, Crompton M. Inhibition of anoxia-induced injury in heart myocytes by cyclosporin A. J Mol Cell Cardiol. 1991;23:1351–1354. doi: 10.1016/0022-2828(91)90181-k. [DOI] [PubMed] [Google Scholar]
- 26.Griffiths EJ, Ocampo CJ, Savage JS, Stern MD, Silverman HS. Protective effects of low and high doses of cyclosporin A against reoxygenation injury in isolated rat cardiomyocytes are associated with differential effects on mitochondrial calcium levels. Cell Calcium. 2000;27:87–95. doi: 10.1054/ceca.1999.0094. [DOI] [PubMed] [Google Scholar]
- 27.Xu M, Wang Y, Hirai K, Ayub A, Ashraf M. Calcium preconditioning inhibits mitochondrial permeability transition and apoptosis. Am J Physiol Heart Circ Physiol. 2001;280:H899–H908. doi: 10.1152/ajpheart.2001.280.2.H899. [DOI] [PubMed] [Google Scholar]
- 28.Villa-Abrille MC, Cingolani E, Cingolani HE, Alvarez BV. Silencing of cardiac mitochondrial NHE1 prevents mitochondrial permeability transition pore opening. Am J Physiol Heart Circ Physiol. 2011;300:H1237–H1251. doi: 10.1152/ajpheart.00840.2010. [DOI] [PubMed] [Google Scholar]
- 29.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 30.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 31.Elrod JW, Wong R, Mishra S, Vagnozzi RJ, Sakthievel B, Goonasekera SA, et al. Cyclophilin D controls mitochondrial pore-dependent Ca(2+) exchange, metabolic flexibility, and propensity for heart failure in mice. J Clin Invest. 2010;120:3680–3687. doi: 10.1172/JCI43171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kajstura J, Cheng W, Reiss K, Clark WA, Sonnenblick EH, Krajewski S, et al. Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest. 1996;74:86–107. [PubMed] [Google Scholar]
- 33.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brocheriou V, Hagege AA, Oubenaissa A, Lambert M, Mallet VO, Duriez M, et al. Cardiac functional improvement by a human Bcl-2 transgene in a mouse model of ischemia/reperfusion injury. J Gene Med. 2000;2:326–333. doi: 10.1002/1521-2254(200009/10)2:5<326::AID-JGM133>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 35.Chen Z, Chua CC, Ho YS, Hamdy RC, Chua BH. Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2001;280:H2313–H2320. doi: 10.1152/ajpheart.2001.280.5.H2313. [DOI] [PubMed] [Google Scholar]
- 36.Imahashi K, Schneider MD, Steenbergen C, Murphy E. Transgenic expression of Bcl-2 modulates energy metabolism, prevents cytosolic acidification during ischemia, and reduces ischemia/reperfusion injury. Circ Res. 2004;95:734–741. doi: 10.1161/01.RES.0000143898.67182.4c. [DOI] [PubMed] [Google Scholar]
- 37.Hochhauser E, Kivity S, Offen D, Maulik N, Otani H, Barhum Y, et al. Bax ablation protects against myocardial ischemia-reperfusion injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2003;284:H2351–H2359. doi: 10.1152/ajpheart.00783.2002. [DOI] [PubMed] [Google Scholar]
- 38.Crompton M, Costi A, Hayat L. Evidence for the presence of a reversible Ca2+-dependent pore activated by oxidative stress in heart mitochondria. Biochem J. 1987;245:915–918. doi: 10.1042/bj2450915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cook SA, Sugden PH, Clerk A. Regulation of bcl-2 family proteins during development and in response to oxidative stress in cardiac myocytes: association with changes in mitochondrial membrane potential. Circ Res. 1999;85:940–949. doi: 10.1161/01.res.85.10.940. [DOI] [PubMed] [Google Scholar]
- 40.Taki J, Higuchi T, Kawashima A, Tait JF, Kinuya S, Muramori A, et al. Detection of cardiomyocyte death in a rat model of ischemia and reperfusion using 99mTc-labeled annexin V. J Nucl Med. 2004;45:1536–1541. [PubMed] [Google Scholar]
- 41.Dumont EA, Reutelingsperger CP, Smits JF, Daemen MJ, Doevendans PA, Wellens HJ, et al. Real-time imaging of apoptotic cell-membrane changes at the single-cell level in the beating murine heart. Nat Med. 2001;7:1352–1355. doi: 10.1038/nm1201-1352. [DOI] [PubMed] [Google Scholar]
- 42.Dumont EA, Hofstra L, van Heerde WL, van den Eijnde S, Doevendans PA, DeMuinck E, et al. Cardiomyocyte death induced by myocardial ischemia and reperfusion: measurement with recombinant human annexin-V in a mouse model. Circulation. 2000;102:1564–1568. doi: 10.1161/01.cir.102.13.1564. [DOI] [PubMed] [Google Scholar]
- 43.Zhao ZQ, Nakamura M, Wang NP, Wilcox JN, Shearer S, Ronson RS, et al. Reperfusion induces myocardial apoptotic cell death. Cardiovasc Res. 2000;45:651–660. doi: 10.1016/s0008-6363(99)00354-5. [DOI] [PubMed] [Google Scholar]
- 44.Zhao ZQ, Velez DA, Wang NP, Hewan-Lowe KO, Nakamura M, Guyton RA, et al. Progressively developed myocardial apoptotic cell death during late phase of reperfusion. Apoptosis. 2001;6:279–290. doi: 10.1023/a:1011335525219. [DOI] [PubMed] [Google Scholar]
- 45.Dunn WA., Jr Autophagy and related mechanisms of lysosome-mediated protein degradation. Trends Cell Biol. 1994;4:139–143. doi: 10.1016/0962-8924(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 46.Elsasser A, Vogt AM, Nef H, Kostin S, Mollmann H, Skwara W, et al. Human hibernating myocardium is jeopardized by apoptotic and autophagic cell death. J Am Coll Cardiol. 2004;43:2191–2199. doi: 10.1016/j.jacc.2004.02.053. [DOI] [PubMed] [Google Scholar]
- 47.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–29787. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 48.Yan L, Vatner DE, Kim SJ, Ge H, Masurekar M, Massover WH, et al. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci USA. 2005;102:13807–13812. doi: 10.1073/pnas.0506843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanamori H, Takemura G, Goto K, Maruyama R, Ono K, Nagao K, et al. Autophagy limits acute myocardial infarction induced by permanent coronary artery occlusion. Am J Physiol Heart Circ Physiol. 2011;300:H2261–H2271. doi: 10.1152/ajpheart.01056.2010. [DOI] [PubMed] [Google Scholar]
- 50.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci USA. 2004;101:15927–15932. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ishihara N, Eura Y, Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci. 2004;117:6535–6546. doi: 10.1242/jcs.01565. [DOI] [PubMed] [Google Scholar]
- 53.Legros F, Lombes A, Frachon P, Rojo M. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol Biol Cell. 2002;13:4343–4354. doi: 10.1091/mbc.E02-06-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoon Y, Krueger EW, Oswald BJ, McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol. 2003;23:5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 57.Dorn GW, 2nd, Clark CF, Eschenbacher WH, Kang MY, Engelhard JT, Warner SJ, et al. MARF and Opa1 control mitochondrial and cardiac function in Drosophila. Circ Res. 2011;108:12–17. doi: 10.1161/CIRCRESAHA.110.236745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Papanicolaou KN, Khairallah RJ, Ngoh GA, Chikando A, Luptak I, O'Shea KM, et al. Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol Cell Biol. 2011;31:1309–1328. doi: 10.1128/MCB.00911-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet. 2000;26:207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- 60.Parra V, Eisner V, Chiong M, Criollo A, Moraga F, Garcia A, et al. Changes in mitochondrial dynamics during ceramide-induced cardiomyocyte early apoptosis. Cardiovasc Res. 2008;77:387–397. doi: 10.1093/cvr/cvm029. [DOI] [PubMed] [Google Scholar]
- 61.Wakabayashi J, Zhang Z, Wakabayashi N, Tamura Y, Fukaya M, Kensler TW, et al. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J Cell Biol. 2009;186:805–816. doi: 10.1083/jcb.200903065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ashrafian H, Docherty L, Leo V, Towlson C, Neilan M, Steeples V, et al. A mutation in the mitochondrial fission gene Dnm1l leads to cardiomyopathy. PLoS Genet. 2010;6:e1001000. doi: 10.1371/journal.pgen.1001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen L, Gong Q, Stice JP, Knowlton AA. Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res. 2009;84:91–99. doi: 10.1093/cvr/cvp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parone PA, James DI, Da Cruz S, Mattenberger Y, Donze O, Barja F, et al. Inhibiting the mitochondrial fission machinery does not prevent Bax/Bak-dependent apoptosis. Mol Cell Biol. 2006;26:7397–7408. doi: 10.1128/MCB.02282-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sugioka R, Shimizu S, Tsujimoto Y. Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J Biol Chem. 2004;279:52726–52734. doi: 10.1074/jbc.M408910200. [DOI] [PubMed] [Google Scholar]
- 66.Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, et al. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol. 2002;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Graber S, et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006;25:3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neuspiel M, Zunino R, Gangaraju S, Rippstein P, McBride H. Activated mitofusin 2 signals mitochondrial fusion, interferes with Bax activation, and reduces susceptibility to radical induced depolarization. J Biol Chem. 2005;280:25060–25070. doi: 10.1074/jbc.M501599200. [DOI] [PubMed] [Google Scholar]
- 69.Shen T, Zheng M, Cao C, Chen C, Tang J, Zhang W, et al. Mitofusin-2 is a major determinant of oxidative stress-mediated heart muscle cell apoptosis. J Biol Chem. 2007;282:23354–23361. doi: 10.1074/jbc.M702657200. [DOI] [PubMed] [Google Scholar]
- 70.Shimomura H, Terasaki F, Hayashi T, Kitaura Y, Isomura T, Suma H. Autophagic degeneration as a possible mechanism of myocardial cell death in dilated cardiomyopathy. Jpn Circ J. 2001;65:965–968. doi: 10.1253/jcj.65.965. [DOI] [PubMed] [Google Scholar]
- 71.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, et al. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou L, Aon MA, Almas T, Cortassa S, Winslow RL, O'Rourke B. A reaction-diffusion model of ROS-induced ROS release in a mitochondrial network. PLoS Comput Biol. 2010;6:e1000657. doi: 10.1371/journal.pcbi.1000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zou H, Li Y, Liu X, Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- 74.Huang C, Andres AM, Ratliff EP, Hernandez G, Lee P, Gottlieb RA. Preconditioning involves selective mitophagy mediated by Parkin and p62/SQSTM1. PLoS ONE. 2011;6:e20975. doi: 10.1371/journal.pone.0020975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, et al. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol. 2008;182:685–701. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 78.Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang NC, Nguyen M, Germain M, Shore GC. Antagonism of Beclin 1-dependent autophagy by BCL-2 at the endoplasmic reticulum requires NAF-1. EMBO J. 2010;29:606–618. doi: 10.1038/emboj.2009.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Regula KM, Ens K, Kirshenbaum LA. Inducible expression of BNIP3 provokes mitochondrial defects and hypoxia-mediated cell death of ventricular myocytes. Circ Res. 2002;91:226–231. doi: 10.1161/01.res.0000029232.42227.16. [DOI] [PubMed] [Google Scholar]
- 81.Yussman MG, Toyokawa T, Odley A, Lynch RA, Wu G, Colbert MC, et al. Mitochondrial death protein Nix is induced in cardiac hypertrophy and triggers apoptotic cardiomyopathy. Nat Med. 2002;8:725–730. doi: 10.1038/nm719. [DOI] [PubMed] [Google Scholar]
- 82.Kubli DA, Quinsay MN, Huang C, Lee Y, Gustafsson AB. Bnip3 functions as a mitochondrial sensor of oxidative stress during myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2008;295:H2025–H2031. doi: 10.1152/ajpheart.00552.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Graham RM, Thompson JW, Wei J, Bishopric NH, Webster KA. Regulation of Bnip3 death pathways by calcium, phosphorylation, and hypoxia-reoxygenation. Antioxid Redox Signal. 2007;9:1309–1315. doi: 10.1089/ars.2007.1726. [DOI] [PubMed] [Google Scholar]
- 84.Diwan A, Krenz M, Syed FM, Wansapura J, Ren X, Koesters AG, et al. Inhibition of ischemic cardiomyocyte apoptosis through targeted ablation of Bnip3 restrains postinfarction remodeling in mice. J Clin Invest. 2007;117:2825–2833. doi: 10.1172/JCI32490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kubasiak LA, Hernandez OM, Bishopric NH, Webster KA. Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc Natl Acad Sci USA. 2002;99:12825–12830. doi: 10.1073/pnas.202474099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vande Velde C, Cizeau J, Dubik D, Alimonti J, Brown T, Israels S, et al. BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. Mol Cell Biol. 2000;20:5454–5468. doi: 10.1128/mcb.20.15.5454-5468.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kubli DA, Ycaza JE, Gustafsson AB. Bnip3 mediates mitochondrial dysfunction and cell death through Bax and Bak. Biochem J. 2007;405:407–415. doi: 10.1042/BJ20070319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen Y, Lewis W, Diwan A, Cheng EH, Matkovich SJ, Dorn GW., 2nd Dual autonomous mitochondrial cell death pathways are activated by Nix/BNip3L and induce cardiomyopathy. Proc Natl Acad Sci USA. 2010;107:9035–9042. doi: 10.1073/pnas.0914013107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rikka S, Quinsay MN, Thomas RL, Kubli DA, Zhang X, Murphy AN, et al. Bnip3 impairs mitochondrial bioenergetics and stimulates mitochondrial turnover. Cell Death Differ. 2011;18:721–731. doi: 10.1038/cdd.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Quinsay MN, Thomas RL, Lee Y, Gustafsson AB. Bnip3-mediated mitochondrial autophagy is independent of the mitochondrial permeability transition pore. Autophagy. 2010;6:855–862. doi: 10.4161/auto.6.7.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schweers RL, Zhang J, Randall MS, Loyd MR, Li W, Dorsey FC, et al. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc Natl Acad Sci USA. 2007;104:19500–19505. doi: 10.1073/pnas.0708818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, Prchal JT, Chen M, et al. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454:232–235. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee Y, Lee HY, Hanna RA, Gustafsson AB. Mitochondrial autophagy by Bnip3 involves Drp1-mediated mitochondrial fission and recruitment of parkin in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2011;301:H1924–H1931. doi: 10.1152/ajpheart.00368.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Novak I, Kirkin V, McEwan DG, Zhang J, Wild P, Rozenknop A, et al. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 2010;11:45–51. doi: 10.1038/embor.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Elmore SP, Qian T, Grissom SF, Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 2001;15:2286–2287. doi: 10.1096/fj.01-0206fje. [DOI] [PubMed] [Google Scholar]
- 97.Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008;15:171–182. doi: 10.1038/sj.cdd.4402233. [DOI] [PubMed] [Google Scholar]
- 98.Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 99.Carreira RS, Lee Y, Ghochani M, Gustafsson AB, Gottlieb RA. Cyclophilin D is required for mitochondrial removal by autophagy in cardiac cells. Autophagy. 2010;6:462–472. doi: 10.4161/auto.6.4.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]