Abstract
Persistent myocardial ischaemia causes cell death if not rescued by early reperfusion. Millions of years in nature's laboratory have evolved protective responses that ‘condition’ the heart (and other tissues) to adapt to stressors, and these responses are applicable to the relatively new societal stress of myocardial ischaemia and reperfusion injury. Conditioning can be applied before (preconditioning), during (perconditioning), or after (postconditioning) the ischaemic stressor by imposing short periods of non-lethal ischaemia separated by brief periods of reperfusion. This conditioning protects multiple cell types and induces or rebalances a number of physiological and molecular pathways that ultimately attenuate necrosis and apoptosis. The seemingly disparate pathways may converge directly or indirectly on the mitochondria as a final effector, but other pathways not affecting mitochondria broaden the mechanisms of cardioprotection. The potential downsides of imposing even brief ischaemia directly on the heart somewhat tempered the enthusiasm for applying conditioning stimuli to the heart, but this hurdle was surmounted by applying ischaemia to remote organs and tissues in pre-, per-, and postconditioning. Although the clinical translation of remote per- and postconditioning has been rapid compared with classical preconditioning, there are numerous basic questions that require further investigation, and wider adoption awaits large-scale randomized clinical trials. Pharmacological mimetics may provide another important therapeutic approach by which to treat evolving myocardial infarction.
Keywords: Reperfusion injury, Preconditioning, Postconditioning, Mitochondrial permeability transition pore, Myocardial infarction, Apoptosis, Endothelium
1. Introduction
It is firmly established that the myocardium can be salvaged by early reperfusion.1 Percutaneous coronary intervention (PCI) has become an important treatment modality to initiate that reperfusion. Smaller infarct size has been associated with lower mortality;2 reduction of infarction size by pharmacological therapy in acute myocardial infarction patients undergoing percutaneous coronary intervention stenting has been associated with improved left ventricular performance and clinical outcomes (death, reinfarction).3 Therefore, a reduction of infarct size is a clinically valuable strategy. However, reperfusion itself may contribute to the pathophysiology of infarction and post-ischaemic complications (reperfusion injury). Reperfusion injury may be responsible for up to 50% or more of the ultimate infarct size4 and is an important contributor to post-surgical mortality and morbidity as well.5 Clinically, the extent of myocardial salvage by early reperfusion may not be realized because of cell injury and death initiated by reperfusion itself.
The numerous mechanisms that contribute to myocardial reperfusion injury have been reviewed in depth elsewhere,4,6 and select mechanisms are discussed in other articles in this focused issue. It is important to recognize that multiple mechanisms are initiated at the onset of reflow and that these mechanisms may act in concert to contribute to necrosis, apoptosis, and dysfunction of organelles, the coronary vascular endothelium, and contractile function. This review will discuss (i) the algorithm and mechanisms of ischaemic postconditioning from data derived from experimental studies, (ii) remote conditioning applied before, during, and after ischaemia, (iii) the clinical studies on direct and remote post- and perconditioning. The sections on clinical studies appear under the relevant conditioning stimulus, rather than discussing them under one section. However, clinical studies are summarized in a table for convenience. Where possible, commonalities between pre-, per-, and postconditioning will be discussed, thereby placing the three cardioprotective manoeuvres under the single umbrella of ‘conditioning’ responses to ischaemia–reperfusion.
2. General comments on ‘conditioning’ of the ischaemic myocardium
Conditioning of the myocardium describes the stimulation of innate cardioprotective mechanisms by short periods of non-lethal ischaemia that target lethal tissue injury caused by a longer period of ischaemia. The conditioning stimulus can be applied before (preconditioning), during (perconditioning), or immediately after (postconditioning) the longer ‘index’ ischaemia. Specifically to the heart, the original studies on preconditioning by Murry et al.7 demonstrated that local intra-organ conditioning exerted by brief periods of coronary artery occlusion applied before the prolonged lethal ischaemia reduced infarct size. However, it has also been shown that ischaemia applied to a distant organ before index ischaemia (remote preconditioning) reduces infarct size. In this article, we will focus on ischaemic postconditioning (PostC) and remote preconditioning (rIPC), perconditioning (rPerC), and postconditioning (rPostC). Pharmacological postconditioning will be discussed elsewhere in this focused series.
3. Ischaemic postconditioning
3.1. Experimental studies
The PostC algorithm involves applying alternating cycles of brief reperfusion (or reoxygenation) interrupted by brief ischaemia (or hypoxia–anoxia) starting at the end of the index ischaemia. This manoeuvre exerts its effects during the immediate PostC period without affecting the pathophysiology of ischaemia. This clearly differs from the time course during which either ischaemic preconditioning (IPC) or perconditioning (PerC) exert cardioprotection, in which there may be a component of biochemical adaptation of the myocardium to ischaemia.
There are three aspects to the PostC cycle: the duration of the initial reperfusion phase, the duration of each subsequent ischaemia and reperfusion cycle, and the number of cycles applied. The PostC procedure should be initiated immediately upon the onset of reperfusion; the first phase of reperfusion is considered the first cycle. The duration of the index ischaemia itself may influence the effect of PostC. PostC may increase infarct size after brief periods of ischaemia while reducing infarct size after longer periods of index ischaemia.8 In addition, there may be a duration of occlusion beyond which PostC does not reduce infarct size.9 Finally, the benefits of PostC are lost if the onset of the first reperfusion cycle is delayed.10
A reduction in infarct size is the signature endpoint of PostC. In 2003, Zhao et al.11 first reported a ∼50% reduction of infarct size (relative to the area at risk) by PostC in an acute canine model of 60 min left anterior descending artery (LAD) occlusion followed by 3 h of reperfusion. Since this initial report, numerous studies have confirmed that PostC reduces infarct size in all species tested (reviewed in Vinten-Johansen et al.12), including humans (summarized in Hansen et al.13 and Hausenloy and Yellon14). PostC has been confirmed in in vitro15 and ex vivo16 models. As with most cardioprotective manoeuvres, there are both positive and negative studies (reviewed in Vinten-Johansen et al.12). The usual suspects may be responsible for such variability, i.e. species variability, differences in the PostC protocol, and types of anaesthesia, but may also include gender, age, and presence of co-morbidities. There is some discrepancy on the magnitude of cardioprotection achieved by PostC relative to IPC. In vivo studies suggest that PostC exerts a similar reduction in either infarct size or enzyme release with IPC and PostC.17,18 In addition, there may be a duration of ischaemia beyond which PostC is not effective, a so-called threshold phenomenon. Extending this threshold to exert cardioprotection after longer durations of ischaemia may require a greater number of cycles, longer durations of reperfusion or ischaemia cycles, or the addition of adjunctive drugs. However, the description and mechanisms of such a threshold remain unresolved.
PostC has also been reported to reduce apoptosis in vivo.19 Taki et al.20 reported that PostC attenuated the uptake of 99mTc-annexin V in the area-at-risk myocardium. In vivo, PostC attenuated known triggers of apoptosis such as oxidants [reactive oxygen species (ROS), peroxynitrite OONO−] and Ca2+ overload, and by attenuating pro-apoptotic pathways21,22 and regulatory factors.23 PostC rebalances the apoptotic regulatory system by increasing anti-apoptotic regulators (Bcl-2)23 and pathways [Janus kinase 2 (JAK2)–signal transducer and activator of transcription 3 (STAT3)24]. However, few of the above studies have confirmed apoptosis by morphological criteria such as appearance of apoptotic bodies. Future studies should include this morphological endpoint.
3.1.1. Mechanisms of postconditioning
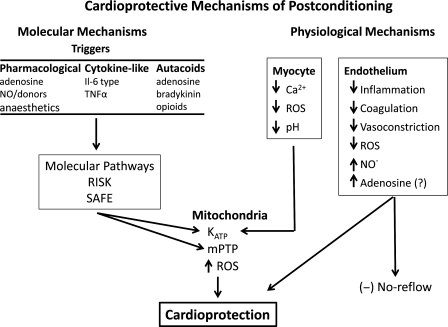
The concept of triggers, mediators, and effectors used in describing IPC is also applicable to PostC. The proximal triggers of PostC include autacoid substances released during PostC that activate cell surface G-protein-coupled receptors or other receptors (Figure 1). The transduction mechanisms then activate intermediary substances or pathways that converge on effectors which may include the mitochondrial permeability transition pore (mPTP) and KATP channel. There are also physiological effects, such as maintenance of tissue acidosis, that may be independent of these molecular pathways that exert protection. Therefore, multiple mechanisms are engaged by PostC, as summarized in Figure 1. Overall, the cardioprotective mechanisms can be divided into physiological mechanisms and prosurvival molecular pathways. The multiplicity and interactions between the physiological and molecular pathways triggered by PostC may explain why PostC successfully attenuates reperfusion injury compared with interventions that trigger a single mechanism or target.
Figure 1.
Overview of physiological and molecular mechanisms involved in postconditioning. The mechanisms are divided into two general categories: molecular and physiological. In the molecular category, the scheme of triggers, mediators, and effectors developed for preconditioning is also applicable to postconditioning, and likely to perconditioning and their respective remote counterparts. Note that elements of both categories converge on the mPTP, but this is not the sole factor leading to cardioprotection. RISK, reperfusion injury salvage kinase; SAFE, survivor activating factor enhancement; mPTP, mitochondrial permeability transition pore; KATP, ATP-sensitive potassium channels; ROS, reactive oxygen species; NO, nitric oxide.
3.1.2. Physiological mechanisms
Adenosine, bradykinin, and endogenous opioids may act as the initial trigger for PostC. These autacoids are present during and modulated by PostC.25,26 Adenosine was the first such autacoid to be associated with PostC.25 All four types of adenosine receptors have been implicated in PostC. However, data on receptor subtype involvement are inconsistent. While a specific A1 receptor inhibitor did not abolish infarct size reduction by PostC,25 specific knock-out of the A1 receptor abolished PostC.27 PostC can also be triggered by activation of other receptors, including the bradykinin B2 receptor, opioid receptor, and the sphingosine-1-phosphate receptor.28,29
3.1.3. Protection of the coronary vascular endothelium in postconditioning
The coronary vascular endothelium is important in regulating blood flow and maintaining a balance in local haemostasis (coagulation) and inflammation, in part through the release of endogenous factors such as adenosine, nitric oxide (NO·), endothelin-1, leucotrienes, and thromboxane A2. Predictably, dysfunction of the coronary vascular endothelium results in (i) local vasoconstriction secondary to the impaired release of NO· and increased release of vasoconstrictors; NO· has potent anti-neutrophil effects which may be lost if NO· generation is reduced or if NO· is neutralized by −O2· to form ONOO−, (ii) increased coagulation and production of thrombotic material, (iii) activation of neutrophils and the inflammatory cascade, in part due to impaired NO· bioavailability, and (iv) loss of barrier function with increased fluid and solute extravasation. The activation of neutrophils during ischaemia–reperfusion has been shown to contribute to the pathophysiological development of infarct size.30,31 A study by Granfeldt et al.32 reported that PostC directly inhibits superoxide anion production by neutrophils sampled from the area-at-risk coronary venous drainage.
PostC attenuates coronary vascular endothelial dysfunction observed after the index ischaemia.11 Increased bioavailability of NO· after PostC may be due to increased phosphorylation of endothelial nitric oxide synthase (eNOS) at Ser1177,9 reduced oxidant generation,15,17 and preservation of endogenous antioxidants.33 The ability of PostC to attenuate neutrophil adhesion and extravascular accumulation mirrors the cardioprotective effects of NO· and adenosine.25 PostC also attenuates the appearance of pro-inflammatory mediators in plasma after ischaemia–reperfusion.19
3.1.4. Maintaining tissue acidosis in postconditioning
Reperfusion quickly reverses tissue acidosis chiefly by activating the sarcolemmal Na+/H+ exchanger, and the Na+ bicarbonate co-transporter, which favours the accumulation of Na+ and secondarily Ca2+, the latter by reverse activity of the Na+/Ca2+ exchanger. The rapid normalization of pH, accumulation of intracellular Ca2+, and a build-up of ROS increase opening of the mPTP. Heusch34 first postulated that the cardioprotection of PostC was related to maintaining tissue acidosis. The studies of Cohen et al.35 suggest that maintaining acidosis during early reperfusion inhibits opening of the mPTP. Reperfusing the ischaemic myocardium with acidic (re)perfusate mimicked PostC protection in isolated rabbit hearts35 and pigs36 while an alkalotic perfusate blocked PostC protection.35 Indeed, PostC maintained tissue acidosis during early reperfusion and thereby delayed the onset of realkalinization.12,37 Neutralizing tissue pH with NaHCO3 not only abrogated the infarct-sparing effects of PostC, but also reduced the phosphorylation of the survival kinases Akt and ERK in the myocardium.38 A recent study using31 P-NMR demonstrates that PostC delayed intracellular pH recovery, reduced protease calpain-dependent α-fodrin proteolysis, improved left ventricular contractility, and decreased LDH release and infarct size. Maintaining acidosis during early reperfusion may also be related to inhibition of the Na+/H+ exchanger via a protein kinase G (PKG)-dependent pathway.39
3.1.5. Activation of survival pathways in postconditioning
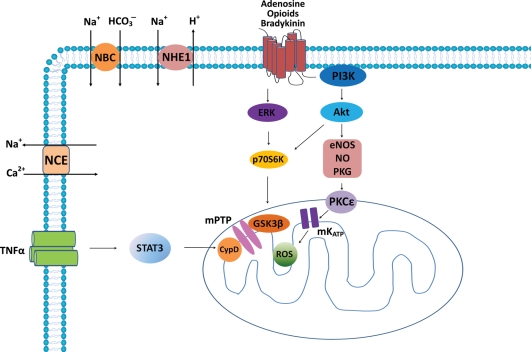
There are two major molecular pathways that are recruited in PostC: the reperfusion injury salvage kinase (RISK) pathway and survivor activating factor enhancement (SAFE) pathway.40,41 The RISK pathway activated by PostC salvages the myocardium in most of mammal species except the porcine model.42 PostC induces phosphorylation of phosphatidylinositol 3-kinases (PI3K) and extracellular signal-regulated kinase (ERK)1/2, and subsequently activates the downstream eNOS and p70S6 kinase, and inhibits (phosphorylates) glycogen synthase kinase-3β (GSK3β) (Figure 2). These molecules are involved in the RISK pathway.40,41 A new study shows that the p85 subunit of PI3K is co-localized with caveolin 3 through MG53 which is also required for PostC.43 The RISK pathway also includes p38MAPK and c-Jun N-terminal kinase (JNK), PKG, and protein kinase C (PKC).40,41
Figure 2.
Major signalling pathways identified in ischaemic postconditioning (PostC). Several autacoid substances (adenosine, bradykinin, opioids, TNFα) are produced endogenously and act as triggers in PostC. These autacoids bind to G-protein-coupled receptors (GPCR) and stimulate RISK pathway which recruits PI3K/Akt and ERK, activates PKCɛ through eNOS/NO/PKG, and phosphorylates (inhibits) GSK3β, thereby delaying opening of mPTP. PKCɛ may stimulate ROS generation through activating mKATP channels, and consequentially inhibit mPTP opening. The RISK pathway also induces some other anti-apoptosis and anti-necrosis pathways. An alternative SAFE pathway recruits TNFα, which binds to TNF receptor resulting in STAT3 activation. STAT3 targets CYP-D leading to mPTP inhibition. PostC also inhibits the Na+/H+ exchanger in a PKG-dependent fashion and reduces Ca2+ accumulation, because the sarcolemmal Na+/H+ exchanger and the Na+ bicarbonate co-transporter are activated at the onset of reperfusion, which causes intracellular Ca2+ accumulation through reverse activation of the Na+/Ca2+ exchanger and induces cell apoptosis and necrosis. CYP-D, cyclophilin-D; eNOS, endothelial nitric oxide synthase; ERK, p42/p44 extracellular-regulated kinase; GPCR, G-protein-coupled receptor; GSK3β, glycogen synthase kinase-3β; mPTP, mitochondrial permeability transition pore; mKATP, mitochondrial KATP channel; NBC, Na+ bicarbonate co-transporter; NCE, Na+/Ca2+ exchanger; NHE1, Na+/H+ exchanger; NO, nitric oxide; PKCɛ, protein kinase C subtype ɛ; PKG, cGMP-dependent protein kinase; ROS, reactive oxygen species; STAT3, signal transducer and activator of transcription-3; TNFR, TNF receptor.
STAT3 is a member of the SAFE pathway and has previously been demonstrated to play a role in IPC.44 A recent study shows that STAT3 is also involved in PostC because: (i) PostC increases STAT3 phosphorylation; (ii) JAK2 inhibition not only reduced STAT3 phosphorylation, but also abolished infarct reduction by PostC; and (iii) STAT3 deletion abolished cardioprotection of PostC.45 Which molecules stimulate STAT3 phosphorylation during PostC is not well understood, but the pro-inflammatory cytokine tumour necrosis factor (TNFα) is a strong candidate.46 Hence, the TNFα–TNF receptor type 2 (TNFR2)–STAT3 axis may underlie, in part, the cardioprotection of PostC by the SAFE pathway.46 The final target of both pathways appears to be the mitochondria, where mPTP may be the final effector.40,41
3.1.6. The role of mitochondria in postconditioning
The above molecular signalling pathways may converge on the mitochondria. Mitochondria play a central role in lethal ischaemia–reperfusion injury largely by actions of the mPTP.47 Many studies suggest that PostC delays mPTP opening potentially by reducing superoxide anion generation and intracellular Ca2+ overload, factors that stimulate opening of the mPTP.48 Hearts deficient in CYP-D (CYP-D−/−), an important regulatory subunit of the mPTP that is targeted by CsA, are resistant to infarct reduction conferred by PostC.49 Therefore, PostC likely prevents opening of mPTP through regulating CYP-D, by decreasing ROS and Ca2+ accumulation, and by maintaining tissue acidosis during early reperfusion. Both RISK and SAFE pathways are linked to regulation of the mPTP in PostC. The direct regulators of the mPTP may be GSK3β (RISK)50 and STAT3 (SAFE)51 (Figure 2).
3.1.7. Mitochondrial KATP channels and ROS in postconditioning
The mitochondrial KATP (mitoKATP) channel also plays a role in PostC.52 PostC induces active signalosome fractions that open mitoKATP channels in isolated-perfused rat hearts. The effect on the mitoKATP channel is PKG-dependent and may also be related to PKCɛ (Figure 2).53 MitoKATP channel activation leads to generation of signalling ROS which may act as a cardioprotective signal.54 PostC stimulates the generation of small concentrations of ROS at the onset of reperfusion which subsequently signal to inhibit the mPTP, probably through another type of PKCɛ.55 On the other hand, PostC reduces the deleterious respiratory burst of ROS and salvages cardiomyocytes during early reoxygenation.15 How the balance of these two actions is struck by PostC is not known.
3.1.8. Postconditioning in co-morbid models
Co-morbidities such as diabetes, hypercholesterolaemia, and hypertension have been reported to attenuate PostC protection. Myocardial protection by PostC was consistently less in different diabetic models.12 The abnormality leading to failure of PostC to reduce infarct size is most likely related to impairment of the RISK pathway components ERK, PI3K, and eNOS. Hypercholesterolaemia affects the efficacy of PostC, but this depends on the disease severity. Animals (rat and swine) fed with 2% cholesterol or cholesterol combined with 6% corn oil were not protected by PostC.56–58 However, animals (rat and rabbit) fed with 1% cholesterol were still protected by PostC.59,60
There is controversy over whether PostC is effective in salvaging the myocardium in aged hearts. While PostC is protective in aged rats,61 it fails to reduce infarct size in isolated-perfused models testing aged murine hearts (>80 weeks of age) and in an in vivo model (>52 weeks of age).45,62 The diminished myocardial salvage could be due to defects in ERK and STAT3 signalling.45,62 The myocardial protection could be restored if the cycle number is increased, and the duration of the index occlusion/reperfusion was reduced, again consistent with a threshold concept.45 However, PostC still improved other endpoints such as post-ischaemic contractile function and reduced ROS generation in the myocardium in senescent working mouse hearts.63
3.1.9. Clinical application of postconditioning
Initial clinical studies on PostC reported by Laskey64 and Staat et al.65 were conducted only 3 short years after its introduction by Zhao et al.11 in experimental studies. To date, clinical studies have shown that PostC increases ST-segment resolution,66,67 improves left ventricular function, and reduces infarct size.65,67–73 A summary of the clinical trial outcomes using PostC is presented in Table 1. In a meta-analyses of the clinical PostC data, infarct reduction was surprisingly consistent among the studies, ranging from 18 to 39%.13,74 Downey and Cohen75 suggested that only 25% of the patients treated by PCI would benefit from pharmacological treatment to reduce infarct size; this 25% would include patients with large areas at risk. Accordingly, Sorensson et al.76 showed that patients with large areas at risk benefited the most from PostC. However, not all clinical studies of PostC have been positive. Freixa et al.77 recently reported a series of 79 first-time ST-segment elevation [ST-segment elevation myocardial infarction (STEMI)] patients presenting for PCI within 12 h of onset of symptoms. There were no group differences in infarct size (% of LV mass) or ejection fraction estimated by cardiac magnetic resonance at 7 days or 6 months. Since most studies on PostC in the setting of PCI have been conducted in patients with symptoms for less than 7 h,13 the 12 h used in this study may represent a limitation in salvage with PostC in patients with prolonged occlusions using a specific algorithm, and perhaps suggests a threshold phenomenon. To date, there has been no report of adverse events reported that are directly attributed to PostC, and none have been referenced in the meta-analysis.13 However, as with preconditioning,78 there is concern that repeated inflations of the angioplasty balloon in the culprit vessel (PCI), or repeated cross-clamping of the aorta (cardiac surgery), could potentially contribute to both showering of atheromatous material and producing localized endothelial injury of the involved vessel. Certainly, PostC as a therapy should be tested for safety and efficacy in large-scale, multicentre randomized trials as concluded by the Working Group of Cellular Biology of the Heart of the European Society of Cardiology.41
Table 1.
Clinical studies of ischaemic postconditioning and remote conditioning
| Reference | Population (n) | Interventions | ST-segment resolution (STR) | Enzymes | Infarct size (SPECT, MRI) | LV function (V-gram, Echo) | Other endpoints | |
|---|---|---|---|---|---|---|---|---|
| Ischaemic postconditioning | 64 | <12 h LAD, RCA, LCx (17) | 90 s R/I × 2 | Improved | → Peak CK | N/A | N/A | ↑ Coronary flow velocity |
| 65 | <6 h LAD or RCA (30) | 60 s R/I × 4 | Improved | ↓ (36%) 72 h CK | N/A | ↑ Myocardial blush grade | — | |
| 115 | <12 h LAD, RCA, LCx (94) | 30 s R/I × 3 | N/A | ↓ 72 h CK (NSD), ↓ MDA | N/A | ↑ Wall motion (8 weeks) | Improved endothelial function | |
| 73 | <12 h LAD, RCA, LCx (41) | 30 s R/I × 3 | N/A | ↓ (27%) 72 h CK | ↓ 27% (1 week) | ↑ EF (NSD) | — | |
| 69 | <6 h LAD or RCA (38) | 60 s R/I × 4 | N/A | ↓ (40%) CK, ↓ (47%) TnI | ↓ 39% (6 months) | ↑ (7%) EF (12 months) | — | |
| 67 | <6 h LAD (24) | 90 s R/I × 2 | Improved | ↓ Peak CK | N/A | → Myocardial blush grade | ↑ Coronary flow velocity | |
| 68 | <12 h LAD, RCA, LCx (75) | 30 s or 60 s R/I × 3 | N/A | N/A | N/A | N/A | ↓ Apoptosis (7 days) | |
| 74 | <12 h LAD, RCA, LCx (118) | 30 s R/I × 4 | N/A | → Peak TnT | ↓ 13% (3 months) | → EF | ↑ (31%) myocardial salvage ratio ↓ (41%) heart failure | |
| 116 | <12 h LAD or RCA (43) | 60 s R/I × 4 | Improved | ↓ CK-MB | ↓ 46% (1 week) | ↑ EF (1 week) | — | |
| 76 | <6 h LAD, RCA, LCx (76) | 60 s R/I × 4 | N/A | → TnT, → CK-MB | ↓ in patients with large AAR | N/A | — | |
| 66 | <12 h (118) | 30 s R/I × 4 | Improved | ↓ Peak TnT in patients with complete STR | ↓ in patients with complete STR (3 months) | ↑ EF in patients with complete STR (3 months) | — | |
| 117 | <12 h LAD, RCA, LCx (75) | 30 or 60 s R/I × 3 | N/A | N/A | N/A | ↑ EF (12 months), ↑ wall motion (12 months) | ↓ TNF-α (1 week) | |
| Remote ischaemic preconditioning | 118 | CABG, LAD, RCA, LCx (8) | Arm (3′–2′) × 2, 300 mmHg | N/A | ↑ LDH at 5 min after declamping | N/A | N/A | — |
| 119 | Children undergoing bypass surgery (37) | Leg (5′–5′) × 4, 15 mmHg > SBP | N/A | ↓ TnI | N/A | N/A | ↓ inotrope scores, ↓ airway resistance | |
| 120 | Elective PCI. LAD, RCA, LCx (41) | Both arms (5′–5′) × 3, 200 mmHg | N/A | ↑ CK-MB, ↑ TnI | N/A | N/A | — | |
| 101 | Elective CABG (58) | Arm (5′–5′) × 3, 200 mmHg | N/A | ↓ TnT | N/A | N/A | — | |
| 121 | Elective abdominal aortic aneurysm repair (82) | Leg (10′–10′) × 2, 200 mmHg | N/A | ↓ TnI | N/A | N/A | ↓ Incidence of MI, ↓ renal injury | |
| 100 | Elective PCI. LAD, RCA, LCx (242) | Arm (5′–5′) × 3, 200 mmHg | N/A | ↓ TnI | N/A | N/A | ↓ EKG change during stent implantation, ↓ MACCE rate at 6 months | |
| rPerC | 107,122 | <12 h LAD, RCA, LCx (251) | Arm (5′–5′) × 4, 200 mmHg or 25 mmHg > SBP | → | N/A | ↓ | → EF | May improve LV function in high-risk patients |
Population, acute ST-elevation myocardial infarction with time from the onset of chest pain in LAD, RCA (right coronary artery), and LCx (left circumflex) artery, or other diseases listed; n, the number of patients; R/I, cycle of reperfusion/ischaemia; CK, creatine kinase; Echo, echocardiogram; EF, ejection fraction; LV, left ventricular; MACCE, major adverse cardiac and cerebral events; MDA, malondialdehyde; MI, myocardial infarction; MRI, magnetic resonance imaging; SBP, systolic blood pressure; SPECT, single-photon emission computed tomography; TnI, troponin I; TnT, troponin T; V-gram, ventriculogram; →, no significant change; ↑, increase; ↓, decrease; N/A, not studied; NSD, no significant difference.
4. Remote conditioning of the myocardium
The disadvantages of directly conditioning the heart are (i) limited access to conditioning protocols under all but in-hospital situations, (ii) the technical resources necessary to apply ischaemia directly to the heart, and (iii) the fear of creating further injury to the heart by repeated balloon inflations. The demonstration that the heart (and other organs) can be protected by brief ischaemia induced in a distant organ79 was a key in overcoming a major conceptual hurdle in translating ischaemic conditioning to clinical applications. The conditioning stimulus can be applied to the distant organ or tissue before (rIPC), during (rPerC), or after (rPostC) the index ischaemic event. rPerC may be applied while the patient is being transported to the hospital by an ambulance or helicopter, in the cath lab before the infarct-related artery is opened, or in cardiac surgery during the period of aortic cross-clamping. rPostC may be applied in any of these cases where reperfusion is predictable.
4.1. Remote ischaemic preconditioning
rIPC was first reported by Przyklenk et al.79 in 1993 using a canine model of LAD occlusion–reperfusion in which the remote site was the myocardium perfused by the left circumflex coronary artery. Infarct size was reduced by 60% compared with a control group with LAD occlusion–reperfusion only. The infarct reduction observed with rIPC is comparable to that reported for classic IPC.80 The paradigm of intracardiac rIPC has subsequently been expanded to include the preconditioning stimulus originating from other organs or tissues, e.g. the kidney and skeletal muscle.
The traditional concept of triggers, mediators, and effectors may pertain to rIPC as it does to classical IPC and PostC. Adenosine, bradykinin, and opioids have been proposed as triggers involved in rIPC.81 Some studies support a ‘second window’ of rIPC in which mesenteric preconditioning cycles applied 24 h before the index ischaemia reduced infarct size.82 The protection is sensitive to inducible NOS inhibitors, suggesting that late rIPC is dependent on inducible NOS. Inhibition of inflammatory gene expression may also contribute to late rIPC.83
4.1.1. Extracellular signal transduction in remote ischaemic preconditioning (rIPC)
There is an intervening communication or transfer step between these triggers from the remote site to the target organ81 that is not required by either direct IPC or PostC. Dickson et al.84 first showed that the myocardium could be preconditioned by transfer of coronary effluent from a preconditioned heart to a virgin isolated perfused acceptor heart, or by transfer of whole blood in a preconditioned rabbit heart to a naive rabbit. The transfer phenomenon for rIPC was confirmed by Shimizu et al.85 in a rabbit model of transient limb ischaemia as the rIPC trigger and cardiac index ischaemia. Shimizu et al.85 also extended the field by showing that plasma from preconditioned humans or rabbits could protect isolated perfused rabbit hearts and isolated rabbit cardiomyocytes, thereby demonstrating the cross-species effect. These communication factors may be humorally borne85,86 or transmitted by neuro-humoral paths. Like direct IPC and PostC, rIPC stimulates receptors for adenosine, bradykinin B2, opioids (δ1 or κ), CB2 cannabinoid, and angiotensin AT1.87 The mechanisms of protection of transferred factors may include suppression of blood-borne leucocyte activation83,88 which would potentially attenuate the neutrophil-mediated component of ischaemia–reperfusion injury.89 This, however, does not explain the transfer of protection in cell-free (isolated-perfused) systems.
There is evidence suggesting that a neural pathway exists in rIPC. For example, the cardioprotection conferred by local administration of bradykinin to the mesenteric artery is abolished by the ganglion blocker hexamethonium.90 A neural pathway was further confirmed by Ding et al.91 who showed that renal rIPC increased renal afferent nerve discharge and renal nerve ablation abolished renal rIPC. Further studies suggest that rIPC may produce adenosine locally which then stimulates afferent sensory nerves or activates capsaicin-sensitive sensory nerves to release calcitonin gene-related peptide.87 However, some recent evidence argues against neural transmission of rIPC triggers. Kingma et al.92 showed in a canine model of renal (remote) preconditioning on left ventricular infarct size that myocardial protection rendered by rIPC was not abrogated either by the autonomic blocking agent hexamethonium or by surgical decentralization of cardiac nerves.92 In agreement, persistence of protection with rIPC by repetitive limb ischaemia–reperfusion in a porcine cardiac transplant model in which the donor heart is denervated also argues against the requirement for a neural pathway.93 Therefore, the evidence supports that neural humour transmission seems adequate to achieve cardioprotection, and neural pathways, to the extent they exist in a given model, may be redundant.
4.1.2. Intracellular signal transduction in remote ischaemic preconditioning
rIPC shares some similar intracellular signal pathways as IPC and PostC. The involved primary kinases include p38, ERK1/2 or JNK1/2,94 PI3K/Akt,95 and PKCɛ.96,97 The downstream target of PKC could be the mitoKATP channel; studies show that KATP channel inhibition abrogates rIPC protection.87 Although inhibition of mPTP opening plays a central role in classical IPC and PostC, it is not understood whether mPTP inhibition contributes to rIPC.87
4.1.3. Clinical applications of remote ischaemic preconditioning
The clinical application of rIPC has recently been reviewed by Kharbanda et al.98 and by Hausenloy and Yellon.14 rIPC is induced clinically by repeatedly inflating and deflating a cuff occluder or tourniquet placed on the upper or lower limbs; therefore, skeletal muscle ischaemia–reperfusion provides the conditioning stimulus.99 Patients with acute coronary occlusions are not candidates for rIPC because the ischaemic event is already underway. The Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study reported by Hoole et al.100 showed that rIPC reduced infarct size estimated by cardiac troponin I (cTnI). Since these patients were elective and had undetectable cTnI levels before reperfusion, one could argue that this was actually rPerC rather than rIPC, but nonetheless remote conditioning reduced post-PCI injury.
In cardiac surgery, aortic cross-clamping with or without cardioplegia is a planned event, and therefore, conditioning before aortic cross-clamping can be strategically applied. Several cardiac surgery studies reported reductions in plasma biomarkers of injury when rIPC was achieved by transient limb occlusion before aortic cross-clamp and cardioplegia.101,102 Applying a rIPC stimulus 18 h before surgery, i.e. within a ‘second window’, reduced cTnI release after cardiac surgery.103 These data suggest that there is room for myocardial protection over and above that exerted by cardioplegia, hypothermia, and inhalational anaesthetics.
4.2. Remote perconditioning
4.2.1. Experimental studies
Practically, neither IPC nor rIPC can be applied to patients undergoing percutaneous or surgical interventions before the ischaemic event; PostC cannot be applied to those 30–40% of the STEMI patients who reperfuse spontaneously or after thrombolysis.104 However, remote conditioning can be applied during ischaemia in the form of rPerC. The rPerC stimulus can be induced by occluding a limb using a tourniquet or inflatable cuff during transport to the emergency room or cath lab. The same communication mechanisms involved in rIPC may also be involved during rPerC.
Using a porcine model of angioplasty balloon catheter-induced LAD occlusion (40 min) and 120 min of reperfusion, Schmidt et al.105 reported that four 5 min cycles of hind limb occlusion–reperfusion applied immediately after LAD occlusion (but before reperfusion) reduced infarct size compared with a control group. Infarct size reduction was reversed by the KATP channel antagonist glibenclamide given before rPerC, suggesting that the KATP channel is involved, which is consistent with both classical IPC and PostC. In the in vivo rat model of ischaemia–reperfusion, both rPerC and direct PostC alone reduced infarct size comparably, but less than that observed with IPC. However, combining rPerC and PostC achieved comparable infarct size reduction observed with IPC.106 The reduction in infarct size has been associated with an increase in phospho-Akt and phospho-ERK1/2.106
4.2.2. Clinical application of perconditioning
rPerC has recently been translated to the clinical setting. Bøtker et al.107 reported that rPerC during transport of patients with STEMI to the hospital reduced infarct size particularly in patients with large areas at risk. Rentoukas et al.108 applied a rPerC stimulus in STEMI patients 10 min prior to estimated reperfusion with or without adjunctive morphine (5 mg) infusion at the onset of reperfusion. This study confirmed the infarct reduction (peak cTnI) observed by Bøtker et al.107 but also showed that the incidence of full ST resolution was higher in the rPerC patients.
In adult patients undergoing cardiac surgery for valve replacement, Li et al.109 applied remote conditioning after either induction of anaesthesia (rIPC) or during aortic cross-clamp (rPerC) by cuff occlusion of the lower limb. Peak plasma nTnI levels at 30 min to 4 h after cross-clamp removal and the incidence of ventricular fibrillation at reanimation were lower in the rPerC group compared with either controls or rIPC. However, whether salvage of myocardium by rPerC is associated with improved clinical outcomes (e.g. length of stay, mortality, or indices of morbidity) has yet to be determined by multicentre studies.
4.3. Remote postconditioning
Direct PostC of the heart may have the disadvantage of inducing additional myocardial ischaemia, although there have been no adverse events reported to date. rPostC was first reported by Kerendi et al.110 in a rat model of prolonged coronary artery occlusion–reperfusion in which a single cycle of 5 min renal artery occlusion–reperfusion applied just prior to the onset of coronary artery reperfusion reduced infarct size by ∼50% relative to controls. This observation was replicated in an elegant closed-chest porcine model using the hind limb as the rPostC stimulus organ.111 rPostC induced by limb ischaemia–reperfusion was also reported to reduce apoptosis.112 A remote conditioning stimulus has also been provided by occlusion of the opposite left coronary artery (intra-organ remote conditioning),113 the carotid artery,113 or femoral artery (limb stimulus).114 Cardioprotection with rPostC has been reported to be equivalent to114 or more potent113 than direct PostC. At the time of writing, there have been no reported clinical trials of rPostC.
Like rIPC and rPerC, the transfer signal between the remote stimulus organ and the target organ may be humoral, neural, or systemically borne (cell signals). Kerendi et al. showed that rPostC could be blocked by the adenosine receptor antagonist 8-SPT, suggesting that adenosine was a humoral communication factor between the kidney and the heart. Adenosine released by the kidney may act directly on the heart or trigger neuronal stimuli that subsequently protect the heart. The communication factor(s) has not been identified as yet.
5. Concluding remarks: unanswered questions, future directions
Conditioning can be applied before, during, or after the ischaemic stressor, protects multiple cell types, and induces or rebalances a number of pathways that attenuate necrosis and apoptosis. This broadness is the conditioning response's strength, which, in the case of PerC and both direct and remote PostC, has allowed it to be successfully translated from bench to bedside in the span of a few years compared with IPC. As with many things, this strength is also its weakness; the need for ischaemia to trigger the cardioprotection is a liability related to the methods of inducing ischaemia. Before conditioning can realize its full therapeutic potential, many questions should to be answered regarding (i) what is the optimal algorithm experimentally and clinically, and can the optimal algorithm extend the threshold effect to longer durations of ischaemia; (ii) is there a real-time marker that can be used to signal that a conditioned state has been achieved; (iii) do the same molecular mechanisms of conditioning observed in animals drive the responses in man; (iv) is there an anti-inflammatory component to conditioning; (v) are the mechanisms common to all three forms of conditioning; (vi) is there an appropriate animal model that accurately reflects the human ‘model’ of multiple co-morbidities and metabolic syndrome? Further, would it not also be useful for the animal model to be treated with the drugs typically prescribed to patients with co-morbidities in order to accurately reflect the clinical? Clinically, one should ask (i) are there specific patient subtypes in which direct or remote conditioning is effective, or is cardioprotection exerted over all disease demographics; (ii) does conditioning of the heart during PCI or surgery protect other organs from multiorgan dysfunction; (iii) what is the duration of coronary artery occlusion (also taking into account the size of area at risk) beyond which the efficacy of conditioning is lost? Answering this latter question will have impact on how patients are triaged as salvageable or non-salvageable.
Both PerC and PostC have been rapidly translated to the clinical setting. This presents unprecedented opportunities for clinicians and basic scientists to work cooperatively and collaboratively, with each contributing to the unravelling of the mechanisms and potential of the conditioning response. However, the use of ischaemia to trigger cardioprotection has certain liabilities which reduce its enthusiasm for clinicians; fewer liabilities exist with remote ischaemia. Ischaemia will likely be replaced by pharmacological agents that exert cardioprotection, although these agents need to address the numerous and often redundant mechanisms involved in ischaemia–reperfusion injury to be effective. The development of pharmacological conditioning mimetics may therefore provide another option to protect the heart at other organs exposed to non-surgical and surgical ischaemia and reperfusion.
Funding
The Cardiothoracic Research Lab is funded in part by grant HL094373 from the National Institutes of Health (National Heart, Lung and Blood Institute) and by the Carlyle Fraser Heart Center.
Acknowledgements
The Cardiothoracic Research Laboratory appreciates the continued support from the Carlyle Fraser Heart Center.
Conflict of interest: none declared.
References
- 1.Anderson JL, Marshall HW, Bray BE, Lutz JR, Frederick PR, Yanowitz FG, et al. A randomized trial of intracoronary streptokinase in the treatment of acute myocardial infarction. N Engl J Med. 1983;308:1312–1318. doi: 10.1056/NEJM198306023082202. [DOI] [PubMed] [Google Scholar]
- 2.Burns RJ, Gibbons RJ, Yi Q, Roberts RS, Miller TD, Schaer GL, et al. The relationships of left ventricular ejection fraction, end-systolic volume index and infarct size to six-month mortality after hospital discharge following myocardial infarction treated by thrombolysis. J Am Coll Cardiol. 2002;39:30–36. doi: 10.1016/s0735-1097(01)01711-9. [DOI] [PubMed] [Google Scholar]
- 3.Schomig A, Kastrati A, Dirschinger J, Mehilli J, Schricke U, Pache J, et al. Coronary stenting plus platelet glycoprotein IIb/IIIa blockade compared with tissue plasminogen activator in acute myocardial infarction. Stent versus Thrombolysis for Occluded Coronary Arteries in Patients with Acute Myocardial Infarction Study Investigators. N Engl J Med. 2000;343:385–391. doi: 10.1056/NEJM200008103430602. [DOI] [PubMed] [Google Scholar]
- 4.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 5.Weman SM, Karhunen PJ, Penttila A, Jarvinen AA, Salminen US. Reperfusion injury associated with one-fourth of deaths after coronary artery bypass grafting. Ann Thorac Surg. 2000;70:807–812. doi: 10.1016/s0003-4975(00)01638-6. [DOI] [PubMed] [Google Scholar]
- 6.Vinten-Johansen J, Jiang R, Reeves JG, Mykytenko J, Deneve J, Jobe LJ. Inflammation, proinflammatory mediators and myocardial ischemia–reperfusion Injury. Hematol Oncol Clin North Am. 2007;21:123–145. doi: 10.1016/j.hoc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 8.Manintveld OC, te Lintel HM, van den Bos EJ, Suurenbroek GM, Dekkers DH, Verdouw PD, et al. Cardiac effects of postconditioning depend critically on the duration of index ischemia. Am J Physiol Heart Circ Physiol. 2007;292:H1551–H1560. doi: 10.1152/ajpheart.00151.2006. [DOI] [PubMed] [Google Scholar]
- 9.Cai M, Li Y, Xu Y, Swartz HM, Chen CL, Chen YR, et al. Endothelial NOS activity and myocardial oxygen metabolism define the salvageable ischemic time window for ischemic postconditioning. Am J Physiol Heart Circ Physiol. 2011;300:H1069–H1077. doi: 10.1152/ajpheart.00694.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kin H, Zhao ZQ, Sun HY, Wang NP, Corvera JS, Halkos ME, et al. Postconditioning attenuates myocardial ischemia–reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res. 2004;62:74–85. doi: 10.1016/j.cardiores.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 12.Vinten-Johansen J, Granfeldt A, Mykytenko J, Undyala VV, Dong Y, Przyklenk K. The multidimensional physiological responses to postconditioning. Antioxid Redox Signal. 2011;14:791–810. doi: 10.1089/ars.2010.3396. [DOI] [PubMed] [Google Scholar]
- 13.Hansen PR, Thibault H, Abdulla J. Postconditioning during primary percutaneous coronary intervention: a review and meta-analysis. Int J Cardiol. 2010;144:22–25. doi: 10.1016/j.ijcard.2009.03.118. [DOI] [PubMed] [Google Scholar]
- 14.Hausenloy DJ, Yellon DM. The therapeutic potential of ischemic conditioning: an update. Nat Rev Cardiol. 2011;8:619–629. doi: 10.1038/nrcardio.2011.85. [DOI] [PubMed] [Google Scholar]
- 15.Sun HY, Wang NP, Kerendi F, Halkos M, Kin H, Guyton RA, et al. Hypoxic postconditioning reduces cardiomyocyte loss by inhibiting ROS generation and intracellular Ca2+ overload. Am J Physiol Heart Circ Physiol. 2005;288:H1900–H1908. doi: 10.1152/ajpheart.01244.2003. [DOI] [PubMed] [Google Scholar]
- 16.Yang XM, Philipp S, Downey JM, Cohen MV. Postconditioning's protection is not dependent on circulating blood factors or cells but involves adenosine receptors and requires PI3-kinase and guanylyl cyclase activation. Basic Res Cardiol. 2005;100:57–63. doi: 10.1007/s00395-004-0498-4. [DOI] [PubMed] [Google Scholar]
- 17.Halkos ME, Kerendi F, Corvera JS, Wang NP, Kin H, Payne CS, et al. Myocardial protection with postconditioning is not enhanced by ischemic preconditioning. Ann Thorac Surg. 2004;78:961–969. doi: 10.1016/j.athoracsur.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 18.Hu X, Jiang H, Ma F, Xu C, Bo C, Wen H, et al. Similarities between ischemic preconditioning and postconditioning in myocardial ischemia/reperfusion injury. Int J Cardiol. 2010;144:135–136. doi: 10.1016/j.ijcard.2008.12.119. [DOI] [PubMed] [Google Scholar]
- 19.Kin H, Wang NP, Mykytenko J, Reeves J, Deneve J, Jiang R, et al. Inhibition of myocardial apoptosis by postconditioning is associated with attenuation of oxidative stress-mediated nuclear factor-kappa B translocation and TNF alpha release. Shock. 2008;29:761–768. doi: 10.1097/SHK.0b013e31815cfd5a. [DOI] [PubMed] [Google Scholar]
- 20.Taki J, Higuchi T, Kawashima A, Fukuoka M, Kayano D, Tait JF, et al. Effect of postconditioning on myocardial 99mTc-annexin-V uptake: comparison with ischemic preconditioning and caspase inhibitor treatment. J Nucl Med. 2007;48:1301–1307. doi: 10.2967/jnumed.106.037408. [DOI] [PubMed] [Google Scholar]
- 21.Wang HC, Zhang HF, Guo WY, Su H, Zhang KR, Li QX, et al. Hypoxic postconditioning enhances the survival and inhibits apoptosis of cardiomyocytes following reoxygenation: role of peroxynitrite formation. Apoptosis. 2006;11:1453–1460. doi: 10.1007/s10495-006-7786-z. [DOI] [PubMed] [Google Scholar]
- 22.Sun HY, Wang NP, Halkos M, Kerendi F, Kin H, Guyton RA, et al. Postconditioning attenuates cardiomyocyte apoptosis via inhibition of JNK and p38 mitogen-activated protein kinase signaling pathways. Apoptosis. 2006;11:1583–1593. doi: 10.1007/s10495-006-9037-8. [DOI] [PubMed] [Google Scholar]
- 23.Penna C, Perrelli MG, Raimondo S, Tullio F, Merlino A, Moro F, et al. Postconditioning induces an anti-apoptotic effect and preserves mitochondrial integrity in isolated rat hearts. Biochim Biophys Acta. 2009;1787:794–801. doi: 10.1016/j.bbabio.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Tian Y, Zhang W, Xia D, Modi P, Liang D, Wei M. Postconditioning inhibits myocardial apoptosis during prolonged reperfusion via a JAK2-STAT3-Bcl-2 pathway. J Biomed Sci. 2011;18:53. doi: 10.1186/1423-0127-18-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kin H, Zatta AJ, Lofye MT, Amerson BS, Halkos ME, Kerendi F, et al. Postconditioning reduces infarct size via adenosine receptor activation by endogenous adenosine. Cardiovasc Res. 2005;67:124–133. doi: 10.1016/j.cardiores.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 26.Zatta AJ, Kin H, Yoshishige D, Jiang R, Wang N, Reeves JG, et al. Evidence that cardioprotection by postconditioning involves preservation of myocardial opioid content and selective opioid receptor activation. Am J Physiol Heart Circ Physiol. 2008;294:H1444–H1451. doi: 10.1152/ajpheart.01279.2006. [DOI] [PubMed] [Google Scholar]
- 27.Xi L, Das A, Zhao ZQ, Merino VF, Bader M, Kukreja RC. Loss of myocardial ischemic postconditioning in adenosine A1 and bradykinin B2 receptors gene knockout mice. Circulation. 2008;118:S32–S37. doi: 10.1161/CIRCULATIONAHA.107.752865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vinten-Johansen J, Shi W. Perconditioning and postconditioning: current knowledge, knowledge gaps, barriers to adoption, and future directions. J Cardiovasc Pharmacol Ther. 2011;16:260–266. doi: 10.1177/1074248411415270. [DOI] [PubMed] [Google Scholar]
- 29.Vessey DA, Li L, Honbo N, Karliner JS. Sphingosine 1-phosphate is an important endogenous cardioprotectant released by ischemic pre- and postconditioning. Am J Physiol Heart Circ Physiol. 2009;297:H1429–H1435. doi: 10.1152/ajpheart.00358.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Litt MR, Jeremy RW, Weisman HF, Winkelstein JA, Becker LC. Neutrophil depletion limited to reperfusion reduces myocardial infarct size after 90 minutes of ischemia: evidence for neutrophil-mediated reperfusion injury. Circulation. 1989;80:1816–1827. doi: 10.1161/01.cir.80.6.1816. [DOI] [PubMed] [Google Scholar]
- 31.Winquist R, Frei P, Harrison P, McFarland M, Letts G, Van G, et al. An anti-CD-18 Mab limits infarct size in primates following ischemia and reperfusion. Circulation. 1990;82:III-701a. [Google Scholar]
- 32.Granfeldt A, Jiang R, Wang N-P, Mykytenko J, Eldaif SM, Deneve J, et al. PMN inhibition contributes to cardioprotection by postconditioning. Acta Anaesth Scand. 2012;56:48–56. doi: 10.1111/j.1399-6576.2011.02577.x. [DOI] [PubMed] [Google Scholar]
- 33.Danielisova V, Nemethova M, Gottlieb M, Burda J. The changes in endogenous antioxidant enzyme activity after postconditioning. Cell Mol Neurobiol. 2006;26:1181–1191. doi: 10.1007/s10571-006-9034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heusch G. Postconditioning: old wine in a new bottle? J Am Coll Cardiol. 2004;44:1111–1112. doi: 10.1016/j.jacc.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 35.Cohen MV, Yang XM, Downey JM. Acidosis, oxygen, and interference with mitochondrial permeability transition pore formation in the early minutes of reperfusion are critical to postconditioning's success. Basic Res Cardiol. 2008;103:464–471. doi: 10.1007/s00395-008-0737-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez-Sinovas A, Cabestrero A, Garcia del Blanco B, Inserte J, Garcia A, Garcia-Dorado D. Intracoronary acid infusion as an alternative to ischemic postconditioning in pigs. Basic Res Cardiol. 2009;104:761–771. doi: 10.1007/s00395-009-0032-4. [DOI] [PubMed] [Google Scholar]
- 37.Inserte J, Barba I, Hernando V, Garcia-Dorado D. Delayed recovery of intracellular acidosis during reperfusion prevents calpain activation and determines protection in postconditioned myocardium. Cardiovasc Res. 2009;81:116–122. doi: 10.1093/cvr/cvn260. [DOI] [PubMed] [Google Scholar]
- 38.Fujita M, Asanuma H, Hirata A, Wakeno M, Takahama H, Sasaki H, et al. Prolonged transient acidosis during early reperfusion contributes to the cardioprotective effects of postconditioning. Am J Physiol Heart Circ Physiol. 2007;292:H2004–H2008. doi: 10.1152/ajpheart.01051.2006. [DOI] [PubMed] [Google Scholar]
- 39.Inserte J, Barba I, Poncelas-Nozal M, Hernando V, Agullo L, Ruiz-Meana M, et al. cGMP/PKG pathway mediates myocardial postconditioning protection in rat hearts by delaying normalization of intracellular acidosis during reperfusion. J Mol Cell Cardiol. 2011;50:903–909. doi: 10.1016/j.yjmcc.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Hausenloy DJ, Lecour S, Yellon DM. Reperfusion injury salvage kinase and survivor activating factor enhancement prosurvival signaling pathways in ischemic postconditioning: two sides of the same coin. Antioxid Redox Signal. 2011;14:893–907. doi: 10.1089/ars.2010.3360. [DOI] [PubMed] [Google Scholar]
- 41.Ovize M, Baxter GF, Di Lisa F, Ferdinandy P, Garcia-Dorado D, Hausenloy DJ, et al. Postconditioning and protection from reperfusion injury: where do we stand? Position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2010;87:406–423. doi: 10.1093/cvr/cvq129. [DOI] [PubMed] [Google Scholar]
- 42.Skyschally A, van Caster P, Boengler K, Gres P, Musiolik J, Schilawa D, et al. Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res. 2009;104:15–18. doi: 10.1161/CIRCRESAHA.108.186429. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Lv F, Jin L, Peng W, Song R, Ma J, et al. MG53 participates in ischaemic postconditioning through the RISK signalling pathway. Cardiovasc Res. 2011;91:108–115. doi: 10.1093/cvr/cvr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hattori R, Maulik N, Otani H, Zhu L, Cordis G, Engelman RM, et al. Role of STAT3 in ischemic preconditioning. J Mol Cell Cardiol. 2001;33:1929–1936. doi: 10.1006/jmcc.2001.1456. [DOI] [PubMed] [Google Scholar]
- 45.Boengler K, Buechert A, Heinen Y, Roeskes C, Hilfiker-Kleiner D, Heusch G, et al. Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ Res. 2008;102:131–135. doi: 10.1161/CIRCRESAHA.107.164699. [DOI] [PubMed] [Google Scholar]
- 46.Lacerda L, Somers S, Opie LH, Lecour S. Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res. 2009;84:201–208. doi: 10.1093/cvr/cvp274. [DOI] [PubMed] [Google Scholar]
- 47.Garlid KD, Costa AD, Quinlan CL, Pierre SV, Dos Santos P. Cardioprotective signaling to mitochondria. J Mol Cell Cardiol. 2009;46:858–866. doi: 10.1016/j.yjmcc.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boengler K, Heusch G, Schulz R. Mitochondria in postconditioning. Antioxid Redox Signal. 2011;14:863–880. doi: 10.1089/ars.2010.3309. [DOI] [PubMed] [Google Scholar]
- 49.Lim SY, Davidson SM, Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: the essential role of the mitochondrial permeability transition pore. Cardiovasc Res. 2007;75:530–535. doi: 10.1016/j.cardiores.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gomez L, Paillard M, Thibault H, Derumeaux G, Ovize M. Inhibition of GSK3beta by postconditioning is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation. 2008;117:2761–2768. doi: 10.1161/CIRCULATIONAHA.107.755066. [DOI] [PubMed] [Google Scholar]
- 51.Boengler K, Hilfiker-Kleiner D, Heusch G, Schulz R. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol. 2010;105:771–785. doi: 10.1007/s00395-010-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mykytenko J, Reeves JG, Kin H, Wang NP, Zatta AJ, Jiang R, et al. Persistent beneficial effect of postconditioning against infarct size: role of mitochondrial K(ATP) channels during reperfusion. Basic Res Cardiol. 2008;103:472–484. doi: 10.1007/s00395-008-0731-2. [DOI] [PubMed] [Google Scholar]
- 53.Quinlan CL, Costa AD, Costa CL, Pierre SV, Dos Santos P, Garlid KD. Conditioning the heart induces formation of signalosomes that interact with mitochondria to open mitoKATP channels. Am J Physiol Heart Circ Physiol. 2008;295:H953–H961. doi: 10.1152/ajpheart.00520.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Penna C, Mancardi D, Rastaldo R, Losano G, Pagliaro P. Intermittent activation of bradykinin B2 receptors and mitochondrial KATP channels trigger cardiac postconditioning through redox signaling. Cardiovasc Res. 2007;75:168–177. doi: 10.1016/j.cardiores.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 55.Costa AD, Jakob R, Costa CL, Andrukhiv K, West IC, Garlid KD. The mechanism by which the mitochondrial ATP-sensitive K+ channel opening and H2O2 inhibit the mitochondrial permeability transition. J Biol Chem. 2006;281:20801–20808. doi: 10.1074/jbc.M600959200. [DOI] [PubMed] [Google Scholar]
- 56.Zhao JL, Yang YJ, You SJ, Cui CJ, Gao RL. Different effects of postconditioning on myocardial no-reflow in the normal and hypercholesterolemic mini-swines. Microvasc Res. 2007;73:137–142. doi: 10.1016/j.mvr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 57.Kupai K, Csonka C, Fekete V, Odendaal L, van Rooyen J, Marais de W, et al. Cholesterol diet-induced hyperlipidemia impairs the cardioprotective effect of postconditioning: role of peroxynitrite. Am J Physiol Heart Circ Physiol. 2009;297:H1729–H1735. doi: 10.1152/ajpheart.00484.2009. [DOI] [PubMed] [Google Scholar]
- 58.Iliodromitis EK, Zoga A, Vrettou A, Andreadou I, Paraskevaidis IA, Kaklamanis L, et al. The effectiveness of postconditioning and preconditioning on infarct size in hypercholesterolemic and normal anesthetized rabbits. Atherosclerosis. 2006;188:356–362. doi: 10.1016/j.atherosclerosis.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 59.Donato M, D'Annunzio V, Berg G, Gonzalez G, Schreier L, Morales C, et al. Ischemic postconditioning reduces infarct size by activation of A1 receptors and K+(ATP) channels in both normal and hypercholesterolemic rabbits. J Cardiovasc Pharmacol. 2007;49:287–292. doi: 10.1097/FJC.0b013e31803c55fe. [DOI] [PubMed] [Google Scholar]
- 60.Zhao H, Wang Y, Wu Y, Li X, Yang G, Ma X, et al. Hyperlipidemia does not prevent the cardioprotection by postconditioning against myocardial ischemia/reperfusion injury and the involvement of hypoxia inducible factor-1alpha upregulation. Acta Biochim Biophys Sin (Shanghai) 2009;41:745–753. doi: 10.1093/abbs/gmp063. [DOI] [PubMed] [Google Scholar]
- 61.Yin Z, Gao H, Wang H, Li L, Di C, Luan R, et al. Ischaemic post-conditioning protects both adult and aged Sprague–Dawley rat heart from ischaemia–reperfusion injury through the phosphatidylinositol 3-kinase-AKT and glycogen synthase kinase-3beta pathways. Clin Exp Pharmacol Physiol. 2009;36:756–763. doi: 10.1111/j.1440-1681.2009.05148.x. [DOI] [PubMed] [Google Scholar]
- 62.Przyklenk K, Maynard M, Darling CE, Whittaker P. Aging mouse hearts are refractory to infarct size reduction with post-conditioning. J Am Coll Cardiol. 2008;51:1393–1398. doi: 10.1016/j.jacc.2007.11.070. [DOI] [PubMed] [Google Scholar]
- 63.Lauzier B, Delemasure S, Debin R, Collin B, Sicard P, Acar N, et al. Beneficial effects of myocardial postconditioning are associated with reduced oxidative stress in a senescent mouse model. Transplantation. 2008;85:1802–1808. doi: 10.1097/TP.0b013e3181775367. [DOI] [PubMed] [Google Scholar]
- 64.Laskey WK. Brief repetitive balloon occlusions enhance reperfusion during percutaneous coronary intervention for acute myocardial infarction: a pilot study. Catheter Cardiovasc Interv. 2005;65:361–367. doi: 10.1002/ccd.20397. [DOI] [PubMed] [Google Scholar]
- 65.Staat P, Rioufol G, Piot C, Cottin Y, Cung TT, L'Huillier I, et al. Postconditioning the human heart. Circulation. 2005;112:2143–2148. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- 66.Lonborg J, Holmvang L, Kelbaek H, Vejlstrup N, Jorgensen E, Helqvist S, et al. ST-segment resolution and clinical outcome with ischemic postconditioning and comparison to magnetic resonance. Am Heart J. 2010;160:1085–1091. doi: 10.1016/j.ahj.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 67.Laskey WK, Yoon S, Calzada N, Ricciardi MJ. Concordant improvements in coronary flow reserve and ST-segment resolution during percutaneous coronary intervention for acute myocardial infarction: a benefit of postconditioning. Catheter Cardiovasc Interv. 2008;72:212–220. doi: 10.1002/ccd.21583. [DOI] [PubMed] [Google Scholar]
- 68.Zhao WS, Xu L, Wang LF, Zhang L, Zhang ZY, Liu Y, et al. A 60-s postconditioning protocol by percutaneous coronary intervention inhibits myocardial apoptosis in patients with acute myocardial infarction. Apoptosis. 2009;14:1204–1211. doi: 10.1007/s10495-009-0387-x. [DOI] [PubMed] [Google Scholar]
- 69.Thibault H, Piot C, Staat P, Bontemps L, Sportouch C, Rioufol G, et al. Long-term benefit of postconditioning. Circulation. 2008;117:1037–1044. doi: 10.1161/CIRCULATIONAHA.107.729780. [DOI] [PubMed] [Google Scholar]
- 70.Darling CE, Solari PB, Smith CS, Furman MI, Przyklenk K. ‘Postconditioning’ the human heart: multiple balloon inflations during primary angioplasty may confer cardioprotection. Basic Res Cardiol. 2007;102:274–278. doi: 10.1007/s00395-007-0643-6. [DOI] [PubMed] [Google Scholar]
- 71.Ma XJ, Zhang XH, Li CM, Luo M. Effect of postconditioning on coronary blood flow velocity and endothelial function in patients with acute myocardial infarction. Scand Cardiovasc J. 2006;40:327–333. doi: 10.1080/14017430601047864. [DOI] [PubMed] [Google Scholar]
- 72.Wang G, Zhang S, Joggerst SJ, McPherson J, Zhao DX. Effects of the number and interval of balloon inflations during primary PCI on the extent of myocardial injury in patients with STEMI: does postconditioning exist in real-world practice? J Invasive Cardiol. 2009;21:451–455. [PubMed] [Google Scholar]
- 73.Yang XC, Liu Y, Wang LF, Cui L, Wang T, Ge YG, et al. Reduction in myocardial infarct size by postconditioning in patients after percutaneous coronary intervention. J Invasive Cardiol. 2007;19:424–430. [PubMed] [Google Scholar]
- 74.Lonborg J, Kelbaek H, Vejlstrup N, Jorgensen E, Helqvist S, Saunamaki K, et al. Cardioprotective effects of ischemic postconditioning in patients treated with primary percutaneous coronary intervention, evaluated by magnetic resonance. Circ Cardiovasc Interv. 2010;3:34–41. doi: 10.1161/CIRCINTERVENTIONS.109.905521. [DOI] [PubMed] [Google Scholar]
- 75.Downey JM, Cohen MV. Why do we still not have cardioprotective drugs? Circ J. 2009;73:1171–1177. doi: 10.1253/circj.cj-09-0338. [DOI] [PubMed] [Google Scholar]
- 76.Sorensson P, Saleh N, Bouvier F, Bohm F, Settergren M, Caidahl K, et al. Effect of postconditioning on infarct size in patients with ST elevation myocardial infarction. Heart. 2010;96:1710–1715. doi: 10.1136/hrt.2010.199430. [DOI] [PubMed] [Google Scholar]
- 77.Freixa X, Bellera N, Ortiz-Perez JT, Jimenez M, Pare C, Bosch X, et al. Ischaemic postconditioning revisited: lack of effects on infarct size following primary percutaneous coronary intervention. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr297. doi:10.1093/eurheartj/ehr297. [DOI] [PubMed] [Google Scholar]
- 78.Kloner RA. Clinical application of remote ischemic preconditioning. Circulation. 2009;119:776–778. doi: 10.1161/CIRCULATIONAHA.108.832832. [DOI] [PubMed] [Google Scholar]
- 79.Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–899. doi: 10.1161/01.cir.87.3.893. [DOI] [PubMed] [Google Scholar]
- 80.Pell TJ, Baxter GF, Yellon DM, Drew GM. Renal ischemia preconditions myocardium: role of adenosine receptors and ATP-sensitive potassium channels. Am J Physiol. 1998;275:H1542–H1547. doi: 10.1152/ajpheart.1998.275.5.H1542. [DOI] [PubMed] [Google Scholar]
- 81.Przyklenk K, Whittaker P. Remote ischemic preconditioning: current knowledge, unresolved questions, and future priorities. J Cardiovasc Pharmacol Ther. 2011;16:255–259. doi: 10.1177/1074248411409040. [DOI] [PubMed] [Google Scholar]
- 82.Wang Y, Xu H, Mizoguchi K, Oe M, Maeta H. Intestinal ischemia induces late preconditioning against myocardial infarction: a role for inducible nitric oxide synthase. Cardiovasc Res. 2001;49:391–398. doi: 10.1016/s0008-6363(00)00266-2. [DOI] [PubMed] [Google Scholar]
- 83.Konstantinov IE, Arab S, Kharbanda RK, Li J, Cheung MM, Cherepanov V, et al. The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics. 2004;19:143–150. doi: 10.1152/physiolgenomics.00046.2004. [DOI] [PubMed] [Google Scholar]
- 84.Dickson EW, Lorbar M, Porcaro WA, Fenton RA, Reinhardt CP, Gysembergh A, et al. Rabbit heart can be ‘preconditioned’ via transfer of coronary effluent. Am J Physiol Heart Circ Physiol. 1999;277:H2451–H2457. doi: 10.1152/ajpheart.1999.277.6.H2451. [DOI] [PubMed] [Google Scholar]
- 85.Shimizu M, Tropak M, Diaz RJ, Suto F, Surendra H, Kuzmin E, et al. Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clin Sci (Lond) 2009;117:191–200. doi: 10.1042/CS20080523. [DOI] [PubMed] [Google Scholar]
- 86.Dickson EW, Reinhardt CP, Renzi FP, Becker RC, Porcaro WA, Heard SO. Ischemic preconditioning may be transferable via whole blood transfusion: preliminary evidence. J Thromb Thrombolysis. 1999;8:123–129. doi: 10.1023/a:1008911101951. [DOI] [PubMed] [Google Scholar]
- 87.Hausenloy DJ, Yellon DM. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res. 2008;79:377–386. doi: 10.1093/cvr/cvn114. [DOI] [PubMed] [Google Scholar]
- 88.Shimizu M, Saxena P, Konstantinov IE, Cherepanov V, Cheung MM, Wearden P, et al. Remote ischemic preconditioning decreases adhesion and selectively modifies functional responses of human neutrophils. J Surg Res. 2010;158:155–161. doi: 10.1016/j.jss.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 89.Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res. 2004;61:481–497. doi: 10.1016/j.cardiores.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 90.Schoemaker RG, van Heijningen CL. Bradykinin mediates cardiac preconditioning at a distance. Am J Physiol Heart Circ Physiol. 2000;278:H1571–H1576. doi: 10.1152/ajpheart.2000.278.5.H1571. [DOI] [PubMed] [Google Scholar]
- 91.Ding YF, Zhang MM, He RR. Role of renal nerve in cardioprotection provided by renal ischemic preconditioning in anesthetized rabbits. Sheng Li Xue Bao. 2001;53:7–12. [PubMed] [Google Scholar]
- 92.Kingma JG, Jr, Simard D, Voisine P, Rouleau JR. Role of the autonomic nervous system in cardioprotection by remote preconditioning in isoflurane-anaesthetized dogs. Cardiovasc Res. 2011;89:384–391. doi: 10.1093/cvr/cvq306. [DOI] [PubMed] [Google Scholar]
- 93.Konstantinov IE, Li J, Cheung MM, Shimizu M, Stokoe J, Kharbanda RK, et al. Remote ischemic preconditioning of the recipient reduces myocardial ischemia–reperfusion injury of the denervated donor heart via a Katp channel-dependent mechanism. Transplantation. 2005;79:1691–1695. doi: 10.1097/01.tp.0000159137.76400.5d. [DOI] [PubMed] [Google Scholar]
- 94.Heidbreder M, Naumann A, Tempel K, Dominiak P, Dendorfer A. Remote vs. ischaemic preconditioning: the differential role of mitogen-activated protein kinase pathways. Cardiovasc Res. 2008;78:108–115. doi: 10.1093/cvr/cvm114. [DOI] [PubMed] [Google Scholar]
- 95.Breivik L, Helgeland E, Aarnes EK, Mrdalj J, Jonassen AK. Remote postconditioning by humoral factors in effluent from ischemic preconditioned rat hearts is mediated via PI3K/Akt-dependent cell-survival signaling at reperfusion. Basic Res Cardiol. 2011;106:135–145. doi: 10.1007/s00395-010-0133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Serejo FC, Rodrigues LF, Jr, da Silva Tavares KC, de Carvalho AC, Nascimento JH. Cardioprotective properties of humoral factors released from rat hearts subject to ischemic preconditioning. J Cardiovasc Pharmacol. 2007;49:214–220. doi: 10.1097/FJC.0b013e3180325ad9. [DOI] [PubMed] [Google Scholar]
- 97.Wolfrum S, Nienstedt J, Heidbreder M, Schneider K, Dominiak P, Dendorfer A. Calcitonin gene related peptide mediates cardioprotection by remote preconditioning. Regul Pept. 2005;127:217–224. doi: 10.1016/j.regpep.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 98.Kharbanda RK, Nielsen TT, Redington AN. Translation of remote ischaemic preconditioning into clinical practice. Lancet. 2009;374:1557–1565. doi: 10.1016/S0140-6736(09)61421-5. [DOI] [PubMed] [Google Scholar]
- 99.Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, et al. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106:2881–2883. doi: 10.1161/01.cir.0000043806.51912.9b. [DOI] [PubMed] [Google Scholar]
- 100.Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG, et al. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study: a prospective, randomized control trial. Circulation. 2009;119:820–827. doi: 10.1161/CIRCULATIONAHA.108.809723. [DOI] [PubMed] [Google Scholar]
- 101.Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet. 2007;370:575–579. doi: 10.1016/S0140-6736(07)61296-3. [DOI] [PubMed] [Google Scholar]
- 102.Venugopal V, Hausenloy DJ, Ludman A, Di Salvo C, Kolvekar S, Yap J, et al. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold-blood cardioplegia: a randomised controlled trial. Heart. 2009;95:1567–1571. doi: 10.1136/hrt.2008.155770. [DOI] [PubMed] [Google Scholar]
- 103.Wagner R, Piler P, Bedanova H, Adamek P, Grodecka L, Freiberger T. Myocardial injury is decreased by late remote ischaemic preconditioning and aggravated by tramadol in patients undergoing cardiac surgery: a randomised controlled trial. Interact Cardiovasc Thorac Surg. 2010;11:758–762. doi: 10.1510/icvts.2010.243600. [DOI] [PubMed] [Google Scholar]
- 104.Kelbaek H, Thuesen L, Helqvist S, Clemmensen P, Klovgaard L, Kaltoft A, et al. Drug-eluting versus bare metal stents in patients with ST-segment-elevation myocardial infarction: eight-month follow-up in the Drug Elution and Distal Protection in Acute Myocardial Infarction (DEDICATION) trial. Circulation. 2008;118:1155–1162. doi: 10.1161/CIRCULATIONAHA.107.758698. [DOI] [PubMed] [Google Scholar]
- 105.Schmidt MR, Smerup M, Konstantinov IE, Shimizu M, Li J, Cheung M, et al. Intermittent peripheral tissue ischemia during coronary ischemia reduces myocardial infarction through a KATP-dependent mechanism: first demonstration of remote ischemic perconditioning. Am J Physiol Heart Circ Physiol. 2007;292:H1883–H1890. doi: 10.1152/ajpheart.00617.2006. [DOI] [PubMed] [Google Scholar]
- 106.Xin P, Zhu W, Li J, Ma S, Wang L, Liu M, et al. Combined local ischemic postconditioning and remote perconditioning recapitulate cardioprotective effects of local ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2010;298:H1819–H1831. doi: 10.1152/ajpheart.01102.2009. [DOI] [PubMed] [Google Scholar]
- 107.Bøtker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–734. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 108.Rentoukas I, Giannopoulos G, Kaoukis A, Kossyvakis C, Raisakis K, Driva M, et al. Cardioprotective role of remote ischemic periconditioning in primary percutaneous coronary intervention: enhancement by opioid action. JACC Cardiovasc Interv. 2010;3:49–55. doi: 10.1016/j.jcin.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 109.Li L, Luo W, Huang L, Zhang W, Gao Y, Jiang H, et al. Remote perconditioning reduces myocardial injury in adult valve replacement: a randomized controlled trial. J Surg Res. 2010;164:e21–e26. doi: 10.1016/j.jss.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 110.Kerendi F, Kin H, Halkos ME, Jiang R, Zatta AJ, Zhao ZQ, et al. Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors. Basic Res Cardiol. 2005;100:404–412. doi: 10.1007/s00395-005-0539-2. [DOI] [PubMed] [Google Scholar]
- 111.Andreka G, Vertesaljai M, Szantho G, Font G, Piroth Z, Fontos G, et al. Remote ischaemic postconditioning protects the heart during acute myocardial infarction in pigs. Heart. 2007;93:749–752. doi: 10.1136/hrt.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu S, Wu XF, Zhang WZ, Sun YX, Cai SL. Remote postconditioning by brief renal ischemia and reperfusion reduces acute myocardial ischemia and reperfusion induced myocardial apoptosis in rabbits. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35:757–760. [PubMed] [Google Scholar]
- 113.Gritsopoulos G, Iliodromitis EK, Zoga A, Farmakis D, Demerouti E, Papalois A, et al. Remote postconditioning is more potent than classic postconditioning in reducing the infarct size in anesthetized rabbits. Cardiovasc Drugs Ther. 2009;23:193–198. doi: 10.1007/s10557-009-6168-5. [DOI] [PubMed] [Google Scholar]
- 114.Li CM, Zhang XH, Ma XJ, Luo M. Limb ischemic postconditioning protects myocardium from ischemia–reperfusion injury. Scand Cardiovasc J. 2006;40:312–317. doi: 10.1080/14017430600925292. [DOI] [PubMed] [Google Scholar]
- 115.Ma X, Zhang X, Li C, Luo M. Effect of postconditioning on coronary blood flow velocity and endothelial function and LV recovery after myocardial infarction. J Interv Cardiol. 2006;19:367–375. doi: 10.1111/j.1540-8183.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- 116.Xue F, Yang X, Zhang B, Zhao C, Song J, Jiang T, et al. Postconditioning the human heart in percutaneous coronary intervention. Clin Cardiol. 2010;33:439–444. doi: 10.1002/clc.20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lin XM, Zhang ZY, Wang LF, Zhang L, Liu Y, Liu XL, et al. Attenuation of tumor necrosis factor-alpha elevation and improved heart function by postconditioning for 60 seconds in patients with acute myocardial infarction. Chin Med J (Engl) 2010;123:1833–1839. [PubMed] [Google Scholar]
- 118.Gunaydin B, Cakici I, Soncul H, Kalaycioglu S, Cevik C, Sancak B, et al. Does remote organ ischaemia trigger cardiac preconditioning during coronary artery surgery? Pharmacol Res. 2000;41:493–496. doi: 10.1006/phrs.1999.0611. [DOI] [PubMed] [Google Scholar]
- 119.Cheung MM, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, Li J, et al. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol. 2006;47:2277–2282. doi: 10.1016/j.jacc.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 120.Iliodromitis EK, Kyrzopoulos S, Paraskevaidis IA, Kolocassides KG, Adamopoulos S, Karavolias G, et al. Increased C reactive protein and cardiac enzyme levels after coronary stent implantation. Is there protection by remote ischaemic preconditioning? Heart. 2006;92:1821–1826. doi: 10.1136/hrt.2006.089060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ali ZA, Callaghan CJ, Lim E, Ali AA, Nouraei SA, Akthar AM, et al. Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: a randomized controlled trial. Circulation. 2007;116:I98–I105. doi: 10.1161/circulationaha.106.679167. [DOI] [PubMed] [Google Scholar]
- 122.Munk K, Andersen NH, Schmidt MR, Nielsen SS, Terkelsen CJ, Sloth E, et al. Remote ischemic conditioning in patients with myocardial infarction treated with primary angioplasty: impact on left ventricular function assessed by comprehensive echocardiography and gated single-photon emission CT. Circ Cardiovasc Imaging. 2010;3:656–662. doi: 10.1161/CIRCIMAGING.110.957340. [DOI] [PubMed] [Google Scholar]