Abstract
Reliable, evaluated human exposure and dose models are important for understanding the health risks from chemicals. A case study focusing on permethrin was conducted because of this insecticide's widespread use and potential health effects. SHEDS-Multimedia was applied to estimate US population permethrin exposures for 3- to 5-year-old children from residential, dietary, and combined exposure routes, using available dietary consumption data, food residue data, residential concentrations, and exposure factors. Sensitivity and uncertainty analyses were conducted to identify key factors, pathways, and research needs. Model evaluation was conducted using duplicate diet data and biomonitoring data from multiple field studies, and comparison to other models. Key exposure variables were consumption of spinach, lettuce, and cabbage; surface-to-skin transfer efficiency; hand mouthing frequency; fraction of hand mouthed; saliva removal efficiency; fraction of house treated; and usage frequency. For children in households using residential permethrin, the non-dietary exposure route was most important, and when all households were included, dietary exposure dominated. SHEDS-Multimedia model estimates compared well to real-world measurements data; this exposure assessment tool can enhance human health risk assessments and inform children's health research. The case study provides insights into children's aggregate exposures to permethrin and lays the foundation for a future cumulative pyrethroid pesticides risk assessment.
Keywords: probabilistic, exposure, model, aggregate, SHEDS, permethrin
Introduction
Reliable, evaluated exposure models are important for improving human health risk assessments. They can answer questions such as: What is the population distribution of exposure, dose, and risk for particular groups and lifestages? What are the time patterns of exposure? What are key media, pathways, and factors to inform how to effectively reduce exposures and address the greatest uncertainties for assessing risk? Implementation of the Food Quality Protection Act of 1996 (FQPA) necessitated developing new methodologies to assess residential exposures as well as refined dietary estimates. Although historically used “lower tier” modeling approaches may be appropriate for obtaining conservatively high screening level estimates of exposure, dose, and risk, higher tier models are needed for more realistic estimates for which uncertainties can be quantified. Probabilistic models have been recommended by the National Academy of Sciences1 and the EPA's Council for Regulatory Environmental Models.2 The Stochastic Human Exposure and Dose Simulation model for multimedia, multipathway chemicals (SHEDS-Multimedia) is a model developed by EPA's Office of Research and Development to help address these needs.
SHEDS-Multimedia is a physically based probabilistic computer model that can simulate aggregate or cumulative human exposure and dose, via dietary and residential routes, to a variety of environmental chemicals (http://www.epa.gov/heasd/products/sheds _multimedia/sheds_mm.html). This model can be used to predict ranges of exposure in a population; to identify critical pathways, factors, and uncertainties; and to enhance dose model estimates.3, 4 The purpose of SHEDS-Multimedia is to improve the understanding of aggregate and cumulative exposures over space and time for enhanced human health risk assessments involving chemicals such as pesticides, metals, and persistent bioaccumulative toxicants. As it uses 2-stage Monte Carlo sampling, SHEDS-Multimedia can quantify variability in population exposure and dose estimates, and the uncertainty associated with different percentiles. Another key feature of the model is the use of a time series approach for simulating dietary and residential exposures, accounting for variability within a day from separate eating occasions and microactivities. The sequential diary-based approach overcomes limitations of summing daily exposures from individual pathways. Tracking sequential dermal hand and body exposures, and linking hand-to-mouth ingestion time series with dermal hand exposures, accounts for both replenishment and removal processes (i.e., surface contact, hand mouthing, hand washing, bathing, absorption into the skin). The newest version of SHEDS-Multimedia (version 4) also includes several longitudinal diary assembly methods, multiple chemical (as well as single chemical) algorithms for conducting cumulative and aggregate assessments, and a number of other unique or advanced features. For example, the model permits correlation of randomly sampled inputs, simulates co-occurrence of chemical usage and application scenarios, includes multiple methods for sensitivity and uncertainty analyses, and is transparent and flexible for simulating different exposure scenarios. SHEDS-Multimedia exposure profiles can be linked to physiologically based pharmacokinetic (PBPK) models for enhanced dose and risk quantification.
Current and earlier versions of SHEDS-Multimedia have been used in EPA, academia, government, and industry for a variety of regulatory and research purposes.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 The most recent versions of SHEDS-Multimedia (versions 3 and 4, respectively) were externally peer reviewed by the EPA's Office of Pesticide Programs (OPP) Federal Insecticide, Fungicide, Rodenticide Act (FIFRA) Scientific Advisory Panel (SAP).17, 18 Evaluation has been conducted on the separate dietary and residential modules, and the combined results, with model-to-model comparisons and comparisons of model estimates against available environmental and biomonitoring data (http://www.epa.gov/heasd/products/sheds _multimedia/files/SHEDS%20Model%20Evaluation.pdf).
This paper presents the application of SHEDS-Multimedia to an aggregate permethrin case study for 3- to 5-year-old children, including variability, sensitivity, and uncertainty analyses, along with model evaluation results. SHEDS is comprised of both a residential module (SHEDS-Residential version 4.0)3, 19, and a dietary module (SHEDS-Dietary version 1.0)6, 20, 21 linked by a methodology presented below. Permethrin, a synthetic pyrethroid insectide, was selected for this model application because of (1) potential health effects,22, 23, 24, 25, 26 and (2) its widespread use, according to national measurement surveys, exposure field studies, National Health and Nutrition Examination Survey (NHANES) biomonitoring data, and use/usage data.27, 28, 29, 30, 31, 32, 33, 34, 35 It is the most commonly used pyrethroid pesticide and the first pyrethroid being reviewed under FQPA.
Methods
The SHEDS technical manuals describe in detail the model algorithms, methodologies, and input and output capabilities.3, 20 This case study quantifies population aggregate exposures for 3- to 5-year-olds (one of the EPA-recommended age groups)36 from both dietary ingestion and nine residential application scenarios of permethrin.
Dietary Exposure Modeling
Model algorithms, key features, and earlier model evaluation efforts of the SHEDS-Dietary module are presented in Xue et al.6 and Xue et al.20 The United States Department of Agriculture (USDA) Continuing Survey of Food Intake by Individuals (CSFII) 1994–1996 and 1998 consumption data and the 1991–2006 USDA Pesticide Data Program (PDP) cis- and trans-permethrin residue database were used in the SHEDS-Dietary module to identify permethrin residue concentration ranges, age-related trends, and foods with higher permethrin residue concentrations. The Diversity and Autocorrelation (D & A) method37 was used to construct longitudinal food consumption diaries. This method creates a population of longitudinal diaries that reproduce target values of the intra- and inter-person variance ratio (diversity, D) and day-to-day autocorrelation (A) for a key diary variable most relevant to exposure. Total caloric consumption was used as the key variable, with D and A statistics set to 0.3 and 0.1, respectively (based on longitudinal data from Lu et al.38 and Alex Lu, personal communication).
Residential Exposure Modeling
The SHEDS Residential module is flexible and can be applied to a wide range of chemical exposure scenarios. For this permethrin case study, nine residential exposure scenarios were selected based on analyses of usage information collected in the 2001–2002 Residential Exposure Joint Venture27 consumer pesticide product use survey provided to the EPA: indoor crack and crevice (aerosol and liquid), indoor flying insect killer (aerosol), indoor fogger (broadcast), lawn (granular — push spreader and liquid – hand wand), pet treatment (liquid and spot-on), and vegetable garden (dust, powder). In addition, all nine of these scenarios were combined in a single simulation. To address the specific exposure scenario(s) of interest, we used the input variables and data provided to the 2010 FIFRA SAP (http://www.regulations.gov/#!documentDetail;D=EPA -HQ-OPP-2010-0383-0015). These model input values are based on peer-reviewed publications, OPP's Residential Exposure Standard Operating Procedures,39 recommendations by OPP's FIFRA SAP, EPA's Exposure Factors Handbook and Child-Specific Exposure Factors Handbook,40, 41 and best Agency-derived estimates (http://www.regulations.gov/#!documentDetail;D =EPA-HQ-OPP-2010-0383-0015). To assemble 1 year longitudinal data, the D (diversity) & A (autocorrelation) method37 was used with indoor awake time as the key variable (D=0.25, A=0.4).42
Linkage of Dietary and Residential Exposure Modeling
The methodology used to combine the dietary and residential module outputs (http://www.regulations.gov/#!documentDetail;D =EPA-HQ-OPP-2010-0383-0023; slides 34 and 35) was externally peer reviewed18 and tested in this permethrin case study. We used the D & A method,37 with total caloric consumption as the key variable to extrapolate the cross-sectional dietary exposure estimates into longitudinal food consumption patterns, and used waking time at home to extrapolate the residential cross-sectional activity patterns into longitudinal patterns. The dietary and residential longitudinal diaries were first binned separately by age and gender, and then matched by percentiles using additional binning variables: total caloric consumption weighted by body weight for dietary and averaged MET (metabolic equivalent of task is an energy expenditure measure, i.e., the ratio of metabolic or energy consumption rate during a specific physical activity to the reference metabolic rate at rest) weighted by body weight for residential. An average of 50–100 data points in each bin was used as a criterion to select the key variables to ensure a large enough sample size in each bin for randomization.
Model Application with Permethrin Case Study
The SHEDS residential and dietary modules were each applied to estimate exposures for 3- to 5-year-olds, including both simulated use and non-use homes (i.e., where permethrin was or was not applied). The built-in pharmacokinetic (PK) model in SHEDS3 along with available absorption rate data (fraction of administered dose) were used for the initial exposure pathway contribution analysis. A sample size of ∼4000 individuals was used for the 1-year variability simulations. Results are reported for an annual averaging time and for separate and aggregated pathways. Sensitivity and uncertainty analyses were conducted to identify key factors, exposure pathways, and data gaps (Glen et al.3 chapters 5 and 6; Xue et al.20 sections 2.6 and 2.7). Uncertainty analyses were conducted to assess whether there were sufficient data for consumption and residue data sources, and to assess which dataset was relatively more important for exposure (assessing impact of residues vs consumption). We applied statistical bootstrapping of certain percentages of both datasets with the SHEDS-Dietary permethrin results.20
Two types of model evaluation were conducted for the permethrin analysis. First, SHEDS-Dietary modeled exposure predictions were compared against Children's Total Exposure to Persistent Pesticides and Other Persistent Organic Pollutants (CTEPP)30 Study duplicate diet data for cis- and trans-permethrin (data were matched by age and gender, based on 246 paired comparisons). Second, aggregate modeled SHEDS-PK dose predictions were compared with NHANES biomonitoring data for the urinary metabolites, cis- and trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid (cis- and trans-DCCA), and 3-phenoxybenzoic acid (3-PBA) (DCCA and 3-PBA are non-specific metabolites for a number of pyrethroid pesticides). In addition, SHEDS was evaluated further against measurements in Tulve et al.5 and Xue et al.6 The SHEDS Residential module compared well against other probabilistic aggregate residential exposure models in a model-to-model comparison using a simulated pyrethroid chemical.43 Dose estimates obtained using SHEDS linked with an aggregate permethrin PBPK model were compared against NHANES DCCA data (http://www.epa.gov/heasd/products/sheds_ multimedia/files/SHEDS%20PBPK%20Permethr in%20Case%20Study.pdf).
Results
Permethrin Population Exposure and Pathway Contribution Analyses
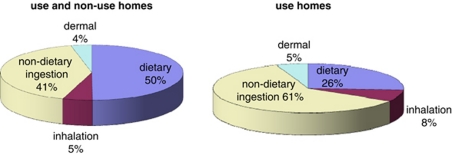
Table 1 shows summary statistics (in both mg/kg/day and μg/day) for the total (aggregated across dietary and residential pathways) annual averaged permethrin absorbed dose population estimates, based on 3825 simulated individuals: 2.4E−4 mg/kg/day (mean), 8.0E−4 mg/kg/day (95th percentile) and 2.0E−3 mg/kg/day (99th percentile). Analyses were conducted for the relative contribution to total absorbed dose by each of the SHEDS exposure routes. Table 1 and Figure 1 present the contribution to annual average daily permethrin dose by exposure pathway for 3- to 5-year-old children. The major exposure pathway for all 3- to 5-year-old simulated children (i.e., including those residing in permethrin use and non-use households) in the US population, based on means, was dietary ingestion (50%), followed by non-dietary ingestion (41%), inhalation (5%), and dermal (4%). For use households (i.e., 3- to 5-year-old children living in homes treated with permethrin). Figure 1 shows that non-dietary ingestion was the key exposure pathway (61%), followed by dietary (26%), inhalation (8%), and dermal (5%).
Table 1. Summary statistics of longitudinal averaged total permethrin absorbed dose by exposure pathways for 3- to 5-year-old children.
| Pathway | Unit | n | Mean | SD | p95 | p99 |
|---|---|---|---|---|---|---|
| Dietary | μg/day | 3825 | 2.07 | 1.57 | 5.15 | 8.17 |
| Inhalation | μg/day | 3825 | 0.22 | 0.95 | 1.10 | 5.56 |
| Non-dietary ingestion | μg/day | 3825 | 1.71 | 6.31 | 8.97 | 27.01 |
| Dermal | μg/day | 3825 | 0.15 | 0.70 | 0.70 | 2.45 |
| Total | μg/day | 3825 | 4.15 | 7.18 | 13.62 | 32.51 |
| Dietary | mg/kg/day | 3825 | 1.2E−04 | 9.3E−05 | 3.0E−04 | 4.8E−04 |
| Inhalation | mg/kg/day | 3825 | 1.3E−05 | 5.6E−05 | 6.5E−05 | 3.2E−04 |
| Non-dietary ingestion | mg/kg/day | 3825 | 1.0E−04 | 3.8E−04 | 5.4E−04 | 1.5E−03 |
| Dermal | mg/kg/day | 3825 | 9.0E−06 | 4.3E−05 | 4.2E−05 | 1.4E−04 |
| Total | mg/kg/day | 3825 | 2.4E−04 | 4.3E−04 | 8.0E−04 | 2.0E−03 |
Figure 1.
Average contribution to total permethrin absorbed dose by pathway (3- to 5-year olds).
On the basis of the mean estimates, dietary and non-dietary ingestion routes are comparable (50% vs 41%) considering all 3- to 5-year-old children (i.e., those residing in permethrin use and non-use households). For permethrin use households, the non-dietary route is predominant (61% vs 26%). At the 95th and 99th percentiles, the non-dietary ingestion route is the predominant route for all 3- to 5-year-old children (use and non-use households) and for the use households only (http://www.epa.gov/heasd/products/sheds_ multimedia/files/SHEDS%20Residential%20Module.pdf; slides 39 and 40).
Evaluation of SHEDS Modeled Permethrin Exposure and Dose Estimates
Table 2 shows that results of SHEDS-Dietary model results and CTEPP measurements match well at the mean, 95th, and 99th percentiles for both cis- and trans-permethrin. Model estimates are much lower than measurements at lower percentiles. The ratio of mean modeled to measured results is 1.09 and 1.01 for the cis- and trans- congeners, respectively; the ratio of the 95th percentile modeled to measured results is 1.06 and 0.99.
Table 2. Comparison of SHEDS dietary model using CSFII with CTEPP duplicate food study data for cis- and trans-permethrin (μg/kg/day).
| Variable | Mean | SD | p5 | p25 | p50 | p75 | p95 | p99 |
|---|---|---|---|---|---|---|---|---|
| SHEDS cis-permethrin | 7.1E−02 | 6.9E−01 | 4.2E−07 | 2.7E−05 | 6.4E−04 | 8.3E−03 | 1.8E−01 | 1.3E+00 |
| CTEPP cis-permethrin | 6.5E−02 | 3.8E−01 | 4.2E−04 | 6.8E−04 | 1.3E−03 | 5.8E−03 | 1.7E−01 | 2.6E+00 |
| SHEDS trans-permethrin | 9.1E−02 | 9.0E−01 | 3.4E−07 | 3.0E−05 | 7.7E−04 | 9.6E−03 | 2.2E−01 | 1.7E+00 |
| CTEPP trans-permethrin | 8.9E−02 | 3.8E−01 | 1.0E−03 | 2.2E−03 | 4.8E−03 | 2.3E−02 | 2.2E−01 | 2.0E+00 |
| Ratio of SHEDS estimates to measured data | ||||||||
| cis-permethrin | 1.09 | 0.50 | 1.44 | 1.06 | 0.51 | |||
| trans-permethrin | 1.01 | 0.16 | 0.42 | 0.99 | 0.85 | |||
Abbreviations: CSFII, Continuing Survey of Food Intake by Individuals; CTEPP, Children's Total Exposure to Persistent Pesticides and Other Persistent Organic Pollutants.
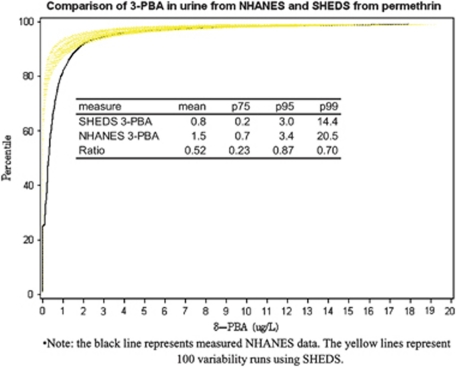
Figure 2 compares SHEDS dose estimates, using the built-in PK model, against NHANES 3-PBA biomarker data. The ratios of modeled to measured estimates are 0.52, 0.23, 0.87, and 0.70 for the mean, 75th, 95th, and 99th percentiles, respectively. Model estimates are lower than measurements at less than approximately the 90th percentile.
Figure 2.
Comparison of 3-phenoxybenzoic acid (3-PBA) in urine from National Health and Nutrition Examination Survey (NHANES) and SHEDS from permethrin.
Table 3 presents results of the SHEDS PK dose estimates against the NHANES cis- and trans-DCCA biomarker data. The ratios of modeled to measured estimates are 0.5–0.6 for the mean and upper percentiles.
Table 3. Comparison of modeled SHEDS-PK estimates with NHANES biomarker data.
| Variable | Mean | SD | p50 | p75 | p95 | p99 | p100 |
|---|---|---|---|---|---|---|---|
| NHANES cis-DCCA (μg/l) | 0.34 | 556.69 | 0.00 | 0.21 | 1.03 | 5.11 | 222.69 |
| NHANES trans-DCCA (μg/l) | 0.89 | 1501.74 | 0.00 | 0.49 | 2.68 | 14.06 | 406.34 |
| SHEDS cis-DCCA (μg/l) | 0.16 | 184.87 | 0.00 | 0.04 | 0.64 | 3.01 | 125.18 |
| SHEDS trans-DCCA (μg/l) | 0.47 | 688.47 | 0.00 | 0.07 | 1.37 | 9.11 | 527.22 |
| Ratio of SHEDS estimates to measured data | |||||||
| cis-DCCA ratio | 0.49 | 0.19 | 0.62 | 0.59 | 0.56 | ||
| trans-DCCA ratio | 0.53 | 0.14 | 0.51 | 0.65 | 1.30 | ||
Abbreviations: cis- and trans-DCCA, cis- and trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid; NHANES, National Health and Nutrition Examination Survey.
Modeled Permethrin Exposure Sensitivity Analyses
The most important commodities contributing to dietary exposure are spinach, lettuce, and cabbage based on total exposure across the population. Around the 99th percentile, the same three dominated, but lettuce is most important (http://www.regulations.gov/#!documentDetail;D= EPA-HQ-OPP-2010-0383-0022).
The key residential exposure variables (based on Sobol sensitivity analysis; http://www.regulations.gov/#!documentDetail; D=EPA-HQ-OPP-2010-0383-0023; slide 42) for this permethrin case study are: usage frequency for crack and crevice aerosol, surface-to-skin transfer efficiency, usage frequency for crack and crevice liquid, usage frequency for indoor fogger, and hand mouthing frequency.
Modeled Permethrin Exposure Uncertainty Analyses
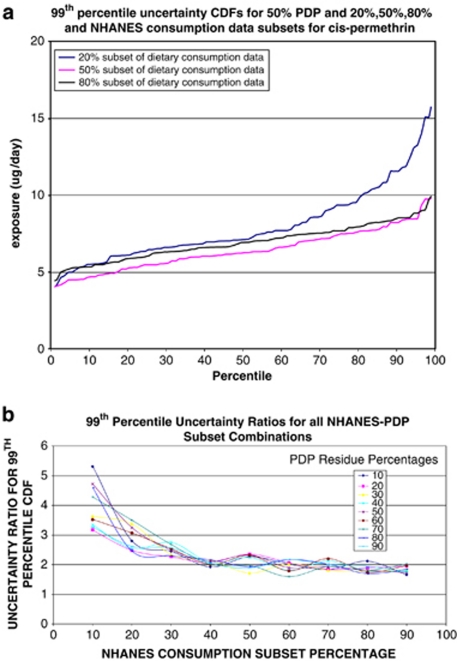
Figure 3a shows the uncertainty for three CDFs based on bootstrap sampling of 50% of PDP cis-permethrin residues and 20%, 50%, and 80% of the NHANES food consumption data (we used the 99th percentile as an indicator). It presents uncertainty results for daily dietary cis-permethrin exposure, based on bootstrapping 100 times. The three lines represent three sampling schemes: the blue line for 50% of residues and 20% consumption data; the pink line for 50% of residues and 50% residue data; and the black line for 50% residues and 80% consumption data. The CDF for the 50% of residues and 20% of consumption data has the biggest uncertainty. The ratio of the 97.5th percentile to the 2.5th percentile (95% confidence interval) is 15.07/4.63=3.3, reflecting the 97.5th/2.5th percentile ratio for an uncertainty run that used subsets: 50% cis-permethrin residue by raw agricultural commodity, and 20% of NHANES dietary consumption data for 3- to 5-year-old children. In the same way, we calculated similar ratios for other bootstrapping schemes to evaluate the major factors contributing to the overall uncertainty (Figure 3b). As the sampling rate of consumption data increases, the ratio decreases until it reaches a plateau at about 60%, but there is no apparent pattern for residue data. Therefore, these uncertainty analyses show we have sufficient consumption data. However, more research is needed on residue uncertainty analyses. See Xue et al.20 section 2.7 for more details.
Figure 3.
(a, b) SHEDS uncertainty analyses for dietary permethrin exposure (3- to 5-year-olds). NHANES, National Health and Nutrition Examination Survey; PDP, Pesticide Data Program.
Residential exposure uncertainty analysis results show that the 97.5th and 2.5th percentiles of the 99th percentile total dose permethrin profile are 119.5 and 17.2, respectively, with a ratio of 17 (http://www.regulations.gov/#!documentDetail; D=EPA-HQ-OPP-2010-0383-0023; slide 43).18 The ratio of the 97.5th to 2.5th percentile of the 95th percentile total dose profile is 6. The ratio of the 97.5th to 2.5th percentile of the 75th percentile total dose profile is 20. Thus, with current inputs, the 75th, 95th, and 99th percentile uncertainty ratios are ∼20, 6, and 17, respectively (from parameter uncertainty). See Glen et al.3 section 6.2, for more details.
Discussion
Real-world data are needed for model inputs and to evaluate model estimates. For example, biological measurements, in combination with other multimedia measurements and supporting information, may be used to estimate aggregate and cumulative exposures and doses, and to compare model predictions. Recent research efforts have collected critical data on potential exposures of young children (<6 years of age) in their homes and child care centers to the current-use pyrethroid pesticides.30, 33, 34 Applying SHEDS to estimate urinary 3-PBA concentrations resulted in a mean and 95th percentile of 0.8 and 3 μg/l; the estimated aggregate absorbed dose of permethrin accounted for ∼50% of the urinary 3-PBA (Figure 2). These modeled estimates compare well with measured results reported from an observational pilot study of 127 young children in Ohio.30 The mean and 95th percentile for measured urinary 3-PBA concentrations were 0.9 and 1.9 μg/l, respectively, and the authors estimated that the aggregate absorbed doses of permethrin accounted for about 60% of the excreted amounts of 3-PBA found in the children's urine.30 PBA is a common metabolite of several pyrethroid compounds.
Using Tulve et al.34 multimedia measurement data as inputs to SHEDS, we compared the measured and predicted urinary 3-PBA metabolite concentrations to further evaluate the ability of SHEDS to estimate urinary 3-PBA concentrations.5 In general, the modeled urinary concentrations compared well with the measured concentrations from this study. SHEDS accurately estimated both the high and low urinary 3-PBA concentrations found in the children's urine samples.5
As presented in the Results section, SHEDS dietary exposure estimates compared well at upper percentiles to the CTEPP duplicate diet data, and SHEDS aggregate (residential + dietary) dose estimates of 3-PBA compared well to the measured NHANES biomarker data at upper percentiles. The lower percentiles (p5 and p25) for SHEDS model estimates in Table 2 are orders of magnitude lower than CTEPP measurements because we used zeroes for non-detect values in SHEDS permethrin residue inputs. In the future, we will use pesticide usage information and detection limits to fill in non-detects, which should yield closer exposure results at lower percentiles; in this paper we focus on higher percentiles. We attribute the higher NHANES concentrations of urinary 3-PBA than SHEDS modeled estimates at lower percentiles to other pyrethroid pesticides besides permethrin. In addition, comparison of linked SHEDS-PBPK modeled estimates to the NHANES cis- and trans-DCCA data showed good agreement at upper percentiles (http://www.epa.gov/heasd/products/sheds_multi media/files/SHEDS%20PBPK%20Permethrin%2 0Case%20Study.pdf).18 These simulations suggest that permethrin accounts for ∼50% of the cis- and trans-DCCA measured in the urine of the NHANES participants at the 75th percentile, and ∼90% of the cis- and trans-DCCA at the 95th percentile. Such results suggest that permethrin exposure accounts for the higher exposures among pyrethroids bearing a DCCA moiety (i.e., cypermethrin, cyfluthrin, and permethrin). This finding is consistent with what is known for real-world dietary and residential/daycare exposures to these three pyrethroids.30, 32, 33, 34 A cumulative pyrethroids assessment is needed to determine the contribution of other pyrethroid pesticides to the cis- and trans-DCCA levels, and 3-PBA levels found in urine samples.
The SHEDS modeling assessment in this paper reveals that considering all homes (i.e., with and without permethrin use) the dietary pathway contributes the most to exposure for 3- to 5-year-old children, followed by non-dietary ingestion, inhalation, and dermal routes. This finding of relative pathway contribution is consistent with the CTEPP OH study.30 SHEDS modeled dermal exposures could be under-predicting based on low skin residue loadings and use of a dermal absorption fraction44; future SHEDS research could adjust the absorption rate as a function of skin loading. Considering only children in homes with residential permethrin use, non-dietary ingestion was found to be more important than dietary and the other routes. The most important food commodities for dietary exposure were found to be lettuce, spinach, and cabbage. Uncertainty analyses show we have sufficient food consumption data for the SHEDS-Dietary module, but more research is needed on residue uncertainty analyses. Uncertainty for the residential module is much greater than for the dietary module. We believe this is because a greater number of inputs are needed for residential exposure modeling, especially for those key variables shown in the sensitivity analyses that are lacking data. These include surface-to-skin transfer efficiency, fraction of hand mouthed, saliva removal efficiency, hand-mouthing frequency, fraction of house treated, and usage frequency. More data collection of these inputs (e.g., updated usage information, mouthing frequencies for different lifestages) would be helpful for refined model estimates.
The focus of SHEDS is exposure, but the model does include a simple PK dose module. Ongoing research involves linking SHEDS exposure time series results with class-oriented PBPK models17 that estimate tissue burden and urinary concentrations, and tissue-based relative potency factors. The results from the linked models (i.e., outputs from SHEDS used as inputs to external PBPK models), various measurement studies, and corresponding data analyses will be used to quantify the cumulative exposure, dose, and risks to populations from pyrethroid mixtures in real-world scenarios. Initial dose estimates with the SHEDS-PK model, and linking SHEDS to a prototype PBPK dose model, have been tested using annual simulations of exposure (by pathway and aggregate) for single chemicals, including permethrin.18 Specific plans for applying SHEDS for a cumulative pyrethroids assessment will include linking it with PBPK pyrethroid dose models, apportioning dietary and residential pathways, and considering the relative contribution of permethrin, cypermethrin, and cyfluthrin to exposure and dose. Further testing of the linkages along with additional model evaluation using NHANES and measurement study data is underway and planned for a combined assessment of these three pyrethroids.
Additional future research activities and planned model refinements include the following: refine algorithms as needed to accommodate new research and regulatory applications, possible incorporation of source-to-concentration module (e.g., fugacity), enhance residential/dietary merging algorithms, refine to allow estimates at local (e.g., census tract) level, and improve cumulative algorithms. More longitudinal activity data from measurement studies are needed for evaluating and refining the D & A method to simulate longitudinal consumption and residential activity patterns, used in these SHEDS analyses. Future modeling efforts could incorporate other pathways, for example, food preparation and handling could contribute to exposures of children who have frequent mouthing behavior in a permethrin-contaminated residential environment. Future case studies may consider different populations, lifestages, chemicals/chemical classes or mixtures, seasons, and regions, and will include PBPK linkage, sensitivity and uncertainty analyses, and further model evaluations. Through these applications of the model, identification of key factors and data gaps will inform future data collection efforts. The methods and models developed through integrated modeling and measurements research will provide new insights and data that will inform and support aggregate and cumulative risk assessments for pyrethroids and other chemical classes.
Conclusions
This paper presents an aggregate permethrin exposure and dose assessment for 3- to 5-year-old children, using EPA's probabilistic SHEDS-Multimedia model. Close comparison of model estimates against measured duplicate diet and biomarker data provided multifaceted evaluation of the SHEDS algorithms and approaches used. Through model sensitivity and uncertainty analyses, we identified key factors and research needs to inform exposure measurement researchers and environmental health decision-makers. Collecting data for key inputs, such as consumption of specific commodities, surface-to-skin transfer efficiency, hand mouthing frequency, fraction of hand mouthed, saliva removal efficiency, fraction of house treated, and residential pesticide usage frequency, will reduce uncertainty for enhancing SHEDS model predictions in future applications. We conclude that the case study presented in this paper provides insights into children's residential and dietary exposures to the insecticide permethrin, illustrates the SHEDS aggregate exposure modeling methodology, and lays the foundation for a future cumulative pyrethroid pesticides risk assessment.
Acknowledgments
We thank Andrew Geller, Brad Schultz, Roy Fortmann, Halûk Õzkaynak, and Kristin Isaacs from the EPA's Office of Research and Development for their support on the SHEDS model. We also acknowledge David Miller, Steve Nako, Matthew Crowley, David Hrdy, Dana Vogel, Charles Smith, Kelly Lowe, Victor Miller and others from the EPA's Office of Pesticide Programs for assisting with permethrin inputs and reviewing an early draft of this paper. We thank Mariko Porter of Alion for assistance with input preparation. The United States Environmental Protection Agency through its Office of Research and Development conducted and partially funded the research described here under Contract EP-D-05-065 to Alion Science and Technology.
The authors declare no conflict of interest.
Footnotes
Disclaimer
This research has been subjected to the US EPA's administrative review and approved for publication.
References
- National Academy of Sciences Models in Environmental Regulatory Decision MakingReport of the Committee on Models in the Regulatory Decision Process. National Research Council, The National Academics Press, Washington, DC; 2007 [Google Scholar]
- US EPA Guidance on the Development, Evaluation and Application of Environmental ModelsDraft.US Environmental Protection Agency, Office of the Science Advisor, Council for Regulatory Environmental Modeling, Washington, DC; 2008 : http://www.epa.gov/crem/library/CREM- Guidance-Public-Review-Draft.pdf . [Google Scholar]
- Glen G., Zartarian V.G., Smith L., Xue J.The Stochastic Human Exposure and Dose Simulation Model for Multimedia, Multipathway Chemicals (SHEDS-Multimedia): Residential Module. Draft Technical Manual US Environmental Protection Agency; 2010 : http://www.epa.gov/heasd/products/sheds_multimedia/files/ SHEDS_Residentialv4_Techmanual_06-16-2010.Final.pdf . [Google Scholar]
- Zartarian V.G., Glen G., Smith L., Xue J.SHEDS-Multimedia Model Version 3 Technical Manual US Environmental Protection Agency; EPA/600/R-08/118,Research Triangle Park; NC, 2008 [Google Scholar]
- Tulve N.S., Egeghy P.P., Fortmann R.C., Xue J., Evans J., Whitaker D.A., Croghan C.W. Methodologies for estimating cumulative human exposures to current-use pyrethroid pesticides. J Expo Sci Environ Epidemiol. 2011;21 (3:317–327. doi: 10.1038/jes.2010.25. [DOI] [PubMed] [Google Scholar]
- Xue J., Zartarian V., Wang S.-W., Liu S.V., Georgopolous P. Probabilistic modeling of dietary arsenic exposure and dose and evaluation with 2003–2004 NHANES data. Environ Health Perspect. 2010;118 (3:345–350. doi: 10.1289/ehp.0901205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J., Zartarian V., Liu S., Geller A.Methyl Mercury Exposure from Fish Consumption in Vulnerable Racial/Ethnic Populations. Sci Tot Env 2012414(1373–379. [DOI] [PubMed]
- Stout D.M., II, Morgan M.K., Egeghy P.P., Xue J.Pesticides in Household, Structural and Residential Pest Management Movement of diazinon residues into homes following applications of a granular formulation to residential lawns American Chemical Society, Washington, DC; 20091015pp 143–162. [Google Scholar]
- Georgopoulos P.G., Wang S.W., Yang Y.C., Xue J., Zartarian V.G., McCurdy T. Biologically based modeling of multimedia, multipathway, multiroute population exposures to arsenic. J Expo Sci Environ Epidemiol. 2008;18:462–476. doi: 10.1038/sj.jes.7500637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- California EPA Assessment of Children's Exposure to Surface Methamphetamine Residues in Former Clandestine Methamphetamine Labs, and Identification of a Risk-Based Cleanup Standard for Surface Methamphetamine Contamination. External Review Draft. Office of Environmental Health Hazard Assessment. Integrated Risk Assessment Branch,2007
- Zartarian V., Xue J., Ozkaynak H., Dang W., Glen G., Smith L., Stallings C. A probabilistic arsenic exposure assessment for children who contact chromated copper arsenate (CCA)-treated playsets and decks, part 1: model methodology, variability results, and model evaluation. Risk Anal. 2006;26 (2:515–531. doi: 10.1111/j.1539-6924.2006.00747.x. [DOI] [PubMed] [Google Scholar]
- Xue J., Zartarian V., Ozkaynak H., Dang W., Glen G., Smith L., Stallings C. A probabilistic arsenic exposure assessment for children who contact CCA-treated playsets and decks, part 2: sensitivity and uncertainty analyses. Risk Analysis. 2006;26 (2:533–541. doi: 10.1111/j.1539-6924.2006.00748.x. [DOI] [PubMed] [Google Scholar]
- Hore P., Zartarian V., Xue J., Ozkaynak H., Wang S.W., Yang Y.C., et al. Children's residential exposure to chlorpyrifos: application of CPPAES field measurements of chlorpyrifos and TCPy within MENTOR/SHEDS-pesticides model. Sci Tot Env. 2006;366:525–537. doi: 10.1016/j.scitotenv.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Stout D.M., II, Mason M.A. The distribution of chlorpyrifos following a crack and crevice type application in the US EPA indoor air quality research house. Atmos Environ. 2003;37:5539–5549. [Google Scholar]
- Buck R.J., Özkaynak H., Xue J., Zartarian V.G., Hammerstrom K. Modeled estimates of chlorpyrifos exposure and dose for minnesota and arizona NHEXAS populations. J Expo Anal Environ Epidemiol. 2001;11 (3:253–268. doi: 10.1038/sj.jea.7500164. [DOI] [PubMed] [Google Scholar]
- Zartarian V.G., Özkaynak H., Burke J.M., Zufall M.J., Rigas M.L., Furtaw E.J., Jr A modeling framework for estimating children's residential exposure and dose to chlorpyrifos via dermal residue contact and non-dietary ingestion. Env Health Perspect. 2000;108 (6:505–514. doi: 10.1289/ehp.00108505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIFRA SAP A Set of Scientific Issues Being Considered by the Environmental Protection Agency Regarding: Review of EPA/ORD/NERL's SHEDS-Multimedia Model Aggregate version 3, SAP Minutes No. 2007-06. 14–15 August 2007 FIFRA Scientific Advisory Panel Meeting, Arlington, VA. http://www.epa.gov/scipoly/sap/meetings/2007 /081407_mtg.htm , 2007
- FIFRA SAP A Set of Scientific Issues Being Considered by the Environmental Protection Agency Regarding: SHEDS-Multimedia version 4, Peer consult on PBPK Modeling, and a SHEDS-PBPK Permethrin study. SAP Minutes No. 2010-06. 20–22 July 2010, FIFRA Scientific Advisory Panel Meeting, Arlington, VA,2010 : http://www.epa.gov/scipoly/sap/meetings/2010 /july/072010minutes.pdf . http://www.regulations.gov/#!docketDetail;D =EPA-HQ-OPP-2010-0383 .
- Isaacs K., Stallings C., Zartarian V.G., Glen G.Stochastic Human Exposure and Dose Simulation (SHEDS) Model for Multimedia, Multipathway Chemicals: Version 4 Residential Module. User GuidePrepared for the 20–22 July 2010EPA FIFRA SAP, Crystal City, VA; 2010 [Google Scholar]
- Xue J., Zartarian V.G., Nako S.The Stochastic Human Exposure and Dose Simulation (SHEDS)-Dietary Model Technical ManualPrepared for the 20–22 July 2010EPA FIFRA SAP, Crystal City, VA; 2010 [Google Scholar]
- Isaacs K., Xue J., Stallings C., Zartarian V.G.Stochastic Human Exposure and Dose Simulation (SHEDS) Model for Multimedia, Multipathway Chemicals: Version 1 SHEDS-Dietary Module User GuidePrepared for the 20–22 July 2010EPA FIFRA SAP, Crystal City, VA; 2010 [Google Scholar]
- US EPA Reregistration Eligibility Decision for PermethrinEPA 738-R-06-017,Office of Prevention, Pesticides and Toxic Substances, Washington, DC; 2006 [Google Scholar]
- US EPA . Chemicals Evaluated for Carcinogenic Potential by the Office of Pesticide Programs. Health Effects Division, Office of Pesticide Programs, US Environmental Protection Agency, Washington, DC; 2007. [Google Scholar]
- Kim S.S., Lee R.D., Lim K.J., Kwack S.J., Rhee G.S., Seok J.H., et al. Potential estrogenic and antiandrogenic effects of permethrin in rats. J Reprod Dev. 2005;51 (2:201–210. doi: 10.1262/jrd.16060. [DOI] [PubMed] [Google Scholar]
- Rusiecki J.A., Padtel R., Koutros S., Beane-Freeman L., Landgren O., Bonner M.R., et al. Cancer incidence among pesticide applicators exposed to permethrin in the Agricultural Health Study. Env Health Perspect. 2009;117 (4:581–586. doi: 10.1289/ehp.11318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecobichon D.J.Toxic effects of pesticidesIn: Klassen CD, Watkins JB III (eds)Casarett & Doull's Essentials of ToxicologyednMcGraw-Hill; NY, 2003 [Google Scholar]
- Jacobs L., Driver J., Pandian M.Residential Exposure Joint Venture: National Pesticide Use Survey--Design, Implementation, Analysis Methods and Results: Lab Project Number: 03-REJV-4M-001: 030-REJV-4M-001: 8D. Unpublished study prepared by NFO WorldGroup and infoscientific.com, Inc. 8515 p., cited in EPA MRID 45870701, 3 March,2003
- CDC Fourth National Report on Human Exposure to Environmental Chemicals http://www.cdc.gov/exposurereport/ , 2009 [PubMed]
- Morgan M.K., Sheldon L.S., Croghan C.W., Jones P.A., Robertson G.L., Chuang J.C., Wilson N.K., Lyu C.W. Exposures of preschool children to chlorpyrifos and its degradation product 3,5,6-trichloro-2-pyridinol in their everyday environments. J Expo Anal Environ Epidemiol. 2005;15 (4:297–309. doi: 10.1038/sj.jea.7500406. [DOI] [PubMed] [Google Scholar]
- Morgan M.K., Sheldon L.S., Croghan C.W., Jones P.A., Chuang J.C., Wilson N.K. An observational study of 127 preschool children at their homes and daycare centers in Ohio: environmental pathways to cis- and trans-permethrin exposure. Environ Res. 2007;104 (2:266–274. doi: 10.1016/j.envres.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Naeher L.P., Tulve N.S., Egeghy P.P., Barr D.B., Adetona O., Fortmann R.C., et al. Organophosphorus and pyrethroid insecticide urinary metabolite concentrations in young children living in a southeastern United States city. Sci Total Environ. 2010;408 (5:1145–1153. doi: 10.1016/j.scitotenv.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Stout D.M., II, Bradham K.D., Egeghy P.P., Jones P.A., Croghan C.W., Ashley P.A., Pinzer E., Friedman W., Brinkman M.C., Nishioka M.G., Cox D.C. American Healthy Homes Survey: a national study of residential pesticides measured from floor wipes. Environ Sci Technol. 2009;43 (12:4294–4300. doi: 10.1021/es8030243. [DOI] [PubMed] [Google Scholar]
- Tulve N.S., Jones P.A., Nishioka M.G., Fortmann R.C., Croghan C.W., Zhou J.Y., Fraser A., Cave C., Friedman W. Pesticides measurements from the first national environmental health survey of child care centers using a multi-residue GC/MS analysis method. Environ Sci Technol. 2006;40 (20:6269–6274. doi: 10.1021/es061021h. [DOI] [PubMed] [Google Scholar]
- Tulve N.S., Egeghy P.P., Fortmann R.C., Whitaker D.A., Nishioka M.G., Naeher L.P., Hilliard A. Multimedia measurements and activity patterns in an observational pilot study of nine young children. J Expo Sci Environ Epidemiol. 2008;18 (1:31–44. doi: 10.1038/sj.jes.7500600. [DOI] [PubMed] [Google Scholar]
- Wilson N.K., Chuang J.C., Iachan R., Lyu C., Gordon S.M., Morgan M.K., et al. Design and sampling methodology for a large study of preschool children's aggregate exposures to persistent organic pollutants in their everyday environments. J Expo Anal Environ Epidemiol. 2004;14 (3:260–274. doi: 10.1038/sj.jea.7500326. [DOI] [PubMed] [Google Scholar]
- US EPA Guidance on Selecting Age Groups for Monitoring and Assessing Childhood Exposures to Environmental Contaminants NCEA, Washington, DC; EPA/630/P-03/003F2005 [Google Scholar]
- Glen G., Smith L., Isaacs K., Mccurdy T., Langstaff J. A new method of longitudinal diary assembly for human exposure modeling. J Expo Sci Environ Epidemiol. 2008;18:299–311. doi: 10.1038/sj.jes.7500595. [DOI] [PubMed] [Google Scholar]
- Lu C., Barr D.B., Pearson M., Bartell S., Bravo R. A longitudinal approach to assessing urban and suburban children's exposure to pyrethroid pesticides. Env Health Perspect. 2006;114 (9:1419–1423. doi: 10.1289/ehp.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA Draft Standard Operating Procedures (SOPs) for Residential Exposure Assessments The Residential Exposure Assessment Work Group, US Environmental Protection Agency, Office of Pesticide Programs, Health Effects Division and Versar, Inc.Contract No. 68-W6-0030. 19 December,1997 , http://www.epa.gov/pesticides/trac/science/ trac6a05.pdf . [Google Scholar]
- US EPA . Exposure Factors Handbook. US Environmental Protection Agency, Office of Research and Development, National Center for Environmental Assessment, Washington, DC; 1997. [Google Scholar]
- US EPA Child-Specific Exposure Factors Handbook US Environmental Protection Agency, Washington, DC; EPA/600/R-06/096F,2008 [Google Scholar]
- Xue J., McCurdy T., Spengler J., Ozkaynak H. Understanding variability in time spent in selected locations for 7–12-year old children. J Expos Anal Environ Epidem. 2004;14:222–233. doi: 10.1038/sj.jea.7500319. [DOI] [PubMed] [Google Scholar]
- Young B.M., Tulve N.S., Egeghy P.P., Driver J., Zartarian V.G., Johnston J., et al. Comparison of four probabilistic models (CARES, Calendex, ConsExpo, SHEDS) to estimate aggregate residential exposures to pesticides J Expos Sci Environ Epidem(submitted). [DOI] [PubMed]
- Kissel J.C. The mismeasure of dermal absorption. J Expo Sci Environ Epidemiol. 2011;21:302–309. doi: 10.1038/jes.2010.22. [DOI] [PubMed] [Google Scholar]