Abstract
In this study we demonstrate that ultrasound (US)-guided injection of thrombin is a safe and effective way to treat iatrogenic pseudoaneurysms as a new treatment modality at a 650-bed urban community hospital. We included retrospective chart review of patients who were treated for iatrogenic pseudoaneurysms from January 2004 to June 2010 at a single institution. All patients' pseudoaneurysms were treated using US-guided thrombin injection. This study demonstrated an overall success rate of 97.1% in treating iatrogenic pseudoaneurysms in 33 of 34 patients. One patient underwent open surgical repair. No mortality or complications were noted. The study was successful in demonstrating that the US-guided injection of thrombin is an efficacious way to treat iatrogenic pseudoaneurysms and can be safely implemented as a new treatment modality by appropriately trained vascular surgeons. A review of different techniques is included. An algorithm for the treatment of iatrogenic pseudoaneurysms is proposed from this study.
Keywords: Pseudoaneurysm, ultrasound-guided thrombin injection, false aneurysm
An arterial pseudoaneurysm is a false aneurysm of the involved blood vessel. Unlike a true aneurysm, it does not consist of all the layers of an arterial wall (intima, media, and adventitia). Instead it is essentially a contained hematoma in the wall of the artery which is being prevented from becoming a free rupture by its surrounding tissue. It results most commonly as a complication from arterial catheterization performed for diagnostic or therapeutic interventions. Other causes of arterial pseudoaneurysms include complications arising as a result of percutaneous hemodialysis access, cardiac catheterization, percutaneous intra-aortic balloon pump placement, and line placement. Their incidence is 1% following diagnostic catheterizations and 3.2 to 7.7% after interventional procedures.1
Diagnosis of iatrogenic pseudoaneurysms relies on a high index of suspicion by the clinician. They are diagnosed clinically with real-time duplex ultrasonography.2 The symptoms of pseudoaneurysm include pain due to increased pressure from swelling or nerve compression, extremity swelling due to venous compression, hematoma, or loss of pulse.1 Presence of new auscultated bruit, widened pulse, or pulsatile mass can also be seen on physical examination.2
There are several modalities of treatment for pseudoaneurysms but thrombin injection is successful in 94 to 100% of attempts, according to peer-reviewed literature.1 The advantages of using ultrasound (US)-guided thrombin injection include minimal discomfort to the patient, high efficacy, lack of influence of concurrent anticoagulation, and rapidity of the procedure.2 We evaluated the outcomes of our new modality treatment over a period of 5 years.
MATERIALS AND METHODS
A retrospective study with institutional review board approval was conducted on all patients who had received treatment for a pseudoaneurysm from January 2004 to June 2010. This study examined data from 34 patients. We tested the hypothesis that the US-guided thrombin injection of pseudoaneurysms in this patient population is as effective as the published literature rate of 94 to 100% for resolution of all pseudoaneurysms when newly started at a facility.
A chart review was performed and the inclusion criteria comprised those patients who were at least 18 years of age and had undergone a percutaneous procedure, and subsequently developed a pseudoaneurysm requiring treatment from January 2004 to June 2010. All patients were treated by vascular surgeons at an urban Level I trauma center. The following variables were collected from the patient's chart:
Service date
Demographics
Type of procedure
Size and location of pseudoaneurysm
Anticoagulation status before and after catheterization
Ankle-brachial index (ABI) pre- and postprocedure
-
Symptoms of presentation of a pseudoaneurysm
- Pain
- Bleeding
- Loss of pulse
- Cold extremities
- Swelling
- Hematoma
Amount of thrombin used
Thrombin efficacy in pseudoaneurysm resolution
Procedural complications
The area of the pseudoaneurysm was prepped and draped in a sterile fashion. Duplex US was used for evaluating the neck, sac of pseudoaneurysm as well as for looking at the native vessel. A 20- or 22-gauge needle was used to inject thrombin, which is reconstituted at a concentration of 1000 U/mL. The US guidance allows placement of the needle within even small and deeply situated pseudoaneurysms. The presence of thrombin solution in the needle tip is more visible because of echogenic thrombus formation on the bevel when it enters the pseudoaneurysm. The visualization of the needle tip location is essential. Thrombosis is generally evident within seconds of injection. If the initial injection is unsuccessful, a second injection is performed. After successful thrombosis, patients are maintained on bed rest for 2 to 4 hours after the procedure. Follow-up US and ABIs are performed to evaluate the treatment at 2 hours and at 24 hours after the procedure.
Descriptive statistics were produced using means, medians, ranges and standard deviations for continuous variables, and percentages for categorical variables. Independent variables included gender, age, resolution rates, information from procedure notes, and complications. Statistical significance was evaluated at the 5% (α = 0.05) level.
RESULTS
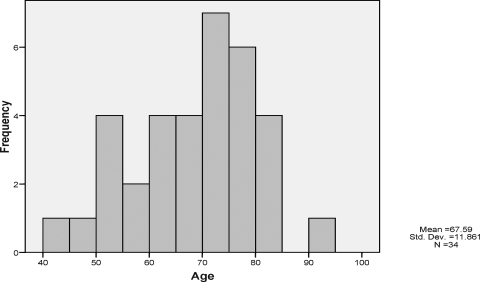
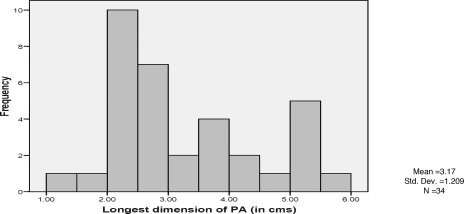
During the 5-year period, 34 patients with pseudoaneurysms were evaluated (Table 1). The study population primarily consisted of women 73.5% (Table 2). The mean age was 67.5 years (Fig. 1). The most common procedural type was lower extremity angiograms which was frequently involved in pseudoaneurysm formation in 55.9% (n = 19). Central line placement resulted in 5.9% (n = 2) of pseudoaneurysm formation. There were also two patients (5.9%) who developed pseudoaneurysm from upper extremity angiogram (Table 3). The size of the aneurysm varied between 1.38 and 5.56 cm, with the average size of 3.17 cm in length (Fig. 2). All of them received US-guided thrombin injection, with a resolution of 97.1% (n = 33). Only one patient did not have complete resolution with US-guided thrombin injection and underwent surgical repair of the pseudoaneurysm (Table 4).
Table 1.
Frequency Distribution for Pseudoaneurysm Patients
| Age | Longest Dimension of PA (in cm) | Amount of Thrombin (in units/mL) | ABI Score Preprocedure (Right) | ABI Score Preprocedure (Left) | ABI Score Postprocedure (Right) | ABI Score Postprocedure (Left) | |
|---|---|---|---|---|---|---|---|
| Mean | 67.5 | 3.17 | 1152.9 | 0.90 | 0.95 | 0.90 | 0.89 |
| Median | 70.0 | 2.65 | 1000.0 | 1.00 | 0.99 | 1.00 | 1.00 |
| Standard deviation | 11.9 | 1.21 | 802.74 | 0.30 | 0.32 | 0.25 | 0.29 |
| Minimum | 42 | 1.38 | 300 | 0.36 | 0.37 | 0.48 | 0.19 |
| Maximum | 95 | 5.56 | 4000 | 1.50 | 1.60 | 1.50 | 1.40 |
PA, pseudoaneurysm; ABI, ankle-brachial index.
Table 2.
Gender Distribution for Pseudoaneurysm Patients
| Gender | Frequency | Percentage |
|---|---|---|
| Male | 9 | 26.5 |
| Female | 25 | 73.5 |
| Total | 34 | 100.0 |
Figure 1.
Histogram of age versus pseudoaneurysm frequency.
Table 3.
Frequency Distribution for Type of Procedure
| Procedure Type | Frequency | Percentage |
|---|---|---|
| Lower extremity angiogram | 19 | 55.9 |
| Heart catheterization | 10 | 29.4 |
| Upper extremity angiogram | 2 | 5.9 |
| Central line placement | 2 | 5.9 |
| Carotid angiogram | 1 | 2.9 |
| Total | 34 | 100.0 |
Figure 2.
Histogram of pseudoaneurysm dimensions by frequency.
Table 4.
Frequency Distribution of Pseudoaneurysm Resolution
| Pseudoaneurysm Resolution | Frequency | Percentage |
|---|---|---|
| No | 1 | 2.9 |
| Yes | 33 | 97.1 |
| Total | 34 | 100.0 |
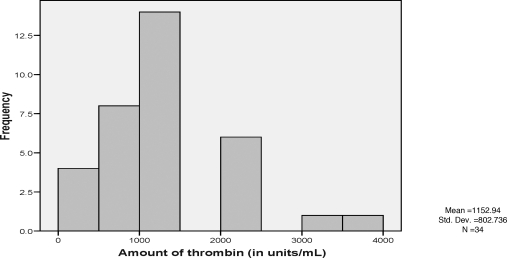
The average amount of thrombin used was 1152 U/mL (range, 300 to 4000 U/mL). Two patients required two injections of thrombin (Fig. 3). Of the 34 patients, 27 (79.4%) were on anticoagulants (antiplatelet and antithrombotic) before and after the US-guided thrombin injection (Tables 5 and 6). There were different reasons for these patients to be on anticoagulants, including recent coronary stents, peripheral intervention, and atrial fibrillation. Anticoagulation, before or after the procedure, did not affect the management of the pseudoaneurysm.
Figure 3.
Histogram of thrombin amount used on pseudoaneurysm patients.
Table 5.
Anticoagulation Status Preprocedure
| Anticoagulation Status Preprocedure | Frequency | Percentage |
|---|---|---|
| Yes | 27 | 79.4 |
| No | 6 | 17.6 |
| Total | 33 | 97.1 |
| Data not available | 1 | 2.9 |
| Total | 34 | 100.0 |
Table 6.
Anticoagulation Status Postprocedure
| Anticoagulation Status Postprocedure | Frequency | Percentage |
|---|---|---|
| Yes | 27 | 79.4 |
| No | 6 | 17.6 |
| Total | 33 | 97.1 |
| Data not available | 1 | 2.9 |
| Total | 34 | 100.0 |
The presenting symptoms of patients diagnosed with pseudoaneurysm are pain, bleeding, decreased pulse, cold extremities, swelling, and hematoma. In this study, pain and swelling were the most common symptoms of presentation followed by a hematoma (Table 7).The majority of pseudoaneurysms (19/34 [55.9%]) were located in the right groin. This was most likely due to the fact that a right femoral route is preferred for ease and reliability of access to the arterial and venous system (Table 8).
Table 7.
Frequency Distribution of the Presentation Symptoms
| Symptoms of Presentation | Frequency | Percentage |
|---|---|---|
| Pain | 9 | 26.5 |
| Hematoma | 4 | 11.8 |
| Pain and swelling | 15 | 44.1 |
| Pain and hematoma | 5 | 14.7 |
| Pain, swelling, and hematoma | 1 | 2.9 |
| Total | 34 | 100.0 |
Table 8.
Frequency Distribution of Pseudoaneurysm Location
| Location of Pseudoaneurysm | Frequency | Percentage |
|---|---|---|
| Left arm | 1 | 2.9 |
| Right groin | 19 | 55.9 |
| Left groin | 14 | 41.2 |
| Total | 34 | 100.0 |
DISCUSSION
A study by Kang et al,3 evaluated 83 femoral pseudoaneurysms and performed US-guided thrombin injections. Of the 83 pseudoaneurysms, 82 were successfully treated using this technique. The only complication experienced was thrombosis of distal brachial artery, which resolved spontaneously.3 There were recurrences in seven patients, four underwent successful reinjection; reinjection failed in two patients, who then underwent surgical repair; and one patient had spontaneous thrombosis on follow-up. After 4 weeks, US examinations were completely normal and there were no recurrent pseudoaneurysms.
A prospective evaluation performed by Khoury et al,4 examined 131 iatrogenic pseudoaneurysms of the lower extremity.4 They were treated with duplex ultrasonography guided thrombin injection and thrombosis was achieved in 126 of these cases. Five cases failed, three of which resulted from complications of the procedures, with two intra-arterial thrombin injections and one pseudoaneurysm rupture after thrombosis. However, they had 96% success rate in treating these lower extremity pseudoaneurysms.
Franklin et al2 compiled multiple studies on the treatment of iatrogenic false aneurysms. The different methods included US-guided compression repair, coil embolization, endovascular repair/stent, US-guided thrombin injection repair, and surgical repair. US-guided compression repair showed a varied success rate of 54 to 98%. Second attempts were often necessary for the final success rate. Coil embolization resulted in 100% success but these were small studies. Various studies used US-guided thrombin repair. Success rate was found to be around 90 to 100% in these studies. A study of open surgical repair resulted in 100% success with two patients suffering limb loss.
Kang et al5 did a prospective study to evaluate the treatment of 21 patients who developed femoral pseudoaneurysms by using percutaneous US-guided thrombin injection. Of 21 patients, 20 patients were successfully treated with thrombin injection. Of 20 pseudoaneurysms, 15 were thrombosed immediately in less than 20 seconds after one injection and 5 had partial thrombosis after first injection and complete thrombosis immediately after second injection.5 They concluded that percutaneous US-guided thrombin injection appears to be safe and expeditious method for treating postcatheterization femoral pseudoaneurysms. It has significant advantages with respect to US-guided compression therapy or surgical repair.
We conducted this 5-year study to evaluate US-guided injection treatment of a pseudoaneurysm at a large urban community hospital. Earlier treatment at our institution consisted of surgical intervention. This procedure was a new means of treatment. Therefore, we wished to evaluate outcomes and safety parameters of the procedure. Risk factors for the development of iatrogenic pseudoaneurysms include age greater than 60 years, female gender, diabetes, femoral puncture, and concurrent anticoagulation.2 In this retrospective study of 34 patients, 73.5% of patients were females, the average age of patients was 67.6 years, and 79.4% were on pre- and postprocedural anticoagulation.
Diagnosis of iatrogenic pseudoaneurysms relies on a high index of suspicion by the clinician. They must be suspected in any patient who has recently undergone arterial catheterization and complain of pain, swelling, ecchymosis, and hematoma. Pain and swelling were the most common presentation in this group of patients. These findings are elicited during a routine physical examination and are critically important after any angiographic procedure.2
Diagnostic imaging techniques such as real-time duplex ultrasonography, computed tomography, and angiography are used to confirm diagnosis. Historically, angiography was used for confirmation but now it has been replaced by real-time duplex ultrasonography and angiography is reserved for atypical cases.2 During ultrasonographic evaluation images are typically obtained with a 4- to 7-MHz probe. The following information can be obtained: size of pseudoaneurysm, presence or absence of surrounding hematoma, length and diameter of the neck of the pseudoaneurysm, velocity of flow in the native vessel and in the pseudoaneurysm cavity, patency of surrounding vasculature, and compression of the vein.2
Treatment options for pseudoaneurysms can be classified into noninvasive and invasive techniques. Noninvasive techniques include observation and compression therapies. The compression therapies are further divided into blind techniques and US-guided compression repair. Invasive treatment plans include open surgical repair and percutaneous techniques include thrombin injection and endovascular coil embolization.
Once diagnosis of false aneurysm is confirmed, observation is recommended if the pseudoaneurysm is 2 cm or less on presentation and without expansion on repeat arterial duplex in 1 week, and patient remains asymptomatic.2
Compression therapy, either blind or US guided is used to augment spontaneous thrombosis. For success using this modality, blood flow in the neck of the pseudoaneurysm must be eliminated. Blind manual compression is applied for 15 to 30 minutes to the pseudoaneurysm site to obtain hemostasis. This manual compression helps form hemostasis in the neck of pseudoaneurysm and the pseudoaneurysm forms a hematoma.2 The disadvantage of this procedure is the inability to visualize the pseudoaneurysm or the native artery, therefore, inadvertent thrombosis can result in the artery as opposed to the pseudoaneurysm during compression.
US-guided compression therapy was developed in 1991 by Fellmeth and colleagues. After visualizing the anatomy the US transducer is positioned to best depict the tract connecting the native arterial lumen and false aneurysm cavity. Then, force is applied with transducer until flow through the tract is eliminated without compromising flow through the normal vasculature. Direct pressure is applied for ~20 to 30 minutes and compression is slowly released. If persistent flow is noted in the pseudoaneurysm the process is repeated again until the process is successful (blood flow into the tract is eliminated). Compression times range from 10 to 150 minutes with a mean of 53 minutes.2 Patients are maintained on bed rest for up to 24 hours and a follow-up US is obtained in 24 to 72 hours after the compression procedure. Contraindications of this procedure are infection, skin ischemia impending compartment syndrome, presence of aneurysms for more than 1 month, and inability to occlude the tract or neck without occluding native vessel.2
According to the literature, the success rate using US-guided compression therapy is only 60%. The other disadvantages include increased time, pain, prolonged hospital stay due to the need for multiple attempts, and increased failure rates with anticoagulation.1 It is also considered inappropriate treatment for long-standing injuries greater than 1 month duration. Whereas, thrombin injection can be used for delayed symptomatic pseudoaneurysm as late as 2 years postcatheterization.1 Advantages of US-guided compression therapy include a lower chance of occlusion of the native vessel and no risk of the side effects associated with the systemic injection of thrombin.
Open surgical repair is the gold standard in treatment of pseudoaneurysms and is usually used as a last resort. Absolute indications for open surgical repair include shock or cardiovascular instability, greater than 100% increase in size of the pseudoaneurysm on duplex US, imminent rupture, and evidence of vascular compromise.2 In this study 1 of the 34 patients needed open surgical repair due to failure of thrombin injection therapy. The disadvantages associated with open repair include potential for bleeding, infection, nerve injury, and lymphatic leaks.
Endovascular transcatheter coil embolization is another modality used for the treatment of pseudoaneurysms. The success rate quoted in the literature ranges from 62 to 100% in visceral pseudoaneurysms.6 Literature on use of this technique in peripheral pseudoaneurysms is sparse. The drawbacks of this technique include technical difficulty of the procedure, use of large number of coils for complete occlusion of the pseudoaneurysm sac, cost of the procedure, possibility of pseudoaneurysm rupture, and peripheral embolization.6
We used US-guided thrombin injection repair of the pseudoaneurysms in this study. In 1997, Liau and associates performed the procedure on five patients with 100% success and no complications. It has become the initial treatment of choice in most centers. In comparison to US-guided compression repair of pseudoaneurysms, thrombin injection is safe, extremely efficacious, and obviates some problems encountered with US-guided compression repair. Larger series report that as many as 10% of patients cannot be treated because flow within pseudoaneurysm cannot be arrested.5 Some patients have pseudoaneurysm necks that are not compressible or cannot be compressed without compressing the native artery. Anticoagulation has no effect on US-guided thrombin repair but increases recurrence rate as much as 4 to 11% after successful US-guided compression repair.5
In this retrospective review, there was no comparison of blind or US-guided compression repair of the treatment of pseudoaneurysms. However, three patients did undergo blind compression for 20 minutes which failed, before treating them using US guidance with thrombin injection. All patients were treated with US-guided thrombin injection. Of 34 patients, 33 were successfully treated with thrombin injection, only 1 patient needed to undergo open surgical repair for resolution of the pseudoaneurysm. There were no complications following the procedure.
The safety of thrombin injection was previously demonstrated in animal studies with doses of 5 U/kg/min for 10 to 15 minutes being tolerated without any adverse effects.2 Before use in the treatment of pseudoaneurysm, the experience with human subjects was previously limited to injection therapy for variceal bleeding. Patients tolerated doses of 10,000 U; doses of 15,000 U were noted to have reversible side effects.2
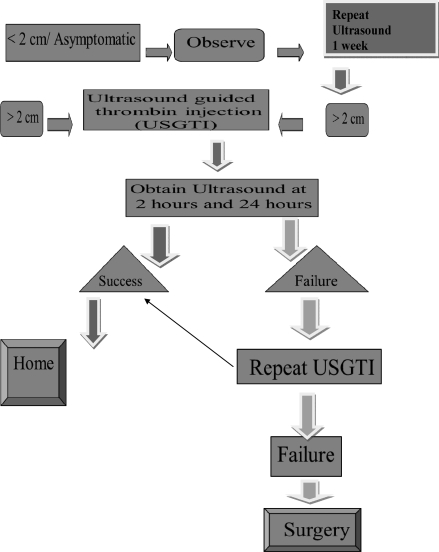
Procedure-related risks of injecting thrombin injection include native arterial thrombosis or embolus, seizures, anaphylaxis, systemic activation of the coagulation cascade, risk of limb ischemia, blue toe syndrome, groin abscess, and crampy buttock pain.1 Inadvertent intra-arterial or venous injection, large total thrombin dose, rate and volume of administration, and size of aneurysm neck are purported risk factors for native arterial thrombosis or complications of thrombin administration.2 The distal pulses were monitored before and after the procedure and found no evidence of thromboembolic events. These patients were followed up at 1 month, 3 months, 6 months, and at 12 months postprocedure and none of these 34 patients had recurrence of the pseudoaneurysm. An algorithm to treat iatrogenic pseudoaneurysms is proposed from this study (Fig. 4).
Figure 4.
Algorithm for treatment of pseudoaneurysms.
One of the limitations of this study was that the number of patients treated with US-guided thrombin injection was only 34. This procedure was performed at a high-volume community teaching hospital where the nursing staff is formally trained on pulling sheaths appropriately which resulted in lower incidence of iatrogenic pseudoaneurysms at our institution. This study included only those patients who were treated for iatrogenic pseudoaneurysms by the vascular surgeons of this 650-bed urban community hospital. The pseudoaneurysms diagnosed by cardiologists and interventional radiologists at this institution were not included in this study.
CONCLUSION
The advantages of using US-guided thrombin injection include minimal discomfort to the patient, high efficacy, lack of influence of concurrent anticoagulation, and rapidity of the procedure.2 This study had overall success rate of 97.1% in treating iatrogenic pseudoaneurysms in 33 out of 34 patients. No patient died or suffered any other complications. Having high clinical suspicion is important in diagnosis. This study was successful in demonstrating that the US-guided injection of thrombin in this patient population is an efficacious way to treat iatrogenic pseudoaneurysms. This study shows that the procedure can be safely started at new facilities by appropriately trained vascular surgeons with good results.
References
- Franz R W, Hughart C. Delayed pseudoaneurysm repair: a case report. Int J Angiol. 2007;16(3):119–120. doi: 10.1055/s-0031-1278263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin J A, Brigham D, Bogey W M, Powell C S. Treatment of iatrogenic false aneurysms. J Am Coll Surg. 2003;197(2):293–301. doi: 10.1016/S1072-7515(03)00375-2. [DOI] [PubMed] [Google Scholar]
- Kang S S, Labropoulos N, Mansour M A, et al. Expanded indications for ultrasound-guided thrombin injection of pseudoaneurysms. J Vasc Surg. 2000;31(2):289–298. doi: 10.1016/s0741-5214(00)90160-5. [DOI] [PubMed] [Google Scholar]
- Khoury M, Rebecca A, Greene K, et al. Duplex scanning-guided thrombin injection for the treatment of iatrogenic pseudoaneurysms. J Vasc Surg. 2002;35(3):517–521. doi: 10.1067/mva.2002.120029. [DOI] [PubMed] [Google Scholar]
- Kang S S, Labropoulos N, Mansour M A, Baker W H. Percutaneous ultrasound guided thrombin injection: a new method for treating postcatheterization femoral pseudoaneurysms. J Vasc Surg. 1998;27(6):1032–1038. doi: 10.1016/s0741-5214(98)70006-0. [DOI] [PubMed] [Google Scholar]
- Loffroy R, Pao P, Ota S, et al. Packing technique for endovascular coil embolization of peripheral arterial pseudo-aneurysms with preservation of the parent artery: safety, efficacy and outcomes. Eur J Endovasc Surg. 2010;40:209–221. doi: 10.1016/j.ejvs.2010.03.009. [DOI] [PubMed] [Google Scholar]