Abstract
The reality of regression of atherosclerotic plaques was established as long ago as 1987 by aggressive cholesterol reduction even before the era of statin therapy. Nevertheless, the most important aspect of patient benefit to prevent cardiovascular (CV) disease events is stabilization of these plaques so they will not rupture. Lowering of low-density lipoproteins is critical to this goal and can be considered the gold standard of preventive CV medicine. The major goal for the high-risk patient and the diabetic patient is lowering these harmful lipoproteins to less than 70 mg/dL. No discussion of CV disease prevention is complete without considering tobacco abuse and its elimination. Even secondhand smoke has been established as harmful. Control of hypertension is another major aspect of CV disease prevention, and a blood pressure less than 120/80 mm Hg is ideal. With obesity a major problem in the developed world, its role in the metabolic syndrome is of major significance as is the high prevalence of this so-called syndrome versus collection of specific risk factors in a population with poor health habits. Control of diabetes mellitus has established benefit from the standpoint of CV disease prevention except that some problems have been reported with extremely tight blood sugar control. Exercise was long considered good but now there are evidence-based reasons to recommend it as essential in CV disease prevention. There are many unforeseen frontiers in CV disease prevention but, for now, everything points to elevation of high-density lipoproteins as the next focus of this prevention.
Keywords: Atherosclerosis, coronary heart disease, peripheral vascular disease, cholesterol, statins
Strides have been made in the prevention or delay of clinical events from the major presentations of atherosclerosis: coronary heart disease (CHD) and peripheral vascular disease (PVD). The lipid hypothesis that cholesterol reduction could benefit CHD and PVD patients was around for many years with limited proof, but was finally established with reduction of cholesterol by cholestyramine in the Lipid Research Clinics Study.1,2 Subsequent confirmation occurred when statins became available and their benefit was supported by successful outcomes studies.3,4,5,6,7 The major benefit of statins is in reducing low-density lipoproteins (LDL), but they also offer additional pleiotropic effects.8 The current status of atherosclerosis prevention is considered in Table 1.
Table 1.
Major Aspects of Atherosclerosis Prevention
| Regression of atherosclerotic plaques | Can occur but for now, not a major goal of prevention.12,13,14 |
| Stabilization of atherosclerotic plaques | This is key goal of prevention for now.18 |
| Lowering of LDL as the gold standard of prevention | Available data support this as the single most important issue of prevention.23 Statin pleiotropic effects also appear of value.8 |
| Tobacco abuse | An established major prevention measure.31,32 |
| Control of hypertension | Now well established to prolong lives.47 |
| MS | Major societal medical problem and treatment of each component is essential. |
| Diabetes mellitus | Treatment of major risk factors is essential but value of very tight control may be problematic.56 |
| Exercise | Makes a definite contribution to atherosclerosis prevention.59 |
| Next frontier in prevention: elevation of HDL | This approach is promising due to risk association with low HDL.73 |
LDL, low-density lipoproteins; MS, metabolic syndrome; HDL, high-density lipoproteins.
ATHEROSCLEROTIC PLAQUE FORMATION AND MECHANISM OF RUPTURE
Chronic inflammation appears to play a major role in atherosclerotic plaque formation and the rupture of that plaque.9 Many factors appear to play a role in this ongoing process. Lipoproteins, especially LDL and even more specifically, oxidized LDL play a major role in causing incipient inflammation and its contribution to plaque formation. Plaque formation and destabilization leading to rupture appear to be closely interrelated with similar basic biochemical and immunological processes. Apolipoprotein E/LDL-receptor-deficient mice are a good model for accelerated atherosclerosis with partial confirmation in human carotid artery studies.10 T-cell activation appears to be a point of integration with possible inciting stimuli from infectious agents and autoantigens. Activated T cells then secrete interferon-γ. This interferon can subsequently impair the synthesis of collagen. Further weakening of the fibrous cap of the plaque can be caused by macrophage and smooth muscle cell activation by cytokines as inflammatory mediators. This macrophage/smooth muscle cell activation can then result in the release of enzymes capable of weakening the tissue framework of the fibrous cap. Therapies that reduce lipids and inhibit inflammation represent potential cellular and molecular mechanisms for reduction of acute CHD events.
REGRESSION OF ATHEROSCLEROTIC PLAQUES
Although regression of atherosclerosis is a reality and established, the major benefit thus far of an aggressive treatment of elevated LDL is atherosclerotic plaque stabilization. A Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden (ASTEROID) trial11 is a recent study in which regression appeared successful after 2 years of rosuvastatin 40 mg/d in patients with plaques determined by intravascular ultrasound, but unfortunately, the goal that would appear most desirable, a significant increase in arterial lumen size, was not achieved, even partially. From the study report it seemed that the achievement of successful regression was a new occurrence; however, review of the literature shows that successful regression of CHD was actually very well established in 1987 by using comparative quantitative coronary angiography as initially reported by Blankenhorn et al.12,13,14 Brown et al also demonstrated regression of CHD shortly thereafter.15,16 Therefore, this approach to atherosclerosis is not an innovative new approach, although it would appear ideal, and it is certainly not as important in clinical medicine as is atherosclerotic plaque stabilization.
DECREASED PROGRESSION, SUPPRESSION, AND STABILIZATION OF ATHEROSCLEROTIC PLAQUES
Initial studies on regression of atherosclerosis also observed and targeted decreased progression as a benefit of treatment. Brown et al looked at the protective increase in high-density lipoprotein (HDL) with simvastatin plus niacin and its attenuation by administration of antioxidants at the same time in CHD patients.15 Average CHD stenosis as determined by quantitative coronary angiography progressed by 3.9% with placebo and 1.8% with antioxidants which was not a significant difference. On the other hand, progression of CHD stenosis was reduced to 0.7% with simvastatin-niacin plus antioxidants (p = 0.004) and improved to an actual regression of 0.4% with simvastatin-niacin alone (p < 0.001). Undoubtedly this improvement to actual regression was associated with the removal of the negative effects of antioxidants in the presence of simvastatin-niacin. The study showed that simvastatin plus niacin resulted in angiographically measurable benefits in patients with CHD and low HDL, extending from decreased progression to regression as modified by antioxidant vitamins. In an earlier study using intensive lipid-lowering therapy with either lovastatin/colestipol or niacin/colestipol, Brown et al found that in men with CHD, an associated increase in apolipoprotein B and therefore at high risk for CV events benefited significantly from their intensive therapy.16 As compared with conventional therapy, both intensive lipid-lowering therapies reduced the angiographically measured progression of CHD lesions and increased the frequency of regression.
Suppression of atherosclerosis is another term used and can basically be associated with reduced progression. However, as used, suppression of atherosclerosis can also refer to suppression of production and activity of such entities as cytokines and platelet-activating factor associated with the development of hypercholesterolemic atherosclerosis.17
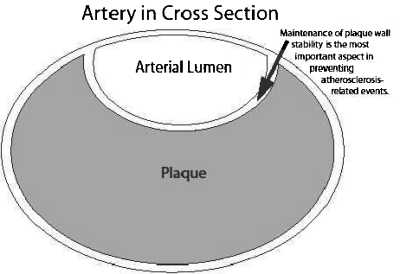
Despite the apparently ideal approach of atherosclerosis regression, atherosclerotic plaque stabilization remains the most clinically relevant result of aggressive atherosclerosis prevention. In 1995, Falk et al reviewed how plaque composition and plaque vulnerability, rather than severity of stenosis, had become the most important factors for the development of thrombus-mediated acute coronary syndromes.18 Also in 1995, Libby discussed the major forms of acute coronary syndrome (ACS) including unstable angina and acute myocardial infarction (AMI) in clinical cardiology.9 In this article, a classic diagram compares the characteristics between vulnerable and stable plaques in which a vulnerable plaque often has a well-preserved lumen due to the tendency of plaques to initially grow outward. This vulnerable plaque typically has a large lipid core with a thin fibrous cap prone to rupture with resultant thrombosis, artery occlusion, and, depending on the extent of thrombosis, an ACS (incomplete artery occlusion) or an ST-segment elevation (transmural) myocardial infarction (MI) with total vessel occlusion. In contrast, a stable plaque is more likely to show vessel narrowing detectable by coronary angiography with that narrowing likely to cause angina. However, a stable plaque is not prone to rupture causing acute events. Therefore, over and above any possible initial prevention, the key to atherosclerosis prevention is stabilization of any plaque (Fig. 1) to avoid progression, showering of plaque emboli, or occlusion. This applies especially to avoidance of plaque rupture and resultant acute vascular events such as thrombotic occlusion.
Figure 1.
The most important aspect of cardiovascular disease prevention is plaque stabilization so that acute rupture can be made less likely to occur.
ROLE OF DIET IN ATHEROSCLEROSIS PREVENTION AND TREATMENT
Diet should always be an initial basic step for a healthy cardiovascular (CV) lifestyle. The problem is that most patients will not follow an extreme diet that might achieve a major cholesterol reduction. Nevertheless, all patients should be encouraged to pay attention to their diet. In addition, there are some specific more practical diets with proven benefit that are worth discussing. In regard to hypertension control as a major contributor to atherosclerosis prevention, the Dietary Approaches to Stop Hypertension (DASH) diet has been shown to have proven benefit.19 The diet is rich in vegetables, fruits, and low-fat dairy products. It appears effective in the presence of high, intermediate, and low levels of sodium with even more dramatic effects with high sodium. DASH diet versus control showed an average systolic blood pressure decrease of 5.9 mm Hg compared with an average systolic blood pressure decrease of 2.2 mm Hg in the presence of low sodium.
Another diet, the so-called Mediterranean diet has a long association with decreased CV risk. As a significant risk factor for CV disease, the metabolic syndrome (MS) is also a major medical problem throughout the world. Kastorini et al performed a meta-analysis on the effect of the Mediterranean diet on the MS and its components, representing an even higher CV risk situation than a general population study.20 The Mediterranean diet is characterized by high consumption of monounsaturated fatty acids, primarily from olives and olive oil as well as encouraging daily consumption of fruits, vegetables, whole grain cereals, and low-fat dairy products as well as a relatively low consumption of red meat. Their meta-analysis of prospective studies and clinical trials confirmed that adherence to the Mediterranean diet decreased the risk of MS (log hazard ratio: −0.69, 95% confidence interval [CI], −1.24 to −1.16). In addition, there was a beneficial effect on components of MS such as decreasing waist circumference, increasing HDL, decreasing triglycerides, decreasing blood pressure, and decreasing plasma glucose. In the Prevención con Dieta Mediterránea (PREDIMED) study from Spain, involving 772 asymptomatic persons (age range, 55 to 80 years) eating Mediterranean diets supplemented with either olive oil or nuts were compared with participants eating a low-fat diet.21 It was found that the two Mediterranean diets had beneficial effects on decreasing CV risk factors such as plasma glucose, systolic blood pressure, and high sensitivity C-reactive protein (CRP) as well as increasing HDL. Also, in a subgroup of the PREDIMED study, there was evidence that the traditional Mediterranean diet caused significant reductions in cellular lipid levels and LDL oxidation.22
CHOLESTEROL TREATMENT GOAL
There is general agreement that the patient at high CV risk should be treated to have an LDL less than 70 mg/dL, and this high CV risk includes diabetic patients. Regarding the LDL level, the evidence appears to support that lower is better, with supporting evidence down to an LDL level of 40 mg/dL.23 In the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction (PROVE IT-TIMI 22) study of high dose statins in ACS, it was found that the patient subgroups with LDL <40 mg/dL or with LDL 40 to 60 mg/dL had fewer major cardiac events.24 A similar result was reported in the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) study in which those patients attaining LDL <50 mg/dL had significantly fewer CV events.25 Much controversy has emerged on the use of ezetimibe in achieving this low LDL goal but there is no question that it contributes significant additional LDL reduction.26 Nevertheless, outcome studies with ezetimibe use are still lacking. Inflammatory risk factors appear significant in contributing to CHD and PVD, especially as evaluated with high sensitivity C-reactive protein (hsCRP). In the JUPITER study, it was pushed that a specific statin, rosuvastatin, has special relevance in lowering hsCRP to decrease CV events, but the reality is that all statins decrease hsCRP relative to potency, and the addition of ezetimibe to a statin also contributes favorably.27
For the most difficult heterozygous and homozygous hypercholesterolemic patients, LDL apheresis is an essential methodology to achieve the LDL goal.28 However, although LDL lowering to less than 70 mg/dL for such patients with high CV risk is established as the gold standard for CHD and PVD prevention,23 it cannot be achieved in this group without LDL apheresis.28 Unfortunately, the technique is very expensive and inadequately covered by health insurance. Therefore, availability is very limited. Clinical attempts to attain the goal of LDL less than 70 mg/dL, whether with medication or use of a drastic and expensive procedure such as LDL apheresis, are all directed at achieving the best possible results. These desired results extend from decreased atherosclerosis progression, suppression of atherosclerosis, essential stabilization of the atherosclerotic plaque as the most practical current goal, and the ultimate ideal of complete regression of atherosclerosis.
A new statin medication, pitavastatin, is now available. This statin is not metabolized via the specific cytochrome P450 system, CYP3A4, like the other statins but instead goes through CYP2C9.29 Pleiotropic effects of especially statins8 are important in prevention, but the best guideline currently available is that the lower the level of LDL achieved, the better.
TOBACCO ABUSE
Tobacco use, especially cigarette smoking, has been well established as a CV risk factor since the Report of the Advisory Committee to the Surgeon General of the United States in 1964.30 Multiple epidemiological studies have shown that cigarette smoking is a major risk factor for CV events.31,32 The occurrence of significantly increased deaths of a CV etiology associated with cigarette smoking is worldwide such as that shown in India.33 Multiple studies through the years have shown that chronic cigarette smokers are at increased risk for sudden death and MI.34,35 Also, compared with nonsmokers who have never smoked, pipe and cigar smokers have also been shown to have a significantly increased risk for major CHD events and stroke events.36 In addition, evidence has continued to accumulate that passive secondhand smoke carries significant risk for CHD, such as a relative risk of CHD for active smokers at 1.78% compared with 1.31% for passive smokers.37 In addition, it has been shown that many important responses of the CV system, such as platelet activation, endothelial dysfunction, inflammation, atherosclerotic plaque instability, increased oxidized LDL, and increased oxidative stress, are very sensitive to toxins in tobacco smoke, whether secondhand or involving the primary smoker. Such mechanisms, rather than an isolated response, interact and compound the risk of CHD. To further extend the relevance of avoiding secondhand smoke, there is now widespread proof of decreased CHD events throughout the world since the advent of smoking bans, including significantly decreased acute coronary events in Italy38 and significantly decreased AMI admissions in Helena, Montana.39 Therefore, there is no doubt that a major factor in atherosclerosis prevention has been established for cigarette smoking cessation as well as for pipe/cigar smoking and also the avoidance of secondhand smoke.
It should be noted that there is an even more specific association of cigarette smoking and PVD than there is with CHD. In the Edinburgh Artery Study involving 1592 subjects (age range, 55 to 74 years) selected at random from age–sex registers from 10 general practices in Edinburgh, Scotland, the effects of cigarette smoking on CHD and PVD were compared.40 By logistic regression, adjusting for a various individual risk factors, it was found that there was no significant impact on the effect of smoking. The investigators found that the age and sex adjusted odds ratios were very significant for PVD but not for CHD. It was concluded that the stronger association between cigarette abuse and PVD as compared with CHD does not appear to be associated with other risk factors as examined and some other mechanism must be found to explain the increased association with PVD.
Cessation of cigarette smoking is associated with a substantial reduction in risk of all-cause mortality among patients with CHD and this reduction appears consistent across age, sex, index cardiac event, country, and year of study commencement in a systematic review by Critchley and Capewell.41 Similar benefit for PVD patients was found by Jonason and Bergström who studied the effect of smoking cessation in intermittent claudication patients.42 In 343 patients with intermittent claudication and involving male and female patients, 1 year after the initial evaluation, 39 (11%) had stopped smoking and 304 (89%) continued to smoke. Rest pain did not develop in any of the nonsmokers but in smokers the cumulative proportion with rest pain was 16% after 7 years (p value less than 0.05). The cumulative proportions with MIs after 10 years were 11 and 53% in nonsmokers versus continued smokers, and the cumulative rates of cardiac deaths was 6 and 43%, respectively. The 10-year survival was 82 and 46% among nonsmokers and smokers. At 1 year follow-up, the association between smoking and mortality was significant (p < 0.05). McRobbie and Thornley evaluated the importance of treating dependence on tobacco and commented on the important long-term benefits of smoking cessation.43 It appears that the risk of AMI is cut in half within a few years of smoking cessation and that patients with preexisting CHD have an even more rapid risk reduction. The authors also commented on a resultant reduced risk of nonfatal stroke and decreased PVD progression. Therefore, there appears to be essentially no doubt of the benefit of smoking cessation for the CHD and PVD patient regardless of sex and age.
CONTROL OF HYPERTENSION
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) appeared in 2004.44 It defined normal blood pressure as less than 120/80 mm Hg, prehypertension from 120/80 to 139/89, stage 1 hypertension as 140/90 to 159/99, and stage 2 hypertension as equal to or greater than 160/100. Also in 2004, data were reported on 4805 adults (age, 18 years and older) surveyed in the 1999–2000 National Health and Nutrition Examination Survey to look at hypertension prevalence by the then new JNC 7 guidelines.45 It was found that ~60% of American adults have prehypertension or hypertension. In addition, there was a disproportionate incidence in some individuals (African-Americans, senior citizens, the obese, and individuals of low socioeconomic status). The authors found that 31% were unaware of their hypertension. Another 31% had their blood pressure controlled and therefore, hypertension control was distressingly low. Only 66% were advised by health caregivers to modify their lifestyle or take medications. Such a result is staggering when there are data to show that CV mortality risk doubles with each increase of 20/10 mm Hg in blood pressure.46
The benefit for CV prevention by treating hypertension was well established long before JNC 7. Evaluation of clinical trials in hypertensive patients with successful blood pressure lowering was shown to confirm documented decreases in risk for stroke, congestive heart failure (CHF), and MI.47 Meta-analyses of various clinical trials indicated a decrease in stroke by over 40% and a reduction in CHF by ~50%. Clinical data following JNC 7 further confirm the benefit of treatment of hypertension including resistant hypertension. Small clinical studies and observational cohorts have suggested increased CV risk for patients with resistant hypertension in contrast to previous reports of questionable benefit to this group. Hypertension control in this high-risk group appears to also offer substantial benefit.48 Also, the importance of treating isolated systolic hypertension has been proven despite previous assertions that this was a normal result of stiff arteries in senior citizens and that treatment was not necessary. In 2000, the Systolic Hypertension in the Elderly Program (SHEP) demonstrated that treating isolated systolic hypertension in the elderly decreased total stroke incidence.49 The Systolic Hypertension in Europe (Syst-Eur) trial included 4695 randomized patients with a minimum age of 60 years.50 Immediate antihypertensive treatment was compared with delayed treatment resulting in a reduced occurrence of stroke and CV complications by 28% (p = 0.01) and 15% (p = 0.03), respectively, with a similar tendency for total mortality (13%, p = 0.09). In 492 diabetic patients, the corresponding estimates of long-term benefit (p < 0.02) were 60, 51, and 38%, respectively. This further supported the benefit of treatment of isolated systolic hypertension. Immediate versus delayed treatment prevented 17 strokes or 25 major CV events per 1000 patients followed-up for 6 years.
MANAGEMENT OF MS
There are specific components that define MS, and obesity is one of these key components. There are actually multiple definitions for MS, but the one prevalent and in use in the United States comes from the National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III as revised by the American Heart Association/National Heart, Lung and Blood Institute in 2005.51 This definition states that MS involves having three of the five following entities: triglycerides ≥150 mg/dL; HDL <40 mg/dL in men and <50 mg/dL in women; blood pressure ≥130/85 mm Hg; waist girth >102 cm for men or >88 cm for women; and fasting glucose ≥100 mg/dL. Controversy over the definitions and etiology of MS as a clinical aggregate or collection of abnormalities in a population with bad health habits continues.52 Efforts continue to elucidate the relationships of the syndrome, the diseases therein, and the clinical aggregate. In any event, for now, the clinician needs to treat all of the components present in MS as would be done in individuals with just one component present.
The Atherothrombosis Intervention in Metabolic Syndrome with Low HDL Cholesterol/High Triglycerides: Impact on Global Health (AIM-HIGH) study was stopped 18 months earlier than planned by the National Institutes of Health.53 This was due to the failure of the study to show that the addition of high dose, extended-release niacin to statin treatment in patients with CV disease reduced CV events, including MIs and strokes. The participants were selected because, despite what was considered a well-controlled LDL, they had increased CV risk due to a history of CV disease and a combination of low HDL and increased triglycerides. During the 32 months of follow-up in the study, the niacin/statin group showed increased HDL and decreased triglycerides in comparison to the participants taking only the statin. Nevertheless, the niacin/statin treatment did not decrease fatal or nonfatal MIs, strokes, hospitalizations for ACS, or revascularization procedures for coronary and cerebral arteries. The results are disappointing but further evaluation and consideration must be made before abandoning niacin as, at the least, it is beneficial in lowering LDL as the gold standard for CV risk management23 and has other studies proving benefit of niacin even though not combined with a statin in those studies.13,54
CONTROL OF DIABETES MELLITUS
Good control of diabetes mellitus appears to be beneficial to the CV patient but very close control has not shown definite benefit as in the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) study55 and some possible harm as in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study.56 Most patients in both studies received drugs from multiples classes, with or without insulin. The issue of tight versus lesser control of insulin-dependent diabetes mellitus (IDDM) has long been a clinical concern. A major study of diabetes control occurred in 1993 in the Diabetes Control and Complication Trial (DCCT)57 where 1441 patients with IDDM were studied including 726 patients with no retinopathy at base line (primary-prevention cohort) and 715 with mild retinopathy (secondary-intervention cohort). They were followed for a mean of 6.5 years. The group randomly assigned to intensive therapy was managed with an external insulin pump or with three or more daily insulin injections, all guided by frequent blood glucose monitoring. The conventional therapy group received one or two daily insulin injections. The major adverse event associated with the intensive therapy was the two to three times increase in severe hypoglycemia. This DCCT study concluded that intensive therapy of IDDM effectively delays the onset of and slows the progression of diabetic retinopathy, diabetic nephropathy, and diabetic neuropathy. It appears appropriate to conclude and recommend that good control of diabetes mellitus is beneficial in the long term but that excessively tight control may lead to more short-term incidences of severe hypoglycemia. The American Diabetes Association asserts that even if DCCT expertise and results are not attainable, any improvement in blood glucose control can slow the development and progression of microvascular complications.58
EXERCISE
The value of exercise for decreasing CV mortality has long been a subject of study, and it now appears that it has become established. In 2007, the National Institutes of Health—American Association of Retired Persons (NIH—AARP) Diet and Health Study evaluated levels of physical activity levels in 252,925 women and men (age range, 50 to 71 years) by questionnaires.59 It was found that moderate activity of at least 30 minutes almost daily or vigorous exercise of at least 20 minutes three times a week was associated with significant decreases in mortality risk. The subjects whose activity levels were equivalent to meeting both recommendations showed an impressive reduction in risk for mortality from any cause (multivariate relative risk [RR], 0.50; 95% CI, 0.46–0.54). There was a similarly impressive decrease in risk for mortality from CV disease (multivariate RR, 0.48; 95% CI, 0.41–0.45). In 1998, the Finnish Twin Cohort Study looked at the association of leisure-time physical activity with mortality.60 Even after accounting for genetic and other familial factors, it was found that such physical activity was associated with improved survival. The reported hazard ratio for death after age/sex adjustment was 0.71 (95% CI, 0.62–0.81) with occasional exercise and 0.57 (95% CI, 0.45–0.74) when conditioning exercise was performed versus those who were sedentary.
Multiple other studies can be cited to support the benefit of exercise for CV disease prevention. The Honolulu Heart Program studied 2678 physically capable, elderly men (age range, 71 to 93 years). The follow-up data suggested that CV disease risk decreased with increases in distance walked.61 Franco et al constructed multistate life tables using data from the Framingham Heart Study and calculated the effects of low, moderate, and high levels of physical activity among populations whose age exceeded 50 years.62 They found that moderate and high levels of physical activity led to 1.3 and 3.7 years more in total life expectancy, respectively. From a CV perspective, these authors found moderate and high physical activity levels resulted in 1.1 and 3.2 years of life without evidence for CV disease, respectively. The Zutphen Elderly Study evaluated self-reported physical activity by a questionnaire in a population-based sample of 802 Dutch men (age range, 64 to 84 years) who walked or cycled for 20 minutes at least three times a week. This was found to be associated with decreased CV mortality (adjusted RR, 0.69; 95% CI, 0.50–0.88).63
Multiple other large and small patient studies could be cited. Previously, the CV benefit of exercise could be called into question but this does not appear to be the case now. It can be stated that exercise has an established benefit in reducing CV disease mortality. Thompson et al reported extensively on possible mechanisms by which exercise may play a role in atherosclerosis prevention.64 Physical activity has been shown to be beneficial for atherosclerosis risk factors including hypertension, insulin resistance, hypertriglyceridemia, low HDL, obesity, and when combined with weight reduction, it may contribute to a decrease in LDL. Other atherosclerosis risk factor benefit from exercise is improved myocardial function, increased vasodilatory capacity, improved vascular tone, and decreased vulnerability to ventricular fibrillation. In addition, there is evidence that exercise training also has been shown to reduce inflammation as an atherosclerosis risk factor as manifest by reduced plasma CRP levels in response to exercise training in sedentary healthy adults with initial high levels.65 However, it must be kept in mind that vigorous physical activity as an acute occurrence appears to increase the risks of sudden cardiac death and occurrence of MI.64 This appears to be the case for individuals with both diagnosed CHD and occult CHD. Nevertheless, this reality is just a blip in the overall favorable benefit/risk ratio for exercise.
MODERATE ALCOHOL INTAKE
Alcohol in and of itself appears to have benefit in prevention of atherosclerosis and its sequelae. There is also evidence that alcohol may decrease insulin resistance66 and that it also has an antiplatelet effect.67 In addition, polyphenols, especially from red wine, including resveratrol as a major red wine polyphenol, appear to offer significant benefit for atherosclerosis and CV disease prevention.68 The increase in endothelial-type nitric oxide synthase (NOS) expression and activity brought about by red wine67 may be a major mechanism of protection, acting through a resultant increase in nitric oxide (NO). Vasoactive NO has significant vasodilatory beneficial effects.69 There is also benefit for atherosclerosis prevention from flavonoids which inhibit LDL oxidation.70 Red wine, dark chocolate, and green tea are the main sources of flavonoids.
NEXT FRONTIER OF ATHEROSCLEROSIS PREVENTION
The major question right now is: What is the next frontier in CHD and PVD prevention? Most experts appear to agree that HDLs represent the next focus of atherosclerosis prevention. This focus appears to involve approaches to HDL elevation but also HDL modification as there is now awareness that there are harmful HDL structures, the alteration of which can convert the lipoprotein into a particle associated with the reduction of CHD and PVD. The best currently available medication to increase HDL favorably is nicotinic acid, the tolerance to which is limited in many patients.13 Laropiprant is already approved in Europe, Mexico, Chile, and some other countries with approval pending in the United States. This medication markedly decreases the adverse flushing associated with nicotinic acid.71 Also regarding the most effective medication class known to raise HDL, the cholesterol ester transfer protein (CETP) inhibitors, the initial one, torcetrapib, turned out to have negative results.72 However, preliminary data for two other CETP inhibitors, anacetrapib73 and dalcetrapib,74 appear beneficial so, fortunately, there does not appear to be a problem for the entire CETP class of medications.
Patient use of alternative medications and natural products is a reality for practicing clinicians, whether or not they are in favor of such usage by their patients. Therefore, this topic is relevant under a current situation and the “next frontier” heading as undoubtedly, there will be newer alternative medications becoming available. Of those alternative medications relevant to CV disease, the following appear to have benefit in different situations and are not associated with any harmful effects: coenzyme Q-10, olive oil, omega fatty acids, policosanol, red wine, resveratrol, red yeast rice, soy protein, stanols,75 and vitamin D.76 Coenzyme Q-10 has some value in decreasing the incidence of statin-related myopathy.75 Olive oil is a key part of the Mediterranean diet. Omega fatty acids decrease triglycerides and act as vasodilators. Policosanol from sugarcane has been shown to decrease LDL. Red wine with its polyphenol resveratrol and its flavonoids inhibits LDL oxidation. Red yeast rice contains lovastatin for the patient who refuses any prescription statin. Soy protein can decrease LDL slightly and decreases saturated fat intake. Stanols (plant sterols) displace cholesterol from micelles and prevent its uptake at the intestinal brush border membrane. This remains a changing field that must be kept up with to advise patients on benefits, harm, and new approaches.
CONCLUSION
The reality of CV disease prevention is now established, at least as far as delayed disease manifestation, which thus far cannot be eliminated as a part of aging. Failure to pay attention to CV disease prevention when managing any such patient whether with surgical/medical problems present or because of family history or presence of established risk factors is malpractice. Evidence-based medicine has established a definite modifiable medical treatment component of CV disease. Efforts should be made to limit disease extension and prevent new disease. This is the standard of care for all physicians and medical practitioners regardless of their area of practice, primary care, highly specialized medicine, or surgery.
References
- The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA. 1984;251(3):351–364. doi: 10.1001/jama.1984.03340270029025. [DOI] [PubMed] [Google Scholar]
- The Lipid Research Clinics Coronary Primary Prevention Trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA. 1984;251(3):365–374. [PubMed] [Google Scholar]
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344(8934):1383–1389. [PubMed] [Google Scholar]
- The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- Downs J R, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- Sacks F M, Pfeffer M A, Moye L A, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- Shepherd J, Cobbe S M, Ford I, et al. West of Scotland Coronary Prevention Study Group Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333(20):1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- Liao J K, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. Molecular bases of the acute coronary syndromes. Circulation. 1995;91(11):2844–2850. doi: 10.1161/01.cir.91.11.2844. [DOI] [PubMed] [Google Scholar]
- Stoll G, Bendszus M. Inflammation and atherosclerosis: novel insights into plaque formation and destabilization. Stroke. 2006;37(7):1923–1932. doi: 10.1161/01.STR.0000226901.34927.10. [DOI] [PubMed] [Google Scholar]
- Nissen S E, Nicholls S J, Sipahi I, et al. ASTEROID Investigators Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295(13):1556–1565. doi: 10.1001/jama.295.13.jpc60002. [DOI] [PubMed] [Google Scholar]
- Blankenhorn D H, Johnson R L, Nessim S A, Azen S P, Sanmarco M E, Selzer R H. The Cholesterol Lowering Atherosclerosis Study (CLAS): design, methods, and baseline results. Control Clin Trials. 1987;8(4):356–387. doi: 10.1016/0197-2456(87)90156-5. [DOI] [PubMed] [Google Scholar]
- Blankenhorn D H, Nessim S A, Johnson R L, Sanmarco M E, Azen S P, Cashin-Hemphill L. Beneficial effects of combined colestipol-niacin therapy on coronary atherosclerosis and coronary venous bypass grafts. JAMA. 1987;257(23):3233–3240. [PubMed] [Google Scholar]
- Blankenhorn D H, Selzer R H, Mack W J, et al. Evaluation of colestipol/niacin therapy with computer-derived coronary end point measures. A comparison of different measures of treatment effect. Circulation. 1992;86(6):1701–1709. doi: 10.1161/01.cir.86.6.1701. [DOI] [PubMed] [Google Scholar]
- Brown B G, Zhao X Q, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345(22):1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- Brown G, Albers J J, Fisher L D, et al. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990;323(19):1289–1298. doi: 10.1056/NEJM199011083231901. [DOI] [PubMed] [Google Scholar]
- Prasad K, Lee P. Suppression of hypercholesterolemic atherosclerosis by pentoxifylline and its mechanism. Atherosclerosis. 2007;192(2):313–322. doi: 10.1016/j.atherosclerosis.2006.07.034. [DOI] [PubMed] [Google Scholar]
- Falk E, Shah P K, Fuster V. Coronary plaque disruption. Circulation. 1995;92(3):657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- Sacks F M, Svetkey L P, Vollmer W M, et al. DASH-Sodium Collaborative Research Group Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- Kastorini C M, Milionis H J, Esposito K, Giugliano D, Goudevenos J A, Panagiotakos D B. The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol. 2011;57(11):1299–1313. doi: 10.1016/j.jacc.2010.09.073. [DOI] [PubMed] [Google Scholar]
- Estruch R, Martínez-González M A, Corella D, et al. PREDIMED Study Investigators Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145(1):1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- Fitó M, Guxens M, Corella D, et al. for the PREDIMED Study Investigators Effect of a traditional Mediterranean diet on lipoprotein oxidation: a randomized controlled trial. Arch Intern Med. 2007;167(11):1195–1203. doi: 10.1001/archinte.167.11.1195. [DOI] [PubMed] [Google Scholar]
- Grundy S M, Cleeman J I, Merz C N, et al. National Heart, Lung, and Blood Institute. American College of Cardiology Foundation. American Heart Association Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- Wiviott S D, Cannon C P, Morrow D A, Ray K K, Pfeffer M A, Braunwald E, PROVE IT-TIMI 22 Investigators Can low-density lipoprotein be too low? The safety and efficacy of achieving very low low-density lipoprotein with intensive statin therapy: a PROVE IT-TIMI 22 substudy. J Am Coll Cardiol. 2005;46(8):1411–1416. doi: 10.1016/j.jacc.2005.04.064. [DOI] [PubMed] [Google Scholar]
- Hsia J, MacFadyen J G, Monyak J, Ridker P M. Cardiovascular event reduction and adverse events among subjects attaining low-density lipoprotein cholesterol <50 mg/dl with rosuvastatin. The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) J Am Coll Cardiol. 2011;57(16):1666–1675. doi: 10.1016/j.jacc.2010.09.082. [DOI] [PubMed] [Google Scholar]
- Kastelein J J, Akdim F, Stroes E S, et al. ENHANCE Investigators Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358(14):1431–1443. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]
- Ridker P M, Danielson E, Fonseca F A, et al. JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- Whayne T F, Jr, Zielke J C, Dickson L G, Winters J L. State of the art treatment of the most difficult low density lipoprotein (LDL) cholesterol problems: LDL apheresis. J Ky Med Assoc. 2002;100(12):535–538. [PubMed] [Google Scholar]
- Kajinami K, Takekoshi N, Saito Y. Pitavastatin: efficacy and safety profiles of a novel synthetic HMG-CoA reductase inhibitor. Cardiovasc Drug Rev. 2003;21(3):199–215. doi: 10.1111/j.1527-3466.2003.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Service: Superintendent of Documents, U.S. Government Printing Office, Washington, D.C., 20402. 1964. pp. 1–387.
- Ambrose J A, Barua R S. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43(10):1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- Benowitz N L. Cigarette smoking and cardiovascular disease: pathophysiology and implications for treatment. Prog Cardiovasc Dis. 2003;46(1):91–111. doi: 10.1016/s0033-0620(03)00087-2. [DOI] [PubMed] [Google Scholar]
- Jha P, Jacob B, Gajalakshmi V, et al. RGI-CGHR Investigators A nationally representative case-control study of smoking and death in India. N Engl J Med. 2008;358(11):1137–1147. doi: 10.1056/NEJMsa0707719. [DOI] [PubMed] [Google Scholar]
- Mulcahy R, Hickey N. Cigarette smoking habits of patients with coronary heart disease. Br Heart J. 1966;28(3):404–408. doi: 10.1136/hrt.28.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle J T, Kannel W B, McNamara P M, Quickenton P, Gordon T. Factors related to suddenness of death from coronary disease: combined Albany-Framingham studies. Am J Cardiol. 1976;37(7):1073–1078. doi: 10.1016/0002-9149(76)90427-6. [DOI] [PubMed] [Google Scholar]
- Shaper A G, Wannamethee S G, Walker M. Pipe and cigar smoking and major cardiovascular events, cancer incidence and all-cause mortality in middle-aged British men. Int J Epidemiol. 2003;32(5):802–808. doi: 10.1093/ije/dyg206. [DOI] [PubMed] [Google Scholar]
- Barnoya J, Glantz S A. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111(20):2684–2698. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- Cesaroni G, Forastiere F, Agabiti N, Valente P, Zuccaro P, Perucci C A. Effect of the Italian smoking ban on population rates of acute coronary events. Circulation. 2008;117(9):1183–1188. doi: 10.1161/CIRCULATIONAHA.107.729889. [DOI] [PubMed] [Google Scholar]
- Sargent R P, Shepard R M, Glantz S A. Reduced incidence of admissions for myocardial infarction associated with public smoking ban: before and after study. BMJ. 2004;328(7446):977–980. doi: 10.1136/bmj.38055.715683.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G C, Lee A J, Fowkes F G, Lowe G D, Housley E. The relationship between cigarette smoking and cardiovascular risk factors in peripheral arterial disease compared with ischaemic heart disease. The Edinburgh Artery Study. Eur Heart J. 1995;16(11):1542–1548. doi: 10.1093/oxfordjournals.eurheartj.a060775. [DOI] [PubMed] [Google Scholar]
- Critchley J A, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA. 2003;290(1):86–97. doi: 10.1001/jama.290.1.86. [DOI] [PubMed] [Google Scholar]
- Jonason T, Bergström R. Cessation of smoking in patients with intermittent claudication. Effects on the risk of peripheral vascular complications, myocardial infarction and mortality. Acta Med Scand. 1987;221(3):253–260. [PubMed] [Google Scholar]
- McRobbie H, Thornley S. [The importance of treating tobacco dependence] Rev Esp Cardiol. 2008;61(6):620–628. [PubMed] [Google Scholar]
- The Seventh Report of the Joint National Committee on Prevention Detection, Evaluation, and Treatment of High Blood Pressure: U.S. Department of Health and Human Services. 2004. pp. 1–86. [PubMed]
- Wang Y, Wang Q J. The prevalence of prehypertension and hypertension among US adults according to the new joint national committee guidelines: new challenges of the old problem. Arch Intern Med. 2004;164(19):2126–2134. doi: 10.1001/archinte.164.19.2126. [DOI] [PubMed] [Google Scholar]
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- Levy D, Merz C N, Cody R J, et al. Hypertension detection, treatment and control: a call to action for cardiovascular specialists. J Am Coll Cardiol. 1999;34(4):1360–1362. doi: 10.1016/s0735-1097(99)00385-x. [DOI] [PubMed] [Google Scholar]
- Doumas M, Papademetriou V, Douma S, et al. Benefits from treatment and control of patients with resistant hypertension. Int J Hypertens. 2011;2011:318–549. doi: 10.4061/2011/318549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry H M, Jr, Davis B R, Price T R, et al. Effect of treating isolated systolic hypertension on the risk of developing various types and subtypes of stroke: the Systolic Hypertension in the Elderly Program (SHEP) JAMA. 2000;284(4):465–471. doi: 10.1001/jama.284.4.465. [DOI] [PubMed] [Google Scholar]
- Staessen J A, Thijisq L, Fagard R, et al. Systolic Hypertension in Europe (Syst-Eur) Trial Investigators Effects of immediate versus delayed antihypertensive therapy on outcome in the Systolic Hypertension in Europe Trial. J Hypertens. 2004;22(4):847–857. doi: 10.1097/00004872-200404000-00029. [DOI] [PubMed] [Google Scholar]
- Grundy S M, Cleeman J I, Daniels S R, et al. American Heart Association. National Heart, Lung, and Blood Institute Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- Whayne T F., Jr In defense of the metabolic syndrome. J Clin Lipidol. 2009;3(4):247–249. doi: 10.1016/j.jacl.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Available at: NIH stops clinical trial on combination cholesterol treatment: lack of efficacy in reducing cardiovascular events prompts decision. May 26, 2011. http://public.nhlbi.nih.gov/newsroom/home/GetPressRelease.aspx?id=2792 http://public.nhlbi.nih.gov/newsroom/home/GetPressRelease.aspx?id=2792
- Clofibrate and niacin in coronary heart disease. JAMA. 1975;231(4):360–381. [PubMed] [Google Scholar]
- Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- Gerstein H C, Miller M E, Byington R P, et al. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association Implications of the diabetes control and complications trial. Diabetes Care. 2003;26(Suppl 1):S25–S27. doi: 10.2337/diacare.26.2007.s25. [DOI] [PubMed] [Google Scholar]
- Leitzmann M F, Park Y, Blair A, et al. Physical activity recommendations and decreased risk of mortality. Arch Intern Med. 2007;167(22):2453–2460. doi: 10.1001/archinte.167.22.2453. [DOI] [PubMed] [Google Scholar]
- Kujala U M, Kaprio J, Sarna S, Koskenvuo M. Relationship of leisure-time physical activity and mortality: the Finnish twin cohort. JAMA. 1998;279(6):440–444. doi: 10.1001/jama.279.6.440. [DOI] [PubMed] [Google Scholar]
- Hakim A A, Curb J D, Petrovitch H, et al. Effects of walking on coronary heart disease in elderly men: the Honolulu Heart Program. Circulation. 1999;100(1):9–13. doi: 10.1161/01.cir.100.1.9. [DOI] [PubMed] [Google Scholar]
- Franco O H, de Laet C, Peeters A, Jonker J, Mackenbach J, Nusselder W. Effects of physical activity on life expectancy with cardiovascular disease. Arch Intern Med. 2005;165(20):2355–2360. doi: 10.1001/archinte.165.20.2355. [DOI] [PubMed] [Google Scholar]
- Bijnen F C, Caspersen C J, Feskens E J, Saris W H, Mosterd W L, Kromhout D. Physical activity and 10-year mortality from cardiovascular diseases and all causes: The Zutphen Elderly Study. Arch Intern Med. 1998;158(14):1499–1505. doi: 10.1001/archinte.158.14.1499. [DOI] [PubMed] [Google Scholar]
- Thompson P D, Buchner D, Pina I L, et al. American Heart Association Council on Clinical Cardiology Subcommittee on Exercise, Rehabilitation, and Prevention. American Heart Association Council on Nutrition, Physical Activity, and Metabolism Subcommittee on Physical Activity Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003;107(24):3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- Lakka T A, Lakka H M, Rankinen T, et al. Effect of exercise training on plasma levels of C-reactive protein in healthy adults: the HERITAGE Family Study. Eur Heart J. 2005;26(19):2018–2025. doi: 10.1093/eurheartj/ehi394. [DOI] [PubMed] [Google Scholar]
- Howard A A, Arnsten J H, Gourevitch M N. Effect of alcohol consumption on diabetes mellitus: a systematic review. Ann Intern Med. 2004;140(3):211–219. doi: 10.7326/0003-4819-140-6-200403160-00011. [DOI] [PubMed] [Google Scholar]
- Wallerath T, Poleo D, Li H, Förstermann U. Red wine increases the expression of human endothelial nitric oxide synthase: a mechanism that may contribute to its beneficial cardiovascular effects. J Am Coll Cardiol. 2003;41(3):471–478. doi: 10.1016/s0735-1097(02)02826-7. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function: role of TNFalpha and vascular oxidative stress. Arterioscler Thromb Vasc Biol. 2009;29(8):1164–1171. doi: 10.1161/ATVBAHA.109.187146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher N D, Hughes M, Gerhard-Herman M, Hollenberg N K. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J Hypertens. 2003;21(12):2281–2286. doi: 10.1097/00004872-200312000-00016. [DOI] [PubMed] [Google Scholar]
- Maron D J. Flavonoids for reduction of atherosclerotic risk. Curr Atheroscler Rep. 2004;6(1):73–78. doi: 10.1007/s11883-004-0119-1. [DOI] [PubMed] [Google Scholar]
- Paolini J F, Mitchel Y B, Reyes R, et al. Effects of laropiprant on nicotinic acid-induced flushing in patients with dyslipidemia. Am J Cardiol. 2008;101(5):625–630. doi: 10.1016/j.amjcard.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Barter P J, Caulfield M, Eriksson M, et al. ILLUMINATE Investigators Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357(21):2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- Yvan-Charvet L, Kling J, Pagler T, et al. Cholesterol efflux potential and antiinflammatory properties of high-density lipoprotein after treatment with niacin or anacetrapib. Arterioscler Thromb Vasc Biol. 2010;30(7):1430–1438. doi: 10.1161/ATVBAHA.110.207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein E A, Roth E M, Rhyne J M, Burgess T, Kallend D, Robinson J G. Safety and tolerability of dalcetrapib (RO4607381/JTT-705): results from a 48-week trial. Eur Heart J. 2010;31(4):480–488. doi: 10.1093/eurheartj/ehp601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whayne T F., Jr What should medical practitioners know about the role of alternative medicines in cardiovascular disease management? Cardiovasc Ther. 2010;28(2):106–123. doi: 10.1111/j.1755-5922.2009.00101.x. [DOI] [PubMed] [Google Scholar]
- Whayne T F. Vitamin D: popular cardiovascular supplement but benefit must be evaluated. Int J Angiol. 2011;20(2):63–72. doi: 10.1055/s-0031-1279679. [DOI] [PMC free article] [PubMed] [Google Scholar]