Abstract
Nitric oxide (NO) formed via endothelial NO synthase (eNOS) plays crucial roles in the regulation of coronary blood flow through vasodilatation and decreased vascular resistance, and in inhibition of platelet aggregation and adhesion, leading to the prevention of coronary circulatory failure, thrombosis, and atherosclerosis. Endothelial function is impaired by several pathogenic factors including smoking, chronic alcohol intake, hypercholesterolemia, obesity, hyperglycemia, and hypertension. The mechanisms underlying endothelial dysfunction include reduced NO synthase (NOS) expression and activity, decreased NO bioavailability, and increased production of oxygen radicals and endogenous NOS inhibitors. Atrial fibrillation appears to be a risk factor for endothelial dysfunction. Endothelial dysfunction is an important predictor of coronary artery disease (CAD) in humans. Penile erectile dysfunction, associated with impaired bioavailability of NO produced by eNOS and neuronal NOS, is also considered to be highly predictive of ischemic heart disease. There is evidence suggesting an important role of nitrergic innervation in coronary blood flow regulation. Prophylactic and therapeutic measures to eliminate pathogenic factors inducing endothelial and nitrergic nerve dysfunction would be quite important in preventing the genesis and development of CAD.
Keywords: Nitric oxide, constitutive nitric oxide synthase, endothelial dysfunction, coronary blood flow, coronary artery disease, asymmetric dimethylarginine
Coronary blood flow is regulated through complex adjustments in the arteriolar tone and resistance of the microcirculation. Impairment of microvascular function leads to organ dysfunction in any body system including the heart. Recent evidence supports the concept that the impairment of endothelial function is an upstream event in the pathophysiology of atherosclerosis, CAD, and myocardial infarction (MI). Nitric oxide (NO) liberated as a paracrine relaxant from the vascular endothelium is known to play a pivotal role in the modulation of microvascular tone and regional blood flow.1 In addition, NO inhibits platelet aggregation and adhesion, inhibits leukocyte adhesion and migration, and reduces vascular smooth muscle proliferation, thus leading to prevention of atherosclerosis. NO produced via neuronal NO synthase (nNOS) is released from parasympathetic postganglionic (nitrergic) neurons and participates in vasodilatation, decreasing vascular resistance, and increasing blood flow.2,3
Abundant and varied data from animal and human studies, performed over the course of more than two decades, indicate that depression of synthesis and bioavailability of NO in the endothelium participates in many cardiovascular diseases, including atherosclerosis,4 coronary heart diseases,5 stroke,3 renal failure,6 and hypertension5,7 and also in insulin resistance and diabetes mellitus.8 Mechanisms underlying impairment of NO-mediated vasodilatation and blood flow increase include the downregulation of endothelial NOS (eNOS) and nNOS expressions, generation of NOS inhibitors and NO scavengers, and upregulation of vasoconstrictor substances, such as endothelin-1 (ET-1), vasoconstrictor prostanoids, and Rho/Rho-kinase.
The literature since the discovery of endothelium-derived relaxing factor by Furchgott and Zawadzki9 contains numerous reports about the interactions between NO and coronary arteries/arterioles or blood flow in health and disease. The present review covers recent advances in these investigations, including those published during these several years, on the roles of endothelial and neurogenic NO in the regulation of coronary circulation in patients with CAD and in some healthy subjects.
SYNTHESIS, DEGRADATION, AND ACTIONS OF NO
NO is produced when L-arginine is transformed to L-citrulline via catalysis by NO synthase (NOS) in the presence of oxygen and cofactors, including calmodulin, tetrahydrobiopterin (BH4), nicotinamide adenine dinucleotide phosphate (NADPH), heme, FAD, and FMN. Calcium ion (Ca2+) is required for the activation of nNOS (NOS I) and eNOS (NOS III) but not inducible NOS (iNOS, NOS II). nNOS is constitutively expressed in the brain, peripheral nerves,10 and kidneys, and eNOS is constitutively expressed mainly in endothelial cells.11 iNOS is not constitutively expressed but is induced mainly in macrophages by bacterial lipopolysaccharide and cytokines.
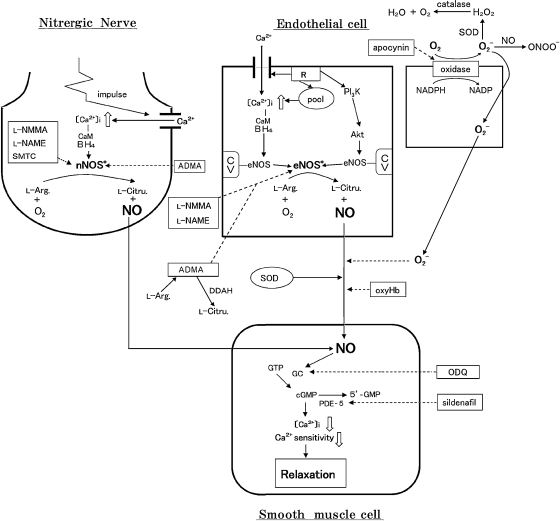
eNOS binds to caveolin-1 in the caveolae, microdomains of the plasma membrane. Caveolin-1 inhibits eNOS activity, and this interaction is regulated by Ca2+/calmodulin.12 The eNOS intracellularly migrates in response to increased cytosolic Ca2+ in the presence of calmodulin (Fig. 1) and is activated for NO synthesis. The transmembrane influx of Ca2+ and its mobilization from intracellular storage sites are caused via stimulation of drug receptors located on the endothelial cell membrane by acetylcholine (ACh), bradykinin (BK), and adenosine diphosphate (ADP) or via mechanical stimuli such as shear stress and vascular smooth muscle stretch. In human conduit coronary arteries, ACh causes contraction rather than relaxation, whereas substance P and histamine induce relaxations mediated by endothelial NO.13 On the other hand, shear stress, BK, or insulin induce the phosphorylation of Ser1177/1179 of eNOS through phosphatidylinositol 3-kinase (PI3K) and the downstream serine/threonine protein kinase Akt, resulting in enhanced NO formation.14 This mechanism does not require the increase in intracellular Ca2+ for NO production (Fig. 1). The alternative pathway through extracellular signal-regulated kinases also plays a role in eNOS activation.15
Figure 1.
Information pathways via NO liberated from endothelial cells and nitrergic neurons to vascular smooth muscle cells. On the endothelial membrane, receptors (R) responding to chemical and physical stimuli; ADMA, asymmetric dimethylarginine; Akt, serine/threonine protein kinase Akt; CaM, calmodulin; cGMP, cyclic GMP; CV, caveolin-1; DDAH, dimethylarginine dimethylaminohydrolase; eNOS*, activated eNOS; GC, soluble guanylyl cyclase; L-Arg., L-arginine; L-Citru., L-citrulline; nNOS*, activated nNOS; O2-, superoxide anion; ODQ, 1H[1,2,4]oxadiazolo [4,3-a]quinoxalin-o1-one; ONOO-, peroxynitrite; oxyHb, oxyhemoglobin; PDE-5, phosphodiesterase-5; PI3K, phosphatidyl inositol 3-kinase pool, Ca2+ storage site; SMTC, S-methyl-L-thiocitrulline; SOD, superoxide dismutase. Solid lines denote stimulation; dotted lines denote inhibition.
Endothelial NO causes vasodilatation, increased blood flow, lowered blood pressure, inhibition of platelet aggregation and adhesion, inhibition of leukocyte adhesion, and reduced smooth muscle proliferation; and it acts to prevent atherosclerosis. These NO actions are mediated by cyclic guanosine monophosphate (cyclic GMP) from GTP synthesized through soluble guanylyl cyclase. Nonadrenergic noncholinergic inhibitory responses to parasympathetic nerve stimulation are mainly mediated through NO synthesized by nNOS; NO plays a crucial role as a neurotransmitter from the peripheral efferent nerves in the blood vessel.2,16
The synthesis of NO by NOS isoforms is inhibited by L-arginine analogs, including NG-monomethyl-L-arginine (L-NMMA), NG-nitro-L-arginine (L-NA), and L-NA methylester (L-NAME). The endogenous NOS inhibitor asymmetric dimethylarginine (ADMA)17 plays a pathogenic role, particularly in the circulation. 7-Nitroindazol (7-NI)18 and S-methyl-L-thiocitrulline (SMTC)19 are promising specific inhibitors of nNOS. Nitro compounds, such as nitroglycerin (GTN) and sodium nitroprusside (SNP), are capable of liberating NO. 1H[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one20 decreases the synthesis of cyclic GMP by inhibiting guanylyl cyclase activity. Insufficiency of BH4 makes NOS uncoupled, which consequentially results in superoxide anions being produced instead of NO. Superoxide anions are also generated by NADPH oxidase and xanthine oxidase. Superoxide dismutase (SOD), catalase, and dimethyl sulfoxide scavenge free radicals. NO reacts with superoxide anions, generating highly toxic compounds such as peroxynitrite (ONOO−).
ROLE OF NO IN CORONARY CIRCULATION
Role of eNOS-Derived NO
Endothelial NO functions as a critical modulator of coronary blood flow by inhibiting smooth muscle contraction and platelet aggregation, and also contributes to angiogenesis and cytoprotection in the heart. The Dietary Approaches to Stop Hypertension (DASH) diet lowers blood pressure and substantially reduces the risk of coronary heart disease.21 In hypertensive and obese hypertensive patients, endothelial function is improved by the DASH diet.22,23
Katz et al24 provided evidence that endothelial dysfunction in patients with chronic heart failure, as assessed by flow-mediated dilatation in the brachial artery and exhaled NO production during submaximal exercise, is associated with an increased mortality risk in subjects with both ischemic and nonischemic chronic heart failure. In patients with slow coronary flow, thrombolysis in myocardial infarction (TIMI) frame count25 was higher, the plasma NO level was lower, and brachial artery endothelium-dependent flow-mediated dilatation was smaller than in subjects with normal coronary flow.26 Endothelial dysfunction appears to contribute to the pathogenesis of slow coronary flow. In patients with normal coronary angiograms, flow-mediated endothelium-dependent vasodilatation in the brachial artery had a negative relation with the intima + media area in coronary artery.27 As the pathogenesis of acute coronary syndrome has been reported to involve plaque rupture even in the patients with normal coronary angiograms, it may be necessary to monitor patients with impaired flow-mediated vasodilatation even if their coronary angiograms show no abnormalities. Flow-mediated brachial artery dilatation, but not GTN-induced vasodilatation, was decreased in children of hypertensive patients as compared with controls, suggesting that children of hypertensive parents appear to have endothelial dysfunction, which may be an early marker for the development of CAD.28 Forearm blood flow increase in response to ACh was smaller in patients with CAD who had periodontitis than in the nonperiodontitis group, SNP-induced vasodilatation was similar in both groups, and circulating levels of C-reactive protein (CRP) and interleukin-6 (IL-6) were higher in the periodontitis group; periodontal therapy reduced serum concentrations of CRP and IL-6, and augmented ACh-induced vasodilatation in patients with periodontitis.29 Periodontitis appears to be associated with endothelial dysfunction in patients with CAD through a decrease in NO bioavailability. In patients without significant CAD, the gradient of F2-isoprostanes between arterial levels and coronary sinus correlated with the change in coronary artery diameter in response to ACh; isoprostanes net production across the left anterior descending (LAD) artery territory correlated with a decrease in SOD activity and a decrease in coronary artery diameter in response to L-NMMA.30 These authors concluded that coronary endothelial dysfunction in humans may be characterized by local enhancement of oxidative stress without a decrease in basal NO release.
In patients with paroxysmal atrial fibrillation, in whom the atrial fibrillation was induced by burst atrial pacing, local (coronary sinus sample) cardiac platelet activation and thrombin generation increased and NO production decreased. However, there was no change in inflammatory markers, suggesting that atrial fibrillation may contribute to the hypercoagulable state within minutes.31 Guazzi and Arena32 summarize evidence that atrial fibrillation is a risk factor for endothelial dysfunction as documented by impaired ACh-induced blood flow increase, reduced plasma nitrite/nitrate levels, and additive impairment of flow-mediated vasodilatation by morbidities causing endothelial dysfunction.
Nitrite levels were increased in the anterior interventricular vein after an anastomosis between the left internal mammary artery and the LAD artery compared with those before the anastomosis, suggesting that the increased production of NO by the internal mammary graft may provide a perpetual vasodilator response.33 The effect of L-NMMA on coronary blood flow, coronary artery diameter, and coronary vascular resistance was attenuated in cyclosporine-treated heart transplant recipients with normal coronary angiograms compared with controls.34 Cardiac allograft epicardial coronary endothelial function is abnormal and may have an impaired endogenous NOS pathway and reduced endothelial NO production in transplant recipients. In cardiac transplant recipients with diabetes mellitus, postprandial hyperglycemia acutely doubled circulating levels of the oxidation product malondialdehyde, but did not affect the ability of ACh to dilate conduit coronary artery segments or accelerate coronary blood flow, suggesting that the oxidative stress associated with an acute episode of hyperglycemia affects neither ACh-mediated coronary endothelial NO release nor subsequent bioavailability.35
Progressive worsening of functional coronary circulatory abnormalities of NO-mediated, endothelium-dependent vasodilatation occurs with increasing severity of insulin resistance and carbohydrate intolerance.36 The levels of homocysteine, ET-1, and circulating endothelial cell in patients with coronary lesions were increased in comparison with patients with no recognizable plaque and/or stenosis, whereas the NO level was lower in those with coronary lesions, suggesting that homocysteine appears to be a predictor for preliminary or active coronary lesion.37
In studies on patients with chronic CAD, it was noted that macrophage-colony stimulating factor (MCSF) and CRP levels were increased in those with T-786C at the promotor region of eNOS or variable nucleotide tandem repeat (VNTR) allele; patients with the combination of VNTR and T-786C had higher MCSF and CRP levels than patients with one or none of these alleles; patients with MCSF >262 pg/mL had lower flow-mediated dilatation of the brachial artery.38 The intron 4-VNTR and T-786C mutation of eNOS appear to enhance the inflammatory process in patients with chronic CAD.
In summary, endothelial dysfunction is a predictor and also one of the important risk factors for CAD. Impaired endothelial function in coronary vasculatures is recognized by attenuated endothelium-dependent vasodilatation induced by chemical stimulation (ACh, BK, and ADP) and physical stimuli, such as flow and shear stress, without affecting the response to NO donors GTN and SNP. Forearm blood flow responses to chemical or physical stimuli and plasma nitrate/nitrite levels appear to reflect coronary arterial/arteriolar endothelial functioning. Decreased production of NO in endothelial cells would be associated with reduced eNOS protein expression and/or activity that might result from pathogenic factors, including smoking,39 chronic alcohol intake,40 high salt intake,41 hyperhomocysteinemia,42 diabetes mellitus,8 hypertension,43 and increased production of endogenous NOS inhibitors such as ADMA. Increased activations of NADPH oxidase and xanthine oxidase, eNOS uncoupling due to BH4 depletion, and SOD deprivation appear to participate in generation of oxidative stress. Table 1 summarizes the synthesis and actions of NO and the possible mechanisms underlying endothelial dysfunction in patients with CAD. Atrial fibrillation may be a risk factor for coronary endothelial dysfunction.
Table 1.
Endothelial Dysfunction in Patients with Coronary Artery Disease
| Author, Year | Disease | Change in Responses | Mechanism |
|---|---|---|---|
| Katz et al, 2005 | Chronic heart failure | FMD↓ exhaled NO↓ | E-dysfunction |
| Sezgin et al, 2005 | Slow coronary flow | FMD↓ plasma NO↓ | E-dysfunction |
| Khalil et al, 2008 | Child of HT parents | FMD↓ GTN-D→ | E-dysfunction, No change in NO response |
| Higashi et al, 2009 | Periodontitis | ACh-D↓ SNP-D→ CRP↑ | E-dysfunction, No change in NO response |
| Levy et al, 2009 | Coronary artery dis. | ACh-R↓ SOD↓ | E-dysfunction, Oxidative stress↑ |
| Guazzi and Arena, 2009 | Atrial fibrillation | ACh-D↓ plasma NO↓ | E-dysfunction |
| Selcuk et al, 2007 | Slow coronary flow | ADMA↑ L-arg./ADMA↓ | E-dysfunction, NOS inhibition by ADMA↑ |
| Okyay et al, 2007 | Syndrome X | ADMA↑ L-arg.→ | E-dysfunction, NOS inhibition by ADMA↑ |
| Tang et al, 2009 | Obst. coronary dis. | L-arginine availability↓ L-citrulline↑ | Global arginine availability ratio↓ |
FMD, flow-mediated vasodilatation; NO, nitric oxide; E-dysfunction, endothelial dysfunction; HT, hypertension; GTN-D, glyceryl trinitrate-induced dilatation; ACh-D, acetylcholine-induced dilatation; SNP-D, sodium nitroprusside-induced dilatation; →, no change; CRP, C-reactive protein; dis., disease; Ach-R, acetylcholine receptor; SOD, superoxide dismutase; ADMA, asymmetric dimethylarginine; L-arg., L-arginine; NOS, nitric oxide synthase; obst., obstructive.
Involvement of ADMA in Blunted NO Availability
ADMA, an endogenous NOS inhibitor, has been known to be a risk factor for cardiovascular diseases17 through impairment of NO synthesis by eNOS and nNOS. Plasma ADMA is accumulated, because the degradation of ADMA through dimethylarginine dimethylaminohydrolase (DDAH)44 and alanine-glyoxylate aminotransferase 2 (AGXT2)45 is reduced.
Patients with slow coronary flow were detected to have higher levels of plasma ADMA and lower L-arginine/ADMA ratio compared with participants with normal coronary flow; both ADMA and L-arginine/ADMA ratio were correlated with coronary flow, as assessed by the TIMI frame count methods, suggesting that endothelial dysfunction may be an important factor in the pathogenesis of slow coronary flow.46 The level of ADMA is suggested to predict survival in patients with chronic heart failure.47 The plasma ADMA levels were higher in patients with typical exertional angina, positive exercise test, and normal coronary arteries diagnosed as cardiac syndrome X than in the control group, whereas plasma L-arginine levels were similar in both groups; patients with abnormal myocardial tissue perfusion had increased plasma ADMA levels compared with those with normal tissue perfusion.48 In the patients with cardiac syndrome X, increased plasma ADMA levels may be associated with impaired myocardial tissue perfusion. Wang et al49 provided evidence suggesting that ADMA, symmetric dimethylarginine (SDMA), and the integrated quantification of arginine methylation provided independent risk prediction for both obstructive CAD and incident major adverse cardiac events in stable patients undergoing cardiac evaluation, and that factors beyond direct NOS inhibition contribute to the clinical association between methylarginines and CAD outcome. Patients with obstructive CAD had a lower global arginine bioavailability ratio (defined as arginine/[ornithine + citrulline] versus plasma L-arginine levels) than those without obstructive CAD.50 After adjusting for Framingham risk score, the lower global arginine availability ratio (but not L-arginine levels) and higher L-citrulline levels remained associated with the prevalence of obstructive CAD; global arginine availability ratio and ADMA showed a negative correlation. The global arginine availability ratio appears to serve as a more comprehensive concept of reduced NO synthetic capacity compared with systemic L-arginine levels. Therefore, Tang et al50 suggested that diminished arginine bioavailability ratio and high citrulline levels are associated with development of obstructive CAD and heightened long-term risk for major adverse cardiovascular events.
Studies on high-risk diabetic men with CAD indicated that plasma ADMA levels were a strong and independent predictor of all-cause mortality; in addition, baseline ADMA values were also an independent predictor of the outcome of all-cause mortality for MI, suggesting that elevated baseline levels of ADMA are an independent predictor of cardiovascular outcomes in patients with diabetes mellitus.51 Coronary flow reserve was reduced in patients with early rheumatoid arthritis compared with that in healthy volunteers; higher levels of plasma ADMA were associated with decreased coronary flow reserve; common carotid intima-media thickness was negatively associated with coronary flow reserve.52
In patients with stable angina, plasma levels of ADMA were related to the severity of CAD and correlated inversely with the number of circulating endothelial progenitor cells (EPCs) and endothelial colony forming units; ADMA repressed in vitro differentiation of EPCs and reduced EPC incorporation into endothelial tube-like structures, suggesting that ADMA is an endogenous inhibitor of mobilization, differentiation, and function of EPCs.53 Surdacki et al54 obtained evidence suggesting that elevated ADMA and EPC deficiency may synergistically contribute to accelerated renal dysfunction and that impairment of the EPC-dependent endothelial renewal may be associated with decreased bioavailability of NO.
Coronary angiogenesis and collateral growth are chronic adaptations to myocardial ischemia to restore coronary blood flow, and increased plasma ADMA levels are related with poor coronary collateral development.55 Collateral development was lower in patients with the Asp variant.56 This may be explained by the decreased eNOS activity in patients with this variant. Patients without CAD, who underwent coronary angiography alone, responded to the angiography with an increase in plasma ADMA, SDMA, and L-ornithine levels, whereas the stent implantation to diseased coronary artery, independent of the stent type used, reduced plasma ADMA levels.57 Plasma ADMA activity in patients, who had diseased saphenous vein grafts was higher than in those with nondiseased saphenous vein grafts; mean platelet volume was also higher in patients with diseased vein grafts, suggesting that increased ADMA activity leads to the acceleration of saphenous vein graft disease and that ADMA may be a precious marker for detecting late saphenous vein graft patency.58
Role of nNOS-Derived NO
Despite the fact that extensive studies have been performed to determine the functional role of autonomic, nitrergic nerves innervating cerebral,2,3 renal,59 and systemic vasculatures,60 there is still a paucity of information concerning the role of nitrergic nerves in the regulation of coronary arterial and arteriolar tone and coronary hemodynamics.
Nerve cell bodies and perivascular neurons containing nNOS immunoreactivity or NADPH diaphorase have been reported in the heart of several species including the rat,61,62,63 guinea-pig,62 and dog.64 However, functional roles of neurogenic NO in the regulation of coronary arterial tone have not been determined. Isolated dog conduit coronary arteries respond to transmural field stimulation and nicotine with relaxations that are abolished by treatment with β-adrenoceptor antagonists. This is in contrast to the findings obtained from isolated cerebral arteries, which respond to electrical and chemical stimulations with relaxations that are sensitive to NOS inhibitors but resistant to β-adrenoceptor blockers. Despite the histological demonstration of NOS-containing neurons in the adventitia of large coronary arteries, evidence for the functional role of nitrergic neurons has not been provided64; however, possible roles of nitrergic nerves in the regulation of coronary arteriolar tone and vascular resistance have been suggested.2
In patients with angiographically normal coronary arteries, intracoronary infusion of the nNOS-selective inhibitor SMTC reduced basal coronary blood flow and epicardial coronary diameter but had no effect on increases in flow evoked by intracoronary substance P that stimulated the release of NO from the endothelium, whereas L-NMMA infusion reduced basal coronary flow and inhibited substance P-induced increases in flow.65 Local nNOS-derived NO, possibly from nitrergic neurons innervating coronary arteries and arterioles (Fig. 1), appears to regulate basal coronary blood flow in humans. Recent studies on anesthetized pigs treated with selective NOS isomer blockade66 and on mice deficient in eNOS, nNOS, and iNOS genes67 provided evidence that NO derived from nNOS alone or in combination with eNOS plays a role in protecting against fatal coronary circulatory disorders, whereas iNOS-derived NO appears to participate in impaired cardiac perfusion and contractility. Whether nNOS involved in the beneficial action is from autonomic nitrergic neurons or other organs and tissues remains to be determined.
Apart from nNOS in nitrergic neurons, Han et al68 provided evidence suggesting that estrogen opens Ca2+-activated K+ channels in human coronary artery smooth muscle cells by stimulating nNOS via a transduction sequence involving PI3K and Akt. This may be a mechanism underlying the estrogen-induced enhancement of coronary blood flow in patients with diseased or damaged coronary arteries.
Role of iNOS-Derived NO
Activation of iNOS during immunological reactions and NO overproduction cause circulatory shock and neurotoxic actions.
Dover et al69 provided evidence that selective iNOS inhibition by intrabrachial infusion of 1400 W {N-[3-(aminomethyl)benzyl]acetamidine} did not influence forearm blood flow in patients with New York Heart Association class II–V heart failure. iNOS activity does not seem to participate in peripheral vascular tone in patients with symptomatic heart failure. On the other hand, patients with heart failure from idiopathic dilated cardiomyopathy, who suffered from adverse events, had a diminished forearm blood flow response to ACh, compared with patients without adverse events. Intrabrachial infusion of aminoguanidine (another selective iNOS inhibitor) decreased forearm blood flow in patients with adverse events, but not in patients without adverse events, indicating that congestive heart failure patients with vascular iNOS activation, as evidenced by a greater vasoconstrictor response to aminoguanidine, had poor outcomes.70 Whether the discrepancy in the actions of iNOS inhibitors is due to different severity or etiology of heart failure, different selectivity of so-called selective iNOS inhibitors used, and different doses of the NOS inhibitor used remains to be determined.
Kawasaki Disease and NO
In Kawasaki disease, a systemic vasculitis of unknown etiology, the intense inflammatory process has a predilection for the coronary arteries and abnormalities of myocardial blood flow appears to be associated with endothelial dysfunction.
Intracoronary infusion of ACh increased the LAD coronary artery area to a lesser extent in Kawasaki disease patients with a normal left coronary artery and patients with a persistent or regressed aneurysm than control subjects, whereas increases in coronary blood flow were similar in these groups, suggesting a persistent endothelial dysfunction in the epicardial but not resistance coronary arteries in patients with Kawasaki disease.71 Long-term coronary artery lesions, even after aneurysm regression, in patients with Kawasaki disease have impaired endothelial function.72 Kurio et al73 obtained evidence suggesting that the endothelial injury in Kawasaki disease is confined to the endothelium of medium-sized arteries and that microvascular endothelial cells are normal after acute Kawasaki disease.
The number of EPCs was higher, the migratory response of EPCs was decreased, and the proliferative and adhesive activities were decreased in patients with Kawasaki disease compared with those in controls: the plasma NO, tumor necrosis factor-α (TNF-α), and high sensitivity CRP levels in the Kawasaki disease group were higher.74 The number of circulating EPCs positively correlated with the level of NO, and the functions of EPCs negatively correlated with the levels of TNF-α and CRP. The two-way regulation of circulating EPCs (an increase in the number and a decrease in the function) in Kawasaki disease patients may be associated with the disorders of cytokines or messengers in these patients.
Neutrophils from patients with the early phase of Kawasaki disease produced higher amount of NO compared with controls; the amount of NO produced by neutrophils in the patients decreased after immunoglobulin treatment; increased production of reactive oxygen species (ROS) was found in both Kawasaki disease and non-Kawasaki disease febrile children.75 The abnormal immune system in Kawasaki disease may be characterized by an overproduction of NO.
PENILE ERECTILE AND CORONARY ENDOTHELIAL DYSFUNCTION
There is growing evidence that erectile dysfunction is a sentinel for future CAD. Erectile dysfunction is associated with impairment of nitrergic neuronal and endothelial functions.76,77 Therefore, interactions between vasculogenic erectile dysfunction and coronary endothelial dysfunction/hemodynamic disorder are inferred.
Significant correlation has been demonstrated between erectile function and the number of occluded coronary vessels in patients with ischemic heart disease in early studies by Greenstein et al.78 The prevalence of erectile dysfunction was relatively high in patients with CAD, and it was related to the extent of CAD.79 The authors suggest that erectile dysfunction may occur before CAD. There was a positive correlation between the severity of erectile dysfunction and coronary artery calcification in men with erectile dysfunction.80,81 As compared with patients without erectile dysfunction, those with erectile dysfunction exhibited higher probability of having coronary atherosclerosis, higher number of coronary stenoses, and higher prevalence of a triple-vessel disease, suggesting that the coincidence of CAD and erectile dysfunction identifies patients at increased risk of severe forms of CAD.82 Erectile dysfunction is also strongly predictive of atherosclerotic cardiovascular events; this is even more striking when erectile dysfunction presents at a younger age.83 According to Böhm et al,84 erectile dysfunction is a potent predictor of all-cause death and the composite of cardiovascular death, MI, and heart failure in men. Vlachopoulos et al85 summarized the pathophysiologic links between erectile dysfunction, endothelial dysfunction, and CAD.
The possible beneficial effect of PDE-5 inhibitors, regarded as promising therapeutics for male sexual dysfunction, in conditions such as MI and endothelial dysfunction has been reviewed by Kapur et al.86 Clinical evidence supports the use of PDE-5 inhibitors as first-line therapy in men with CAD.87
Taken together, pathogenic mechanisms underlying endothelial dysfunction in the corpus cavernosum appear to contribute to impairment of coronary arterial endothelial function; therefore, early signs of penile erectile dysfunction are regarded as an important predictor of CAD. It is hypothesized that neurogenic NO derived from parasympathetic nitrergic nerves plays a pivotal role in the intracavernous pressure increase and immediate penile erection, and endothelially generated NO and neurogenic NO act together to maintain penile erection.76,77 Despite this fact, only little information is available about the role of nitrergic nerves in the regulation of coronary blood flow and its dysfunction in human subjects.
THERAPEUTIC MEASURES
L-Arginine and BH4
In patients with CAD, the intracoronary application of L-arginine (150 μmol/min) increased the luminal diameter of the stenotic segment without affecting other coronary artery segments and also increased the poststenotic coronary blood flow; the NO donor isosorbide dinitrate dilated all segments with a predominance of the stenotic coronary artery segment, suggesting a therapeutic potential of L-arginine in patients with coronary stenosis.88 Compared with that in preischemia, the endothelium-dependent vasodilatation induced by ACh was reduced by reperfusion when saline was infused, but not following intrabrachial infusion of L-arginine (20 mg/min) and BH4 (500 μg/min) in patients with type II diabetes mellitus and CAD; vasodilatation induced by SNP was unaffected by ischemia/reperfusion, suggesting that L-arginine and BH4 supplementation may be a novel treatment strategy to limit ischemia/reperfusion injury in these patients.89 On the other hand, in patients with noncritical CAD or following percutaneous coronary intervention, coronary microvascular endothelial function, as assessed by intracoronary infusions of ACh, was not improved by administration of BH4 (250 and 500 μg/min).90
Abnormal zone myocardial blood flow reserve treated with adenosine and sildenafil exceeded that with adenosine and placebo, suggesting that PDE-5 inhibition appears to improve the myocardial blood flow response to adenosine in abnormal zones, possibly by augmenting NO-mediated increase in cyclic GMP.91
Antioxidants
Inhibition of xanthine oxidase activity by oxypurinol attenuated ACh-induced coronary vasoconstriction and increased coronary blood flow in patients with CAD compared with patients with preserved coronary endothelial function; flow-mediated dilatation of the brachial artery was also increased.92 Xanthine oxidase-derived ROS may contribute to impaired coronary NO bioavailability in CAD. In patients with chronic heart failure, allopurinol lowered ROS and ADMA concentrations and improved postischemic vasodilatation and endothelium-dependent vasodilatation.47 Recent studies by Noman et al93 on patients with chronic stable angina showed that high-dose (600 mg/d) alloprinol increased the mean time to ST depression, median total exercise time, and the time to chest pain from the baseline. Alloprinol seems to be a useful, inexpensive, well-tolerated, and safe anti-ischemic drug for patients with chronic stable angina.
Folic Acid
According to Moat et al,94 both 400 μg/d and 5 mg/d of folic acid (for 6 weeks) increased plasma folate and decreased plasma homocysteine in patients with CAD; flow-mediated vasodilatation of the brachial artery was improved after treatment with 5 mg/d folic acid, but this did not correlate with the reduction of homocysteine; there was no change in flow-mediated vasodilatation in the 400 μg/d folic acid or placebo group; folic acid promoted eNOS dimerization in cultured porcine aortic endothelial cells. Folic acid appears to improve endothelial function in CAD via a promotion of eNOS dimerization but not through a mechanism dependent on homocysteine lowering. On the other hand, Shirodaria et al95 obtained evidence that low-dose folic acid treatment (400 μg/d for 7 weeks) improved vascular function via an increase in enzymatic coupling of eNOS through availability of BH4 and a decrease in vascular oxidative stress in patients with CAD undergoing coronary artery bypass grafting surgery and that high-dose (5 mg/d) treatment provided no additional benefit. Despite the similar study designs used by these groups (Moat et al94 versus Shirodaria et al95), there is quite a difference in the effective doses of folic acid. Whether this is due to the use of patients with bypass grafting by the latter group remains to be determined. According to Dragoni et al,96 treatment of healthy volunteers with folic acid (10 mg/d for 7 days) did not protect the vascular endothelium from ischemia/reperfusion injury.
3-Hydroxy-3-Methylglutaryl Coenzyme: A Reductase Inhibitor (Statin)
Beneficial pleiotropic effects of statins including improvement of endothelial dysfunction, increased NO bioavailability, antioxidant properties, and stabilization of atherosclerotic plaques have been summarized in previous review articles.97,98 In patients with nonischemic chronic heart failure, the area under the curve ratio during ACh infusion increased in resistance vessels to a greater extent with atorvastatin treatment (40 mg/d for 6 weeks) compared with that without treatment; in conduit arteries, flow-mediated vasodilatation increased more with statin.99 Young et al100 noted that short-term (6 weeks) atorvastatin treatment in patients with nonischemic chronic heart failure improved endothelial function but had no effect on ADMA or the L-arginine/ADMA ratio. In 35 out of 46 patients with CAD, EPCs and EPC colony-forming units increased after a cardiac rehabilitation program and treatment for 1 month with statin compared with before the program and therapy, but the remaining 11 patients had no increase in either measure; those patients whose EPCs increased from baseline showed increases in plasma nitrite and decreases in annexin-V staining, a marker of apoptosis, in EPCs; over the course of the program, EPCs increased before the nitrite increase in the blood.101 Most, but not all, patients responded to the cardiac rehabilitation and statin therapy with increases in EPC number, EPC survival, and endothelial differentiation potential, possibly associated with increased NO in the blood. In patients with established CAD, abrupt discontinuation of simvastatin treatment led to a rebound of serum total cholesterol and low-density lipoprotein (LDL) cholesterol levels and decreased endothelial dependent flow-mediated dilatation of the brachial artery; in human umbilical vein endothelial cells, the NO production and eNOS expression were decreased after stopping statin treatment.102 Abrupt withdrawal of simvastatin treatment appears to not only abrogate its beneficial effects on endothelial function but also induce further vascular injury.
Angiotensin II Type 1 Receptor Blocker (ARB)
In patients with type II diabetes, serum ADMA concentrations decreased and coronary flow velocity reserve increased after a 4-week treatment with temocapril, an angiotensin-converting enzyme inhibitor.103 Decrease in ADMA may be related to improvement of coronary circulation. Oral administration of the ARB losartan tended to improve endothelium-dependent brachial artery flow-mediated vasodilatation compared with the baseline (although statistically insignificant), while combination therapy with losartan and intravenous L-arginine significantly improved flow-mediated vasodilatation; urinary NO excretion after losartan alone and combined therapy was correlated with improved hemodynamic variables.104 According to Koh et al,105 ARBs decrease the incidence of CAD, because they inhibit angiotensin II-induced increases in superoxide anion generation and oxidative stress, leading to activation of nuclear transcription factor and endothelial dysfunction.
Vasodilating β-Adrenoceptor Blockers (β-Blockers of the Third Generation)
β-Adrenoceptor blockers with vasodilatory action associated with the release of NO (celiprolol, nebivolol, and nipradilol) or the suppression of ROS (carvedilol) are expected to counteract the possible β-blockade-induced coronary vasoconstriction.106 Celiprolol was suggested to be potentially useful in patients with angina pectoris and hypertension, complicated by other conditions associated with advanced age, impaired glucose tolerance or diabetes mellitus, peripheral vascular disease, and hyperlipidemia.107,108 Coronary flow reserve at rest was less in patients with CAD than in control individuals; intracoronary administration of nebivolol increased coronary flow reserve both in the controls and patients; collateral flow index decreased with nebivolol and correlated to changes in heart rate.109 It appears that intracoronary nebivolol is associated with an increase in coronary flow reserve due to an increase in maximal coronary flow and that the collateral flow index decreases with nebivolol parallel to the reduction in myocardial oxygen consumption. Changes of coronary flow reserve due to vasodilator β-blockers improve microvascular angina pectoris or silent ischemia in patients without epicardial artery stenosis.110 Akçay et al111 provided evidence suggesting that nebivolol is beneficial for improving oxidative stress parameters in patients with slow coronary flow.
Herbal Agents
During aged-garlic extract supplementation, flow-mediated endothelium-dependent brachial artery dilatation increased from the baseline in patients with CAD that were currently being treated with aspirin and a statin; markers of oxidative stress (plasma oxidized LDL and peroxides), systemic inflammation, and endothelial activation did not change during the study.112 Intravenous administration of Ginkgo biloba extract to patients with CAD increased LAD coronary artery blood flow and brachial artery flow-dependent dilatation.113 In addition, plasma NO increased and ET-1 decreased after 2 weeks of ginkgo extract treatment; a linear correlation was obtained between the percentage change in LAD coronary artery blood flow and in NO, ET-1, or NO/ET-1 ratio following extract treatment, suggesting that the ginkgo extract led to an increase in coronary blood flow, which may be related to improvement of NO/ET-1 imbalance.114
Exercise and External Counterpulsation
After 8 weeks of exercise training in patients with congestive heart disease, forearm blood flow responses to ACh and SNP increased as compared with the control group (usual living); the clearance of L-arginine also increased in the training group.115 The authors suggested that an increase in the transport of L-arginine may contribute to the augmentation of endothelial function by exercise. In addition, increased NO actions appear to be involved, since endothelium-independent NO-mediated vasodilatation was also augmented. According to Duncker and Bache,116 exercise training augments endothelium-dependent vasodilatation through the coronary microcirculation, possibly through an increased expression of NOS; during exercise, endothelium-derived NO, prostanoids, and β-adrenergic activity exert vasodilator influences on coronary collateral vessels.
Enhanced external counterpulsation (EECP) is a noninvasive, pneumatic technique that provides beneficial effects for patients with chronic, symptomatic angina pectoris. EECP elicited increases in intracoronary pressure with a decrease in systolic pressure, intracoronary Doppler flow velocity, and coronary flow, as assessed by TIMI frame count, suggesting that EECP may serve as a potential mechanical assist device.117 During the course of EECP therapy in patients with CAD, plasma nitrate/nitrite progressively increased and plasma ET-1 decreased, suggesting that EECP improves endothelial function.118 In symptomatic patients with CAD, EECP increased flow-mediated vasodilatation and plasma levels of nitrate/nitrite and 6-keto-prostaglandin F1α, whereas it decreased plasma levels of ET-1, ADMA, and proinflammatory cytokines.119
Miscellaneous
Treatment with the α-adrenoceptor antagonist urapidil improved coronary flow, myocardial perfusion, and left ventricular function following percutaneous coronary intervention in patients with ST-elevation acute coronary syndrome; myocardial NO concentrations in the urapidil group was higher than that of the control group.120 These beneficial effects appear to be associated with an enhanced biosynthesis of NO. Patients with coronary vasospasm had lower endothelium-dependent flow-mediated vasodilatation as compared with normal individuals; benidipine, but not diltiazem and verapamil, increased flow-mediated vasodilatation and plasma cyclic GMP levels; none of the treatments affected GTN-induced vasodilatation, suggesting that upregulation of the NO-cyclic GMP system by benidipine may partly contribute to the improvement of endothelial dysfunction.121 Short-term (4 weeks) trimetazidine therapy improved heart rate variability parameters and endothelial products such as NO and ET-1 as well as anginal symptom in patients with slow coronary artery flow; this improvement was correlated with increased NO and decreased ET-1 levels.122 The Rho kinase inhibitor fasudil increased endothelium-dependent vasodilatation in patients with CAD but not in healthy controls and also reduced Rho kinase activity in the patients, suggesting that inhibition of the Rho/Rho kinase pathway appears to provide a useful strategy to restore NO bioavailability in humans with atherosclerosis.123
In patients with symptomatic coronary disease and long-term aspirin therapy, vascular function tests showed improvement of ACh-induced vasodilatation and L-NMMA responses in the clopidogrel-added group, while SNP-induced vasodilatation was not altered; urinary excretion of 8-iso-prostaglandin F2α and plasma levels of inflammation products were reduced in patients on additional treatment with clopidogrel but not in patients on placebo.124 Beyond inhibition of platelet aggregation, adenosine diphosphate–receptor blockade may have promising vasoprotective effects, such as improvement of endothelial NO bioavailability and diminishment of biomarkers of oxidative stress and inflammation in these patients. The increase in leukocyte-derived myeloperoxidase plasma content on bolus heparin was higher in patients with CAD; heparin treatment improved endothelial NO bioavailability, as evidenced by flow-mediated vasodilatation and by ACh-induced increase in forearm blood flow, suggesting that mobilization of vessel-associated myeloperoxidase may represent a mechanism by which heparins exert anti-inflammatory effects and increase vascular NO bioavailability.125
Aldehyde dehydrogenase-2 may confer cardioprotection through metabolism of reactive aldehydes and through its role in the bioconversion of nitrates to NO. Therefore, Budas et al126 suggest that aldehyde dehydrogenase-2 is a key mediator of endogenous survival signaling in the heart and its agonists, such as an aldehyde dehydrogenase-2 activator 1, may lead to novel therapeutics, which limit injury during MI or bypass surgery.
SUMMARY
This review article summarizes information concerning recent advances in research on coronary blood flow regulation by NO generated mainly through eNOS and also nNOS or iNOS in patients with CAD. The mechanisms underlying endothelial dysfunction in CAD cannot be fully discussed because of limited information from studies on healthy and diseased individuals, in which ethical problems must be avoided. Endothelial dysfunction is undoubtedly one of the important risk factors for CAD. In addition, the imbalance between vasodilator factors, such as NO, endothelium-derived hyperpolarizing factor, and prostacyclin, and vasoconstrictors, including ET-1, thromboxane A2, Rho-Rho-kinase, and endothelium-derived contracting factors, must be kept in mind for treatment of CAD. Studies on the physiological role of nitrergic neurons in the coronary blood flow regulation in humans are still insufficient; however, together with neurogenic coronary vasodilatation mediated by the β-adrenergic mechanism, the nitrergic vasodilatation is expected to play a role in the control of coronary circulation. Penile erectile dysfunction of both nitrergic neural and endothelial origins is an independent predictor of coronary insufficiency and the severity of erectile dysfunction appears to reflect the extents of coronary dysfunction and histological damage. Maintenance of healthy endothelial cells through controlled daily life, including quitting smoking, balanced diet, decreasing body weight to a healthy level, and adequate physical exercise, together with prophylactic and therapeutic measures to augment constitutive NOS expression, increase NO availability, degrade oxygen radicals, and inhibit the production of endogenous NOS inhibitors would provide us with an important way to prevent or treat impairments of endothelial and nitrergic neural functions and then CAD.
ACKNOWLEDGMENT
The authors thank Ms. M. Bandou for her assistance in computer graphics of Fig. 1. The authors have no potential conflict of interest relevant to the contents of this article.
References
- Moncada S, Palmer R M, Higgs E A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43(2):109–142. [PubMed] [Google Scholar]
- Toda N, Okamura T. The pharmacology of nitric oxide in the peripheral nervous system of blood vessels. Pharmacol Rev. 2003;55(2):271–324. doi: 10.1124/pr.55.2.3. [DOI] [PubMed] [Google Scholar]
- Toda N, Ayajiki K, Okamura T. Cerebral blood flow regulation by nitric oxide: recent advances. Pharmacol Rev. 2009;61(1):62–97. doi: 10.1124/pr.108.000547. [DOI] [PubMed] [Google Scholar]
- Sitia S, Tomasoni L, Atzeni F, et al. From endothelial dysfunction to atherosclerosis. Autoimmun Rev. 2010;9(12):830–834. doi: 10.1016/j.autrev.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Tang E H, Vanhoutte P M. Endothelial dysfunction: a strategic target in the treatment of hypertension? Pflugers Arch. 2010;459(6):995–1004. doi: 10.1007/s00424-010-0786-4. [DOI] [PubMed] [Google Scholar]
- Malyszko J. Mechanism of endothelial dysfunction in chronic kidney disease. Clin Chim Acta. 2010;411(19-20):1412–1420. doi: 10.1016/j.cca.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Dharmashankar K, Widlansky M E. Vascular endothelial function and hypertension: insights and directions. Curr Hypertens Rep. 2010;12(6):448–455. doi: 10.1007/s11906-010-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda N, Imamura T, Okamura T. Alteration of nitric oxide-mediated blood flow regulation in diabetes mellitus. Pharmacol Ther. 2010;127(3):189–209. doi: 10.1016/j.pharmthera.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Furchgott R F, Zawadzki J V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Bredt D S, Snyder S H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förstermann U, Pollock J S, Schmidt H H, Heller M, Murad F. Calmodulin-dependent endothelium-derived relaxing factor/nitric oxide synthase activity is present in the particulate and cytosolic fractions of bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991;88(5):1788–1792. doi: 10.1073/pnas.88.5.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel J B, Feron O, Sacks D, Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem. 1997;272(25):15583–15586. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- Toda N, Okamura T. Endothelium-dependent and -independent responses to vasoactive substances of isolated human coronary arteries. Am J Physiol. 1989;257(3 Pt 2):H988–H995. doi: 10.1152/ajpheart.1989.257.3.H988. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher A M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399(6736):601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Bernier S G, Haldar S, Michel T. Bradykinin-regulated interactions of the mitogen-activated protein kinase pathway with the endothelial nitric-oxide synthase. J Biol Chem. 2000;275(39):30707–30715. doi: 10.1074/jbc.M005116200. [DOI] [PubMed] [Google Scholar]
- Toda N, Okamura T. Possible role of nitric oxide in transmitting information from vasodilator nerve to cerebroarterial muscle. Biochem Biophys Res Commun. 1990;170(1):308–313. doi: 10.1016/0006-291x(90)91275-w. [DOI] [PubMed] [Google Scholar]
- Vallance P, Leiper J. Cardiovascular biology of the asymmetric dimethylarginine: dimethylarginine dimethylaminohydrolase pathway. Arterioscler Thromb Vasc Biol. 2004;24(6):1023–1030. doi: 10.1161/01.ATV.0000128897.54893.26. [DOI] [PubMed] [Google Scholar]
- Moore P K, Babbedge R C, Wallace P, Gaffen Z A, Hart S L. 7-Nitro indazole, an inhibitor of nitric oxide synthase, exhibits anti-nociceptive activity in the mouse without increasing blood pressure. Br J Pharmacol. 1993;108(2):296–297. doi: 10.1111/j.1476-5381.1993.tb12798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furfine E S, Harmon M F, Paith J E, et al. Potent and selective inhibition of human nitric oxide synthases. Selective inhibition of neuronal nitric oxide synthase by S-methyl-L-thiocitrulline and S-ethyl-L-thiocitrulline. J Biol Chem. 1994;269(43):26677–26683. [PubMed] [Google Scholar]
- Garthwaite J, Southam E, Boulton C L, Nielsen E B, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharmacol. 1995;48(2):184–188. [PubMed] [Google Scholar]
- Chen S T, Maruthur N M, Appel L J. The effect of dietary patterns on estimated coronary heart disease risk: results from the Dietary Approaches to Stop Hypertension (DASH) trial. Circ Cardiovasc Qual Outcomes. 2010;3(5):484–489. doi: 10.1161/CIRCOUTCOMES.109.930685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Solaiman Y, Jesri A, Mountford W K, Lackland D T, Zhao Y, Egan B M. DASH lowers blood pressure in obese hypertensives beyond potassium, magnesium and fibre. J Hum Hypertens. 2010;24(4):237–246. doi: 10.1038/jhh.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal J A, Babyak M A, Hinderliter A, et al. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch Intern Med. 2010;170(2):126–135. doi: 10.1001/archinternmed.2009.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S D, Hryniewicz K, Hriljac I, et al. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation. 2005;111(3):310–314. doi: 10.1161/01.CIR.0000153349.77489.CF. [DOI] [PubMed] [Google Scholar]
- Gibson C M, Cannon C P, Daley W L, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93(5):879–888. doi: 10.1161/01.cir.93.5.879. [DOI] [PubMed] [Google Scholar]
- Sezgin N, Barutcu I, Sezgin A T, et al. Plasma nitric oxide level and its role in slow coronary flow phenomenon. Int Heart J. 2005;46(3):373–382. doi: 10.1536/ihj.46.373. [DOI] [PubMed] [Google Scholar]
- Kawano H, Yoshida T, Miyao Y, et al. The relationship between endothelial function in the brachial artery and intima plus media thickening of the coronary arteries in patients with chest pain syndrome. Atherosclerosis. 2007;195(2):361–366. doi: 10.1016/j.atherosclerosis.2006.09.033. [DOI] [PubMed] [Google Scholar]
- Khalil A, Sareen R, Mallika V, Chowdhury V. Non-invasive evaluation of endothelial function, arterial mechanics and nitric oxide levels in children of hypertensive parents. Indian Heart J. 2008;60(1):34–38. [PubMed] [Google Scholar]
- Higashi Y, Goto C, Hidaka T, et al. Oral infection-inflammatory pathway, periodontitis, is a risk factor for endothelial dysfunction in patients with coronary artery disease. Atherosclerosis. 2009;206(2):604–610. doi: 10.1016/j.atherosclerosis.2009.03.037. [DOI] [PubMed] [Google Scholar]
- Lavi S, Yang E H, Prasad A, et al. The interaction between coronary endothelial dysfunction, local oxidative stress, and endogenous nitric oxide in humans. Hypertension. 2008;51(1):127–133. doi: 10.1161/HYPERTENSIONAHA.107.099986. [DOI] [PubMed] [Google Scholar]
- Akar J G, Jeske W, Wilber D J. Acute onset human atrial fibrillation is associated with local cardiac platelet activation and endothelial dysfunction. J Am Coll Cardiol. 2008;51(18):1790–1793. doi: 10.1016/j.jacc.2007.11.083. [DOI] [PubMed] [Google Scholar]
- Guazzi M, Arena R. Endothelial dysfunction and pathophysiological correlates in atrial fibrillation. Heart. 2009;95(2):102–106. doi: 10.1136/hrt.2007.135277. [DOI] [PubMed] [Google Scholar]
- Tarr F I, Sasvári M, Tarr M, Rácz R. Evidence of nitric oxide produced by the internal mammary artery graft in venous drainage of the recipient coronary artery. Ann Thorac Surg. 2005;80(5):1728–1731. doi: 10.1016/j.athoracsur.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Raichlin E, Kushwaha S S, Lennon R J, et al. Features of cardiac allograft coronary endothelial dysfunction. Am J Cardiol. 2009;103(8):1154–1158. doi: 10.1016/j.amjcard.2008.12.039. [DOI] [PubMed] [Google Scholar]
- McNulty P H, Tulli M A, Robertson B J, et al. Effect of simulated postprandial hyperglycemia on coronary blood flow in cardiac transplant recipients. Am J Physiol Heart Circ Physiol. 2007;293(1):H103–H108. doi: 10.1152/ajpheart.00779.2006. [DOI] [PubMed] [Google Scholar]
- Prior J O, Quiñones M J, Hernandez-Pampaloni M, et al. Coronary circulatory dysfunction in insulin resistance, impaired glucose tolerance, and type 2 diabetes mellitus. Circulation. 2005;111(18):2291–2298. doi: 10.1161/01.CIR.0000164232.62768.51. [DOI] [PubMed] [Google Scholar]
- Chen Z, Li C S, Zhang J, Pang B S, Xia C Q, Liu X F. Relationship between endothelial dysfunction and serum homocysteine in patients with coronary lesions. Chin Med Sci J. 2005;20(1):63–66. [PubMed] [Google Scholar]
- Lekakis J P, Ikonomidis I, Tsibida M, et al. Genetic variations of the endothelial nitric oxide synthase gene are related to increased levels of C-reactive protein and macrophage-colony stimulating-factor in patients with coronary artery disease. Thromb Haemost. 2006;96(4):520–528. [PubMed] [Google Scholar]
- Toda N, Toda H. Nitric oxide-mediated blood flow regulation as affected by smoking and nicotine. Eur J Pharmacol. 2010;649(1-3):1–13. doi: 10.1016/j.ejphar.2010.09.042. [DOI] [PubMed] [Google Scholar]
- Toda N, Ayajiki K. Vascular actions of nitric oxide as affected by exposure to alcohol. Alcohol Alcohol. 2010;45(4):347–355. doi: 10.1093/alcalc/agq028. [DOI] [PubMed] [Google Scholar]
- Toda N, Arakawa K. Salt-induced hemodynamic regulation mediated by nitric oxide. J Hypertens. 2011;29(3):415–424. doi: 10.1097/HJH.0b013e328341d19e. [DOI] [PubMed] [Google Scholar]
- Lentz S R, Rodionov R N, Dayal S. Hyperhomocysteinemia, endothelial dysfunction, and cardiovascular risk: the potential role of ADMA. Atheroscler Suppl. 2003;4(4, Suppl):61–65. doi: 10.1016/s1567-5688(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Busse R, Fleming I. Nitric oxide, nitric oxide synthase, and hypertensive vascular disease. Curr Hypertens Rep. 1999;1(1):88–95. doi: 10.1007/s11906-999-0078-6. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Kimoto M, Sasaoka K. Purification and properties of a new enzyme, NG,NG-dimethylarginine dimethylaminohydrolase, from rat kidney. J Biol Chem. 1989;264(17):10205–10209. [PubMed] [Google Scholar]
- Ogawa T, Kimoto M, Sasaoka K. Dimethylarginine:pyruvate aminotransferase in rats. Purification, properties, and identity with alanine:glyoxylate aminotransferase 2. J Biol Chem. 1990;265(34):20938–20945. [PubMed] [Google Scholar]
- Selcuk M T, Selcuk H, Temizhan A, et al. Asymmetric dimethylarginine plasma concentrations and L-arginine/asymmetric dimethylarginine ratio in patients with slow coronary flow. Coron Artery Dis. 2007;18(7):545–551. doi: 10.1097/MCA.0b013e3282eff1c6. [DOI] [PubMed] [Google Scholar]
- von Haehling S, Bode-Böger S M, Martens-Lobenhoffer J, et al. Elevated levels of asymmetric dimethylarginine in chronic heart failure: a pathophysiologic link between oxygen radical load and impaired vasodilator capacity and the therapeutic effect of allopurinol. Clin Pharmacol Ther. 2010;88(4):506–512. doi: 10.1038/clpt.2010.116. [DOI] [PubMed] [Google Scholar]
- Okyay K, Cengel A, Sahinarslan A, et al. Plasma asymmetric dimethylarginine and L-arginine levels in patients with cardiac syndrome X. Coron Artery Dis. 2007;18(7):539–544. doi: 10.1097/MCA.0b013e3282f08ece. [DOI] [PubMed] [Google Scholar]
- Wang Z, Tang W H, Cho L, Brennan D M, Hazen S L. Targeted metabolomic evaluation of arginine methylation and cardiovascular risks: potential mechanisms beyond nitric oxide synthase inhibition. Arterioscler Thromb Vasc Biol. 2009;29(9):1383–1391. doi: 10.1161/ATVBAHA.109.185645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W H, Wang Z, Cho L, Brennan D M, Hazen S L. Diminished global arginine bioavailability and increased arginine catabolism as metabolic profile of increased cardiovascular risk. J Am Coll Cardiol. 2009;53(22):2061–2067. doi: 10.1016/j.jacc.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavusoglu E, Ruwende C, Chopra V, et al. Relation of baseline plasma ADMA levels to cardiovascular morbidity and mortality at two years in men with diabetes mellitus referred for coronary angiography. Atherosclerosis. 2010;210(1):226–231. doi: 10.1016/j.atherosclerosis.2009.10.034. [DOI] [PubMed] [Google Scholar]
- Turiel M, Atzeni F, Tomasoni L, et al. Non-invasive assessment of coronary flow reserve and ADMA levels: a case-control study of early rheumatoid arthritis patients. Rheumatology (Oxford) 2009;48(7):834–839. doi: 10.1093/rheumatology/kep082. [DOI] [PubMed] [Google Scholar]
- Thum T, Tsikas D, Stein S, et al. Suppression of endothelial progenitor cells in human coronary artery disease by the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine. J Am Coll Cardiol. 2005;46(9):1693–1701. doi: 10.1016/j.jacc.2005.04.066. [DOI] [PubMed] [Google Scholar]
- Surdacki A, Marewicz E, Wieczorek-Surdacka E, et al. Synergistic effects of asymmetrical dimethyl-L-arginine accumulation and endothelial progenitor cell deficiency on renal function decline during a 2-year follow-up in stable angina. Nephrol Dial Transplant. 2010;25(8):2576–2583. doi: 10.1093/ndt/gfp439. [DOI] [PubMed] [Google Scholar]
- Kocaman S A. Asymmetric dimethylarginine, NO and collateral growth. Anadolu Kardiyol Derg. 2009;9(5):417–420. [PubMed] [Google Scholar]
- Lamblin N, Cuilleret F J, Helbecque N, et al. A common variant of endothelial nitric oxide synthase (Glu298Asp) is associated with collateral development in patients with chronic coronary occlusions. BMC Cardiovasc Disord. 2005;5:27. doi: 10.1186/1471-2261-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajtay Z, Scalera F, Cziráki A, et al. Stent placement in patients with coronary heart disease decreases plasma levels of the endogenous nitric oxide synthase inhibitor ADMA. Int J Mol Med. 2009;23(5):651–657. doi: 10.3892/ijmm_00000176. [DOI] [PubMed] [Google Scholar]
- Cagirci G, Cay S, Karakurt O, et al. Association between plasma asymmetrical dimethylarginine activity and saphenous vein graft disease in patients with coronary bypass. Coron Artery Dis. 2010;21(1):20–25. doi: 10.1097/MCA.0b013e328332a6da. [DOI] [PubMed] [Google Scholar]
- Toda N, Okamura T. Modulation of renal blood flow and vascular tone by neuronal nitric oxide synthase-derived nitric oxide. J Vasc Res. 2011;48(1):1–10. doi: 10.1159/000317395. [DOI] [PubMed] [Google Scholar]
- Toda N, Ayajiki K, Okamura T. Control of systemic and pulmonary blood pressure by nitric oxide formed through neuronal nitric oxide synthase. J Hypertens. 2009;27(10):1929–1940. doi: 10.1097/HJH.0b013e32832e8ddf. [DOI] [PubMed] [Google Scholar]
- Klimaschewski L, Kummer W, Mayer B, et al. Nitric oxide synthase in cardiac nerve fibers and neurons of rat and guinea pig heart. Circ Res. 1992;71(6):1533–1537. doi: 10.1161/01.res.71.6.1533. [DOI] [PubMed] [Google Scholar]
- Sosunov A A, Hassall C J, Loesch A, Turmaine M, Burnstock G. Ultrastructural investigation of nitric oxide synthase-immunoreactive nerves associated with coronary blood vessels of rat and guinea-pig. Cell Tissue Res. 1995;280(3):575–582. doi: 10.1007/BF00318361. [DOI] [PubMed] [Google Scholar]
- Sequeira I M, Haberberger R V, Kummer W. Atrial and ventricular rat coronary arteries are differently supplied by noradrenergic, cholinergic and nitrergic, but not sensory nerve fibres. Ann Anat. 2005;187(4):345–355. doi: 10.1016/j.aanat.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Okamura T, Kimura H, Bredt D S, Snyder S H, Toda N. Nitric oxide synthase-immunoreactive nerve fibers in dog cerebral and peripheral arteries. Brain Res. 1993;629(1):67–72. doi: 10.1016/0006-8993(93)90482-3. [DOI] [PubMed] [Google Scholar]
- Seddon M, Melikian N, Dworakowski R, et al. Effects of neuronal nitric oxide synthase on human coronary artery diameter and blood flow in vivo. Circulation. 2009;119(20):2656–2662. doi: 10.1161/CIRCULATIONAHA.108.822205. [DOI] [PubMed] [Google Scholar]
- Adams J A, Wu D, Bassuk J, et al. Nitric oxide synthase isoform inhibition before whole body ischemia reperfusion in pigs: vital or protective? Resuscitation. 2007;74(3):516–525. doi: 10.1016/j.resuscitation.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Nakata S, Tsutsui M, Shimokawa H, et al. Spontaneous myocardial infarction in mice lacking all nitric oxide synthase isoforms. Circulation. 2008;117(17):2211–2223. doi: 10.1161/CIRCULATIONAHA.107.742692. [DOI] [PubMed] [Google Scholar]
- Han G, Ma H, Chintala R, Miyake K, Fulton D J, Barman S A, White R E. Nongenomic, endothelium-independent effects of estrogen on human coronary smooth muscle are mediated by type I (neuronal) NOS and PI3-kinase-Akt signaling. Am J Physiol Heart Circ Physiol. 2007;293:H314–H321. doi: 10.1152/ajpheart.01342.2006. [DOI] [PubMed] [Google Scholar]
- Dover A R, Chia S, Ferguson J W, et al. Inducible nitric oxide synthase activity does not contribute to the maintenance of peripheral vascular tone in patients with heart failure. Clin Sci (Lond) 2006;111(4):275–280. doi: 10.1042/CS20060104. [DOI] [PubMed] [Google Scholar]
- Ishibashi Y, Takahashi N, Tokumaru A, et al. Activation of inducible NOS in peripheral vessels and outcomes in heart failure patients. J Card Fail. 2008;14(9):724–731. doi: 10.1016/j.cardfail.2008.06.450. [DOI] [PubMed] [Google Scholar]
- Mitani Y, Okuda Y, Shimpo H, et al. Impaired endothelial function in epicardial coronary arteries after Kawasaki disease. Circulation. 1997;96(2):454–461. [PubMed] [Google Scholar]
- Yamakawa R, Ishii M, Sugimura T, et al. Coronary endothelial dysfunction after Kawasaki disease: evaluation by intracoronary injection of acetylcholine. J Am Coll Cardiol. 1998;31(5):1074–1080. doi: 10.1016/s0735-1097(98)00033-3. [DOI] [PubMed] [Google Scholar]
- Kurio G H, Zhiroff K A, Jih L J, Fronek A S, Burns J C. Noninvasive determination of endothelial cell function in the microcirculation in Kawasaki syndrome. Pediatr Cardiol. 2008;29(1):121–125. doi: 10.1007/s00246-007-9077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M G, Men L N, Zhao C Y, et al. The number and function of circulating endothelial progenitor cells in patients with Kawasaki disease. Eur J Pediatr. 2010;169(3):289–296. doi: 10.1007/s00431-009-1014-0. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Tatsumi K, Iharada A, et al. Increased nitric oxide production by neutrophils in early stage of Kawasaki disease. Eur J Pediatr. 2009;168(9):1037–1041. doi: 10.1007/s00431-008-0872-1. [DOI] [PubMed] [Google Scholar]
- Andersson K E. Pharmacology of penile erection. Pharmacol Rev. 2001;53(3):417–450. [PubMed] [Google Scholar]
- Toda N, Ayajiki K, Okamura T. Nitric oxide and penile erectile function. Pharmacol Ther. 2005;106(2):233–266. doi: 10.1016/j.pharmthera.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Greenstein A, Chen J, Miller H, Matzkin H, Villa Y, Braf Z. Does severity of ischemic coronary disease correlate with erectile function? Int J Impot Res. 1997;9(3):123–126. doi: 10.1038/sj.ijir.3900282. [DOI] [PubMed] [Google Scholar]
- Foroutan S K, Rajabi M. Erectile dysfunction in men with angiographically documented coronary artery disease. Urol J. 2007;4(1):28–32. [PubMed] [Google Scholar]
- Lee J H, Ngengwe R, Jones P, Tang F, O'Keefe J H. Erectile dysfunction as a coronary artery disease risk equivalent. J Nucl Cardiol. 2008;15(6):800–803. doi: 10.1007/BF03007361. [DOI] [PubMed] [Google Scholar]
- Yaman O, Gulpinar O, Hasan T, Ozdol C, Ertas F S, Ozgenci E. Erectile dysfunction may predict coronary artery disease: relationship between coronary artery calcium scoring and erectile dysfunction severity. Int Urol Nephrol. 2008;40(1):117–123. doi: 10.1007/s11255-007-9293-8. [DOI] [PubMed] [Google Scholar]
- Meluzín J, Vasků A, Kincl V, Panovský R, Srámková T. Association of coronary artery disease, erectile dysfunction, and endothelial nitric oxide synthase polymorphisms. Heart Vessels. 2009;24(3):157–163. doi: 10.1007/s00380-008-1097-y. [DOI] [PubMed] [Google Scholar]
- Chew K K, Finn J, Stuckey B, et al. Erectile dysfunction as a predictor for subsequent atherosclerotic cardiovascular events: findings from a linked-data study. J Sex Med. 2010;7(1 Pt 1):192–202. doi: 10.1111/j.1743-6109.2009.01576.x. [DOI] [PubMed] [Google Scholar]
- Böhm M, Baumhäkel M, Teo K, et al. ONTARGET/TRANSCEND Erectile Dysfunction Substudy Investigators Erectile dysfunction predicts cardiovascular events in high-risk patients receiving telmisartan, ramipril, or both: The ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial/Telmisartan Randomized AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (ONTARGET/TRANSCEND) Trials. Circulation. 2010;121(12):1439–1446. doi: 10.1161/CIRCULATIONAHA.109.864199. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C, Ioakeimidis N, Terentes-Printzios D, Stefanadis C. The triad: erectile dysfunction—endothelial dysfunction—cardiovascular disease. Curr Pharm Des. 2008;14(35):3700–3714. doi: 10.2174/138161208786898716. [DOI] [PubMed] [Google Scholar]
- Kapur V, Chien C V, Fuess J E, Schwarz E R. The relationship between erectile dysfunction and cardiovascular disease. Part II: The role of PDE-5 inhibition in sexual dysfunction and cardiovascular disease. Rev Cardiovasc Med. 2008;9(3):187–195. [PubMed] [Google Scholar]
- Jackson G, Boon N, Eardley I, et al. Erectile dysfunction and coronary artery disease prediction: evidence-based guidance and consensus. Int J Clin Pract. 2010;64(7):848–857. doi: 10.1111/j.1742-1241.2010.02410.x. [DOI] [PubMed] [Google Scholar]
- Lauer T, Kleinbongard P, Rath J, Schulz R, Kelm M, Rassaf T. L-arginine preferentially dilates stenotic segments of coronary arteries thereby increasing coronary flow. J Intern Med. 2008;264(3):237–244. doi: 10.1111/j.1365-2796.2008.01943.x. [DOI] [PubMed] [Google Scholar]
- Settergren M, Böhm F, Malmström R E, Channon K M, Pernow J. L-arginine and tetrahydrobiopterin protects against ischemia/reperfusion-induced endothelial dysfunction in patients with type 2 diabetes mellitus and coronary artery disease. Atherosclerosis. 2009;204(1):73–78. doi: 10.1016/j.atherosclerosis.2008.08.034. [DOI] [PubMed] [Google Scholar]
- Worthley M I, Kanani R S, Sun Y H, et al. Effects of tetrahydrobiopterin on coronary vascular reactivity in atherosclerotic human coronary arteries. Cardiovasc Res. 2007;76(3):539–546. doi: 10.1016/j.cardiores.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Tawakol A, Aziz K, Migrino R, et al. Effects of sildenafil on myocardial blood flow in humans with ischemic heart disease. Coron Artery Dis. 2005;16(7):443–449. doi: 10.1097/00019501-200510000-00005. [DOI] [PubMed] [Google Scholar]
- Baldus S, Köster R, Chumley P, et al. Oxypurinol improves coronary and peripheral endothelial function in patients with coronary artery disease. Free Radic Biol Med. 2005;39(9):1184–1190. doi: 10.1016/j.freeradbiomed.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noman A, Ang D S, Ogston S, Lang C C, Struthers A D. Effect of high-dose allopurinol on exercise in patients with chronic stable angina: a randomised, placebo controlled crossover trial. Lancet. 2010;375(9732):2161–2167. doi: 10.1016/S0140-6736(10)60391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moat S J, Madhavan A, Taylor S Y, et al. High- but not low-dose folic acid improves endothelial function in coronary artery disease. Eur J Clin Invest. 2006;36(12):850–859. doi: 10.1111/j.1365-2362.2006.01739.x. [DOI] [PubMed] [Google Scholar]
- Shirodaria C, Antoniades C, Lee J, et al. Global improvement of vascular function and redox state with low-dose folic acid: implications for folate therapy in patients with coronary artery disease. Circulation. 2007;115(17):2262–2270. doi: 10.1161/CIRCULATIONAHA.106.679084. [DOI] [PubMed] [Google Scholar]
- Dragoni S, Gori T, Di Stolfo G, Sicuro S, Forconi S, Parker J D. Folic Acid does not limit endothelial dysfunction induced by ischemia and reperfusion: a human study. J Cardiovasc Pharmacol. 2005;46(4):494–497. doi: 10.1097/01.fjc.0000177983.68563.d1. [DOI] [PubMed] [Google Scholar]
- Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109(23, Suppl 1):III39–III43. doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- Almuti K, Rimawi R, Spevack D, Ostfeld R J. Effects of statins beyond lipid lowering: potential for clinical benefits. Int J Cardiol. 2006;109(1):7–15. doi: 10.1016/j.ijcard.2005.05.056. [DOI] [PubMed] [Google Scholar]
- Strey C H, Young J M, Lainchbury J H, et al. Short-term statin treatment improves endothelial function and neurohormonal imbalance in normocholesterolaemic patients with non-ischaemic heart failure. Heart. 2006;92(11):1603–1609. doi: 10.1136/hrt.2005.082560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J M, Strey C H, George P M, et al. Effect of atorvastatin on plasma levels of asymmetric dimethylarginine in patients with non-ischaemic heart failure. Eur J Heart Fail. 2008;10(5):463–466. doi: 10.1016/j.ejheart.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Paul J D, Powell T M, Thompson M, et al. Endothelial progenitor cell mobilization and increased intravascular nitric oxide in patients undergoing cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2007;27(2):65–73. doi: 10.1097/01.HCR.0000265031.10145.50. [DOI] [PubMed] [Google Scholar]
- Chen H, Ren J Y, Xing Y, et al. Short-term withdrawal of simvastatin induces endothelial dysfunction in patients with coronary artery disease: a dose-response effect dependent on endothelial nitric oxide synthase. Int J Cardiol. 2009;131(3):313–320. doi: 10.1016/j.ijcard.2007.10.044. [DOI] [PubMed] [Google Scholar]
- Kawata T, Daimon M, Hasegawa R, et al. Effect of angiotensin-converting enzyme inhibitor on serum asymmetric dimethylarginine and coronary circulation in patients with type 2 diabetes mellitus. Int J Cardiol. 2009;132(2):286–288. doi: 10.1016/j.ijcard.2007.08.066. [DOI] [PubMed] [Google Scholar]
- Koifman B, Topilski I, Megidish R, et al. Effects of losartan + L-arginine on nitric oxide production, endothelial cell function, and hemodynamic variables in patients with heart failure secondary to coronary heart disease. Am J Cardiol. 2006;98(2):172–177. doi: 10.1016/j.amjcard.2006.01.085. [DOI] [PubMed] [Google Scholar]
- Koh K K, Quon M J, Han S H, Chung W J, Kim J A, Shin E K. Vascular and metabolic effects of candesartan: insights from therapeutic interventions. J Hypertens Suppl. 2006;24(1):S31–S38. doi: 10.1097/01.hjh.0000220404.38622.6a. [DOI] [PubMed] [Google Scholar]
- Toda N. Vasodilating β-adrenoceptor blockers as cardiovascular therapeutics. Pharmacol Ther. 2003;100(3):215–234. doi: 10.1016/j.pharmthera.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Cleophas T J, 't Leven M van, Kauw F H, Remmert H P, Kuijper A, Zwinderman K. Celiprolol vs propranolol in unstable angina pectoris: a double-blind, randomized, parallel-group study. Angiology. 1995;46(2):137–144. doi: 10.1177/000331979504600207. [DOI] [PubMed] [Google Scholar]
- Dunn C J, Spencer C M. Celiprolol. An evaluation of its pharmacological properties and clinical efficacy in the management of hypertension and angina pectoris. Drugs Aging. 1995;7(5):394–411. doi: 10.2165/00002512-199507050-00006. [DOI] [PubMed] [Google Scholar]
- Togni M, Vigorito F, Windecker S, et al. Does the beta-blocker nebivolol increase coronary flow reserve? Cardiovasc Drugs Ther. 2007;21(2):99–108. doi: 10.1007/s10557-006-0494-7. [DOI] [PubMed] [Google Scholar]
- Galderisi M, D'Errico A. Beta-blockers and coronary flow reserve: the importance of a vasodilatory action. Drugs. 2008;68(5):579–590. doi: 10.2165/00003495-200868050-00002. [DOI] [PubMed] [Google Scholar]
- Akçay A, Acar G, Kurutaş E, et al. Beneficial effects of nebivolol treatment on oxidative stress parameters in patients with slow coronary flow. Turk Kardiyol Dern Ars. 2010;38(4):244–249. [PubMed] [Google Scholar]
- Williams M J, Sutherland W H, McCormick M P, Yeoman D J, de Jong S A. Aged garlic extract improves endothelial function in men with coronary artery disease. Phytother Res. 2005;19(4):314–319. doi: 10.1002/ptr.1663. [DOI] [PubMed] [Google Scholar]
- Wu Y, Li S, Cui W, Zu X, Wang F, Du J. Ginkgo biloba extract improves coronary blood flow in patients with coronary artery disease: role of endothelium-dependent vasodilation. Planta Med. 2007;73(7):624–628. doi: 10.1055/s-2007-981536. [DOI] [PubMed] [Google Scholar]
- Wu Y Z, Li S Q, Zu X G, Du J, Wang F F. Ginkgo biloba extract improves coronary artery circulation in patients with coronary artery disease: contribution of plasma nitric oxide and endothelin-1. Phytother Res. 2008;22(6):734–739. doi: 10.1002/ptr.2335. [DOI] [PubMed] [Google Scholar]
- Parnell M M, Holst D P, Kaye D M. Augmentation of endothelial function following exercise training is associated with increased L-arginine transport in human heart failure. Clin Sci (Lond) 2005;109(6):523–530. doi: 10.1042/CS20050171. [DOI] [PubMed] [Google Scholar]
- Duncker D J, Bache R J. Regulation of coronary blood flow during exercise. Physiol Rev. 2008;88(3):1009–1086. doi: 10.1152/physrev.00045.2006. [DOI] [PubMed] [Google Scholar]
- Michaels A D, Accad M, Ports T A, Grossman W. Left ventricular systolic unloading and augmentation of intracoronary pressure and Doppler flow during enhanced external counterpulsation. Circulation. 2002;106(10):1237–1242. doi: 10.1161/01.cir.0000028336.95629.b0. [DOI] [PubMed] [Google Scholar]
- Akhtar M, Wu G F, Du Z M, Zheng Z S, Michaels A D. Effect of external counterpulsation on plasma nitric oxide and endothelin-1 levels. Am J Cardiol. 2006;98(1):28–30. doi: 10.1016/j.amjcard.2006.01.053. [DOI] [PubMed] [Google Scholar]
- Braith R W, Conti C R, Nichols W W, et al. Enhanced external counterpulsation improves peripheral artery flow-mediated dilation in patients with chronic angina: a randomized sham-controlled study. Circulation. 2010;122(16):1612–1620. doi: 10.1161/CIRCULATIONAHA.109.923482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao D K, Jia S Q, Wang L, et al. Therapeutic effect of urapidil on myocardial perfusion in patients with ST-elevation acute coronary syndrome. Eur J Intern Med. 2009;20(2):152–157. doi: 10.1016/j.ejim.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Miwa Y, Masai H, Shimizu M. Differential effects of calcium-channel blockers on vascular endothelial function in patients with coronary spastic angina. Circ J. 2009;73(4):713–717. doi: 10.1253/circj.cj-08-0188. [DOI] [PubMed] [Google Scholar]
- Topal E, Ozdemir R, Barutcu I, et al. The effects of trimetazidine on heart rate variability in patients with slow coronary artery flow. J Electrocardiol. 2006;39(2):211–218. doi: 10.1016/j.jelectrocard.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Nohria A, Grunert M E, Rikitake Y, et al. Rho kinase inhibition improves endothelial function in human subjects with coronary artery disease. Circ Res. 2006;99(12):1426–1432. doi: 10.1161/01.RES.0000251668.39526.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzer T, Rudolph V, Schwedhelm E, et al. Clopidogrel improves systemic endothelial nitric oxide bioavailability in patients with coronary artery disease: evidence for antioxidant and antiinflammatory effects. Arterioscler Thromb Vasc Biol. 2006;26(7):1648–1652. doi: 10.1161/01.ATV.0000225288.74170.dc. [DOI] [PubMed] [Google Scholar]
- Baldus S, Rudolph V, Roiss M, et al. Heparins increase endothelial nitric oxide bioavailability by liberating vessel-immobilized myeloperoxidase. Circulation. 2006;113(15):1871–1878. doi: 10.1161/CIRCULATIONAHA.105.590083. [DOI] [PubMed] [Google Scholar]
- Budas G R, Disatnik M H, Mochly-Rosen D. Aldehyde dehydrogenase 2 in cardiac protection: a new therapeutic target? Trends Cardiovasc Med. 2009;19(5):158–164. doi: 10.1016/j.tcm.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]