Abstract
The activation of purinergic receptors modulates central pattern generators controlling rhythmic motor behaviors, including respiration in rodents and swimming in frog tadpoles. The present study aimed to determine whether purinergic signaling also modulates the mammalian locomotor central pattern generator. This was investigated by using isolated spinal cord preparations obtained from neonatal mice in which locomotor-related activity can be induced pharmacologically. The application of either ATP or adenosine led to a reduction in the frequency of locomotor activity recorded from ventral roots. ATP had no effect when applied in the presence of both the adenosine receptor antagonist theophylline and the ectonucleotidase inhibitor ARL67156, demonstrating that the effects of ATP application result from the breakdown of ATP to adenosine and subsequent activation of adenosine receptors. The application of theophylline or the A1-specific antagonist cyclopentyl dipropylxanthine, but not the A2A-receptor antagonist SCH58261, caused an increase in locomotor burst frequency, demonstrating that endogenously derived adenosine activates A1 receptors during locomotor network activity. Furthermore, theophylline had no effect in the presence of the ectonucleotidase inhibitor ARL67156 or the glial toxins methionine sulfoximine or ethyl fluoracetate, suggesting that endogenous adenosine is derived from ATP, which is released from glia. Finally, adenosine had no effect on slow rhythmic activity recorded upon blockade of all inhibitory transmission, suggesting that adenosine may act via the modulation of inhibitory transmission. Together, these data highlight endogenous purinergic gliotransmission, involving activation of A1 receptors, as an important intrinsic modulatory system controlling the frequency of activity generated by spinal locomotor circuitry in mammals.
Keywords: spinal cord, motor control, neuromodulation
the modulation of neuronal networks is critical for the production of complex behaviors that are flexible and adaptable. Within motor control systems, neuromodulation allows central pattern generators (CPGs) controling rhythmic motor behaviors, such as locomotion, to respond to the varying biomechanical demands of different states, developmental stages, or environments (Grillner 2006). Neuromodulatory inputs can originate from both “extrinsic” supraspinal systems (reviewed by Heckman et al. 2009; Rekling et al. 2000) and less studied “intrinsic” intraspinal systems (Dale and Gilday 1996; El Manira et al. 2008; Katz and Frost 1996; Zagoraiou et al. 2009).
Purines, particularly ATP and adenosine, represent one group of intrinsic modulators that may be important in motor control (Dale and Gilday 1996; Huxtable et al. 2009). Although primarily considered an energy source, ATP can be released by neurons and glia to act as a signaling molecule via binding to P2 receptors, which exist as two main subtypes, ionotropic P2X receptors and G protein-coupled metabotropic P2Y receptors (Burnstock 2007). Adenosine, which is mainly derived from the ectonucleotidase-mediated hydrolysis of extracellular ATP, also acts as a signaling molecule via its binding to P1 receptors, of which there are four subtypes: A1, A2A, A2B, and A3 (Burnstock 2007; Cunha 2001). The downstream effects of P2- and P1-receptor activation are diverse and typically oppose each other. P2-receptor activation most often leads to neuronal excitation, while P1-receptor activation normally inhibits synaptic transmission and neuronal activity (Burnstock 2007).
Data concerning a role for purines in the control of locomotion are limited to studies of Xenopus tadpoles, where ATP facilitates swimming by increasing the excitability of neurons within the locomotor CPG, most likely via the activation of P2Y receptors (Brown and Dale 2002; Dale and Gilday 1996). In contrast, adenosine, most likely derived from the ectonucleotidase-mediated breakdown of ATP, reduces CPG excitability via the activation of A1 receptors (Brown and Dale 2000; Dale and Gilday 1996). Extracellular ATP levels, which are linked to activity, are highest at the beginning of locomotor episodes, while adenosine production is delayed by feed-forward inhibition of ectonucleotidases. Because of their opposing actions, a gradually changing balance between ATP and adenosine is thought to contribute to the run-down and eventual cessation of swimming in Xenopus tadpoles (Dale 1998; Dale and Gilday 1996).
Although the role of purines in the control of mammalian locomotion remains to be addressed, purines have been shown to modulate mammalian respiration, another CPG-driven rhythmic motor behavior. In medullary slice preparations obtained from neonatal mice, ATP acts via P2Y receptors to increase the frequency of inspiratory-related activity recorded from motor nerve roots (Lorier et al. 2007). As in the tadpole locomotor system, adenosine-mediated inhibition appears to follow ATP-mediated excitation of respiratory activity (Lorier et al. 2007). In addition, adenosine is reported to have a tonic depressive effect on the frequency of mammalian respiration, which appears strongest at fetal stages (Herlenius and Lagercrantz 1999; Huxtable et al. 2009; Kawai et al. 1995; Mironov et al. 1999; Schmidt et al. 1995). Adding to growing evidence of an important role for purinergic gliotransmission in the modulation of neuronal networks (Halassa et al. 2009), glia cells appear to contribute to the ATP sensitivity of the respiratory CPG (Huxtable et al. 2010), as well as central respiratory chemosensitivity (Gourine et al. 2010). In addition to modulating the frequency of respiratory activity, purines also modulate the intensity of respiratory-related motor output. ATP excites both phrenic and hypoglossal motoneurons (Funk et al. 1997; Miles et al. 2002), most likely via activation of P2X receptors, to increase their inspiratory-related output. This effect is again followed by inhibition thought to reflect the actions of adenosine derived from the breakdown of ATP.
Given the importance of purines in the control of tadpole locomotion and the demonstration that purines can modulate mammalian CPG networks, we aimed to determine whether purines also contribute to the control of mammalian locomotion via modulation of spinal locomotor networks. Using rhythmically active isolated mouse spinal cord preparations, we demonstrate that endogenous purines modulate the locomotor CPG. Furthermore, we provide evidence that this modulation involves gliotransmission.
METHODS
In vitro whole spinal cord preparation.
All methods required to obtain tissue for in vitro experiments were conducted in accordance with the UK Animals (Scientific Procedures) Act 1986 and were reviewed and approved by the University of St. Andrews Animal Welfare and Ethics Committee. Spinal cord preparations were obtained from postnatal day 0–5 C57BL/6 mice using techniques similar to those described previously (Jiang et al. 1999). Briefly, animals were killed via cervical dislocation, decapitated, and eviscerated before spinal cords were isolated from the midcervical to upper sacral segments in a chamber containing artificial cerebrospinal fluid (aCSF; equilibrated with 95% oxygen, 5% carbon dioxide, ∼4°C). Dorsal and ventral roots were trimmed, and the preparation was pinned ventral side up to Slygard resin in a recording chamber superfused with oxygenated aCSF, with a flow rate of 8–10 ml/min.
Ventral root recordings.
Glass suction electrodes were attached to the first or second lumbar (L1/L2) ventral roots on both the left and right sides of isolated spinal cord preparations. N-methyl-d-aspartic acid (NMDA; 5 μM), 5-hydroxytryptamine (5-HT; 10 μM), and dopamine (DA; 50 μM) were added to the aCSF to induce rhythmic, left-right alternating bursts of locomotor-related ventral root activity (Jiang et al. 1999; Miles et al. 2007).Tonic ventral root activity was first recorded several minutes after NMDA, 5-HT, and DA were applied. This was followed by irregular bursts of ventral root activity (variable burst amplitude, duration, and frequency). This irregular activity was gradually replaced by regular, left-right alternating, ventral root bursts. Locomotor-related activity was considered stable when the amplitude, duration, and frequency of bursts showed no observable changes over several minutes of recordings (also confirmed offline via time course plots of control data). Subsequent drug applications were performed after locomotor-related activity had been allowed to stabilize (∼1 h). All signals were amplified and filtered (30–3,000 Hz) (Qjin Design, ON, Canada) before being acquired at 1 kHz using a Digidata 1440A A/D board and AxoScope software (version 7.0, Molecular Devices, Sunnyvale, CA). A subset of signals was also rectified and integrated (Qjin Design) before acquisition, such that both raw and rectified/integrated traces were recorded for each ventral root.
Data analysis.
Data were analyzed offline using DataView software (courtesy of Dr. W. J. Heitler, University of St. Andrews). Integrated traces were used to detect locomotor bursts. The frequency and peak-to-peak amplitude of these bursts was then measured from raw traces. Unless otherwise stated in the results section, all sample sizes (n) refer to the number of preparations in which drugs were applied. For time course plots, mean values are presented normalized to control levels ± SE to facilitate comparison between preparations. Time course plots were constructed from data averaged in 1-min time bins. Statistical comparisons between different experimental conditions were performed on raw data averaged over 10-min periods, and these data are presented in bar graph form. Differences in means were assessed from raw data using paired, two-tailed Student's t-tests (Microsoft Excel) or analysis of variance with Tukey post hoc tests (SPSS). Values of P < 0.05 were considered significant. The phase relationship between activity recorded from the left and right sides of the spinal cord was assessed using circular plots and Rayleigh's test for uniformity (Kjaerulff and Kiehn 1996; Zagoraiou et al. 2009) (statistiXL software, Nedlands, WA, Australia). In circular plots, the point labeled 0 represents the beginning of the locomotor cycle (defined as the onset of left ventral root activity). If left and right ventral root activity is out of phase and therefore appropriate alternation is present, left ventral root activity should terminate and right ventral root activity should begin near point 0.5 on the circle. The individual data points plotted around the circle represent the onset of individual locomotor bursts recorded from right ventral roots. Arrows point to the mean burst onset time, and their length represents the concentration of the data points around the mean.
Solution and drugs.
The aCSF used for dissecting and recording contained the following (in mM): 127 NaCl, 3 KCl, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, 26 NaHCO3, 10 glucose. The adenosine A2A-receptor antagonist SCH58261 and the ectonucleotidase inhibitor ARL67156 were obtained from Tocris; all other drugs were obtained from Sigma-Aldrich, UK: NMDA, 5-HT, DA, adenosine, adenosine triphosphate disodium salt (ATP), theophylline (nonselective adenosine receptor antagonist), bicuculline (GABAA-receptor antagonist), strychnine (glycine receptor antagonist), picrotoxin (blocker of the GABAA-receptor chloride channel), glutamine, cyclopentyl dipropylxanthine (DPCPX; A1-receptor antagonist), fluoroacetate (FA; glial aconitase inhibitor), and methionine sulfoximine (MSO; glial-specific glutamine synthetase inhibitor). All drugs were made up fresh using aCSF, apart from bicuculline and strychnine, which were stored as frozen aliquots before their use.
RESULTS
Modulation of spinal locomotor networks by exogenously applied purines.
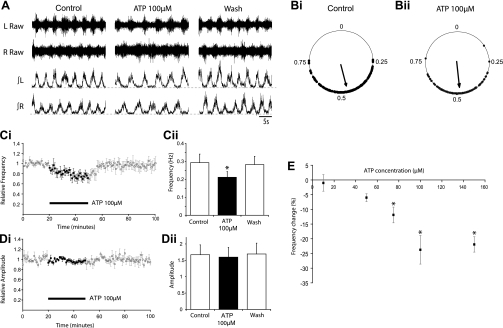
To determine whether activation of P2 (ATP) receptors modulates the activity of spinal motor networks controlling mammalian locomotion, we bath applied ATP at a variety of doses (10 μM, n = 3 preparations; 50 μM, n = 8; 75 μM, n = 5; 100 μM, n = 6; 150 μM, n = 4; duration 30 min) to isolated mouse spinal cord preparations while recording pharmacologically induced (5 μM NMDA, 10 μM 5-HT, 50 μM DA) rhythmic, left-right alternating, locomotor-related activity from lumbar ventral roots. At doses of 75–150 μM, application of ATP caused a significant decrease in the frequency of bursts of locomotor-related ventral root activity, while lower doses had no significant effect (Fig. 1, A, C, and E). The maximum effect of ATP on burst frequency was reached at a concentration of 100 μM (24 ± 4.9%, n = 6; Fig. 1, C and E). Locomotor burst frequency decreased gradually throughout the application of 75–150 μM ATP, reaching a minimum toward the end of the drug application, before returning to control levels after drug washout (Fig. 1C). At all doses utilized, ATP application had no significant effect on the amplitude of locomotor bursts (Fig. 1, A and D). Circular plots were used to assess the degree of alternation between activity recorded from segmentally aligned left and right ventral roots in control conditions and in the presence of ATP. Rayleigh's test for uniformity (Kjaerulff and Kiehn 1996; Zagoraiou et al. 2009) was used to statistically analyze the phase relationships of at least 100 bursts during both control and drug conditions within individual spinal cord preparations. These analyses revealed that left-right coordination of locomotor activity remained in the presence of ATP (n = 3 preparations; Fig. 1B). Together these results indicate that exogenously applied ATP affects components of the CPG for locomotion to alter locomotor frequency, but not the intensity of locomotor-related motoneuron output or the left-right coordination of motor activity.
Fig. 1.
ATP reduces locomotor burst frequency, but does not affect burst amplitude or left-right alternation. A: sample raw (top) and rectified/integrated (bottom) L2 ventral root recordings during control, 100 μM ATP application, and washout. Traces represent 30 s of each condition. B: circular plots depicting the phasing of the onset of locomotor bursts recorded from the right L2 root in relation to the onset of activity recorded from the left L2 root in control conditions (Bi) and during 100 μM ATP application (Bii). In both cases, the points are clustered around 0.5, suggesting that bursting alternates between the left and right sides with little change upon the application of ATP. Ci: pooled time course plot showing changes in the normalized frequency of locomotor bursts due to ATP application (n = 8). Cii: graph showing average burst frequency during the final 10-min period of each condition (control, 100 μM ATP application, and washout; n = 8). A significant decrease in frequency (P < 0.01) was observed in the presence of ATP. D: time course plot (Di) and bar chart (Dii) showing no change in the amplitude of locomotor bursts following the application of ATP (n = 8). E: dose-response relationship for ATP showing the dose-dependency of the reduction in locomotor-related burst frequency. *Significantly different from control.
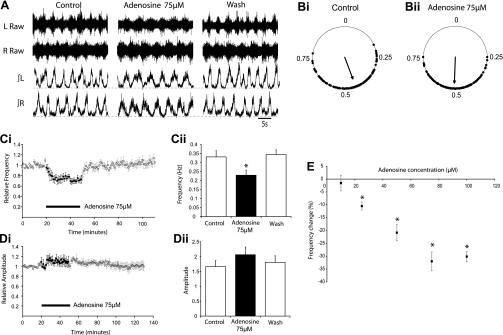
Interestingly, a reduction in locomotor burst frequency contrasts the excitatory effects of ATP on the respiratory rhythm in mice (Lorier et al. 2007) and locomotor rhythm in Xenopus tadpoles (Dale and Gilday 1996). However, in both of these systems, adenosine derived from the breakdown of ATP has inhibitory effects. We, therefore, hypothesized that the reduction in locomotor frequency and lack of any transient excitatory effects observed following the application of ATP might involve breakdown to adenosine and subsequent activation of adenosine receptors. We began investigating this by assessing the effects of exogenously applied adenosine, at a range of doses (10 μM, n = 3; 25 μM, n = 4; 50 μM, n = 5; 75 μM, n = 12; 100 μM, n = 3; 150 μM, n = 5; duration 30 min), on locomotor activity recorded from isolated spinal cord preparations. Similar to ATP, application of adenosine (25–100 μM) caused a significant reduction in locomotor burst frequency, while lower doses had no significant effect (Fig. 2, A, C, and E). The maximum effect of adenosine on burst frequency was reached at a concentration of 75 μM (32 ± 3.7%; n = 12; Fig. 2, C and E). At all doses utilized, adenosine had no significant effect on burst amplitude (Fig. 2, A and D). Left-right alternation, assessed via the analysis of at least 100 locomotor bursts under each experimental condition, within three different preparations, also remained intact in the presence of adenosine (75 μM; Fig. 2B). The effects of adenosine on locomotor bursts followed a faster time course than those mediated by ATP. The effect of adenosine (75 μM) plateaued ∼7 min after drug application, while the maximum effect of ATP (100 μM) occurred ∼18 min after the drug was applied (Fig. 2C vs. Fig. 1C).
Fig. 2.
Adenosine reduces locomotor burst frequency, but does not affect burst amplitude or left-right alternation. A: sample raw (top) and rectified/integrated (bottom) L2 ventral root recordings during control, 75 μM adenosine application, and washout. Traces represent 30 s of each condition. B: circular plots demonstrating that left-right alternation is unaffected by adenosine application. Ci: pooled time course plot showing changes in the normalized frequency of locomotor bursts following the application of adenosine (n = 12). Cii: graph of the average burst frequency during 10-min periods of control, 75 μM adenosine application, and washout (n = 12). Adenosine caused a significant reduction in frequency (P < 0.01). D: time course plot (Di) and bar chart (Dii) showing no significant change in burst amplitude following the application of adenosine. E: dose-response relationship for adenosine showing the dose-dependency of the reduction in locomotor-related burst frequency. *Significantly different from control.
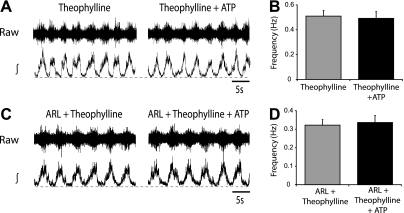
These data demonstrate that activation of adenosine receptors modulates components of the spinal locomotor CPG to regulate locomotor frequency. Furthermore, given that applications of adenosine and ATP both led to a decrease in locomotor frequency and that the effects of ATP had a slower time course than those of adenosine, it seemed likely that ATP-mediated effects reflect the breakdown of ATP to adenosine and subsequent activation of adenosine receptors. To test this further, we applied ATP (100 μM) in the presence of the general adenosine receptor antagonist theophylline (20 μM). When adenosine receptors were blocked by theophylline, ATP had no significant effect on locomotor burst frequency (n = 6; Fig. 3, A and B). In addition, when the ectonucleotidase inhibitor ARL67156 was applied to reduce ATP breakdown, ATP still had no effect on locomotor activity (n = 6; Fig. 3, C and D). Thus adenosine, rather than ATP, seems to be the primary purinergic modulator of the mammalian locomotor CPG.
Fig. 3.
The effects of exogenously applied ATP reflect breakdown to adenosine and activation of P1 receptors. A: raw (top) and rectified/integrated (bottom) L2 ventral root recordings in the presence of the adenosine receptor antagonist theophylline (20 μM) and during the subsequent application of ATP (100 μM). B: averaged frequency values from the final 10 min in theophylline alone and ATP with theophylline, showing no significant change in burst frequency (n = 6). C: raw (top) and rectified/integrated (bottom) L2 ventral root recordings in the presence of theophylline and the ectonuclotidase inhibitor ARL67156 (50 μM; ARL) and then upon addition of ATP. D: averaged data showing no change in frequency with ATP applications in the presence of both theophylline and ARL (n = 8).
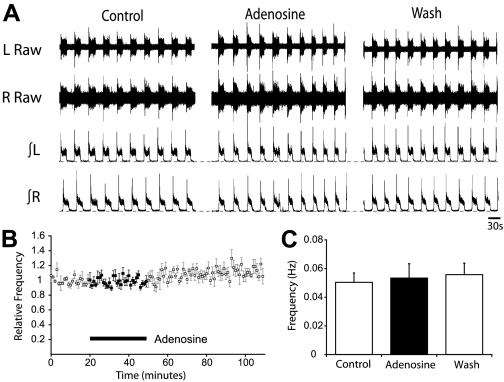
To begin to elucidate the mechanisms by which adenosine receptor activation modulates the locomotor CPG, we next investigated whether adenosine affects excitatory or inhibitory components of spinal motor circuitry. Given that isolated spinal cord preparations can generate a motor rhythm in the absence of inhibition (Bracci et al. 1996; Taccola et al. 2004), we investigated whether the modulatory actions of adenosine remain when inhibitory transmission is blocked. When NMDA (5 μM), 5-HT (10 μM), and DA (50 μM) were applied in conjunction with blockers of inhibitory transmission (strychnine, 1 μM, and bicuculline, 10 μM), we recorded slow, rhythmic bursts of synchronous activity from left and right lumbar ventral roots (Fig. 4A). Application of adenosine (75 μM; duration 30 min; n = 8) had no significant effect on the rhythmic activity recorded under these conditions (Fig. 4, A–C). In addition to blocking GABAergic transmission, bicuculline has also been shown to affect the after-hyperpolarization (AHP); (Khawaled et al. 1999). Thus the lack of an adenosine effect in the presence of bicuculline may suggest that adenosine modulates the AHP rather than inhibitory transmission. To control for this possibility, we substituted bicuculline with a blocker of the GABAA-receptor chloride channel, picrotoxin, which does not affect the AHP in spinal neurons (Pflieger et al. 2002). Slow, rhythmic bursts of synchronous activity from left and right lumbar ventral roots was again observed in the presence of strychnine (1 μM) and picrotoxin (60 μM). In these preparations, adenosine still had no effect on locomotor frequency (n = 4; data not shown). Together these data suggest that adenosine receptor activation affects the locomotor CPG by modulating inhibitory transmission or the activity of inhibitory interneurons.
Fig. 4.
Adenosine modulates mammalian locomotion via effects on inhibitory transmission. A: sample raw (top) and rectified/integrated (bottom) traces showing the lack of effect of adenosine (75 μM) on rhythmic activity recorded from ventral roots when the locomotion inducing drugs [N-methyl-d-aspartic acid (NMDA), 5-hydroxytryptamine (5-HT), and dopamine (DA)] were applied in the presence of the GABAA receptor antagonist bicuculline (10 μM) and the glycine receptor antagonist strychnine (1 μM). B: pooled time course plot of burst frequency over the course of the adenosine application (n = 8). C: graphical representation of average frequency, during the final 10 min from control, 75 μM adenosine application, and washout, showing no change in the frequency of activity.
Modulation of spinal locomotor networks by endogenously derived adenosine.
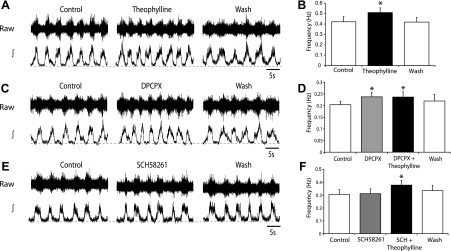
Having demonstrated that the activation of adenosine receptors by exogenous adenosine can modulate the mammalian locomotor CPG, we next assessed whether endogenously released adenosine contributes to the control of the locomotor CPG. This was investigated by observing the effects of adenosine receptor antagonists on locomotor activity recorded from isolated spinal cord preparations. We first examined the effects of the general adenosine receptor antagonist theophylline. Application of theophylline (20 μM; duration 30 min) caused a significant increase in locomotor burst frequency (21 ± 4.5%; n = 6), which persisted throughout the drug application and reversed following drug washout (Fig. 5, A and B). Theophylline had no significant effect on burst amplitude, and left-right coordination remained in the presence of the drug (data not shown; n = 6).
Fig. 5.
Endogenous adenosine modulates the locomotor central pattern generator (CPG) via activation of A1 receptors. A: sample raw (top) and rectified/integrated (bottom) ventral root recordings in control, theophylline (20 μM) application, and washout. B: averaged data of the final 10-min periods of control, theophylline application, and washout, showing a significant increase in locomotor frequency in the presence of theophylline (n = 6). C: traces showing the effects of the A1-receptor antagonist cyclopentyl dipropylxanthine (DPCPX; 50 μM) on locomotor activity. D: average frequency data showing an increase in locomotor frequency in the presence of DPCPX and no further change with the subsequent addition of theophylline (20 μM; n = 6). E: traces showing that the A2A-receptor antagonist SCH58261 (25 μM) has no effect on locomotor activity. F: average frequency data also show no significant effect of SCH58261 on locomotor frequency, while the subsequent addition of theophylline (20 μM) led to a significant increase in frequency (n = 6). *Significantly different from control.
Next, we assessed the actions of antagonists specific for the two adenosine receptor subtypes most prevalent in the central nervous system (CNS) (A1 and A2A; Cunha 2001). Bath application of the selective A1-receptor antagonist DPCPX (50 μM; duration 30 min) caused a significant increase in locomotor burst frequency (22 ± 1.7%; n = 6), which persisted throughout the drug application and reversed following drug washout (Fig. 5, C and D). The magnitude of the effects of DPCPX and theophylline was very similar, suggesting that adenosine-mediated modulation of locomotor activity primarily involves A1 receptors. To test this further, we next applied theophylline (20 μM) in the presence of DPCPX (50 μM). When A1 receptors were blocked by DPCPX, theophylline had no significant effect (n = 6; Fig. 5D), confirming that endogenous adenosine primarily acts via A1 receptors. Furthermore, although theophylline has nonspecific effects [for example on phosphodiesterases (Banner and Page 1995) and internal calcium stores (Munakata and Akaike 1994)], these data demonstrate that the actions of theophylline on locomotor frequency are specific to the blockade of adenosine receptors. We next went on to test whether there was any involvement of A2A receptors using the A2A-specific antagonist SCH58261. Application of SCH58261 (25 μM, duration 30 min) had no significant effect on locomotor burst frequency (n = 6; Fig. 5, E and F). The subsequent addition of theophylline (20 μM), in the presence of SCH58261, led to a significant increase in burst frequency (24 ± 3.7%; n = 6; Fig. 5F). Together these data reveal that release of adenosine from endogenous sources within the spinal cord regulates the frequency of on-going locomotor activity. Furthermore, the effects of endogenous adenosine appear to be primarily mediated by A1-receptor activation.
Glia as a source of neuromodulatory adenosine.
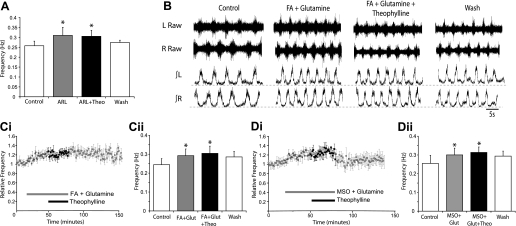
Next, we sought to determine the source of endogenous adenosine, which modulates the spinal locomotor network. Extracellular adenosine is thought to be primarily derived from the enzymatic breakdown of extracellular ATP (Cunha 2001), which can be released from either neurons or glia via exocytosis (Burnstock 2007). Given the established link between purinergic signaling and glia within mammalian respiratory control networks (Gourine et al. 2010; Huxtable et al. 2010), we hypothesized that ATP is released from glia, then converted to adenosine, which, in turn, modulates components of the spinal locomotor network. We first examined whether inhibition of ATP breakdown removes the endogenous modulatory actions of adenosine. Bath application of the ectonucleotidase inhibitor ARL67156 (50 μM) caused a significant increase in locomotor frequency (20 ± 6.5%; n = 9; Fig. 6A). When theophylline (20 μM) was subsequently applied in the presence of ARL67156, no further change in frequency was observed (n = 9; Fig. 6A). These data support that neuromodulatory adenosine is primarily derived from the breakdown of extracellular ATP.
Fig. 6.
Glia-derived ATP is the source of endogenous modulatory adenosine. A: bar chart showing averaged frequency data from the final 10 min of control, ARL (50 μM) application, addition of theophylline (20 μM), and washout. ARL increases burst frequency, while theophylline has no effect in the presence of ARL, indicating that breakdown of ATP is the primary source of adenosine. B: sample raw (top) and rectified/integrated (bottom) ventral root recordings in control, during coapplication of the glial toxin fluoroacetate (FA; 5 mM) and glutamine (1.5 mM), the addition of theophylline (20 μM), and then washout. Ci: pooled time course data showing the frequency of locomotor activity during control, the application of FA and glutamine, addition of theophylline, and washout (n = 6). Cii: averaged data of the final 10-min time periods from each condition (control; FA and glutamine; FA, glutamine and theophylline, washout). Di: pooled time course plot showing the frequency of locomotor activity during control, the application of methionine sulfoximine (MSO; 100 μM) and glutamine, addition of theophylline, and washout (n = 6). Dii: averaged data of the final 10-min time periods from each condition (control; MSO and glutamine; MSO, glutamine and theophylline, washout). Theophylline has no effect on the frequency of locomotor activity when applied in the presence of the glial toxins FA and MSO, suggesting that ATP is released from glia, then converted to adenosine to provide tonic P1-receptor mediated modulation of the locomotor CPG. *Significantly different from control.
Next, we investigated whether glial cells represent the primary source of extracellular ATP, which is converted to adenosine. We utilized two compounds that disturb glial cell function: the glialaconitase inhibitor FA (Fonnum et al. 1997), and the glial-specific glutamine synthetase inhibitor MSO (Ronzio et al. 1969). Bath application of either FA (5 mM; n = 6) or MSO (100 μM; n = 6) caused a gradual decline in both the amplitude and frequency of drug-induced locomotor activity (data not shown). Activity was disrupted after 30 min and eventually ceased after 120 min of FA or MSO application. Given that loss of glial function will perturb the glutamate-glutamine cycle, we next attempted to recover locomotor activity using glutamine (1.5 mM). When FA or MSO was applied together with glutamine, stable locomotor activity, indistinguishable in pattern from the activity recorded without toxins, could be maintained for the duration of recordings (Fig. 6B). Coapplications of FA or MSO with glutamine were associated with a significant increase in locomotor frequency (FA, 19 ± 3.5%, n = 6; MSO, 18 ± 8.8%; n = 6; Fig. 6, B–D), which is likely to reflect increases in extracellular glutamate, but may also reflect the loss of gliotransmission. Once stable locomotor activity was obtained in the presence of FA or MSO and glutamine, we bath applied theophylline (20 μM) to assess whether the effects of endogenous adenosine were blocked. Theophylline had no significant effect on locomotor activity in the presence of FA (Fig. 6, B and C; n = 6) or MSO (Fig. 6D; n = 6), suggesting a lack of endogenous adenosine when glial function is perturbed. In a subset of experiments, we verified that theophylline increased locomotor activity in control conditions before the application of glial toxins (n = 2, data not shown). We also assessed whether exogenously applied adenosine could modulate locomotor-related activity in the presence of glial toxins. When adenosine (75 μM) was applied in the presence of MSO and glutamine, a rapid and significant reduction in locomotor frequency was observed (9 ± 1.2%; n = 6; data not shown). Although this reduction was smaller than that observed in the absence of glial toxins, it may represent an underestimation due to the gradual increase in locomotor frequency observed in the presence of MSO and glutamine (as seen in Fig. 6Di), which continued during the application of adenosine. Together these data demonstrate that, most likely via the release of ATP, glia are involved in the production of endogenous adenosine, which modulates spinal locomotor networks.
DISCUSSION
Purines are known to modulate CPGs controlling Xenopus tadpole swimming (Dale and Gilday 1996) and mammalian respiration (Lorier et al. 2007). In the present study, we provide the first report of purinergic modulation of the mammalian locomotor CPG. We demonstrate that endogenous adenosine modulates the frequency of locomotor-related activity generated by spinal motor networks in mice. This neuromodulatory adenosine appears to derive from the breakdown of extracellular ATP, which is itself released by glial cells.
Previous work in Xenopus tadpoles and rodents suggest that the purines ATP and adenosine work in concert, with opposing effects, to modulate rhythmic motor patterns (Dale and Gilday 1996; Lorier et al. 2007). In tadpole swimming, ATP excites CPG neurons, leading to an increase in the frequency of rhythmic motor activity, while adenosine, derived from ATP-breakdown, then inhibits CPG neurons, causing a secondary slowing in motor rhythm (Dale and Gilday 1996). Initial studies of the respiratory control network in rats indicated that purines have similar, biphasic effects on respiratory frequency, with ATP-mediated excitation preceding adenosine-mediated inhibition (Lorier et al. 2007). However, more recent studies suggest that purinergic modulation of the respiratory rhythm is complicated by species-specific ectonucleotidase expression, such that ATP-mediated excitation dominates in rats, while adenosine-mediated inhibition dominates in mice (Huxtable et al. 2009; Zwicker et al. 2011). In the present study, we found no evidence of ATP-mediated modulation of spinal locomotor circuitry in mice, even when ATP breakdown was reduced using an ectonucleotidase inhibitor, and potentially opposing effects of adenosine receptor activation were blocked using a general adenosine receptor antagonist. Adenosine, therefore, appears to be the primary purinergic modulator of the mouse locomotor CPG. Although our findings contrast those of tadpole locomotion and rat respiration, adenosine-dominated modulation is consistent with studies of respiratory rhythm generation in mice and purinergic modulation in other regions of the CNS, including the hippocampus (Pascual et al. 2005; Zhang et al. 2003), retina (Newman 2003), and cortex (Fellin et al. 2009).
The application of adenosine to isolated mouse spinal cord preparations demonstrated significant modulatory effects of this purine on rhythmic, locomotor-related output. Activation of adenosine receptors was associated with a rapid and persistent reduction in the frequency of locomotor activity. These data indicate that adenosine acts on neurons within the locomotor CPG, which are involved in controlling the frequency of rhythmic output from the network. In contrast, adenosine receptor activation had no effect on the amplitude of bursts of locomotor-related motoneuron output recorded from ventral roots. Thus adenosine does not appear to directly modulate the activity of motoneurons, which determine the intensity of motor output from the CNS. It should be noted, however, that adenosine has been shown to modulate N-type, high-voltage-activated Ca2+ currents in cultured embryonic motoneurons (Mynlieff and Beam. 1994). Data demonstrating modulatory effects of adenosine on interneurons involved in locomotor rhythm generation parallel findings in the mammalian respiratory system where activation of adenosine receptors within the rhythm generating pre-Bötzinger complex of the medulla also leads to a slowing in the frequency of respiratory activity (Lorier et al. 2007). However, in contrast to the locomotor system, adenosine also modulates the output of respiratory motoneurons to affect the intensity of respiratory-related motor output and the activation of respiratory muscles (Funk et al. 1997; Miles et al. 2002).
We also found that applications of the general adenosine receptor antagonist theophylline led to an increase in the frequency of locomotor activity. These data demonstrate that spinally derived, endogenous adenosine modulates the locomotor CPG and highlight adenosine as an important intrinsic modulator of locomotor circuitry. By using adenosine receptor subtype-specific antagonists (DPCPX, A1; SCH58261, A2A), we then established that the modulatory actions of endogenous adenosine predominantly result from activation of A1-type adenosine receptors. Consistent with a primary role for A1 receptors in adenosine-mediated modulation, significant expression of A1 receptors has been reported throughout the rodent spinal cord, including in the ventral horn (Deuchars et al. 2001).
In the present study, exogenously applied adenosine had no effect on the frequency of rhythmic activity recorded from isolated spinal cord preparations in which inhibitory transmission was blocked. Despite indirect evidence supporting the involvement of common motor circuitry in the generation of disinhibited bursting and fictive locomotor activity (Bracci et al. 1996; Kiehn 2006), it remains unknown whether the same populations of neurons underlie these two rhythmic outputs. Nevertheless, our data suggest a link between the activity of inhibitory interneurons and/or the strength of inhibitory transmission within spinal motor circuitry and the modulatory effects of adenosine on locomotor output. Given that endogenous adenosine reduces locomotor frequency without affecting left-right coordination (Fig. 2B), adenosine may alter locomotor frequency by modulating ipsilaterally projecting inhibitory interneurons. Interestingly, the ablation or inactivation of the V1 class of ipsilaterally projecting inhibitory interneurons (Alvarez et al. 2005) leads to a decrease in the frequency of locomotor activity (Gosgnach et al. 2006). Adenosine could, therefore, modulate locomotor activity by reducing the excitability of V1 interneurons or inhibiting their synaptic transmission. An alternative mechanism by which inhibition might be involved in adenosine-mediated modulation has been described in the hippocampus where GABA, released by inhibitory neurons, activates GABAB receptors on glial cells to simulate the release of ATP. Glial-derived ATP is then broken down to extracellular adenosine, which inhibits synaptic transmission (Serrano et al. 2006). Similar mechanisms could contribute to adenosine-mediated modulation of spinal locomotor circuitry, since glycine- and GABA-activated currents have been observed in spinal glial cells (Pastor et al. 1995). To provide more definitive information regarding the role of inhibitory transmission in adenosine-mediated modulation of spinal locomotor circuitry, and to identify the exact cellular mechanisms involved, future studies will need to focus on individual classes of inhibitory interneurons within the locomotor CPG. Such experiments will become more feasible with continued advances in the definition of distinct subclasses of ventral horn interneurons using developmental genetics (Goulding 2009; Kiehn et al. 2010).
The primary source of extracellular adenosine is thought to be the breakdown of ATP by ectonucleotidases. However, adenosine may also be released directly from neurons and glial cells via equilibrative nucleoside transporters or possibly by exocytosis (Hamilton and Attwell 2010; Wall and Dale 2008). In the present study, we sought to determine the source of the endogenous adenosine, which modulates spinal locomotor circuitry. In the presence of the ectonucleotidase inhibitor ARL67156, the general adenosine receptor antagonist theophylline had no effect on the frequency of locomotor activity. Thus, when ATP breakdown is reduced, endogenous adenosine-mediated modulation is lost, indicating that spinal neuromodulatory adenosine is derived from the breakdown of ATP. Next, we investigated the source of this ATP. ATP can be released by neurons, often as a cotransmitter (Burnstock 1995) and from glial cells, specifically astrocytes (Hamilton and Attwell 2010). We utilized compounds that selectively interfere with glial function, commonly referred to as glial toxins, to probe involvement of glial cells in the purinergic modulation of spinal motor circuitry. The first of these was FA, which, via its toxic metabolite fluorocitrate, acts as a glial aconitase inhibitor, leading to accumulation of citrate and a reduction in the formation of glutamine and, of particular relevance to the present study, ATP (Fonnum et al. 1997). We also utilized the glial toxin MSO, a glial glutamine synthetase enzyme inhibitor (Ronzio et al. 1969). Both of these glial toxins have been widely used to demonstrate the glial origin of purines and other signaling molecules and the role of glial cells in neuronal function (e.g., Clarke 1991; Fonnum et al. 1997; Huxtable et al. 2010; Zhang et al. 2003).
In keeping with studies of respiratory rhythm-generating networks (Hülsmann et al. 2000; Huxtable et al. 2010), we found that FA and MSO inhibited locomotor circuitry, such that rhythmic activity eventually ceased in the presence of glial toxins. Locomotor activity could, however, be rescued by the coapplication of glutamine, most likely because exogenous glutamine replaces the glial-derived glutamine that is normally taken up by neurons and converted to glutamate for subsequent release (Hülsmann et al. 2000). In the presence of glial toxins (supplemented with glutamine), the adenosine receptor antagonist theophylline no longer affected locomotor activity. Thus glial toxins prevent the endogenous modulatory influence of adenosine, supporting that neuromodulatory adenosine is derived from ATP, which is released from glial cells.
It should be noted that FA and MSO have additional effects within the CNS that may not relate to their actions on glia. Such effects include increasing extracellular potassium levels (Largo et al. 1997), effecting calcium chelation (Clarke 1991), and causing glycogen deposition in cranial motoneurons (Young et al. 2005). In addition, although FA and MSO are primarily taken up by glial cells, they can also cross neuronal membranes (Hassel et al. 1992). Studies in the rodent respiratory system have shown that FA depolarizes glia but not XII motoneurons (Hülsmann et al. 2000), and that modulation of the respiratory rhythm by substance P, which is thought to act neuronally, remains intact in the presence of FA and MSO (Huxtable et al. 2010). We found that adenosine could still modulate the locomotor network in the presence of glial toxins. Together, these data support the glial specificity of FA and MSO and suggest that the function of respiratory and locomotor networks is not substantially perturbed by the potential nonglial actions of these toxins.
Our data highlight purinergic gliotransmission as an important intrinsic modulatory system affecting spinal motor circuitry and add another level of complexity to the regulation of the mammalian locomotor CPG by supporting a role for nonneuronal cells. Purinergic gliotransmission, particularly that culminating in adenosine receptor activation, is emerging as an important mechanism for the control of neuronal networks throughout the brain, including those of the retina (Newman 2003), hippocampus (Pascual et al. 2005; Zhang et al. 2003), and cortex (Fellin et al. 2009). At the behavioral level, purinergic gliotransmission has recently been shown to be important in regulating sleep homeostasis (Fellin et al. 2009; Halassa et al. 2009). However, the exact stimuli that induce the release of purines from glia, the mechanisms of purine release, and the temporal relationship between purine release and neuronal activity remain unclear. In the case of the spinal locomotor CPG, tonic neuromodulation by adenosine may act as a negative feedback mechanism, limiting and stabilizing the frequency of locomotor-related output. Alternatively, given the demonstration that glial-derived adenosine contributes to activity-dependent heterosynaptic depression in hippocampal circuits (Pascual et al. 2005; Serrano et al. 2006), temporally dynamic control of adenosine levels within the spinal cord could help to reduce synaptic noise and ensure the synchronicity of neuronal components of the locomotor CPG. To fully understand the role of glial-derived adenosine in the control of spinal locomotor circuitry, it will be important to determine the exact stimuli that lead to purine release from glia and to study the activity patterns of glia during locomotion. Given the neuroprotective potential of adenosine (Cunha 2001) and the involvement of glial cells in neurodegenerative diseases, such as amyotrophic lateral sclerosis (Di Giorgio et al. 2007; Nagai et al. 2007), a greater understanding of glial and adenosine-mediated modulation is likely to benefit both our basic understanding of motor control and ultimately the treatment of diseases that affect motor systems.
GRANTS
We are grateful for support from the Wellcome Trust and Biotechnology and Biological Sciences Research Council UK.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.C.W., K.M.P., and G.B.M. conception and design of research; E.C.W. and K.M.P. performed experiments; E.C.W. and K.M.P. analyzed data; E.C.W., K.M.P., and G.B.M. interpreted results of experiments; E.C.W. and G.B.M. prepared figures; E.C.W. and G.B.M. drafted manuscript; E.C.W., K.M.P., and G.B.M. edited and revised manuscript; E.C.W., K.M.P., and G.B.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Keith Sillar for helpful comments on the manuscript.
REFERENCES
- Alvarez FJ, Jonas PC, Sapir T, Hartley R, Berrocal MC, Geiman EJ, Todd AJ, Goulding M. Postnatal phenotype and localization of spinal cord V1 derived interneurons. J Comp Neurol 493: 177–192, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banner KH, Page CP. Theophylline and selective phosphodiesterase inhibitors as anti-inflammatory drugs in the treatment of bronchial asthma. Eur Respir J 8: 996–1000, 1995 [PubMed] [Google Scholar]
- Bracci E, Ballerini L, Nistri A. Spontaneous rhythmic bursts induced by pharmacological block of inhibition in lumbar motoneurons of the neonatal rat spinal cord. J Neurophysiol 75: 640–647, 1996 [DOI] [PubMed] [Google Scholar]
- Brown P, Dale N. Adenosine A1 receptors modulate high voltage-activated Ca2+ currents and motor pattern generation in the xenopus embryo. J Physiol 525: 655–667, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Dale N. Modulation of K(+) currents in Xenopus spinal neurons by p2y receptors: a role for ATP and ADP in motor pattern generation. J Physiol 540: 843–850, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Noradrenaline and ATP: cotransmitters and neuromodulators. J Physiol Pharmacol 46: 365–384, 1995 [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87: 659–797, 2007 [DOI] [PubMed] [Google Scholar]
- Clarke DD. Fluoroacetate and fluorocitrate: mechanism of action. Neurochem Res 16: 1055–1058, 1991 [DOI] [PubMed] [Google Scholar]
- Cunha RA. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int 38: 107–125, 2001 [DOI] [PubMed] [Google Scholar]
- Dale N. Delayed production of adenosine underlies temporal modulation of swimming in frog embryo. J Physiol 511: 265–272, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N, Gilday D. Regulation of rhythmic movements by purinergic neurotransmitters in frog embryos. Nature 383: 259–263, 1996 [DOI] [PubMed] [Google Scholar]
- Deuchars SA, Brooke RE, Deuchars J. Adenosine A1 receptors reduce release from excitatory but not inhibitory synaptic inputs onto lateral horn neurons. J Neurosci 21: 6308–6320, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci 10: 608–614, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Manira A, Kyriakatos A, Nanou E, Mahmood R. Endocannabinoid signaling in the spinal locomotor circuitry. Brain Res Rev 57: 29–36, 2008 [DOI] [PubMed] [Google Scholar]
- Fellin T, Halassa MM, Terunuma M, Succol F, Takano H, Frank M, Moss SJ, Haydon PG. Endogenous nonneuronal modulators of synaptic transmission control cortical slow oscillations in vivo. Proc Natl Acad Sci U S A 106: 15037–15042, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F, Johnsen A, Hassel B. Use of fluorocitrate and fluoroacetate in the study of brain metabolism. Glia 21: 106–113, 1997 [PubMed] [Google Scholar]
- Funk GD, Kanjhan R, Walsh C, Lipski J, Comer AM, Parkis MA, Housley GD. P2 receptor excitation of rodent hypoglossal motoneuron activity in vitro and in vivo: a molecular physiological analysis. J Neurosci 17: 6325–6337, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosgnach S, Lanuza GM, Butt SJ, Saueressig H, Zhang Y, Velasquez T, Riethmacher D, Callaway EM, Kiehn O, Goulding M. V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature 440: 215–219, 2006 [DOI] [PubMed] [Google Scholar]
- Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci 10: 507–518, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science 329: 571–575, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron 52: 751–766, 2006 [DOI] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology 57: 343–346, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci 11: 227–238, 2010 [DOI] [PubMed] [Google Scholar]
- Hassel B, Paulsen RE, Johnsen A, Fonnum F. Selective inhibition of glial cell metabolism in vivo by fluorocitrate. Brain Res 576: 120–124, 1992 [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Mottram C, Quinlan K, Theiss R, Schuster J. Motoneuron excitability: the importance of neuromodulatory inputs. Clin Neurophysiol 120: 2040–2054, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlenius E, Lagercrantz H. Adenosinergic modulation of respiratory neurones in the neonatal rat brainstem in vitro. J Physiol 518: 159–172, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hülsmann S, Oku Y, Zhang W, Richter DW. Metabolic coupling between glia and neurons is necessary for maintaining respiratory activity in transverse medullary slices of neonatal mouse. Eur J Neurosci 12: 856–862, 2000 [DOI] [PubMed] [Google Scholar]
- Huxtable AG, Zwicker JD, Alvares TS, Ruangkittisakul A, Fang X, Hahn LB, Posse de Chaves E, Baker GB, Ballanyi K, Funk GD. Glia contribute to the purinergic modulation of inspiratory rhythm-generating networks. J Neurosci 30: 3947–3958, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable AG, Zwicker JD, Poon BY, Pagliardini S, Vrouwe SQ, Greer JJ, Funk GD. Tripartite purinergic modulation of central respiratory networks during perinatal development: the influence of ATP, ectonucleotidases, and ATP metabolites. J Neurosci 29: 14713–14725, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Carlin KP, Brownstone RM. An in vitro functionally mature mouse spinal cord preparation for the study of spinal motor networks. Brain Res 816: 493–499, 1999 [DOI] [PubMed] [Google Scholar]
- Katz PS, Frost WN. Intrinsic neuromodulation: altering neuronal circuits from within. Trends Neurosci 19: 54–61, 1996 [DOI] [PubMed] [Google Scholar]
- Kawai A, Okada Y, Muckenhoff K, Scheid P. Theophylline and hypoxic ventilatory response in the rat isolated brainstem-spinal cord. Respir Physiol 100: 25–32, 1995 [DOI] [PubMed] [Google Scholar]
- Khawaled R, Bruening-Wright A, Adelman JP, Maylie J. Bicuculline block of small-conductance calcium-activated potassium channels. Pflügers Arch 438: 314–321, 1999 [DOI] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci 29: 279–306, 2006 [DOI] [PubMed] [Google Scholar]
- Kiehn O, Dougherty KJ, Hagglund M, Borgius L, Talpalar A, Restrepo CE. Probing spinal circuits controlling walking in mammals. Biochem Biophys Res Commun 396: 11–18, 2010 [DOI] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci 16: 5777–5794, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largo C, Ibarz JM, Herreras O. Effects of the gliotoxin fluorocitrate on spreading depression and glial membrane potential in rat brain in situ. J Neurophysiol 78: 295–307, 1997 [DOI] [PubMed] [Google Scholar]
- Lorier AR, Huxtable AG, Robinson DM, Lipski J, Housley GD, Funk GD. P2Y1 receptor modulation of the pre-Botzinger complex inspiratory rhythm generating network in vitro. J Neurosci 27: 993–1005, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Hartley R, Todd AJ, Brownstone RM. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci U S A 104: 2448–2453, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Parkis MA, Lipski J, Funk GD. Modulation of phrenic motoneuron excitability by ATP: consequences for respiratory-related output in vitro. J Appl Physiol 92: 1899–1910, 2002 [DOI] [PubMed] [Google Scholar]
- Mironov SL, Langohr K, Richter DW. A1 adenosine receptors modulate respiratory activity of the neonatal mouse via the cAMP-mediated signaling pathway. J Neurophysiol 81: 247–255, 1999 [DOI] [PubMed] [Google Scholar]
- Munakata M, Akaike N. Theophylline affects three different potassium currents in dissociated rat cortical neurons. J Physiol 471: 599–616, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mynlieff M, Beam KG. Adenosine acting at an A1 receptor decreases N-type calcium current in mouse motoneurons. J Neurosci 14: 3628–3634, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci 10: 615–622, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Glial cell inhibition of neurons by release of ATP. J Neurosci 23: 1659–1666, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science 310: 113–116, 2005 [DOI] [PubMed] [Google Scholar]
- Pastor A, Chvátal A, Syková E, Kettenmann H. Glycine- and GABA-activated currents in identified glial cells of the developing rat spinal cord slice. Eur J Neurosci 7: 1188–1198, 1995 [DOI] [PubMed] [Google Scholar]
- Pflieger J-F, Clarac F, Vinay L. Picrotoxin and bicuculline have different effects on lumbar spinal networks and motoneurons in the neonatal rat. Brain Res 935: 81–86, 2002 [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev 80: 767–852, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronzio RA, Rowe WB, Meister A. Mechanism of inhibition of glutamine synthetase by methionine sulfoximine. Biochemistry 8: 1066–1075, 1969 [DOI] [PubMed] [Google Scholar]
- Schmidt C, Bellingham MC, Richter DW. Adenosinergic modulation of respiratory neurones and hypoxic responses in the anaesthetized cat. J Physiol 483: 769–781, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano A, Haddjeri N, Lacaille JC, Robitaille R. GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J Neurosci 26: 5370–5382, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taccola G, Marchetti C, Nistri A. Modulation of rhythmic patterns and cumulative depolarization by group I metabotropic glutamate receptors in the neonatal rat spinal cord in vitro. Eur J Neurosci 19: 533–541, 2004 [DOI] [PubMed] [Google Scholar]
- Wall M, Dale N. Activity-dependent release of adenosine: a critical re-evaluation of mechanism. Curr Neuropharm 6: 329–337, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JK, Dreshaj IA, Wilson CG, Martin RJ, Zaidi SIA, Haxhiu MA. An astrocyte toxin influences the pattern of breathing and the ventilatory response to hypercapnia in neonatal rats. Respir Physiol Neurobiol 147: 19–30, 2005 [DOI] [PubMed] [Google Scholar]
- Zagoraiou L, Akay T, Martin JF, Brownstone RM, Jessell TM, Miles GB. A cluster of cholinergic premotor interneurons modulates mouse locomotor activity. Neuron 64: 645–662, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, Duan S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron 40: 971–982, 2003 [DOI] [PubMed] [Google Scholar]
- Zwicker JD, Rajani V, Hahn LB, Funk GD. Purinergic modulation of preBotzinger complex inspiratory rhythm in rodents: the interaction between ATP and adenosine. J Physiol 589: 4583–4600, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]