Abstract
Acetylcholine profoundly affects neocortical function, being involved in arousal, attention, learning, memory, sensory and motor function, and plasticity. The majority of cholinergic afferents to neocortex are from neurons in nucleus basalis. Nucleus basalis also contains projecting neurons that release other transmitters, including GABA and possibly glutamate. Hence, electrical stimulation of nucleus basalis evokes the release of a mixture of neurotransmitters in neocortex, and this lack of selectivity has impeded research on cholinergic signaling in neocortex. We describe a method for the selective stimulation of cholinergic axons in neocortex. We used the Cre-lox system and a viral vector to express the light-activated protein channelrhodopsin-2 in cholinergic neurons in nucleus basalis and their axons in neocortex. Labeled neurons depolarized on illumination with blue light but were otherwise unchanged. In anesthetized mice, illumination of neocortex desynchronized the local field potential, indicating that light evoked release of ACh. This novel technique will enable many new studies of the cellular, network, and behavioral physiology of ACh in neocortex.
Keywords: nucleus basalis, basal forebrain, acetylcholine
neocortex is densely innervated by cholinergic axons. ACh, released by these axons, profoundly affects neocortical function, being involved in arousal and attention (Buzsáki and Gage 1989; Herrero et al. 2008; Parikh et al. 2008), learning and memory (Conner et al. 2003; Kilgard 2003; Ramanathan et al. 2009; Winkler et al. 1995) and sensory and motor function and plasticity (Berg et al. 2005; Conner et al. 2003; Kilgard and Merzenich 1998). The majority of cholinergic axons in neocortex project from somata in the basal forebrain, primarily in nucleus basalis, with a minority originating from cholinergic interneurons, other nuclei within the basal forebrain complex (e.g., medial septum, which projects to cingulate cortex; Bigl et al. 1982), and possibly brain stem cholinergic nuclei, such as laterodorsal tegmentum (Wainer and Mesulam 1990). Release of ACh in neocortex can be evoked by stimulation of nucleus basalis, but the ascending projection from nucleus basalis to cortex includes not only cholinergic axons, but also GABAergic and glutamatergic axons (Henny and Jones 2008). Within neocortex, these axons form a diffuse network, intermingled with axons that release other transmitters. Hence, a stimulating electrode placed in nucleus basalis or in neocortex results in the release of multiple transmitters (Chu et al. 2000; Roerig et al. 1997).
Much of what we understand of the cellular effects of ACh in neocortex originates from experiments in which ACh was applied to neurons in vitro either by addition to the perfusing solution or pressure ejection onto the soma. In these experiments, ACh increases the excitability of pyramidal neurons through the activation of muscarinic ACh receptors (mAChRs). mAChR activation causes a slow (seconds) depolarization of the plasma membrane by inhibiting several potassium currents (McCormick and Prince 1985, 1986; McCormick 1992), including M-type current (Delmas and Brown 2005; Halliwell 1989; McCormick and Williamson 1989), the current underlying the slow afterhyperpolarization (McCormick and Williamson 1989; Schwindt et al. 1988), A-type current (Hoffman and Johnston 1999), and inward rectifier current (Carr and Surmeier 2007), and by opening a nonspecific cation channel (Haj-Dahmane and Andrade 1996). A mAChR-mediated membrane hyperpolarization preceding this slow depolarization has also been reported (Gulledge and Stuart 2005). Unfortunately, exogenous application is unlikely to mimic accurately the spatiotemporal profile of ACh release from cholinergic axons, and a method for the selective stimulation of cholinergic axons would therefore aid research into the actions of ACh in neocortex, both in vitro and in vivo.
Here, we describe a method for selective stimulation of cholinergic neurons and axons via optogenetics. We drive selective expression of channelrhodopsin-2 (ChR2; Nagel et al. 2003, 2005) in cholinergic neurons by stereotaxic injection of a double-floxed virus into nucleus basalis in a choline-acetyltransferase (ChAT)-Cre mouse line (Gu and Yakel 2011; Witten et al. 2010; Zhao et al. 2011). We characterize the timing and pattern of expression of ChR2 in nucleus basalis and in neocortex and establish that infected neurons depolarize on illumination with blue light but are otherwise unchanged. Finally, we show that stimulation of cholinergic axons in neocortex with blue light results in desynchronization of the local field potential (LFP) in anesthetized mice, indicating that ChR2 can activate cholinergic afferents from nucleus basalis. This novel methodology makes possible many new studies of the cellular, network, and behavioral physiology of synaptically released ACh in neocortex.
MATERIALS AND METHODS
Stereotaxic injections.
All experiments and procedures were approved by the Northwestern University Institutional Animal Care and Use Committee. Expression of ChR2 was limited to cholinergic neurons in nucleus basalis using the Cre-lox system. Tg(ChAT-Cre)60Gsat mice (GENSAT) express Cre recombinase on a ChAT promoter, resulting in Cre expression in cholinergic neurons throughout the brain (Gong et al. 2007). Of the three ChAT-Cre lines currently available through the GENSAT project, two [Tg(ChAT-Cre)60Gsat and Tg(ChAT-Cre)24Gsat] express Cre in nucleus basalis. These two lines have similar distributions of Cre within nucleus basalis. We chose to use the Tg(ChAT-Cre)60Gsat line. Into this mouse, we injected adeno-associated virus [8 × 1012 gc/ml; rAAV5/EF1a-DIO-hChR2(H134R)eYFP; Virus Vector Core, University of North Carolina] with a double-floxed inverse open-reading frame (Sohal et al. 2009), which drives expression of the H134R variant of ChR2 (Nagel et al. 2005) as a ChR2-yellow fluorescent protein fusion protein (ChR2-YFP) in neurons containing Cre recombinase. In preliminary experiments, we tested several virus serotypes and found, empirically, that this adeno-associated virus type 5 (AAV5) construct drove expression of ChR2-YFP within several hundred micrometers of the injection site, which was appropriate for localization of ChR2-YFP to nucleus basalis and consistent with previous measurements with AAV5 (Paterna et al. 2004). Virus (400 or 800 nl) was injected into nucleus basalis in mice under deep anesthesia (2.5% isoflurane, inhaled) using stereotaxic coordinates (0.2–0.35 mm anterior-posterior, 1.7 mm medial-lateral, 4.2–4.7 mm dorsal-ventral). Stereotaxic surgeries were performed with a Kopf model 940 small animal stereotaxic instrument (David Kopf Instruments, Tujunga, CA) under a Leica S6D dissecting microscope (Leica Microsystems, Wetzlar, Germany) using glass pipettes with a tip diameter of ∼10 μm, pulled on a Sutter P-97 puller (Sutter Instrument, Novato, CA). Core body temperature was monitored and maintained at ∼38°C using a rectal probe and temperature controller (FHC, Bowdoin, ME).
We injected mice with virus at postnatal day 21 (P21). Injections at P21 enabled us to study cholinergic signaling in adult mice, several weeks after viral injection, while minimizing the length of time that the mice were held in the animal facility and allowing us to obtain healthy brain slices from young adult mice expressing ChR2-YFP. The cholinergic system may not be fully mature at P21, but injections at P21 were nonetheless effective in our experiments.
Immunofluorescence.
Mice were deeply anesthetized with isoflurane (2.5%, inhaled) and then ketamine-xylazine (90 and 10 mg/kg ip, respectively) and transcardially perfused with PBS (0.1 M NaH2PO4, 0.1 M Na2HPO4, pH 7.4, room temperature) followed by 4% (wt/vol) paraformaldehyde (PFA) in PBS (at 4°C). For anti-ChAT immunohistochemistry, brains were cryoprotected in 15 and then 30% sucrose (wt/vol) in PBS, and 75-μm thick coronal sections were cut on a freezing microtome (Spencer Lens, Buffalo, NY). For all other immunofluorescence procedures, brains were postfixed for 24–48 h in 4% PFA before cutting 100-μm thick coronal sections with a vibrating microtome (Vibratome 1000 series; Vibratome, St. Louis, MO).
For anti-green fluorescent protein (GFP) and anti-glial fibrillary acidic protein (GFAP) immunofluorescence, free-floating sections were blocked for 1.5 h in 1% (wt/vol) bovine serum albumin, 2% (vol/vol) rabbit serum, and 1% (vol/vol) Triton X-100 in PBS, incubated overnight in primary antibodies against GFP (ab13970; 1:10,000; Abcam) and GFAP (MAB3402; 1:2,500; Millipore, Billerica, MA), incubated for 2 h in secondary antibodies (GFP secondary: rabbit anti-chicken FITC, 63-3111, 1:100, Jackson ImmunoResearch, West Grove, PA; GFAP secondary: rabbit anti-mouse Alexa Fluor 594, A-21201, 1:750, Invitrogen, Carlsbad, CA), and mounted in Mowiol4-88 with glycerol and Tris·HCl (Calbiochem, San Diego, CA).
For anti-ChAT immunofluorescence, free-floating sections were incubated for 10 min in 0.1 M boric acid in PBS, washed with 0.2% (vol/vol) Tween 20 in PBS, blocked for 1.5 h in 1% (wt/vol) bovine serum albumin, 2% (vol/vol) rabbit serum, and 1% (vol/vol) Triton in PBS, and incubated overnight at room temperature in primary antibodies against GFP and ChAT (anti-ChAT: AB144P; 1:200; Millipore). Endogenous peroxidases were quenched for 10 min with 0.3% hydrogen peroxide and 0.1% (wt/vol) sodium azide in PBS, and sections were incubated for 2 h at room temperature in secondary antibodies (GFP secondary: rabbit anti-chicken FITC, 63-3111, 1:100, Jackson ImmunoResearch; ChAT secondary: rabbit anti-mouse Alexa Fluor 594, A-21201, 1:750, Invitrogen).
Image analysis.
The mean location and area of neurons infected with ChR2-YFP (Fig. 1C) was calculated as described by Hedrick and Waters (2010). From each mouse, we took one representative section and manually drew an outline around the infected somata. We converted this outline to a binary mask. Masks from multiple mice were averaged to create a probability density distribution.
Fig. 1.
Expression of channelrhodopsin-2-yellow fluorescent protein fusion protein (ChR2-YFP) in nucleus basalis and neocortex. A: schematic illustration of the injection site in nucleus basalis, in coronal section, ∼0.3 mm posterior to bregma. The injection tract (dashed line) passes vertically through neocortex, caudate putamen (CP), and striatum en route to nucleus basalis (red). Our approach labeled cholinergic neurons in nucleus basalis and their axons that project to neocortex. LV, lateral ventricle. B: wide-field fluorescence images of a coronal section from an injected mouse, 4 wk after injection, with choline-acetyltransferase (ChAT) immunoreactivity and ChR2-YFP fluorescence in nucleus basalis. Globus pallidus (GP), caudate putamen (CP), neocortex (ctx), and nucleus basalis (NB) are labeled. Nucleus basalis, defined from anti-ChAT staining, is outlined on the ChAT image. The injection site, defined as the extent of somata labeled with ChR2-YFP, is outlined on the ChR2-YFP image. C: mean location and dimensions of the injection site from 5 mice. The density of anti-ChAT immunofluorescence was calculated as described in materials and methods. The relative intensities of staining along the major and minor axes of the injection site (dashed lines) are displayed in the inset. D: 2 images of ChR2-YFP-labeled neurons in nucleus basalis 3 wk after viral injection. Maximum intensity projections were derived from 2-photon z-stacks.
Laminar distributions of ChR2-YFP-labeled axons (Fig. 3) were calculated from wide-field images. Intensity in a 1-mm-wide region extending from the white matter to beyond the pia was measured in ImageJ. For each section, a similar measurement was obtained from the hemisphere contralateral to the injection site, as a measure of tissue autofluorescence, and this was subtracted from the ipsilateral measurement.
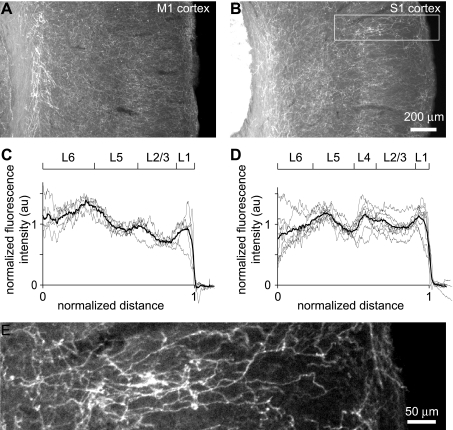
Fig. 3.
Laminar distribution of ChR2-YFP-labeled axons in neocortex. A and B: wide-field images of primary motor (A) and primary somatosensory (B) neocortex, showing many ChR2-YFP-labeled axons throughout all layers. C and D: laminar distribution of axon density, measured as fluorescence intensity, in primary somatosensory (C) and primary motor (D) neocortices. The approximate positions of laminar boundaries are indicated, based on the Allen Brain Atlas. Gray line: individual sections. Black lines: mean of 6 mice. au, Arbitrary units. E: axons are clearly visible in a higher-magnification image of a subregion (white rectangle) of B.
Anti-GFP and anti-ChAT colabeling (Fig. 4) were examined in two-photon z-stacks. The somata in nucleus basalis and axons in neocortex were identified and counted manually. In nucleus basalis, we counted the somata in a single section near the center of the injection site, identified by ChR2-YFP immunofluorescence intensity. In neocortex, we counted axons in a single section from the appropriate cortical region. We counted axons with a minimum length of ∼100 μm. In some preparations, there was a correlation between ChAT immunofluorescence intensity and ChR2-YFP fluorescence intensity. Axon counts are likely to have been biased toward clearly identifiable axons. When selecting ChAT-positive axons, which are extremely dense in neocortex, this bias may have led us to select axons that were particularly intensely immunoreactive for ChAT and therefore for ChR2-YFP. Hence, our measurements may have been weighted toward axons expressing ChR2-YFP, resulting in an elevated percentage of cholinergic axons that were labeled with ChR2-YFP.
Fig. 4.
Selectivity of ChR2-YFP for cholinergic somata and axons. A: example of ChAT immunoreactivity and ChR2-YFP fluorescence in nucleus basalis. Maximum intensity projection was derived from a 2-photon z-stack. B: example of ChAT immunoreactivity and ChR2-YFP fluorescence in a coronal section of neocortex. A single 2-photon optical section is shown. For both A and B, images were acquired from a coronal section. The location of the images within the section is illustrated schematically above the images. AC, anterior commissure.
To measure the increase in ChR2-YFP expression with time after virus injection (Fig. 2), we calculated the average intensity of a 150- × 150-μm region of nucleus basalis and for neocortex counted the axon density along a randomly selected line of length 200 μm. Fluorescence intensities or axon densities were normalized to the mean 3 wk after virus injection.
Fig. 2.
Time course of expression of ChR2-YFP. A: example of ChR2-YFP fluorescence in nucleus basalis and primary motor cortex 1, 2, and 3 wk after viral injection. Wide-field images are shown. B: quantification of ChR2-YFP fluorescence in nucleus basalis and primary motor cortex 0–6 wk after viral injection. For measurements from nucleus basalis, we measured the mean fluorescence in a region of interest placed over nucleus basalis; for measurements from neocortex, we manually counted axons labeled with ChR2-YFP (see Image Analysis in materials and methods). In both, data were normalized to those at 3 wk of age. Nucleus basalis measurements are from 25 mice, cortical measurements from 13 mice.
Whole cell recording.
Whole cell recordings were obtained from cholinergic neurons in acute slices of nucleus basalis. Slices were prepared as described previously (Hedrick and Waters 2010). During recordings, slices were continuously perfused with artificial cerebrospinal fluid at 36 ± 1°C (in mM): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 20 NaHCO3, 5 HEPES, 25 glucose, 2 CaCl2, 1 MgCl2, pH 7.3, oxygenated with 95% O2-5% CO2. ChR2-YFP-labeled neurons were first identified by wide-field fluorescence microscopy, and somatic whole cell recordings were subsequently obtained under visual control using infrared difference interference contrast optics (IR-DIC), an Olympus BX51 microscope body with a ×40, 0.8-numerical aperture (NA) water-immersion objective and a Hamamatsu ORCA-285 CCD camera (Hamamatsu Photonics, Hamamatsu City, Japan) and using Sutter MP-225 manipulators (Sutter Instrument). Pipettes were pulled from borosilicate glass (Sutter Instrument) and were 4–9 MΩ when filled with intracellular solution. Recordings were obtained with an Axoclamp-2A amplifier (Molecular Devices, Sunnyvale, CA) and the following intracellular solution (in mM): 135 potassium gluconate, 4 KCl, 10 HEPES, 4 MgATP, 0.3 NaGTP, 10 Na2-phosphocreatine, 0.2% (wt/vol) biocytin (pH 7.3, 291–293 mosM). In slice experiments, ChR2 was activated by wide-field illumination through a ×40/0.8-NA or ×20/0.95-NA microscope objective (Olympus America, Center Valley, PA) using a blue light-emitting diode (LED; Thorlabs LEDC5 and LEDD1 driver; Newton, NJ). The maximum steady-state intensity obtained with this LED was 6.5 mW/mm2 (×20/0.95-NA objective), which is below the damage threshold of ∼100 mW/mm2 for blue light (Cardin et al. 2010).
Two-photon microscopy.
Two-photon microscopy was performed using a microscope designed and built by J. Waters and based on an Olympus BX51 frame. The specimen was illuminated with 840-nm light from a Ti:sapphire laser (80-MHz repetition rate, 100- to 150-fs pulse width; Chameleon Ultra; Coherent, Santa Clara, CA). Excitation light was focused onto the specimen using a ×40/0.8-NA or ×20/1.0-NA water-immersion objective (Olympus America). Emitted light was collected in the epifluorescence configuration through a 680-nm dichroic reflector and an infrared-blocking emission filter (ET700sp-2p; Chroma Technology, Bellows Falls, VT). Fluorescence was split into red and green channels with a secondary dichroic reflector (570 DCXR; Chroma Technology) and detected via 490- to 560- and 575- to 645-nm band-pass filters (Chroma Technology) using photomultiplier tubes (R6357; Hamamatsu Photonics). Scanning and image acquisition were controlled using custom software written in LabVIEW (National Instruments, Austin, TX).
Anesthetized mouse preparation and recording.
Postnatal days 40–50 mice, 3–4 wk after virus injection, were anesthetized with isoflurane (2.5%, inhaled) and urethane (1 g/kg ip). Depth of anesthesia was sufficient to eliminate pinch withdrawal, palpebral reflex, and vibrissal movements and was assessed periodically during the experiment with supplementary doses of urethane administered to maintain deep anesthesia. Core body temperature of 37°C was maintained with a heating pad and rectal probe. A steel head plate was attached to the skull with dental acrylic, and a cranial window was opened above primary somatosensory cortex (2 × 2 mm, centered 1.5 mm posterior and 3 mm lateral to bregma). The craniotomy was covered with, in mM, 135 NaCl2, 5.4 KCl, 1 MgCl2, 1.8 CaCl2, 5 HEPES, pH 7.4. LFPs were recorded with a glass electrode (<1 MΩ when filled with the above solution) and Axopatch 200B amplifier (Molecular Devices) and low-pass filtered at 100 Hz with a four-pole RC filter (Krohn-Hite model 3202). Recordings were acquired at 20 kHz with a National Instruments board (NI-PCI6259) and software custom-written by J. Waters in LabVIEW (National Instruments).
Desynchronization was evoked using several stimuli. Tail pinch was performed manually and lasted approximately 1–2 s. Electrical stimulation of nucleus basalis was performed with 100–300 μA, 0.1-ms pulses at 100 Hz, delivered through a bipolar tungsten electrode with tip separation of 0.5–1 mm (WPI A365 stimulus isolator and TM31A10 electrodes; World Precision Instruments, Sarasota, FL). The electrode was placed stereotaxically, 0.2 mm posterior to bregma, 1.7 mm lateral, and 4.5 mm deep. For blue light illumination of the neocortical surface, light from a 473-nm laser (Optotronics model VA-I-100-473) was directed via an optic fiber (core diameter 550 μm; Thorlabs). The end of the fiber was positioned ∼1 cm from the craniotomy such that the laser illuminated an area of approximately 1 × 1.5 mm on the cortical surface. Illumination intensity was ∼60 mW/mm2 at the cortical surface, which is below the damage threshold of ∼100 mW/mm2 for blue light (Cardin et al. 2010). Desynchronization was evoked with 50 10-ms pulses of 473-nm light at 20 Hz.
Analysis of LFP traces was performed in Igor (WaveMetrics, Lake Oswego, OR) or MATLAB (MathWorks, Natick, MA). Traces were downsampled to 1 kHz, and spectrograms were computed for 5-s sections of the LFP. Mean power in the 0.4- to 1-Hz band was calculated for each section of LFP.
RESULTS
Pattern of ChR2-YFP expression in nucleus basalis and neocortex.
Our aim was to express ChR2-YFP in cholinergic neurons in nucleus basalis and their axons that project to neocortex (Fig. 1A). Nucleus basalis is readily identified in coronal sections as a cluster of cholinergic somata, which can be visualized with anti-ChAT immunocytochemistry (Fig. 1B). We stereotaxically injected double-floxed virus into nucleus basalis of ChAT-Cre mice at P21. This drove expression of ChR2-YFP in neurons near the injection site (Fig. 1B) with ChR2-YFP-labeled somata distributed over 1.58 ± 0.15 × 1.23 ± 0.15 mm (range 1.12–2.2 × 0.8–1.9 mm; n = 7 mice), generally in the ventral aspect of nucleus basalis (Fig. 1, B and C). Diffuse ChR2-YFP fluorescence was visible in the somata of labeled cells, with greater fluorescence associated with the plasma membrane (Fig. 1D), suggesting that ChR2-YFP accumulated in the plasma membrane.
One week after injection of virus, ChR2-YFP fluorescence was weak in nucleus basalis (Fig. 2). Fluorescence in nucleus basalis increased through weeks 2 and 3 and stabilized ∼3 wk after viral injection (Fig. 2). The number of ChR2-YFP-labeled axons in primary motor (M1) and somatosensory (S1) regions of neocortex increased with a similar time course (Fig. 2). In our experiments, we therefore used mice at postnatal days 40-52, 3–4 wk after injection with virus. At this age, a dense network of ChR2-YFP-labeled axons extended throughout all layers of primary somatosensory and motor cortices, with local increases in axonal fluorescence in layer 1 and along the upper and lower borders of layer 5 (Fig. 3).
ChR2-YFP-labeled somata in nucleus basalis were almost all cholinergic (Fig. 4A): 98.01 ± 2.15% of ChR2-YFP-labeled somata were ChAT-positive (range 94.25–100%, n = 4,418 somata in a single section from each of 7 mice). Of ChAT-positive somata in the injection site, 27.7 ± 2.15% (range 21.5–35.2%) were labeled with ChR2-YFP. Similarly, almost all neocortical axons labeled with ChR2-YFP were cholinergic (Fig. 4B): 94.4 ± 1.3% of ChR2-YFP-labeled axons were ChAT-positive (range 93–98%, n = 400 axons in 4 mice). Of ChAT-positive axons in neocortex, 56.4 ± 10.15% (range 40.6–77.0%, n = 100 axons in 4 mice) were labeled with ChR2-YFP.
To determine whether our injections caused loss of cholinergic neurons in nucleus basalis, we compared the dimensions of nucleus basalis and the numbers of ChAT-positive somata in opposing hemispheres. The dimensions of nucleus basalis were 2.36 ± 0.13 × 1.39 ± 0.16 mm (range 1.9–2.7 × 1.1–1.7 mm; n = 7 mice) in the injected hemisphere and 2.15 ± 0.08 × 1.44 ± 0.05 mm (range 1.9–2.45 × 1.3–1.6 mm; n = 7 mice) in the contralateral hemisphere, indicating that our injections did not alter the size of nucleus basalis (the dimension in major and minor axes were both similar; P > 0.1, 2-tailed unpaired t-test). ChR2-YFP-labeled somata were all within nucleus basalis, with the occasional exception of a few labeled somata in ventral striatum, close to the injection tract. In each mouse, we examined a single 100-μm thick section with dense somatic ChR2-YFP expression, finding 308 ± 42 (range 181–424) ChAT-positive somata in nucleus basalis (n = 7 mice). The contralateral hemisphere contained 323 ± 49 somata (range 184–555; not significantly different, P > 0.1, 2-tailed unpaired t-test). Hence, no cholinergic somata were lost from nucleus basalis as a result of viral injection.
Both opening a craniotomy and inserting a glass pipette into the brain can provoke an immune response that may include long-lasting changes in astrocyte morphology (e.g., Holtmaat et al. 2009; Xu et al. 2007; Yan et al. 2009). We therefore looked for evidence of an immune response as a result of viral injections. Astrocyte number and morphology have previously been used as a marker for inflammation in the CNS (Holtmaat et al. 2009; Xu et al. 2007; Yan et al. 2009), and we therefore visualized astrocytes by immunocytochemistry for GFAP. We compared GFAP immunofluorescence intensity and astrocyte morphology near the injection site and in contralateral nucleus basalis and also in neocortex ipsi- and contralateral to the injection site. Astrocyte density and morphology in both nucleus basalis and neocortex were unaffected 1 wk (4 mice), 2 wk (4 mice), and 3 wk (7 mice) after injections (Fig. 5). We conclude that viral injection and subsequent expression of ChR2-YFP in cholinergic neurons, as described here, do not cause inflammation in nucleus basalis or neocortex.
Fig. 5.
Astrocyte morphology is unchanged by ChR2-YFP expression. A: example of glial fibrillary acidic protein (GFAP) immunohistochemistry. Middle: wide-field image of a coronal section. The extent of ChR2-YFP labeling is outlined. Peripheral images: 2-photon maximum intensity projections from ipsi- and contralateral nucleus basalis and neocortex. B: intensity of anti-GFAP immunofluorescence as a function of time after viral injection, expressed as a ratio in intensity in ipsi- and contralateral hemispheres. The ratio of ∼1 indicates that there was no increase in anti-GFAP immunofluorescence near the injection site. Points are the means ± SE from 3, 4, 4, and 1 mice at 1, 2, 3, and 4 wk, respectively.
In summary, our injections resulted in expression of ChR2-YFP in a substantial minority of cholinergic somata in nucleus basalis and axons in neocortex, with little labeling of noncholinergic cells in nucleus basalis or of cells outside nucleus basalis and without cell loss or an immune response.
Health of ChAT-YFP-positive neurons in nucleus basalis.
We prepared acute coronal slices of nucleus basalis as described previously (Hedrick and Waters 2010) and obtained whole cell recordings from ChR2-YFP-labeled neurons (Fig. 6A). Virally infected neurons displayed the characteristic spiking pattern, large slow afterhyperpolarization, and limited spiking rate characteristic of cholinergic neurons in nucleus basalis and other nuclei of the basal forebrain complex (Fig. 6, B and C; Gorelova and Reiner 1996; Griffith 1988; Griffith et al. 1986, 1991; Hedrick and Waters 2010; Markram and Segal 1990). To determine whether viral infection and ChR2-YFP expression affected the physiology of infected neurons, we compared a large number of physiological parameters with those we reported previously for cholinergic nucleus basalis neurons in mice that had not received injections (Hedrick and Waters 2010). Three weeks after a 400-nl viral injection, none of the measured cellular properties differed from those of uninfected neurons (Table 1). In contrast, after 800-nl injections, many cellular parameters differed from those of uninfected neurons (Table 1). Hence, expression of ChR2-YFP via viral infection can alter the cellular physiology of cholinergic neurons, but in our experiments these deleterious effects were not observed when the volume of injected virus was no more than 400 nl.
Fig. 6.
Health of neurons in nucleus basalis expressing ChR2-YFP. A: images of an infected neuron in an acute slice from nucleus basalis. The infected neuron expresses ChR2-YFP (green) and was filled with Alexa Fluor 594 (red) via a whole cell recording. Images are maximum intensity projections acquired by 2-photon microscopy using excitation wavelengths of 840 nm (Alexa Fluor 594 image) and 910 nm (ChR2-YFP image). B: example voltage recordings from a neuron in nucleus basalis labeled with ChR2-YFP. Note the pronounced slow afterhyperpolarization (arrowheads), which is characteristic of cholinergic neurons in nucleus basalis (Hedrick and Waters 2010). Current injections were 25 pA for 300 ms and 900 pA for 1 ms. C: mean ± SE spiking frequency of 9 infected neurons as a function of current injected via the somatic recording pipette.
Table 1.
Cellular properties of infected cholinergic neurons
| 400-nl Injections | 800-nl Injections | |
|---|---|---|
| Resting membrane potential, mV | −48 ± 1.8 (n = 9; P > 0.1) | −43 ± 1.8* (n = 13; P < 0.01) |
| Resting input resistance, MΩ | 268 ± 42 (n = 9; P > 0.1) | 152 ± 27* (n = 13; P < 0.05) |
| Rectification (CAR), MΩ/nA | 400 ± 137 (n = 9; P > 0.1) | 124 ± 61* (n = 13; P < 0.05) |
| Resting membrane time constant, ms | 28.2 ± 3.5 (n = 9; P > 0.1) | 15.3 ± 3.1 (n = 13; P > 0.05) |
| Sag ratio | 0.97 ± 0.01 (n = 9; P > 0.1) | |
| Rheobase, pA | 33 ± 4.6 (n = 9; P > 0.1) | 32 ± 3.2 (n = 13; P > 0.1) |
| Action potential threshold, mV | −31.9 ± 0.8 (n = 9; P > 0.1) | −26.8 ± 2.2 (n = 13; P > 0.1) |
| Action potential amplitude, mV | 66.7 ± 4.5 (n = 9; P > 0.1) | 55.6 ± 5.0 (n = 13; P > 0.1) |
| Action potential half-width, ms | 0.52 ± 0.03 (n = 9; P > 0.1) | 0.69 ± 0.02* (n = 13; P < 0.01) |
| Action potential 10-90% rise time, ms | 0.25 ± 0.02 (n = 9; P > 0.1) | 0.31 ± 0.02* (n = 13; P < 0.05) |
| Action potential 10-90% decay time, ms | 0.57 ± 0.05 (n = 9; P > 0.1) | 0.67 ± 0.04* (n = 13; P < 0.01) |
| fAHP amplitude, mV | 16 ± 2.6 (n = 9; P > 0.1) | 12 ± 1.8 (n = 13; P > 0.1) |
| sAHP amplitude, mV | 8.7 ± 2.1 (n = 8; P > 0.1) | 5.5 ± 1.5* (n = 9; P < 0.01) |
| sAHP latency, ms | 34 ± 3.0 (n = 8; P > 0.1) | 54 ± 12 (n = 9; P > 0.1) |
Values are means ± SE. n Indicates number of neurons.
Significant difference from that of cholinergic neurons in nucleus basalis in uninfected mice [unpaired t-test; comparison with results in Table 1 of Hedrick and Waters (2010)]. fAHP, fast afterhyperpolarization; sAHP, slow afterhyperpolarization.
Blue light drives depolarization and spiking of ChAT-YFP-positive cholinergic neurons in nucleus basalis.
To activate ChR2, we illuminated the slice via the microscope objective with a blue LED. We first measured the temporal characteristics of the LED using a photomultiplier tube to measure its output. The LED was triggered with a square-step voltage command. After an initial delay of ∼180 μs, the LED provided illumination with an extremely brief initial transient, followed by a sustained intensity (Fig. 7A). The rise time of the illumination was extremely rapid, the intensity reaching a steady-state in ≤10 μs. At the end of the step-voltage command, the decay was approximately exponential with a time constant of 33 μs. hChR2 opens and closes with time constants in the 1- to 10-ms range at 37°C (Lin et al. 2009; Nagel et al. 2005; Yizhar et al. 2011). Hence, the LED switches on and off extremely quickly relative to the kinetics of ChR2.
Fig. 7.
Blue light depolarizes infected neurons in nucleus basalis. A: output of a blue light-emitting diode (LED) in response to 2-ms commands of 1, 3, and 5 V. Output was measured with a photomultiplier tube. Intensity at the focal plane (y-axis) was calculated for a ×40/0.8-numerical aperture (NA) objective after measuring the intensity of illumination through the objective using a handheld light meter. B: example voltage recordings from the neuron shown in Fig. 6, showing responses to blue light illumination of 300 and 5 ms. The timing of blue light illumination is indicated below each voltage trace. Illumination intensity was 6.5 mW/mm2. C: voltage responses of an infected neuron to trains of 5-ms blue light stimuli at different frequencies. Illumination intensity was 6.5 mW/mm2. D: spiking probability as a function of stimulus frequency during trains of 5-ms blue light stimuli. Points are for the neuron in E. The line and shaded area are the mean relationship and 95% confidence interval, respectively, for 5 neurons. Illumination intensity was 6.5 mW/mm2.
Infected neurons depolarized in response to wide-field illumination of the slice (Fig. 7B), whereas blue light never evoked responses in uninfected neurons (data not shown). When exposed to 5-ms pulses of blue light, infected neurons failed to follow at high frequencies, with 50% of pulses evoking a spike at 11.4 ± 3 Hz (Fig. 7, C and D; n = 5 neurons). This failure is expected and probably results from the large afterhyperpolarization, which places a limit of ∼15 Hz on the maximum frequency at which these neurons can spike (Hedrick and Waters 2010).
Optogenetic desynchronization of the neocortical network.
During some sleep states and under many general anesthetics, waves of activity sweep across neocortex, driving synchronous activation of many neurons. This synchronous activity is visible as deflections in the neocortical LFP at approximately 0.5–2 Hz and can be disrupted by activation of the cholinergic projection from nucleus basalis, causing desynchronization of the LFP in neocortex, in which the periodic deflections are temporarily eliminated (Metherate et al. 1992). Hence, desynchronization is commonly used to test for cholinergic activity in neocortex when placing stimulating electrodes in nucleus basalis, for example. To test whether blue light could activate ChR2-YFP-labeled axons in neocortex, we monitored the neocortical LFP during illumination of the neocortical surface with blue light.
In an anesthetized mouse, expressing ChR2-YFP in cholinergic axons, we opened a 2- × 2-mm cranial window over primary motor or primary somatosensory neocortex. We measured the LFP with a glass electrode, the tip of which was placed ∼400 μm below the pial surface of neocortex. To activate ChR2, we directed light from a 473-nm laser into a multimode optical fiber with a 550 μm-diameter core and positioned the flat-cleaved end of the fiber ∼1 cm from the craniotomy such that an approximately 1.5- × 1-mm region within the craniotomy was illuminated with 473-nm light.
We first verified that a strong sensory stimulus such as a tail pinch (Fig. 8A) or electrical stimulation of nucleus basalis (Fig. 8B) could desynchronize the neocortical LFP, as described previously (Metherate et al. 1992). Illumination of the neocortical surface with 473-nm light also evoked desynchronization of the LFP in virally injected mice (Fig. 8C). Tail pinch and electrical stimulation of nucleus basalis evoked desynchronization in uninjected mice, but in uninjected mice illumination of the neocortical surface had no effect on the LFP (5 uninjected mice; data not shown).
Fig. 8.
Optogenetic desynchronization of the neocortical local field potential. A–C: examples of desynchronization of the local field potential measured in primary motor cortex, evoked by a brief (∼2-s) manual pinch of the animal's tail (A), by electrical stimulation of nucleus basalis (B), and by illumination of the neocortical surface with blue (473-nm) light (C; 10-ms pulses at 20 Hz). Each example is from a different mouse. D: power-spectral densities during 3 5-s sections of the local field recording illustrated in C, starting 10, 5, and 0 s before onset of blue light illumination. The 3 sections are color-coded and numbered (inset), with red representing the 1st section from 10 to 5 s before illumination (section 1), green representing the 2nd section from 5 to 0 s before illumination (section 2), and illumination beginning at the start of the blue section (section 3). Power in the 0.4- to 1-Hz band (shaded area) was used to monitor desynchronization. E–G: traces in A–C band-pass filtered at 0.4–1 Hz. H–J: mean (±SE) changes in power-spectral density at 0.4–1 Hz for tail pinch, electrical stimulation of nucleus basalis, and 473-nm illumination of neocortex, respectively. Each bar represents the power in a 5-s long section of the trace, with the stimulus beginning at the start of the 3rd section. In each trial, the power was normalized to the average of the 10-s long prestimulus period. Tail pinch: manual tail pinch for 2 s, n = 8 mice. Electrical stimulation: 50 pulses at 100 Hz, n = 5 mice. 473-nm Illumination: 50- × 10-ms pulses at 20 Hz, n = 9 mice. K: effect of blue light on 0.4- to 1-Hz power, expressed as a ratio of power after and before illumination (power in section 3 ÷ power in section 1). In this animal, neocortex was illuminated 19 times over ∼1 h, and the power ratio is plotted for each trial. As an internal control, the power ratio during sections 1 and 2 is also plotted for each trial. L: frequency histogram of power ratios for the animal in E, with each trial counted once. Power ratio was reduced in response to illumination with 473-nm light. M: frequency histogram summarizing results from 25 mice. In each mouse, desynchronization was monitored in multiple trials, yielding a median power ratio for the effect of blue light (ratio 3:1) and for the prestimulus period (ratio 2:1) for each mouse.
To quantify desynchronization, we compared the spectral power density of the LFP before and after the onset of blue light illumination. Desynchronization reduced spectral power density at low frequencies, up to 1–2 Hz (Fig. 8D). For each trial, we calculated the power in the 0.4–1 Hz band in 5-s long sections of the LFP recording (Fig. 8, H–J). Desynchronization in response to illumination lasted for only a few seconds, which is briefer than typically observed following tail pinch or electrical stimulation (Fig. 8, H–J), but could be evoked repeatedly (Fig. 8K). For each trial, we calculated the ratio of power before and after the onset of illumination (3:1 ratio = power in section 3 ÷ power in section 1; see Fig. 8D, inset). In the example shown in Fig. 8, this ratio was consistently <1 over ∼1 h of recording (Fig. 8K). As a control, we calculated the power ratio for two sections of local field recording before blue light illumination (2:1 ratio = power in section 2 ÷ power in section 1; see inset in Fig. 8D). As expected, the mean of this control ratio, over multiple trials, was ∼1 (Fig. 8L). These two power ratios were significantly different (P < 0.0001, Wilcoxon matched-pairs signed-rank test), leading us to conclude that blue light illumination reproducibly evoked desynchronization in this mouse. Using this approach, we observed significant desynchronization (P < 0.05) in ∼70% of mice examined (17 of 25 mice; Fig. 8M). Desynchronization in response to blue light was suppressed by addition of cholinergic receptor antagonists to the surface of neocortex (Fig. 9; 3:1 ratio of 0.59 ± 0.09 before and 0.96 ± 0.07 after addition of 100 μM atropine and 3 mM mecamylamine, n = 9 mice), indicating that desynchronization can be evoked by optogenetic activation in neocortex of cholinergic axons from nucleus basalis.
Fig. 9.
Blue light fails to evoke desynchronization in ACh receptor antagonists. A: example trace under control conditions, showing desynchronization is response to blue (473-nm) light (50 10-ms pulses at 20 Hz). B: power-spectral densities during 3 5-s sections of the local field recording illustrated in A, starting 10, 5, and 0 s before onset of blue light illumination. The 3 sections are color-coded and numbered (inset), with red representing the 1st section from 10 to 5 s before illumination (section 1), green representing the 2nd section from 5 to 0 s before illumination (section 2), and illumination beginning at the start of the blue section (section 3). Power in the 0.4- to 1-Hz band (shaded area) was used to monitor desynchronization. C and D: example trace and corresponding power-spectral densities after application of cholinergic receptor antagonists to the brain surface. E: mean ± SE desynchronization (3:1 ratio, 0.4- to 1-Hz band) before and after addition of 100 μM atropine and 3 mM mecamylamine to the brain surface in the experiment illustrated in A–D; n = 20 and 25 trials under control conditions (ctrl) and in cholinergic receptor antagonists (+ ant.), respectively. F: pooled results showing the mean (±SE) of 3:1 ratio before and after addition of cholinergic receptor antagonists in 9 mice.
DISCUSSION
We have described a method for the selective stimulation of the neocortical axons of cholinergic neurons in nucleus basalis. Previous studies of the effects of this projection on neocortical neurons have used electrical stimulation to drive release of ACh (e.g., Berg et al. 2005; Metherate and Ashe 1993; Metherate et al. 1992; Sachdev et al. 1998). However, cholinergic and noncholinergic somata are not segregated in nucleus basalis nor are their projections to neocortex (Henry and Jones 2008; Wainer and Mesulam 1990). In addition, nucleus basalis contains fiber tracts from neighboring nuclei (e.g., hypothalamus; Cullinan and Záborszky 1991), some of which may project to neocortex. Hence, electrical stimulation results in the activation of cholinergic and noncholinergic projecting neurons. We have shown that optogenetics provides a method to evoke neocortical desynchronization via the selective activation of only the cholinergic projection from nucleus basalis.
We drove selective expression of ChR2 in the cholinergic projection from nucleus basalis to neocortex, using viral injection to localize ChR2 expression to nucleus basalis and the Cre-lox system to limit ChR2 expression to cholinergic neurons. ChR2 has previously been expressed in cholinergic neurons in the rodent CNS. We used a similar approach to that described by Witten et al. (2010), who employed ChAT-Cre mice and a floxed adeno-associated virus to express ChR2-YFP in cholinergic interneurons in nucleus accumbens. This approach has also been employed to drive expression of ChR2 in the cholinergic projection from medial septum to hippocampus (Bell et al. 2011; Gu and Yakel 2011). Presumably, neither of these approaches labeled neurons in nucleus basalis or their axons in neocortex. Expression of ChR2 in nucleus basalis and other populations of cholinergic neurons has been reported in transgenic mouse lines in which ChR2 expression was driven by the ChAT promoter (Zhao et al. 2011). ChR2-YFP was observed in neocortex in these mice but was expressed by local cholinergic interneurons and probably in projections from nucleus basalis and other extracortical regions. It is therefore unclear whether it would be possible to stimulate selectively the projection from nucleus basalis in these mice. Hence, our methodology is similar to several strategies that have been employed previously to stimulate cholinergic neurons but offers greater selectivity for the cholinergic projection from nucleus basalis to neocortex.
In our experiments, expression of ChR2-YFP in nucleus basalis and in neocortex developed over the 1st 3 wk after viral injection, comparable with previous reports using AAVs to drive ChR2 expression, in which expression was generally permitted to develop for ≥2 wk (Carter et al. 2010; Gu and Yakel 2011; Petreanu et al. 2009; Witten et al. 2010).
We found expression of ChR2 was almost exclusively in cholinergic neurons within nucleus basalis. This specificity is similar to that in a previous report in which similar techniques were used to express ChR2 in cholinergic interneurons in nucleus accumbens, where 91% of ChR2-expressing neurons were cholinergic (Witten et al. 2010). In contrast to this previous report, in which 93.5% of cholinergic interneurons expressed ChR2, we found that only ∼30% of cholinergic neurons in nucleus basalis expressed ChR2-YFP. The reason for the lower infection efficiency in our experiments is unknown, but increasing the volume of injected virus, to increase the number of viral infections per neuron and thereby expression of ChR2-YFP, resulted in unhealthy neurons in nucleus basalis. Importantly, expression in this significant minority of ∼30% of cholinergic neurons was enough to desynchronize the neocortical LFP.
We observed no effects on cellular physiology following 400-nl injections of virus, but 800-nl injections were deleterious. Whether deleterious effects were due to viral infection or to the subsequent expression of ChR2-YFP is unclear, but previous authors using AAV to drive ChR2 expression have injected comparable volumes of virus with a similar titer to that used here (e.g., 500 nl to 1 μl, >1012 gc/ml; Carter et al. 2010; Gu and Yakel 2011) with no reported ill effects. These previous results and the lack of inflammation apparent in our GFAP staining suggest that the volume of injected material is unlikely to have been problematic. Within each preparation, the number of infecting viral particles and density of expression of ChR2-YFP probably differ considerably between neurons, resulting in considerable cell-to-cell variability in the effects of viral infection. It is likely, therefore, that some neurons in nucleus basalis were adversely affected after 400-nl injections, although the majority (>90%) of neurons in the nucleus basalis were unaffected.
Previous studies have typically activated ChR2 by delivering blue light to labeled somata via an optic fiber, often pushed deep into the brain (e.g., Tsai et al. 2009). Where neurons in neocortex express ChR2, illumination of the neocortical surface with blue light is a viable alternative (e.g., Ayling et al. 2009). In brain slice preparations, transmitter release can be evoked by illumination of axons expressing ChR2 (e.g., Cruikshank et al. 2010; Petreanu et al. 2007). We therefore reasoned that in our preparation, in which axons expressing ChR2-YFP ramify throughout neocortex, illumination of the surface of neocortex with blue light should evoke release of ACh within neocortex. Surface illumination is less invasive than an implanted fiber and provides simple, accurate targeting of blue light to cholinergic axons in the neocortical area of interest. In contrast, an implanted fiber might illuminate much or perhaps all of nucleus basalis, and if there were significant interanimal variability in the pattern or levels of expression of ChR2 in cholinergic axons, illumination of the somata in nucleus basalis might offer less interexperiment variability. Nevertheless, we were routinely able to activate cortical axons by surface illumination with blue light. Use of the H134R variant of ChR2 and a double-floxed virus were important in this regard, driving high levels of expression of ChR2 in axons without significant expression in noncholinergic neurons.
The spatial distribution of cholinergic axons activated in our experiments is unknown. Activation of axons by blue light is likely to have been largely restricted to the region of neocortex under the craniotomy since a neuron in nucleus basalis innervates only a small region of neocortex, no more than 1–1.5 mm in diameter (Price and Stern 1983). In our experiments, we illuminated an approximately 1- × 1.5-mm area on the surface of neocortex. If activated axons may extend up to 1.5 mm from the site of activation, this would result in activation of axons over an area of ≤ 4 × 4.5 mm.
A limiting factor inherent to optical techniques is poor penetration of light into brain tissue, with the power density of blue light declining precipitously over hundreds of micrometers (Yizhar et al. 2011). In our experiments, cholinergic neurons in slices of nucleus basalis spiked in response to a few milliwatts per square millimeter of blue light. In vivo, we applied ∼60 mW/mm2 blue light to the surface of neocortex, but the power density of blue light would decline to less than a few milliwatts per square millimeter within approximately 200–300 μm of the neocortical surface (Aravanis et al. 2007; Yizhar et al. 2011). It is likely, therefore, that the blue light intensity was sufficient to initiate spikes in cholinergic axons in layer 1 and perhaps 2 and 3 but not in deeper layers of neocortex or in other brain regions. However, many cholinergic axons from nucleus basalis traverse all layers of neocortex, from white matter to layer 1 (Eckenstein et al. 1988), so action potentials that initiate in superficial branches of cholinergic axons may propagate antidromically, releasing ACh in all layers of neocortex. The high density of cholinergic axons in layer 1, as observed here and as expected for cholinergic axons from nucleus basalis (Avendanǒ et al. 1996; Eckenstein et al. 1988; Lysakowski et al. 1989; Mechawar et al. 2000; Wainer and Mesulam 1990), may facilitate this process by maximizing the number of cholinergic axons exposed to blue light near the brain surface.
In summary, here we have described the application of optogenetics to allow selective simulation of the cholinergic projection from nucleus basalis to neocortex. Selectivity is a key advantage of optogenetics that will facilitate the study of the ascending cholinergic system in neocortex and to the study of other connections throughout the brain. With a large and expanding library of Cre mice and viral tools now both commercially available, this general approach will also be widely applicable to the study of other projections within the brain.
GRANTS
This work was supported by the National Institute of Mental Health (5T32MH067564-08 and 5R21MH085117-02) and the Brain Research Foundation (BRF SG 2010-13).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.K. and T.H. performed viral injections; A.K., T.H., and J.W. acquired and analyzed the results; J.W. supervised the project; J.W. and A.K. wrote the manuscript.
ACKNOWLEDGMENTS
We thank Rebecca Imhoff for expert technical assistance.
REFERENCES
- Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng 4: S143–S156, 2007 [DOI] [PubMed] [Google Scholar]
- Avendanǒ C, Umbriaco D, Dykes RW, Descarries L. Acetylcholine innervation of sensory and motor neocortical areas in adult cat: a choline acetyltransferase immunohistochemical study. J Chem Neuroanat 11: 113–130, 1996 [DOI] [PubMed] [Google Scholar]
- Ayling OG, Harrison TC, Boyd JD, Goroshkov A, Murphy TH. Automated light-based mapping of motor cortex by photoactivation of channelrhodopsin-2 transgenic mice. Nat Methods 6: 219–224, 2009 [DOI] [PubMed] [Google Scholar]
- Bell KA, Shim H, Chen CK, McQuiston AR. Nicotinic excitatory postsynaptic potentials in hippocampal CA1 interneurons are predominantly mediated by nicotinic receptors that contain α4 and β2 subunits. Neuropharmacology 61: 1379–1388, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg RW, Friedman B, Schroeder LF, Kleinfeld D. Activation of nucleus basalis facilitates cortical control of a brain stem motor program. J Neurophysiol 94: 699–711, 2005 [DOI] [PubMed] [Google Scholar]
- Bigl V, Woolf NJ, Butcher LL. Cholinergic projections from the basalforebrain to frontal, parietal, temporal, occipital, and cingulate cortices: a combined fluorescent tracer and acetylcholinesterase analysis. Brain Res Bull 8: 727–749, 1982 [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Gage FH. The cholinergic nucleus basalis: a key structure in neocortical arousal. EXS 57: 159–171, 1989 [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of channelrhodopsin-2. Nat Protoc 5: 247–254, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Surmeier DJ. M1 muscarinic receptor modulation of Kir2 channels enhances temporal summation of excitatory synaptic potentials in prefrontal cortex pyramidal neurons. J Neurophysiol 97: 3432–3438, 2007 [DOI] [PubMed] [Google Scholar]
- Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci 13: 1526–1535, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu ZG, Zhou FM, Hablitz JJ. Nicotinic acetylcholine receptor-mediated synaptic potentials in rat neocortex. Brain Res 887: 399–405, 2000 [DOI] [PubMed] [Google Scholar]
- Conner JM, Culberson A, Packowski C, Chiba AA, Tuszynski MH. Lesions of the basal forebrain cholinergic system impair task acquisition and abolish cortical plasticity associated with motor skill learning. Neuron 38: 819–829, 2003 [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Urabe H, Nurmikko AV, Connors BW. Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron 65: 230–245, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan WE, Záborszky L. Organization of ascending hypothalamic projections to the rostralforebrain with special reference to the innervation of cholinergic projection neurons. J Comp Neurol 306: 631–667, 1991 [DOI] [PubMed] [Google Scholar]
- Delmas P, Brown DA. Pathways modulating neural KCNQ/M(Kv7) potassium channels. Nat Rev Neurosci 6: 850–862, 2005 [DOI] [PubMed] [Google Scholar]
- Eckenstein FP, Baughman RW, Quinn J. An anatomical study of cholinergic innervation in rat cerebral cortex. Neuroscience 25: 457–474, 1988 [DOI] [PubMed] [Google Scholar]
- Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci 27: 9817–9823, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelova N, Reiner PB. Histamine depolarizes cholinergic septal neurons. J Neurophysiol 75: 707–714, 1996 [DOI] [PubMed] [Google Scholar]
- Griffith WH. Membrane properties of cell types within guinea pig basal forebrain nuclei in vitro. J Neurophysiol 59: 1590–1612, 1988 [DOI] [PubMed] [Google Scholar]
- Griffith WH, Matthews RT. Electrophysiology of AChE-positive neurons in basal forebrain slices. Neurosci Lett 71: 169–174, 1986 [DOI] [PubMed] [Google Scholar]
- Griffith WH, Sim JA, Matthews RT. Electrophysiologic characteristics of basal forebrain neurons in vitro. Adv Exp Med Biol 295: 143–155, 1991 [DOI] [PubMed] [Google Scholar]
- Gu Z, Yakel JL. Timing-dependent septal cholinergic induction of dynamic hippocampal synaptic plasticity. Neuron 71: 155–165, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Stuart GJ. Cholinergic inhibition of neocortical pyramidal neurons. J Neurosci 25: 10308–10320, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Dahmane S, Andrade R. Muscarinic activation of a voltage dependent cation nonselective current in rat association cortex. J Neurosci 16: 3848–3861, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell JV. Cholinergic responses in human neocortical neurones. EXS 57: 138–149, 1989 [DOI] [PubMed] [Google Scholar]
- Hedrick T, Waters J. Physiological properties of cholinergic and non-cholinergic magnocellular neurons in acute slices from adult mouse nucleus basalis. PLoS One 5: e11406, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henny P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neurosci 27: 654–670, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero JL, Roberts MJ, Delicato LS, Gieselmann MA, Dayan P, Thiele A. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature 454: 1110–1114, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DA, Johnston D. Neuromodulation of dendritic action potentials. J Neurophysiol 81: 408–411, 1999 [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Bonhoeffer T, Chow DK, Chuckowree J, De Paola V, Hofer SB, Hübener M, Keck T, Knott G, Lee WC, Mostany R, Mrsic-Flogel TD, Nedivi E, Portera-Cailliau C, Svoboda K, Trachtenberg JT, Wilbrecht L. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat Protoc 4: 1128–1144, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard M. Cholinergic modulation of skill learning and plasticity. Neuron 38: 678–680, 2003 [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science 279: 1714–1718, 1998 [DOI] [PubMed] [Google Scholar]
- Lin JY, Lin MZ, Steinbach P, Tsien RY. Charcaterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys J 96: 1803–1814, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysakows A, Wainer BH, Bruce G, Hersh LB. An atlas of the regional and laminar distribution of choline acetyltransferase immunoreactivity in rat cerebral cortex. Neuroscience 28: 291–336, 1989 [DOI] [PubMed] [Google Scholar]
- Markram H, Segal M. Electrophysiological characteristics of cholinergic and non-cholinergic neurons in the rat medial septum-diagonal band complex. Brain Res 513: 171–174, 1990 [DOI] [PubMed] [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol 39: 337–388, 1992 [DOI] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Two types of muscarinic response to acetylcholine in mammalian cortical neurons. Proc Natl Acad Sci USA 82: 6344–6348, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Mechanisms of action of acetylcholine in the guinea-pig cerebral cortex in vitro. J Physiol 375: 169–194, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Williamson A. Convergence and divergence of neurotransmitter action in human cerebral cortex. Proc Natl Acad Sci USA 86: 8098–8102, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechawar N, Cozzari C, Descarries L. Cholinergic innervation in adult rat cerebral cortex: a quantitative immunocytochemical description. J Comp Neurol 428: 305–318, 2000 [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Ionic flux contributions to neocortical slow waves and nucleus basalis-mediated activation: whole-cell recordings in vivo. J Neurosci 13: 5312–5323, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: modulation of neuraloscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci 12: 4701–4711, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol 15: 2279–2284, 2005 [DOI] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directlylight-gated cation-selective membrane channel. Proc Natl Acad Sci USA 100: 13940–13945, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Sarter M. Cholinergic mediation of attention: contributions of phasic and tonic increases in prefrontal cholinergic activity. Ann NY Acad Sci 1129: 225–235, 2008 [DOI] [PubMed] [Google Scholar]
- Paterna JC, Feldon J, Büeler H. Transduction profiles of recombinant adeno-associated virus vectors derived from serotypes 2 and 5 in the nigrostriatal system of rats. J Virol 78: 6808–6817, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci 10: 663–668, 2007 [DOI] [PubMed] [Google Scholar]
- Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature 457: 1142–1146, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Stern R. Individual cells in the nucleus basalis-diagonal band complex have restricted axonal projections to the cerebral cortex in the rat. Brain Res 269: 352–356, 1983 [DOI] [PubMed] [Google Scholar]
- Ramanathan D, Tuszynski MH, Conner JM. The basal forebrain cholinergic system is required specifically for behaviorally mediated cortical map plasticity. J Neurosci 29: 5992–6000, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerig B, Nelson DA, Katz LC. Fast synaptic signaling by nicotinic acetylcholine and serotonin 5-HT3 receptors in developing visual cortex. J Neurosci 17: 8353–8362, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev RN, Lu SM, Wiley RG, Ebner FF. Role of the basal forebrain cholinergic projection in somatosensory cortical plasticity. J Neurophysiol 79: 3216–3228, 1998 [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Spain WJ, Foehring RC, Chubb MC, Crill WE. Slow conductances in neurons from cat sensorimotor cortex in vitro and their role in slow excitability changes. J Neurophysiol 59: 450–467, 1988 [DOI] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459: 698–702, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324: 1080–1084, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainer BH, Mesulam MM. Ascending cholinergic pathways in the rat brain. In: Brain Cholinergic Systems, edited by Steriade M, Biesold D. New York: Oxford Univ. Press, 1990 [Google Scholar]
- Winkler J, Suhr ST, Gage FH, Thal LJ, Fisher LJ. Essential role of neocortical acetylcholine in spatial memory. Nature 375: 484–487, 1995 [DOI] [PubMed] [Google Scholar]
- Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C, Deisseroth K. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science 330: 1677–1681, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HT, Pan F, Yang G, Gan WB. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat Neurosci 10: 549–551, 2007 [DOI] [PubMed] [Google Scholar]
- Yan P, Bero AW, Cirrito JR, Xiao Q, Hu X, Wang Y, Gonzales E, Holtzman DM, Lee JM. Characterizing the appearance and growth of amyloid plaques in APP/PS1 mice. J Neurosci 29: 10706–10714, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron 71: 9–34, 2011 [DOI] [PubMed] [Google Scholar]
- Zhao S, Ting JT, Atallah HE, Qiu Li Tan J, Gloss B, Augustine GJ, Deisseroth K, Luo M, Graybiel AM, Feng G. Cell type-specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat Methods 8: 745–752, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]