Abstract
Different types of retinal ganglion cells represent distinct spatiotemporal filters that respond selectively to specific features in the visual input. Much about the circuitry and synaptic mechanisms that underlie such specificity remains to be determined. This study examines how N-methyl-d-aspartate (NMDA) receptor signaling combines with other excitatory and inhibitory mechanisms to shape the output of small-field OFF brisk-sustained ganglion cells (OFF-BSGCs) in the rabbit retina. We used voltage clamp to separately resolve NMDA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and inhibitory inputs elicited by stimulation of the receptive field center. Three converging circuits were identified. First is a direct glutamatergic input, arising from OFF cone bipolar cells (CBCs), which is mediated by synaptic NMDA and AMPA receptors. The NMDA input was saturated at 10% contrast, whereas the AMPA input increased monotonically up to 60% contrast. We propose that NMDA inputs selectively enhance sensitivity to low contrasts. The OFF bipolar cells, mediating this direct excitatory input, express dendritic kainate (KA) receptors, which are resistant to the nonselective AMPA/KA receptor antagonist, 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt (NBQX), but are suppressed by a GluK1- and GluK3-selective antagonist, (S)-1-(2-amino-2-carboxyethyl)-3-(2-carboxy-thiophene-3-yl-methyl)-5-methylpyrimidine-2,4-dione (UBP-310). The second circuit entails glycinergic crossover inhibition, arising from ON-CBCs and mediated by AII amacrine cells, which modulate glutamate release from the OFF-CBC terminals. The third circuit also comprises glycinergic crossover inhibition, which is driven by the ON pathway; however, this inhibition impinges directly on the OFF-BSGCs and is mediated by an unknown glycinergic amacrine cell that expresses AMPA but not KA receptors.
Keywords: visual system, synaptic transmission, crossover inhibition, OFF cone bipolar cell, ionotropic glutamate receptors
as in other sensory systems, the retina encodes information in parallel channels, which constitute the spiking output from multiple types of retinal ganglion cells (RGCs). Each RGC type represents a distinct spatiotemporal filter that encodes visual information as trains of action potentials. Recent work has identified multiple retinal circuits, using different glutamate receptors, and inhibitory networks, which contribute to the diversity of RGC receptive field characteristics (reviewed by Oesch et al. 2011). However, it remains unclear how synaptic drive from several circuits converges to shape the spike output in a single RGC type. Here, we sought to define the synaptic circuits that generate the center receptive fields of rabbit brisk-sustained RGCs (BSGCs), which have a center-surround receptive-field organization and do not exhibit complex response properties. These cells are likely homologues of the X or β RGCs, studied extensively in the cat and other mammals (Boycott and Wässle 1974; Cleland et al. 1971; Enroth-Cugell and Robson 1966; Hochstein and Shapley 1976; Shapley and Victor 1978; Troy 1983; Vaney et al. 1981). As in the cat, there are two populations of BSGCs in rabbit—OFF-BSGCs and ON-BSGCs—which are activated by negative and positive contrasts, respectively. This paper will focus exclusively on the OFF-BSGCs.
BSGCs have small dendritic arbors and correspondingly small receptive fields and occur at a density sufficient to generate a complete, high-acuity representation of the visual field (Devries and Baylor 1997; Pu and Amthor 1990). Another ganglion cell, the local edge detector (LED), also provides a high-acuity representation of the visual field; however, its temporal response properties are sluggish relative to the OFF-BSGC. Unlike LEDs, in which α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate (AMPA/KA) receptor currents are primary drivers of excitation, at least in rabbit (van Wyk et al. 2006), cat homologues of BSGCs receive a significant N-methyl-d-aspartate (NMDA) receptor-mediated synaptic input (Cohen 1998, 2000). NMDA receptors have been detected in many types of vertebrate ganglion cell (Cohen and Miller 1994, 1999; Diamond and Copenhagen 1993; Kittila and Massey 1997; Manookin et al. 2010; Massey and Miller 1990; Matsui et al. 1998; Mittman et al. 1990; Tjepkes and Amthor 2000), and recent studies have begun to elucidate the role of these inputs in retinal processing (Manookin et al. 2010; Venkataramani and Taylor 2010). One of our goals was to establish that rabbit OFF-BSGCs also receive a significant NMDA receptor-mediated input and to investigate its functional significance.
Another goal was to determine whether inhibitory circuits, involving narrow-field amacrine cells, contribute to generating the excitatory drive to OFF-BSGCs. Inhibition originating in the ON pathway has been shown to modulate excitatory drive in OFF-RGCs (Chen and Linsenmeier 1989b; Wässle et al. 1986; Zaghloul et al. 2003), an effect termed “crossover inhibition”, since the signals are crossing between the pathways. One such crossover circuit is mediated by direct inputs from narrow-field AII amacrine cells to OFF-RGCs in the guinea pig and mouse (Manookin et al. 2008; van Wyk et al. 2009). Suppression of this input disinhibits the ganglion cells, contributing to excitatory drive when responding to low-contrast stimuli. A similar circuit involving AII amacrine cells feeds back, suppressing the output of glutamate from the OFF cone bipolar cells (CBCs), which make input to the ganglion cells (Liang and Freed 2010; Molnar and Werblin 2007; O'Brien et al. 2003). Crossover inhibition in the retina may be involved in diverse functions, ranging from spatial summation to temporal contrast sensitivity and motion detection (Liang and Freed 2010; Molnar et al. 2009; Munch et al. 2009). These circuits have not been described in narrow-field ganglion cells and to further investigate their prevalence and possible functional roles, we sought evidence for crossover inhibition in OFF-BSGCs.
MATERIALS AND METHODS
Tissue preparation and maintenance.
Procedures involving animals were performed in accordance with National Institutes of Health guidelines and with approval from the Oregon Health & Science University Institutional Animal Care and Use Committee. Pigmented rabbits, aged 5 wk and older, were dark adapted for at least 1 h before sedation by intramuscular injection of ketamine (50 mg/kg) and xylazine (10 mg/kg), followed by surgical anesthesia using intravenous sodium pentobarbital (100 mg/kg). After the eyes were removed, the animal was euthanized by sodium pentobarbital and potassium chloride injection. A central portion of inferior retina was excised, placed photoreceptor side down in a glass-bottom recording chamber, and held down by a platinum-iridium wire harp with nylon strings. The recording chamber was perfused continuously at a rate of 5 mL/min with bicarbonate-buffered, pH 7.4, Ames medium (US Biological, Swampscott, MA), equilibrated with a mixture of 95% O2 and 5% CO2 and maintained at 36–37°C. All manipulations were carried out under dim-red illumination.
Ganglion cell recording and morphology.
BSGCs were targeted based on extracellular spike recordings made with borosilicate patch electrodes, 4–6 MΩ resistance. The electrodes were filled with Ames medium. Ganglion cell somas within ∼2 mm of the visual streak were targeted based on their small size, ≤15 μm diameter. The ganglion cells were visualized through a 40×/0.75-NA water-immersion objective, using a video camera mounted on an upright Olympus BX51 microscope with infrared (IR; 900 nm) differential interference contrast optics. For voltage-clamp recordings, the intracellular solution contained (in mM): 128 Cs-methylsulfonate, 6 CsCl, 10 Na-HEPES, 1 EGTA, 2 Mg-ATP, 1 Na-GTP, 2.5 Na2 phosphocreatine, and 3 lidocaine-N-ethyl-Cl. The lidocaine was included to block spikes generated by voltage-gated Na channels. Recordings were performed using a MultiClamp 700A patch-clamp amplifier (Molecular Devices, Sunnyvale, CA). Signals were digitized at 5 kHz and filtered at 2 kHz through the 4-pole Bessel filter in the amplifier. Currents were further digitally filtered during offline analysis with a −3-dB corner frequency of 100 Hz.
Holding potentials were corrected for a −13-mV liquid-junction potential. In approximately one-half of the recordings, up to 75% of the series resistance error was compensated online. The average series resistance for 74 OFF-BSGCs was 19 ± 6 MΩ (mean ± SD), and the resting input resistance measured over the linear range (−100 mV to ∼−30 mV) was 356 ± 135 MΩ. Additionally, voltages were corrected for uncompensated series resistance offline, as described below. With the Cs-rich intracellular solution used, the absolute amplitude of the whole-cell current at the extreme holding potentials was only a few hundred picoamperes, and therefore, uncompensated series resistance errors were, at most, ∼10 mV. Due to the residual, outward rectification of the whole-cell current-voltage (I-V) relation, the largest errors would occur at the most positive potentials. The dendritic arbors of OFF-BSGCs are small and are expected to be electrically compact; therefore, with application of online and offline compensation, series resistance errors should not have a significant impact on the accuracy of our conductance estimates.
In some experiments, 0.4% Alexa-594 hydrazide or 0.4% Alexa-488 hydrazide (Invitrogen, Carlsbad, CA) was included in the intracellular solution to visualize cell morphology. Images were recorded online using a microscope-mounted charge-coupled device camera (Roper Scientfic, Tucson, AZ). In experiments where dendritic stratification was confirmed, the retinas were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer for 45 min, rinsed, and then incubated for 7 days (25°C) in a goat anti-choline acetyltransferase antibody (Catalogue #AB144P; Millipore, Billerica, MA), diluted 1:200 in an incubation buffer containing 3% normal horse serum, 1% Triton X-100, and 0.025% NaN3 in 0.1 M PBS (pH 7.4). The retina was rinsed for 3–5 h and then incubated overnight (25°C) with an Alexa-488-conjugated donkey anti-goat secondary antibody, diluted 1:500 in incubation buffer without Triton X-100 (Invitrogen). Wholemounts were rinsed, mounted, and coverslipped for confocal imaging on an Olympus FluoView 1000 confocal microscope equipped with a 60×/1.42 oil objective.
Bath-applied drugs included L-(+)-2-amino-4-phosphonobutyric acid (L-AP4; 50 μM), D-(−)-2-amino-5-phosphonopentanoic acid (D-AP5; 50 μM), 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt (NBQX; 0.3–100 μM), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 25 μM; Ascent Scientific, Princeton, NJ, now Abcam Biochemicals), (S)-1-(2-amino-2-carboxyethyl)-3-(2-carboxy-thiophene-3-yl-methyl)-5-methylpyrimidine-2,4-dione (UBP 310; 10 μM), 1-(4-aminophenyl)-3-methylcarbamyl-4-methyl-3,4-dihydro-7,8-methylenedioxy-5H-2,3-benzodiazepine hydrochloride (GYKI 53655; 50–100 μM; Tocris Bioscience, Minneapolis, MN), and strychnine (0.5–1 μM; Sigma-Aldrich, St. Louis, MO). In experiments where NMDA (Tocris Cookson) was puffed onto the ganglion cell, synaptic transmission was blocked with 100 μM CdCl2, and a standard patch-electrode was filled with 2 mM NMDA, dissolved in Ames medium, and positioned above a hole in the inner-limiting membrane, ∼30 μm from the cell body. Puffs, 100 ms in duration, were generated using a Picospritzer microinjector (Parker Hannifin, Cleveland, OH).
Light stimulation.
Light stimuli were generated on a computer monitor with a 60-Hz refresh rate, using custom procedures implemented in IGOR Pro (WaveMetrics, Lake Oswego, OR). The screen intensity was linearized in the software using a look-up table. During light stimulation, the 40× objective was replaced by a 10×/0.25-NA water-immersion objective, which provided a 2-mm diameter stimulus area focused onto the photoreceptor layer. The screen intensity was attenuated by placing calibrated neutral density filters in the light path.
The area to be used for recording was adapted for 30 min to the background luminance level through the 10× objective. The standard stimulus comprised a 100- to 120-μm diameter-centered spot, square-wave modulated at 1 Hz on a steady background of ∼3 × 103 photons·s−1·μm−2 at the retina, and measured for the green phosphor of the cathode ray tube monitor. The background light intensity was an order of magnitude greater than steady-state rod photoreceptor saturation in the rabbit, and therefore, stimuli were in the photopic range (Dacheux and Raviola 1986; Nakatani et al. 1991). Contrast was defined as C = 100·(Lmax − Lmin)/(Lmax + Lmin), where Lmax and Lmin are the maximum and minimum intensities of the spot. Variations in the diameter and contrast of the stimulus are detailed in results.
Conductance analysis.
Stimulus-activated synaptic conductance was measured from I-V relations obtained over a range of holding potentials between −98 and +27 mV or −102 mV and +34 mV. The net light-activated synaptic I-V relation was obtained by subtracting the mean current during the 200 ms prior to stimulus onset from the current during the light response. At positive potentials, the intracellular Cs appeared insufficient to completely suppress outward rectification, and during the voltage steps, the currents often displayed a slow inactivation, reminiscent of potassium currents. This sloping baseline was subtracted from the records at positive potentials to obviate errors in measuring the amplitudes of the net light-activated synaptic currents. I-V relations were measured at 10-ms intervals to determine the time course of the synaptic conductances. At each time point, the voltages were corrected for series resistance errors—using the whole-cell current—prior to correcting for the sloping baseline.
Synaptic inputs comprised excitation, with a reversal potential VE = 0 mV, and inhibition, with a reversal potential at the chloride equilibrium potential, ECl, which was calculated as −68 mV under our conditions. Linearity of the inhibitory synaptic I-V relation was evident during the ON phase of the stimulus, which largely activated inhibitory inputs, since it reversed close to ECl (see Figs. 5B, 6, B and E, 7B, 8B, and 9B). Similarly, linearity of the excitatory AMPA/KA component of the synaptic I-V relation was evident during recordings where NMDA receptors were blocked (see Fig. 6, B and E). In addition to these two linear, voltage-independent conductances, part of the excitatory input to OFF-BSGCs was mediated by NMDA receptors, which have a nonlinear I-V relation due to voltage-dependent channel block by extracellular Mg2+ ions (Mayer et al. 1984; Nowak et al. 1984). Analysis of the NMDA component of the synaptic conductance was performed as described previously (Venkataramani and Taylor 2010). The magnitude of the synaptic conductances was obtained by performing a least-squares fit to synaptic I-V relations using the equation
| (1) |
where V is the membrane potential, Gi is the inhibitory conductance, ECl is the chloride reversal potential, Ge is the linear AMPA/KA excitatory conductance, VE is the excitatory reversal potential, GNMDA is the NMDA conductance, and f(V) is the fraction of conducting NMDA channels as a function of voltage. The function, f(V), which accounts entirely for any nonlinearity of the synaptic I-V relations, was evaluated as
| (2) |
where the extracellular magnesium concentration ([Mg]) = 1.2 mM, KMg is the apparent Mg-binding affinity at 0 mV, and Vδ is proportional to the fraction of the membrane electric field sensed by the Mg2+ ion at the binding site. The shape of the NMDA I-V relation was measured from responses to NMDA, applied to the dendrites using pneumatic pulses through a microelectrode containing 2 mM NMDA (see Fig. 4). KMg and Vδ were evaluated from fits of Eq. 1 to these I-V relations, with Gi and Ge set to zero (KMg = 14 mM; Vδ = 21 mV; see Fig. 4B). These parameters were held constant in all analyses of synaptic I-V relations. With these parameter values, one-half of the NMDA channels is blocked at −51 mV. The value for Vδ is equivalent to Mg2+ binding, 63% across the membrane electric field, which is within the range of previous estimates for NMDA channels (Chen and Huang 1992; Mayer and Westbrook 1985). The apparent KMg is approximately twofold higher than GluN2A- or GluN2B-containing NMDA receptors and may be consistent with an alternative subunit composition, having a lower Mg2+ sensitivity (Monyer et al. 1994). Lower Mg2+ sensitivity for NMDA receptors has been reported for other RGC types (Manookin et al. 2010; Venkataramani and Taylor 2010). To take into account the Mg2+ block and better compare the excitatory effects of the NMDA and AMPA conductances near resting potential, the NMDA conductance in all figures has been scaled to show the chord conductance at −70 mV; i.e., GNMDA(−70) = GNMDA × f(−70), where f(−70) = 0.29 from Eq. 2.
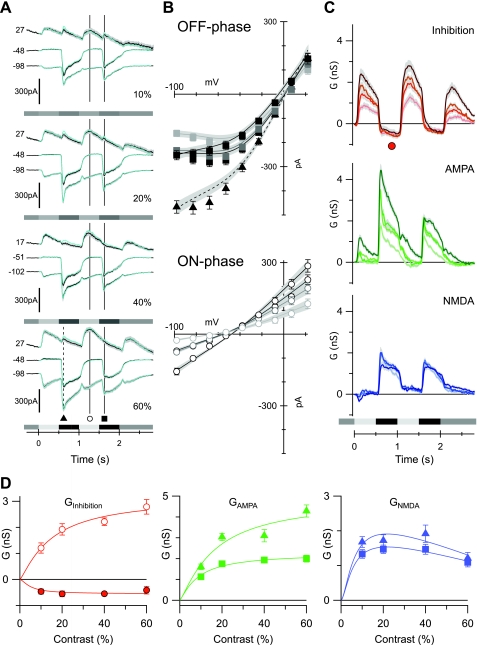
Fig. 5.
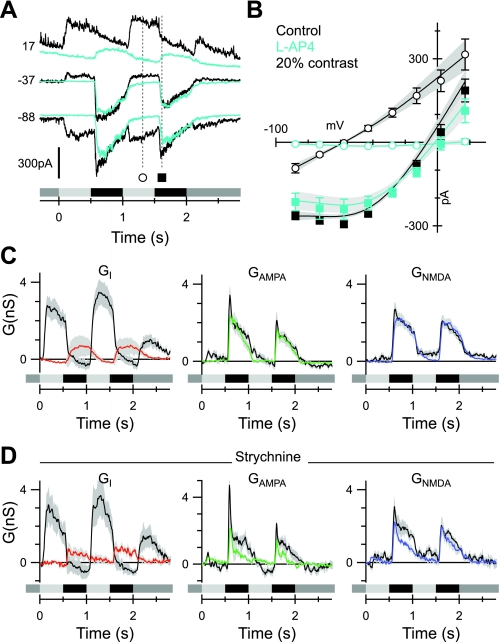
Contrast dependence of the excitatory and inhibitory synaptic inputs to OFF-BSGCs. The stimulus, a 100- to 120-μm diameter spot centered on the receptive field, was square-wave modulated at 1 Hz. Stimulus timing is indicated below the records in A and C. This stimulus applies to this and all subsequent figures. The contrast is shown at the bottom right of each panel in A. A: average currents from 36, 41, 28, and 33 cells at 10%, 20%, 40%, and 60% contrasts, respectively, at the holding potentials (mV) shown to the left of the traces. The superimposed cyan lines show the predicted currents reconstructed from the conductances shown in C. B: average light-evoked, series-resistance, corrected I-V relations obtained at the time points indicated in A. ▴, average currents at the indicated time point during the OFF phase of the 1st stimulus cycle; ○ and ■, ON phase and OFF phase of the 2nd stimulus cycle, respectively. The error bars show the SE, where they are larger than the symbols. The shading indicates increases in stimulus contrast (darkest indicates the highest contrast). The least-squares fits to the individual I-Vs were averaged from all of the cells and are shown by the solid lines; shading shows SE. This format is followed for the I-Vs in all subsequent figures. C: conductance components calculated every 10 ms for the duration of the light stimulus, from fits to average I-Vs, as illustrated in B for a single time point. The shaded regions show the SE. The Gi (Inhibition) is shown in red, the linear AMPA conductance (Glinear) is shown in green, and the NMDA conductance (GNMDA) is shown in blue, here and in all subsequent figures. D: the average amplitude of the inhibitory, AMPA, and NMDA conductance components, measured as a function of stimulus contrast. Mean conductances were measured over a 50-ms period at the time points shown by the corresponding symbols in A and C. The lines show empirical fits to the data.
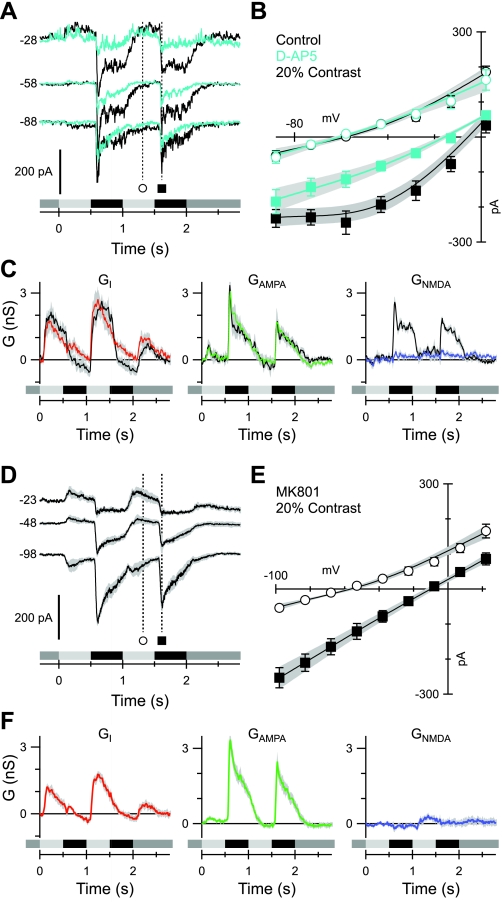
Fig. 6.
Blocking NMDA receptors blocks the nonlinearity of the I-V relations. A: currents recorded at the indicated voltages (mV) in a representative cell before (black) and during (cyan) application of 50 μM D-AP5. B: example of average light-evoked I-V relations from 7 cells at the time points indicated in A. C: average conductances corresponding to the cells in B. The colored traces were obtained in the presence of D-AP5; control records are shown in black. Note that D-AP5 completely suppressed the NMDA component. D: average currents recorded from 5 cells at 20% contrast during whole-cell recording with 2 mM MK-801 in the recording electrode. At least 2 min were allowed for the MK-801 to equilibrate with the interior of the recorded cell before data collection. E: average I-V relations from the cells shown in D. F: conductances corresponding to the I-Vs shown in E. Note that the NMDA component is suppressed completely.
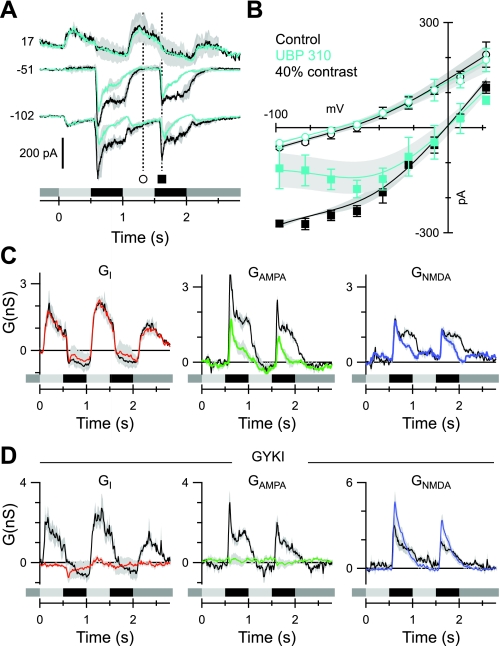
Fig. 7.
KA and AMPA receptor contribution to OFF-BSGC excitatory inputs. The format is similar to Fig. 6, A–C. A: average currents (n = 3), evoked by a 40% contrast stimulus, recorded at the indicated voltages (mV) before (black) and during (cyan) application of 10 μM UBP 310. B: example of average light-evoked I-V relations, measured at the time points indicated in A. C: average conductances corresponding to the currents in A. The colored traces were obtained in the presence of UBP 310. Control records are shown in black. UBP 310 suppressed the excitatory components but left the inhibition unaffected. D: average conductances as in C (n = 4) but in the presence of 100 μM GYKI 53655. The linear component of the excitation (green trace) is suppressed completely, indicating that it is mediated by AMPA receptors.
Fig. 8.
Blocking the OFF pathway reveals presynaptic crossover input from the ON pathway. The format is similar to Fig. 6, A–C. Data in B and C show averages from 3 cells. A: currents recorded at the indicated voltages (mV) in a representative cell before (black) and during (cyan) application of 100 μM GYKI 53655 plus 10 μM UBP 310. B: fits to the light-evoked I-V relations at the time points indicated in A. C: conductance components calculated from fits to the average I-Vs as illustrated in B. The colored traces were obtained in the presence of the blocking drugs. Control records are shown in black. Note the complete suppression of the linear inhibitory and excitatory components. D: average current traces (n = 4) showing that the addition of 50 μM L-AP4 blocks the residual NMDA inputs seen in the presence of 100 μM GYKI 53655 plus 10 μM UBP 310 (A). E: similarly, either 50 μM L-AP4 (cyan) or 0.5 μM strychnine (yellow) blocks the residual NMDA input seen in the presence of 100 μM NBQX plus 10 μM UBP 310. In this example, we successfully applied and washed out both L-AP4 and strychnine (gray).
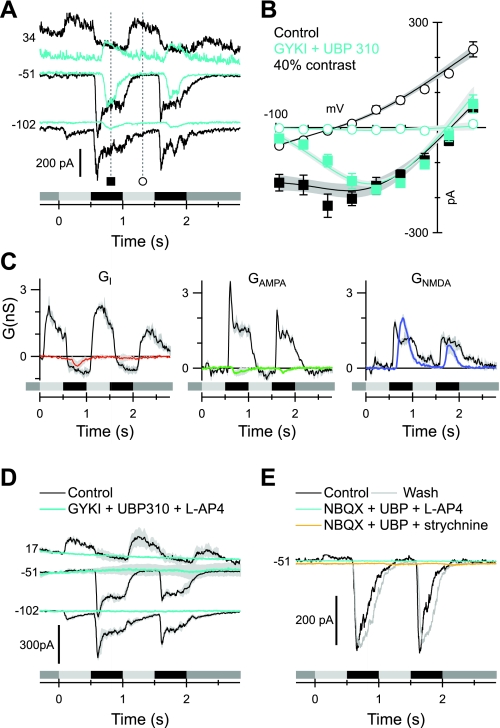
Fig. 9.
Blocking the ON pathway suppresses direct crossover inhibition. The format is similar to Fig. 6, A–C. Data in B and C show averages from 10 cells. A: currents recorded at the indicated voltages (mV) in a representative cell before (black) and during (cyan) application of 50 μM L-AP4. B: average light-evoked I-V relations at the time points indicated in A. Note the complete suppression of the inhibition during the ON phase of the stimulus (open symbols). C: conductance components calculated from fits to the average I-Vs as illustrated in B. The colored traces were obtained in the presence of the blocking drugs; control records are shown in black. D: effects of 1 μM strychnine on the average conductances (n = 4 cells). The strychnine completely suppresses the inhibition during the ON phase.
Fig. 4.
Determination of the current-voltage (I-V) relation for the N-methyl-d-aspartate (NMDA) conductance in OFF-BSGCs. A: currents evoked by 2 mM NMDA puffs at indicated holding potentials ECl. The black bar, labeled “NMDA”, indicates the timing and duration of the puff; the gray bar indicates the period where the I-V in B was measured. B: leak-subtracted I-Vs of individual puff responses in 9 OFF-BSGCs (small ◊; ▴, data for the cell illustrated in A). Individual I-Vs have been normalized to have the average slope conductance for the dataset calculated for the most positive 3 data points. Large ◊, mean values ± SD. The solid, black line shows the fit to the mean data of the function describing the voltage dependence of NMDA receptor conductance [see materials and methods, Eq. 1, inhibitory conductance (Gi) = 0, and linear α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate (KA) excitatory conductance (Ge) = 0)].
The combination of the NMDA, AMPA/KA, and inhibitory conductances accounted for the current responses over the full voltage range under most conditions; however, sometimes, deviations were evident, in that the data points were systematically below the line of best fit (e.g., see Fig. 5B). The deviations invariably occurred at potentials between ∼−85 and −60 mV, which encompasses the region of negative-slope conductance for the NMDA receptors (see Fig. 4B). Therefore, it seems likely that the deviations result from space-clamp errors in the dendrites that allowed NMDA receptor-mediated, regenerative depolarization to create larger-than-expected inward currents. In line with this interpretation, fits to the I-V relations tended to be better for low-contrast stimuli and during the second cycle of the stimulus than for the first, presumably because excitation was smaller under these conditions and thus would be less likely to involve regenerative activation of NMDA receptors. In an effort to obviate errors from such effects, we excluded the data points at −83 and −68 mV when fitting the I-V relations. This improved the fit to the most negative point in the I-Vs and allowed us to better estimate the contribution of the linear AMPA receptor conductance. In most cases, however, the deviations were small, and fitting while excluding these data points had no noticeable effect on the amplitudes of the three conductance components. Although the basic conclusions of this study are unaffected either way, in most of the analyses, we used the second response cycle to more accurately quantify the contributions of the excitatory and inhibitory conductance components.
Bipolar cell recordings.
Vertical slices of dark-adapted rabbit retina were prepared using methods similar to those described previously for mouse retina (Puthussery et al. 2009). All manipulations were performed under IR or dim-red illumination. The retina was isolated from the retinal pigment epithelium, placed ganglion-cell side down on a cellulose membrane filter (Millipore), and sliced at ∼300 μm using a Vibratome 600 tissue chopper. Slices were oriented vertically, transferred to the recording chamber, and perfused at ∼2 mL/min with bicarbonate-buffered Ames medium (US Biologicals), continuously bubbled with 95% O2, 5% CO2. All recordings were performed at 32–33°C. Slices were viewed with an Olympus BX51 upright microscope fitted with a 40×/0.8-NA water-immersion objective and IR gradient contrast optics. Patch electrodes were pulled from thick-walled borosilicate glass to have a resistance of 10–12 MΩ. For bipolar cell recordings, the intracellular solution contained in (mM): 135 K-methylsulfonate, 6 KCl, 10 Na-HEPES, 1 EGTA, 2 Na-ATP, 1 Na-GTP, 2 MgCl2, and 0.05 Alexa-488 hydrazide. The pH was adjusted to 7.33 with KOH. Whole-cell voltage-clamp recordings were made with a EPC 10 patch-clamp amplifier (HEKA Elektronic, Germany). For voltage-clamp recordings, cells were clamped at a holding potential of −70 mV. Series resistance ranged from 17 to 55 MΩ and was compensated up to 75% online. Responses were digitally sampled at 5 kHz and filtered at 2 kHz. Current traces were further filtered offline with a −3-dB corner frequency of 100 Hz. Offline filtering and analysis were performed using custom-analysis routines in IGOR Pro (WaveMetrics). Full-field light stimuli were applied with a light-emitting diode (peak emission at 525 nm), which illuminated the back of a white plastic diffuser. The light projected through the microscope objective was collimated to produce even illumination at the retinal preparation. The identity of cells as OFF-CBCs was confirmed by their characteristic light response and by visualizing the level of axonal stratification at the conclusion of the recordings.
Statistical analysis.
Conductance analysis was performed on current traces from individual cells. Mean conductances for groups of cells are shown with SE. The average I-V relations (see Figs. 5B, 6, B and E, 7B, 8B, and 9B) were generated by averaging I-Vs from the individual cells. Paired Students' t-tests were used to assess the statistical significance of drug effects for ganglion cell experiments, and nonpaired t-tests were used to compare drug effects for bipolar cell experiments. An α-level of 0.05 was used for all statistical tests.
RESULTS
Identifying OFF-BSGCs.
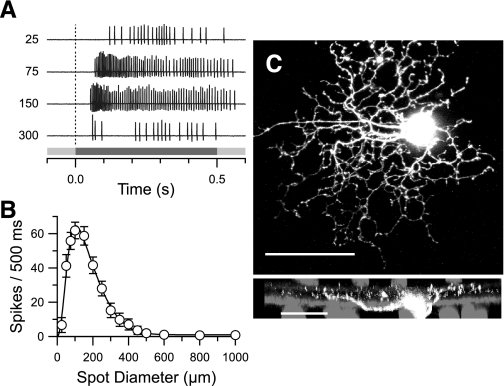
We targeted OFF-BSGCs in the whole-mount retina by selecting cells with somata <15 μm in diameter. The concentric, center-surround organization of the receptive field was confirmed by flashing centered spots of increasing diameter (Fig. 1A). Latency to the peak-firing rate was ∼85 ms. As with many rabbit RGC types in the whole-mount preparation, BSGCs did not fire action potentials in the presence of steady, bright-background illumination (Amthor et al. 1989). The diameters of the receptive field center and surround were estimated from the space constants obtained by fitting a difference of Gaussians function to the responses as a function of stimulus diameter (Fig. 1B). The center diameter averaged 130 ± 12 μm (mean ± SD; n = 141) and closely matched the anatomical dendritic extent [Fig. 1C, and see Amthor et al. (1989)]. The surround diameter averaged 460 ± 36 μm. These center and surround estimates are consistent with previous measurements for brisk-sustained cells (Devries and Baylor 1997; Vaney et al. 1981). The dendritic branching patterns of 14 physiologically identified OFF-BSGCs were consistent and easily recognizable from previous reports (Amthor et al. 1989; Roska et al. 2006; Roska and Werblin 2001). Examination of confocal micrographs in three cells revealed irregularly branching dendrites, often overlapping each other at multiple levels in the OFF sublamina of the inner plexiform layer (IPL). Fine, terminal dendrites were punctuated by varicosities (Fig. 1C). These anatomical features correspond to cell type G4, described by Rockhill and colleagues (2002), or cell type III.3, described by Famiglietti (2005). The density and receptive field size indicate that OFF-BSGCs represent the visual input at a high spatial resolution. Previous studies examining the physiological roles of ON pathway crossover inhibition and NMDA receptor-mediated transmission have targeted wide-field RGCs, which represent low spatial-resolution pathways (Manookin et al. 2008, 2010; van Wyk et al. 2009; Venkataramani and Taylor 2010). Here, we explore the possible role of these inputs for visual processing in a high spatial-resolution pathway.
Fig. 1.
Physiological and anatomical properties of OFF brisk-sustained ganglion cells (BSGCs). A: spikes in an OFF-BSGC, evoked by a dark spot flashed for 0.5 s. The stimulus timing is shown by the gray bar (bottom). The stimulus spot diameter (μm) is shown to the left of each response. Firing rates peaked within 85 ms of stimulus onset and were sustained for optimally sized spots, 75 and 150 μm in diameter. B: the mean spike count during the stimulus presentation is plotted vs. stimulus diameter (±SE; n = 141). The continuous line shows the best fit to a difference-of-Gaussians function, with a center size (2σ center Gaussian) of 130 ± 12 μm and a surround of 460 ± 36 μm. C: confocal z-projection of an OFF-BSGC filled with Alexa-594 hydrazide. Dendrites from an adjacent-filled BSGC are visible (top; original scale bar = 50 μm). Bottom: side projection, which illustrates the diffuse vertical stratification in the outer 1/2 of the inner plexiform layer. For reference, the ON and OFF cholinergic amacrine cells, which stratify at 22% and 69% (Brandon 1987; Famiglietti 1987), were labeled with an antibody for choline acetyltransferase (gray; original scale bar = 25 μm).
Light-evoked spiking in OFF-BSGCs is maintained in the presence of NBQX.
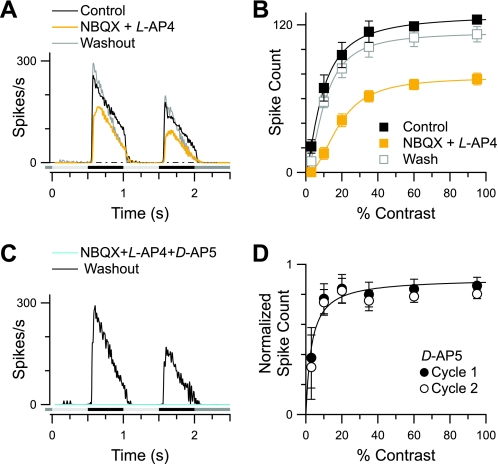
Initial attempts to reveal an ON pathway crossover component to the spiking responses used the potent, nonselective AMPA/KA receptor antagonist NBQX to suppress OFF pathway transmission by blocking photoreceptor input to OFF-CBCs. The stimulus was a saturating, circular spot, 100–125 μm in diameter, square-wave modulated at 1 Hz. We found that high concentrations of NBQX (50 μM) had modest effects on stimulus-driven spiking in OFF-BSGCs (data not shown); however, the spiking in the presence of NBQX was not mediated via crossover inhibition, since it persisted when NBQX was coapplied with 50 μM L-AP4, which completely suppresses ON pathway transmission. Under these conditions, the number of spikes was suppressed by only 40% of control (n = 6; P = 0.031; Fig. 2, A and B). Similar results were obtained with another, less-selective quinoxaline AMPA/KA receptor antagonist, CNQX (data not shown). Subsequent addition of the NMDA antagonist, D-AP5 (50 μM), completely suppressed the spiking, suggesting that it was mediated by NMDA receptors on BSGCs (n = 4; Fig. 2C). Consistent with this idea, application of D-AP5 alone suppressed spiking during both the first and second stimulus cycles by 20–25% at the higher contrasts and by 62 ± 20% (n = 5) at the lowest contrast (3%; Fig. 2D). These results suggest that center excitation of OFF-BSGCs is mediated by both NMDA and AMPA/KA receptors and unexpectedly, that signal transmission from cones to OFF-CBCs is resistant to high concentrations of NBQX.
Fig. 2.
2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt (NBQX)-resistant inputs in OFF-BSGCs. A: average spike-time histograms at the highest stimulus intensity (95% contrast) show the NBQX and L-(+)-2-amino-4-phosphonobutyric acid (L-AP4)-resistant response (yellow trace). B: mean spike count vs. stimulus contrast is reduced significantly but not blocked by 50 μM NBQX and 50 μM L-AP4 (n = 6). C: the addition of D-(−)-2-amino-5-phosphonopentanoic acid (D-AP5) in the presence of NBQX and L-AP4 reversibly eliminated all spiking (cyan trace). D: mean spike count in the presence of D-AP5 alone, normalized to control. D-AP5 suppressed spiking most strongly at the lowest contrast (3%; n = 5).
Light-evoked inputs to OFF-CBCs are not completely blocked by NBQX.
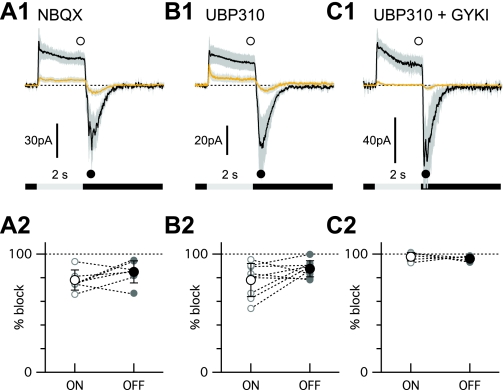
To directly test whether glutamate inputs to OFF-CBCs are resistant to NBQX, we recorded currents in OFF-CBCs in response to steps of light before and during application of NBQX (Fig. 3A1). The bipolar cells were targeted randomly in retinal slice preparations and filled with fluorescent dye during the recordings. At the conclusion of the recordings, the fluorescently labeled cells were identified as OFF-CBCs by the level of the axonal stratification in the IPL (MacNeil et al. 2004) and if they projected dendrites into the outer plexiform layer (OPL). Since these experiments were technically demanding, we did not attempt to acquire a complete dataset of recordings from each morphological OFF-CBC, and therefore, data from morphologically distinct bipolar cell classes were pooled together for analysis. On average, NBQX (50 μM) suppressed the outward, light-evoked current at the end of a 2-s diffuse light step (ON response; Fig. 3A1) by 77.2 ± 8.5% and the rebound tail currents at the termination of the step (OFF response; Fig. 3A1) by 85.9 ± 9.3% (n = 7; Fig. 3A1). The summary data shown in Fig. 3A2 show that some cells were more sensitive to NBQX than others. In a second set of OFF-CBCs, the KA receptor antagonist UBP 310 (10 μM) suppressed the average ON response by 78.0 ± 14.0% and the OFF response by 87.6 ± 3.8% (n = 9; Fig. 3, B1 and B2). Similarly to NBQX, the sensitivity to UBP 310 varied across individual cells (Fig. 3B2). Coapplication of UBP 310 and GYKI 53655 (100 μM), a selective AMPA receptor antagonist, completely suppressed the ON response (97.7 ± 3.6%) and OFF response (96.1 ± 2.2%) in a third group of cells (n = 5; Fig. 3, C1 and C2). The suppression observed with the combined application of UBP 310 and GYKI 53655 was significantly greater than that seen with NBQX alone (ON response, P < 0.001; OFF response, P = 0.048) or with UBP 310 alone (ON response, P = 0.006; OFF response, P = 0.002). These results indicate that some KA or AMPA receptors on OFF-CBCs are not blocked by high concentrations of the nonselective antagonist NBQX. The residual OFF responses seen in BSGCs in the presence of NBQX and L-AP4 likely result from signals mediated via these receptors.
Fig. 3.
NBQX-resistant, light-evoked inputs to OFF bipolar cells. A1, B1, C1: average responses to 2-s diffuse light steps before (black traces) and after (yellow traces) the wash-in of 50 μM NBQX (A1), 10 μM (S)-1-(2-amino-2-carboxyethyl)-3-(2-carboxy-thiophene-3-yl-methyl)-5-methylpyrimidine-2,4-dione (UBP 310; B1), or a combination of 10 μM UBP 310 and 100 μM 1-(4-aminophenyl)-3-methylcarbamyl-4-methyl-3,4-dihydro-7,8-methylenedioxy-5H-2,3-benzodiazepine hydrochloride (GYKI 53655; C1). Shading shows the 95% confidence interval. The initial baseline current has been offset to 0 for comparison. The symbols show the time points used for the measurements shown in the lower panels. A2, B2, C2: summary of percentage block of the ON and OFF response components in individual bipolar cells treated with NBQX (A2), 10 μM UBP 310 (B2), or a combination of 10 μM UBP 310 and 100 μM GYKI 53655 (C2). A2: NBQX, n = 8 cells. Five cells that had similar response kinetics were averaged for the traces shown. B2: UBP 310. Average of 7 of the 8 cells recorded. One outlier was suppressed by only ∼20% and was omitted to perform the statistical comparison with the data in C2. C2: UBP 310 + GYKI 53655. Average from 5 cells.
Contrast sensitivity of excitatory and inhibitory drive to OFF-BSGCs.
The extracellular recordings suggested the presence of a significant NMDA-mediated input to OFF-BSGCs, particularly during low-contrast stimuli. Our next step was to measure the light-evoked synaptic inputs to the cells directly to resolve the NMDA, AMPA/KA receptor-mediated, and inhibitory components and determine their contrast sensitivity. To do this, we measured light-evoked currents at a series of holding potentials and fitted the I-V relations to extract the linear AMPA/KA and inhibitory components and the nonlinear NMDA component (see materials and methods). To account for the nonlinear NMDA component when fitting the I-V relations, we needed to determine the shape of the NMDA I-V in OFF-BSGCs. This was achieved by measuring the I-V relations for currents produced by puff application of 2 mM NMDA in the presence of 100 μM CdCl2 (Fig. 4, A and B; see materials and methods for further details). The parameters obtained from the fit of Eq. 1 to the data (Fig. 4B) were used to describe the NMDA-basis function for fitting I-Vs in all subsequent analysis.
We recorded light-evoked, whole-cell currents in 41 OFF-BSGCs at a series of voltages in response to the same stimulus as was used for extracellular recordings (Fig. 5A). After subtracting the leak current measured just prior to the light stimulus, the net light-evoked I-V relation during the OFF phase of the stimulus showed strong outward rectification, consistent with a significant NMDA receptor contribution (Fig. 5B). The I-V relations were fitted with the sum of the three conductances: the linear AMPA/KA component that reversed at 0 mV, the linear inhibitory component that reversed at the chloride equilibrium potential (ECl), and the nonlinear NMDA component that reversed at +5 mV, as determined empirically from the data illustrated in Fig. 4. The time courses of the conductances were calculated by fitting the I-V relations, constructed every 10 ms for the full duration of the light stimulus (Fig. 5C).
The magnitude of the conductances as a function of contrast was measured at the time points shown in Fig. 5, A and C, and plotted against contrast in Fig. 5D. The AMPA/KA component was significantly larger during the first stimulus cycle than the second, indicating rapid adaptation (Fig. 5D). The amplitude of the NMDA component changed little from the first to the second stimulus cycles (Fig. 5D). The mechanisms of this adaptation were not investigated further. The NMDA component was only activated during the OFF phase of the stimulus, and since it varied little in magnitude between 10% and 60% contrast, it appeared to be saturated already at the lowest contrast tested (Fig. 5, C and D). The linear AMPA/KA component during the OFF phase increased monotonically with contrast (Fig. 5, C and D) and was not activated during the ON phase, except at the highest contrast (Fig. 5C). This high-contrast AMPA/KA excitation during the ON phase of the stimulus likely represents input from the ON pathway, as it was blocked by L-AP4 (see Fig. 9C). The diffuse stratification of OFF-BSGCs (Fig. 1C) raises the possibility that they receive input from the ON pathway, as reported for other ganglion cells in the mouse (Renteria et al. 2006), but the possible roles of such inputs are not yet known. Under physiological conditions, the strong, coincident ON inhibition will presumably obviate the excitatory effects of this ON excitation. Since this ON excitation was generally small or absent at the nonsaturating contrasts, which are most significant for normal physiological activity, we have not analyzed it further in this paper.
Inhibition dominated the synaptic drive during the ON phase of the stimulus, as evident from the linear I-V relations that reversed close to ECl (Fig. 5B). The ON phase inhibition increased monotonically with stimulus contrast (Fig. 5, B–D). During the OFF phase, a decrease of the inhibitory input below the prestimulus baseline was observed, which appeared to be saturated at the lowest contrast tested (Fig. 5, C and D). Such disinhibition presumably reflects suppression of a tonic inhibitory input. The disinhibition was small and variable compared with the magnitude of the excitation and therefore, is unlikely to play a large role in driving spiking.
In summary, whereas the linear excitatory and inhibitory components increased monotonically from 10% to 60% contrast, the NMDA component was already saturated at 10% contrast (Fig. 5D). These results suggest that NMDA receptors contribute relatively more to the excitatory input at low contrast.
Nonlinear excitatory inputs to OFF BSGSs are mediated by postsynaptic NMDA receptors.
To determine whether the observed nonlinearity in the I-V relation was driven purely by NMDA inputs, light-activated currents were measured in the presence of the NMDA receptor antagonist D-AP5 (50 μM; Fig. 6A). The I-V relation became linear when NMDA receptors were blocked (Fig. 6B), and the calculated NMDA conductance was suppressed completely (Fig. 6C), whereas there was no significant change in the peak amplitudes of the AMPA/KA (n = 7; P = 0.9; Fig. 6C) and inhibitory inputs (P = 0.1; Fig. 6C). Similarly, the time courses of the AMPA/KA and inhibitory conductances were essentially unchanged. These results indicate that NMDA receptors are not involved in the presynaptic circuitry, which drives or modulates the AMPA/KA and inhibitory synaptic inputs. To confirm that the D-AP5 linearizes the I-V relation by blocking NMDA receptors located on the OFF-BSGCs, we restricted the NMDA receptor block to the recorded cell by including 2 mM MK-801 in the recording pipette (Berretta and Jones 1996). After at least 2 min of recording with MK-801, the I-V relations became linear (Fig. 6, D and E), and the calculated NMDA component was suppressed completely (Fig. 6F).
The roles of KA and AMPA receptors in the excitatory pathways to OFF-BSGCs.
Earlier work has proposed that KA receptors mediate relatively sustained inputs to specific OFF-CBCs (DeVries 2000), which suggests that KA receptors might mediate sustained bipolar cell output to ganglion cells. This hypothesis led us to examine whether blocking KA receptors would affect the temporal properties of the excitatory inputs in OFF-BSGCs. UBP 310, which is a selective antagonist for KA receptors containing GluK1 or -3 subunits, partially blocked both the linear excitatory input and the NMDA input to OFF-BSGCs (n = 3; Fig. 7, A–C). We assessed the effects of UBP on the amplitude of the combined excitatory input [AMPA conductance (Glinear) + GNMDA] near the peak and late in the stimulus cycle. UBP caused a larger reduction late in the stimulus cycle (last 200 ms of OFF response) than early in the stimulus cycle (30 ± 3% peak vs. 74 ± 4% late time point; P = 0.0001), consistent with the notion that KA receptors tend to mediate sustained responses. The antagonist had no effect on the time course or amplitude of the inhibition during either the ON of OFF phases of the stimulus (Fig. 7, A–C). The partial block of both the linear excitatory input and the NMDA input is consistent with the partial block seen at the input to the OFF-CBCs (Fig. 3), but it is unclear whether KA receptors are located directly on the BSGCs. Therefore, to reveal a possible, direct KA input, we applied GYKI 53655 (100 μM), which selectively blocks AMPA receptors. GYKI completely suppressed the linear input to the OFF-BSGCs, indicating that this component is mediated entirely by AMPA receptors (Fig. 7D). Similar suppression of the linear excitatory input was observed using NBQX or CNQX at concentrations that tend to be selective for AMPA over KA receptors (1–3 μM; data not shown). In addition, the peak amplitude of the NMDA component in the presence of GYKI became larger (143 ± 6% of control; n = 4; P = 0.045; Fig. 7D). This effect may have been due to the block of AMPA receptors in the upstream inhibitory network. However, we did not further test this possibility. In summary, the linear excitatory input to OFF-BSGCs is likely mediated entirely by AMPA receptors (see Fig. 10).
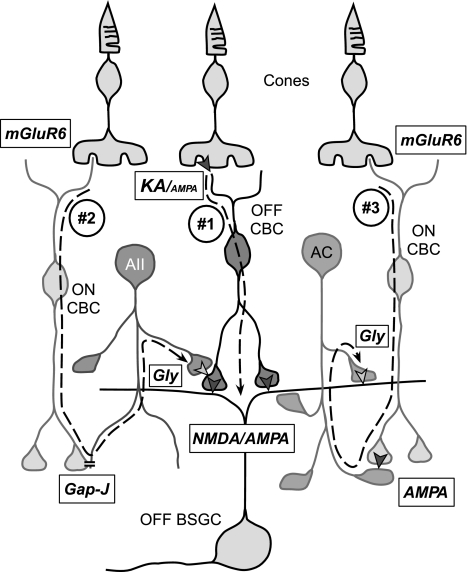
Fig. 10.
Diagram showing the 3 OFF and ON pathways proposed to converge onto the OFF-BSGC. #1: direct excitatory inputs from OFF cone bipolar cells (CBCs), mediated by a combination of NMDA and AMPA receptors. The OFF-CBCs, providing the glutamatergic input to the OFF-BSGCs, are likely to receive a strong input mediated by synaptic GluK1-containing KA receptors in the outer plexiform layer. #2: crossover inhibition from the ON pathway proposed to modulate release of glutamate from OFF cone bipolar terminals. The crossover effect was insensitive to AMPA antagonists and thus is likely mediated via AII amacrine cells. #3: crossover inhibition, impinging directly onto the OFF-BSGC, is mediated by a glycinergic amacrine cell (AC) other than the AII, because it is abolished by AMPA receptor antagonists. The receptors mediating synaptic transmission are shown in the boxes: Gap-J, electrical gap junction-mediated synapse; Gly, glycinergic synapse; KA/AMPA, synapses mediated by a mix of KA and AMPA receptors; NMDA/AMPA, synapses mediated by a mix of NMDA and AMPA receptors; AMPA, synapse mediated purely by AMPA receptors; mGluR6, glutamatergic synapse mediated by type 6 metabotropic glutamate receptors.
Suppression of the OFF pathway reveals presynaptic crossover input from the ON pathway.
Our recordings from OFF-CBCs indicate that a combination of selective AMPA and KA receptor blockers completely suppresses synaptic transmission through the OFF pathway (Fig. 3C, and see Fig. 10). To determine whether excitatory synaptic input to OFF-BSGCs originates exclusively in the OFF pathway, we applied 100 μM GYKI 53655 with 10 μM UBP 310 to suppress the photoreceptor input to the OFF-CBCs. Complete block of the OFF pathway may reveal excitatory inputs driven by the ON pathway, since transmission from cones to ON-CBCs is mediated by metabotropic glutamate receptors (mGluRs), which are not affected by AMPA/KA receptor antagonists. In concurrence with the idea that ON pathway-driven inhibition may modulate glutamate release from OFF bipolar cell terminals (Liang and Freed 2010), a significant NMDA receptor-mediated synaptic input remained in the presence of GYKI and UBP, although the linear inhibitory and excitatory inputs were abolished completely (n = 3; Fig. 8, A–C). This residual NMDA input was delayed and more transient when compared with control. We tested whether it was driven by crossover inhibition from the ON pathway by adding L-AP4 to selectively suppress ON pathway signaling.
Application of L-AP4 (50 μM) in the presence of GYKI and UBP or NBQX and UBP completely suppressed all synaptic input to the cell (n = 4; Fig. 8, D and E). In one cell, we were able to apply and wash out two drug combinations: NBQX + UBP + 0.5 μM strychnine and NBQX + UBP + L-AP4. In both cases, all input was suppressed completely (Fig. 8E), indicating that a glycinergic amacrine cell drives the NMDA input that remained in the presence of GYKI and UBP through presynaptic inhibition (Fig. 10). This inhibition most likely arises from ON pathway-driven AII amacrine cells, since under the photopic conditions used here, this crossover inhibitory circuit is insensitive to GluR antagonists (Manookin et al. 2008; Roska et al. 2006; van Wyk et al. 2009). The insensitivity arises because AII amacrine cells are driven by ON-CBCs via gap junctions (McGuire et al. 1984; Mills and Massey 1995; Vaney et al. 1998; Veruki and Hartveit 2002). We next applied L-AP4 alone to determine the role of this presynatpic disinhibition under more physiological conditions. A peak in the excitatory conductance at the beginning of the OFF phase was suppressed consistently (Fig. 9, A and C). The effect was significant for the linear component of excitation during the first cycle of the response (24 ± 3% reduction; n = 10; P = 0.033). Although the reduction in the peak amplitude of the NMDA component was not significant, the total excitatory conductance was also suppressed significantly (20 ± 1% reduction; P = 0.002). This suppression is also evident during the second cycle as a reduction in the slope of the I-V relation (Fig. 9B).
Direct glycinergic crossover inhibition is mediated via a distinct circuit.
The inhibition observed in OFF-BSGCs during the ON phase of the stimulus was blocked by application of 50 μM L-AP4, indicating that it also represents crossover from the ON pathway (Fig. 9C). Application of 0.5 μM strychnine blocked the ON phase inhibition as well, consistent with input from a glycinergic amacrine cell (Fig. 9D). However, in contrast to the presynaptic disinhibition described above, the postsynaptic ON phase inhibitory input was blocked by application of GYKI and UBP (Fig. 8C) or by GYKI alone (Fig. 7D), which indicates that it does not arise via the gap junction-driven AII amacrine cell. Rather, the direct glycinergic input to OFF-BSGCs likely arises from amacrine cells, which receive only AMPA receptor-mediated inputs from ON-CBCs (Fig. 10).
DISCUSSION
The study provides an analysis of the converging synaptic pathways that drive excitatory and inhibitory responses in the receptive field center of OFF-BSGCs. We have identified the expected, direct excitatory drive from cone photoreceptors via OFF-CBCs and two additional novel circuits. These three circuits are summarized in Fig. 10: 1) OFF-BSGCs receive glutamatergic inputs from OFF-CBCs, which are mediated by AMPA and NMDA receptors. By contrast, transmission at the preceding synapse, between cone photoreceptors and OFF-CBCs, is driven to a significant extent by KA receptors, which are relatively insensitive to NBQX. 2) Glycinergic crossover inhibition from the ON pathway, mediated by ON-CBC connections to AII amacrine cells, modulates glutamate release from OFF-CBCs to the OFF-BSGCs. 3) Crossover inhibition impinges directly onto OFF-BSGCs and is mediated by an unidentified glycinergic amacrine cell. The unknown glycinergic amacrine cell is driven via AMPA receptors. The evidence supporting this proposed circuitry is discussed in light of previous findings.
OFF-CBCs provide a mixed AMPA/NMDA input to OFF-BSGCs.
The OFF-BSGCs receive a direct, excitatory drive from OFF-CBC terminals via NMDA receptors and AMPA receptors (Fig. 10). The evidence for the NMDA input is twofold; first is the finding that an excitatory component to the I-V relation displays the characteristic nonlinearity typical of NMDA receptors, which could be blocked by the NMDA antagonist, D-AP5, and second, inclusion of intracellular MK-801, a use-dependent blocker of NMDA channels (Berretta and Jones 1996; Humeau et al. 2003), also blocked the nonlinear component of the I-V, thus ruling out any contribution from uncontrolled network effects. The linear component of the direct excitatory input was partially suppressed by the GluK1 and -3 antagonist UBP 310, an effect that is attributable to the block of KA receptors in the OPL, but was suppressed completely by the AMPA receptor-selective antagonist, GYKI 53655, indicating that AMPA and not KA receptors mediate this direct synaptic input.
Evidence for functional NMDA receptor expression on RGCs is long standing and has been demonstrated either by exogenous application or from measurements of excitatory postsynaptic currents (Chen and Diamond 2002; Cohen 2000; Cohen and Miller 1994; Diamond and Copenhagen 1993; Matsui et al. 1998; Mittman et al. 1990), but the effects of NMDA antagonists on light-evoked spiking were often weak (Cohen and Miller 1994; Diamond and Copenhagen 1993; Kittila and Massey 1997). More recent analyses of light-evoked activity, obtained in the absence of inhibitory blockers, have shown that NMDA receptors can represent a major component of the center responses to specific ganglion cell types (Manookin et al. 2010; Venkataramani and Taylor 2010). However, the presence of NMDA receptors is not always conserved across species. For example, NMDA receptors contribute much less to excitation in OFF and ON α-cells in the mouse (Manookin et al. 2010; van Wyk et al. 2009) but represent a major component in the homologous, brisk-transient ganglion cells in the guinea pig (Manookin et al. 2010). The present study extends the analysis of NMDA receptor function in the retina by showing that similar to the homologous cells in the cat (Cohen 1998, 2000), NMDA receptors comprise a major component of the center response in rabbit OFF-BSGCs. The results indicate that NMDA receptors are particularly important for driving spiking at the lowest contrast tested, 3%, which is close to the threshold contrast for RGCs (Dhingra et al. 2003; Linsenmeier et al. 1982). Blocking NMDA receptors in the retina did not significantly affect the inhibitory or linear excitatory inputs to OFF-BSGCs, consistent with a direct effect on the NMDA receptor input, rather than secondary effects due to blocking NMDA receptors within the presynaptic circuitry.
The conventional view of NMDA receptor activation is that it occurs secondary to AMPA-mediated depolarization, which relieves the voltage-dependent magnesium block (reviewed by Kerchner and Nicoll 2008). However, in OFF-BSGCs, similar to NMDA inputs to orientation-selective ganglion cells (Venkataramani and Taylor 2010) and guinea pig α-cells (Manookin et al. 2010), NMDA receptors can operate independently, since the currents near typical resting potentials are relatively larger, likely due to the predicted, twofold-lower Mg2+ affinity, than commonly found for NMDA receptors in cortical neurons (Johnson and Ascher 1990). Receptors with a low Mg2+ sensitivity, relative to those containing GluN2A and GluN2B subunits, such as GluN3-containing receptors, have been found in RGCs (Brandstätter et al. 1998; Sucher et al. 2003).
NBQX-resistant OFF inputs are mediated by OFF-CBC KA receptors.
A surprising outcome of these experiments was the finding that OFF-BSGCs maintained robust, light-evoked firing in the presence of the quinoxaline antagonist, NBQX, at concentrations several orders of magnitude above commonly reported IC50 values for AMPA and KA receptors (Jane et al. 2009; Sheardown et al. 1990). Although such quinoxaline insensitivity in mammalian OFF-RGCs has been reported, the mechanism was not addressed directly but was suggested to involve either an ON pathway input or NMDA receptors at the first visual synapse (Cohen and Miller 1994, 1999; Yang et al. 2011). Here, we have ruled out the possibility that ON pathway inputs mediate the residual input, as spiking was observed in OFF-BSGCs, even when the ON pathway blocker L-AP4 was coapplied with NBQX. Moreover, our finding that the more-selective AMPA and KA receptor antagonists, GYKI 53655 and UBP 310, respectively, could completely block OFF-CBC light responses suggests an absence of NMDA receptors on OFF bipolar cell dendrites, consistent with previous studies (DeVries and Schwartz 1999; Hartveit 1997). The most likely explanation for the insensitivity of the OFF pathway to NBQX is that OFF-CBCs express AMPA/KA-type glutamate receptors, which show relatively low sensitivity to quinoxaline antagonists. The variable sensitivity to NBQX, evident as differences in the percentage block amongst the bipolar cells tested, seems consistent with the idea that glutamate receptor subunit expression is different across bipolar cell subtypes (DeVries 2000).
Based on the pharmacology of the light responses, which we observed in OFF-CBCs, the potential subunit composition of the NBQX-insensitive, AMPA/KA-type receptors can be inferred. Although NBQX has low selectivity for AMPA over most KA receptors at the 30- to 100-μM concentrations used in this study, its IC50 for certain GluK subunit-containing receptors can be relatively high, ranging from below 25 μM (GluK1 subunit-containing) to as high as 90 μM for GluK2/5-containing receptors (Alt et al. 2004), making it likely that any insensitive input would be KA receptor mediated. However, GluK2 subunit contribution is unlikely, because UBP 310, which blocked most of the excitatory input to the OFF-CBCs that we sampled, is highly selective for the GluK1 and -3 subunits over all GluA-containing receptors and GluK2 or the GluK2/3 and GluK2/5 heteromers, whereas GYKI 53655, the AMPA-selective antagonist, also does not affect GluK2 subunits, only blocking GluK3-containing receptors by ∼50% at the concentration we used (100 μM) (Atlason et al. 2010; Dolman et al. 2007; Perrais et al. 2009). Furthermore, although immunohistochemical studies have demonstrated the presence of both GluK1 and GluK2/3 receptors in OFF-CBC dendrites, these subunits do not colocalize and therefore, are likely to be expressed at discrete synaptic sites (Haverkamp et al. 2001). In summary, the previous immunohistochemical data, taken together with our pharmacological findings, suggest that the KA receptors, which contribute to OFF-BSGC excitation, may be homomeric GluK1 or a GluK1/4 or GluK1/5 combination.
It is worth emphasizing that blocking GluK1 and -3 receptors did not affect the inhibitory inputs to OFF-BSGCs, which suggests that these KA receptors are unlikely to contribute to signal transmission beyond the photoreceptor–OFF-CBC synapse (Fig. 7). Indeed, in the primate and mouse, GluK1 subunits have not been localized to the IPL (Haverkamp et al. 2001). It is also noteworthy that blocking KA receptors resulted in more transient, excitatory responses in the OFF-BSGCs. Since KA receptors are not present on the OFF-BSGCs, this result suggests an effect at the level of the bipolar cells and is consistent with the notion that KA receptors may selectively mediate transmission at low temporal frequencies (DeVries 2000). Our findings suggest that GluK-selective antagonists may prove useful for determining how KA receptors at the bipolar cell level contribute to encoding temporal features of visual stimuli in different types of OFF-RGC.
ON pathway-driven inhibition mediated by AII amacrine cells.
A component of the excitatory input to OFF-BSGCs is driven by modulation of glutamate release from OFF-CBC axon terminals through crossover inhibition from the ON pathway (Fig. 10). This is evident from the NMDA receptor-mediated input to OFF-BSGCs, which remains in the presence of GYKI 53655 and UBP 310 and is blocked by L-AP4. The insensitivity to GYKI and sensitivity to strychnine are consistent with glycinergic inputs from AII amacrine cells, which receive sign-conserving input from ON-CBCs via gap junctions (Liang and Freed 2010; Manookin et al. 2008; Munch et al. 2009; van Wyk et al. 2009). Liang and Freed (2010) showed that such AII-mediated inhibition tonically hyperpolarizes OFF-CBC terminals and increases rectification in the OFF pathway. In OFF-BSGCs, however, we did not detect an increase in tonic excitation when we blocked the ON pathway with L-AP4 alone. This suggests that presynaptic effects of ON-driven inhibition on the OFF pathway may be cell-type specific or depend on experimental conditions, such as light adaptation. Earlier studies looking at the effect of ON pathway block on spike output in cat OFF X and Y RGCs demonstrated a simultaneous increase in tonic firing and suppression of stimulus modulation-evoked responses (Chen and Linsenmeier 1989b), which varied with stimulus temporal frequency (Chen and Linsenmeier 1989a).
ON pathway-driven inhibition mediated by other amacrine cells.
The direct inhibitory input to the OFF-BSGCs, which is present during the ON phase of a flickering light stimulus, is likely mediated by an ON center glycinergic amacrine cell, since it was eliminated completely by L-AP4 and was also blocked by strychnine. Unlike the ON pathway-driven inhibition, which modulates excitatory inputs to the OFF-BSGCs, this direct inhibitory input was blocked both by GYKI and NBQX, which indicates that it is mediated by an amacrine cell driven by conventional AMPA receptor-mediated inputs (Fig. 10), not via gap-junction connections with ON-CBCs, as has been shown for the AII amacrine cell. There are approximately eight types of narrow-field glycinergic amacrine cells in the rabbit, in addition to the AII, which stratify in both the ON and OFF sublamina of the IPL (MacNeil et al. 1999), and the present study is the first to demonstrate a putative role for such amacrine cells. Previously, direct ON pathway-crossover inputs have only been studied in wide-field α and δ OFF-RGCs, where they are mediated by AII amacrine cells (Manookin et al. 2008; van Wyk et al. 2009), and comprise a large tonic inhibitory current, which is shut off during center stimulation. The combination of direct excitation and ON pathway-mediated disinhibition produces a “push-pull” arrangement for center excitation. However, in the OFF-BSGCs, where the putative glycinergic amacrine cell is not the AII, the crossover inhibition is rectified more strongly, since only a small and variable disinhibition is observed during the OFF phase of the stimulus, suggesting that there is very little tonic glycinergic input. These differences suggest that ON inhibitory inputs may serve distinct functions in different OFF-RGC types. One possible role in OFF-BSGCs is to prevent firing in response to the seemingly aberrant ON excitatory inputs seen with high-contrast stimuli (Fig. 5C). Others have proposed that ON crossover inhibition is necessary for OFF ganglion cells with linear spatial summation properties for integrating excitatory inputs from bipolar cells with rectified, nonlinear output (Molnar et al. 2009). Further work will be required to determine the physiological importance of diverse crossover pathways.
GRANTS
Support for the work was provided by the National Eye Institute (EY014888) and an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology, Oregon Health & Science University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: I.B., T.P., and W.R.T. conception and design of research; I.B. and T.P. performed experiments; I.B., T.P., and W.R.T. analyzed data; I.B., T.P., and W.R.T. interpreted results of experiments; I.B., T.P., and W.R.T. prepared figures; I.B., T.P., and W.R.T. drafted manuscript; I.B., T.P., and W.R.T. edited and revised manuscript; I.B., T.P., and W.R.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Jacqueline Gayet for excellent technical assistance and Sowmya Venkataramani for helpful discussion and suggestions.
REFERENCES
- Alt A, Weiss B, Ogden AM, Knauss JL, Oler J, Ho K, Large TH, Bleakman D. Pharmacological characterization of glutamatergic agonists and antagonists at recombinant human homomeric and heteromeric kainate receptors in vitro. Neuropharmacology 46: 793–806, 2004 [DOI] [PubMed] [Google Scholar]
- Amthor FR, Takahashi ES, Oyster CW. Morphologies of rabbit retinal ganglion cells with concentric receptive fields. J Comp Neurol 280: 72–96, 1989 [DOI] [PubMed] [Google Scholar]
- Atlason PT, Scholefield CL, Eaves RJ, Mayo-Martin MB, Jane DE, Molnar E. Mapping the ligand binding sites of kainate receptors: molecular determinants of subunit-selective binding of the antagonist [3H]UBP310. Mol Pharmacol 78: 1036–1045, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta N, Jones RS. Tonic facilitation of glutamate release by presynaptic N-methyl-d-aspartate autoreceptors in the entorhinal cortex. Neuroscience 75: 339–344, 1996 [DOI] [PubMed] [Google Scholar]
- Boycott BB, Wässle H. The morphological types of ganglion cells of the domestic cat's retina. J Physiol 240: 397–419, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon C. Cholinergic neurons in the rabbit retina: dendritic branching and ultrastructural connectivity. Brain Res 426: 119–130, 1987 [DOI] [PubMed] [Google Scholar]
- Brandstätter JH, Koulen P, Wässle H. Diversity of glutamate receptors in the mammalian retina. Vision Res 38: 1385–1397, 1998 [DOI] [PubMed] [Google Scholar]
- Chen EP, Linsenmeier RA. Centre components of cone-driven retinal ganglion cells: differential sensitivity to 2-amino-4-phosphonobutyric acid. J Physiol 419: 77–93, 1989a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EP, Linsenmeier RA. Effects of 2-amino-4-phosphonobutyric acid on responsivity and spatial summation of X cells in the cat retina. J Physiol 419: 59–75, 1989b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Huang LY. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature 356: 521–523, 1992 [DOI] [PubMed] [Google Scholar]
- Chen S, Diamond JS. Synaptically released glutamate activates extrasynaptic NMDA receptors on cells in the ganglion cell layer of rat retina. J Neurosci 22: 2165–2173, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland BG, Dubin MW, Levick WR. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol 217: 473–496, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ED. Interactions of inhibition and excitation in the light-evoked currents of X type retinal ganglion cells. J Neurophysiol 80: 2975–2990, 1998 [DOI] [PubMed] [Google Scholar]
- Cohen ED. Light-evoked excitatory synaptic currents of X-type retinal ganglion cells. J Neurophysiol 83: 3217–3229, 2000 [DOI] [PubMed] [Google Scholar]
- Cohen ED, Miller RF. The network-selective actions of quinoxalines on the neurocircuitry operations of the rabbit retina. Brain Res 831: 206–228, 1999 [DOI] [PubMed] [Google Scholar]
- Cohen ED, Miller RF. The role of NMDA and non-NMDA excitatory amino acid receptors in the functional organization of primate retinal ganglion cells. Vis Neurosci 11: 317–332, 1994 [DOI] [PubMed] [Google Scholar]
- Dacheux RF, Raviola E. The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. J Neurosci 6: 331–345, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron 28: 847–856, 2000 [DOI] [PubMed] [Google Scholar]
- Devries SH, Baylor DA. Mosaic arrangement of ganglion cell receptive fields in rabbit retina. J Neurophysiol 78: 2048–2060, 1997 [DOI] [PubMed] [Google Scholar]
- DeVries SH, Schwartz EA. Kainate receptors mediate synaptic transmission between cones and “Off” bipolar cells in a mammalian retina. Nature 397: 157–160, 1999 [DOI] [PubMed] [Google Scholar]
- Dhingra NK, Kao YH, Sterling P, Smith RG. Contrast threshold of a brisk-transient ganglion cell in vitro. J Neurophysiol 89: 2360–2369, 2003 [DOI] [PubMed] [Google Scholar]
- Diamond JS, Copenhagen DR. The contribution of NMDA and non-NMDA receptors to the light-evoked input-output characteristics of retinal ganglion cells. Neuron 11: 725–738, 1993 [DOI] [PubMed] [Google Scholar]
- Dolman NP, More JC, Alt A, Knauss JL, Pentikainen OT, Glasser CR, Bleakman D, Mayer ML, Collingridge GL, Jane DE. Synthesis and pharmacological characterization of N3-substituted willardiine derivatives: role of the substituent at the 5-position of the uracil ring in the development of highly potent and selective GLUK5 kainate receptor antagonists. J Med Chem 50: 1558–1570, 2007 [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C, Robson JG. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol 187: 517–552, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti EV. “Small-tufted” ganglion cells and two visual systems for the detection of object motion in rabbit retina. Vis Neurosci 22: 509–534, 2005 [DOI] [PubMed] [Google Scholar]
- Famiglietti EV. Starburst amacrine cells in cat retina are associated with bistratified, presumed directionally selective, ganglion cells. Brain Res 413: 404–408, 1987 [DOI] [PubMed] [Google Scholar]
- Hartveit E. Functional organization of cone bipolar cells in the rat retina. J Neurophysiol 77: 1716–1730, 1997 [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Grunert U, Wässle H. Localization of kainate receptors at the cone pedicles of the primate retina. J Comp Neurol 436: 471–486, 2001 [DOI] [PubMed] [Google Scholar]
- Hochstein S, Shapley RM. Quantitative analysis of retinal ganglion cell classifications. J Physiol 262: 237–264, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humeau Y, Shaban H, Bissiere S, Luthi A. Presynaptic induction of heterosynaptic associative plasticity in the mammalian brain. Nature 426: 841–845, 2003 [DOI] [PubMed] [Google Scholar]
- Jane DE, Lodge D, Collingridge GL. Kainate receptors: pharmacology, function and therapeutic potential. Neuropharmacology 56: 90–113, 2009 [DOI] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Voltage-dependent block by intracellular Mg2+ of N-methyl-d-aspartate-activated channels. Biophys J 57: 1085–1090, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci 9: 813–825, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittila CA, Massey SC. Pharmacology of directionally selective ganglion cells in the rabbit retina. J Neurophysiol 77: 675–689, 1997 [DOI] [PubMed] [Google Scholar]
- Liang Z, Freed MA. The ON pathway rectifies the OFF pathway of the mammalian retina. J Neurosci 30: 5533–5543, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmeier RA, Frishman LJ, Jakiela HG, Enroth-Cugell C. Receptive field properties of x and y cells in the cat retina derived from contrast sensitivity measurements. Vision Res 22: 1173–1183, 1982 [DOI] [PubMed] [Google Scholar]
- MacNeil MA, Heussy JK, Dacheux RF, Raviola E, Masland RH. The population of bipolar cells in the rabbit retina. J Comp Neurol 472: 73–86, 2004 [DOI] [PubMed] [Google Scholar]
- MacNeil MA, Heussy JK, Dacheux RF, Raviola E, Masland RH. The shapes and numbers of amacrine cells: matching of photofilled with Golgi-stained cells in the rabbit retina and comparison with other mammalian species. J Comp Neurol 413: 305–326, 1999 [PubMed] [Google Scholar]
- Manookin MB, Beaudoin DL, Ernst ZR, Flagel LJ, Demb JB. Disinhibition combines with excitation to extend the operating range of the OFF visual pathway in daylight. J Neurosci 28: 4136–4150, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manookin MB, Weick M, Stafford BK, Demb JB. NMDA receptor contributions to visual contrast coding. Neuron 67: 280–293, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey SC, Miller RF. N-methyl-d-aspartate receptors of ganglion cells in rabbit retina. J Neurophysiol 63: 16–30, 1990 [DOI] [PubMed] [Google Scholar]
- Matsui K, Hosoi N, Tachibana M. Excitatory synaptic transmission in the inner retina: paired recordings of bipolar cells and neurons of the ganglion cell layer. J Neurosci 18: 4500–4510, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. The action of N-methyl-d-aspartic acid on mouse spinal neurones in culture. J Physiol 361: 65–90, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature 309: 261–263, 1984 [DOI] [PubMed] [Google Scholar]
- McGuire BA, Stevens JK, Sterling P. Microcircuitry of bipolar cells in cat retina. J Neurosci 4: 2920–2938, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills SL, Massey SC. Differential properties of two gap junctional pathways made by AII amacrine cells. Nature 377: 734–737, 1995 [DOI] [PubMed] [Google Scholar]
- Mittman S, Taylor WR, Copenhagen DR. Concomitant activation of two types of glutamate receptor mediates excitation of salamander retinal ganglion cells. J Physiol 428: 175–197, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar A, Hsueh HA, Roska B, Werblin FS. Crossover inhibition in the retina: circuitry that compensates for nonlinear rectifying synaptic transmission. J Comput Neurosci 27: 569–590, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar A, Werblin F. Inhibitory feedback shapes bipolar cell responses in the rabbit retina. J Neurophysiol 98: 3423–3435, 2007 [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12: 529–540, 1994 [DOI] [PubMed] [Google Scholar]
- Munch TA, da Silveira RA, Siegert S, Viney TJ, Awatramani GB, Roska B. Approach sensitivity in the retina processed by a multifunctional neural circuit. Nat Neurosci 12: 1308–1316, 2009 [DOI] [PubMed] [Google Scholar]
- Nakatani K, Tamura T, Yau KW. Light adaptation in retinal rods of the rabbit and two other nonprimate mammals. J Gen Physiol 97: 413–435, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature 307: 462–465, 1984 [DOI] [PubMed] [Google Scholar]
- O'Brien BJ, Richardson RC, Berson DM. Inhibitory network properties shaping the light evoked responses of cat alpha retinal ganglion cells. Vis Neurosci 20: 351–361, 2003 [DOI] [PubMed] [Google Scholar]
- Oesch NW, Kothmann WW, Diamond JS. Illuminating synapses and circuitry in the retina. Curr Opin Neurobiol 21: 238–244, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrais D, Pinheiro PS, Jane DE, Mulle C. Antagonism of recombinant and native GluK3-containing kainate receptors. Neuropharmacology 56: 131–140, 2009 [DOI] [PubMed] [Google Scholar]
- Pu ML, Amthor FR. Dendritic morphologies of retinal ganglion cells projecting to the lateral geniculate nucleus in the rabbit. J Comp Neurol 302: 675–693, 1990 [DOI] [PubMed] [Google Scholar]
- Puthussery T, Gayet-Primo J, Pandey S, Duvoisin RM, Taylor WR. Differential loss and preservation of glutamate receptor function in bipolar cells in the rd10 mouse model of retinitis pigmentosa. Eur J Neurosci 29: 1533–1542, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria RC, Tian N, Cang J, Nakanishi S, Stryker MP, Copenhagen DR. Intrinsic ON responses of the retinal OFF pathway are suppressed by the ON pathway. J Neurosci 26: 11857–11869, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockhill RL, Daly FJ, MacNeil MA, Brown SP, Masland RH. The diversity of ganglion cells in a mammalian retina. J Neurosci 22: 3831–3843, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roska B, Molnar A, Werblin FS. Parallel processing in retinal ganglion cells: how integration of space-time patterns of excitation and inhibition form the spiking output. J Neurophysiol 95: 3810–3822, 2006 [DOI] [PubMed] [Google Scholar]
- Roska B, Werblin F. Vertical interactions across ten parallel, stacked representations in the mammalian retina. Nature 410: 583–587, 2001 [DOI] [PubMed] [Google Scholar]
- Shapley RM, Victor JD. The effect of contrast on the transfer properties of cat retinal ganglion cells. J Physiol 285: 275–298, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheardown MJ, Nielsen EO, Hansen AJ, Jacobsen P, Honore T. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline: a neuroprotectant for cerebral ischemia. Science 247: 571–574, 1990 [DOI] [PubMed] [Google Scholar]
- Sucher NJ, Kohler K, Tenneti L, Wong HK, Grunder T, Fauser S, Wheeler-Schilling T, Nakanishi N, Lipton SA, Guenther E. N-methyl-d-aspartate receptor subunit NR3A in the retina: developmental expression, cellular localization, and functional aspects. Invest Ophthalmol Vis Sci 44: 4451–4456, 2003 [DOI] [PubMed] [Google Scholar]
- Tjepkes DS, Amthor FR. The role of NMDA channels in rabbit retinal directional selectivity. Vis Neurosci 17: 291–302, 2000 [DOI] [PubMed] [Google Scholar]
- Troy JB. Spatial contrast sensitivities of X and Y type neurones in the cat's dorsal lateral geniculate nucleus. J Physiol 344: 399–417, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyk M, Taylor WR, Vaney DI. Local edge detectors: a substrate for fine spatial vision at low temporal frequencies in rabbit retina. J Neurosci 26: 13250–13263, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyk M, Wässle H, Taylor WR. Receptive field properties of ON- and OFF-ganglion cells in the mouse retina. Vis Neurosci 26: 297–308, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaney DI, Levick WR, Thibos LN. Rabbit retinal ganglion cells. Receptive field classification and axonal conduction properties. Exp Brain Res 44: 27–33, 1981 [DOI] [PubMed] [Google Scholar]
- Vaney DI, Nelson JC, Pow DV. Neurotransmitter coupling through gap junctions in the retina. J Neurosci 18: 10594–10602, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataramani S, Taylor WR. Orientation selectivity in rabbit retinal ganglion cells is mediated by presynaptic inhibition. J Neurosci 30: 15664–15676, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veruki ML, Hartveit E. Electrical synapses mediate signal transmission in the rod pathway of the mammalian retina. J Neurosci 22: 10558–10566, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H, Schafer-Trenkler I, Voigt T. Analysis of a glycinergic inhibitory pathway in the cat retina. J Neurosci 6: 594–604, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Nemargut JP, Wang GY. The roles of ionotropic glutamate receptors along the On and Off signaling pathways in the light-adapted mouse retina. Brain Res 1390: 70–79, 2011 [DOI] [PubMed] [Google Scholar]
- Zaghloul KA, Boahen K, Demb JB. Different circuits for ON and OFF retinal ganglion cells cause different contrast sensitivities. J Neurosci 23: 2645–2654, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]