Abstract
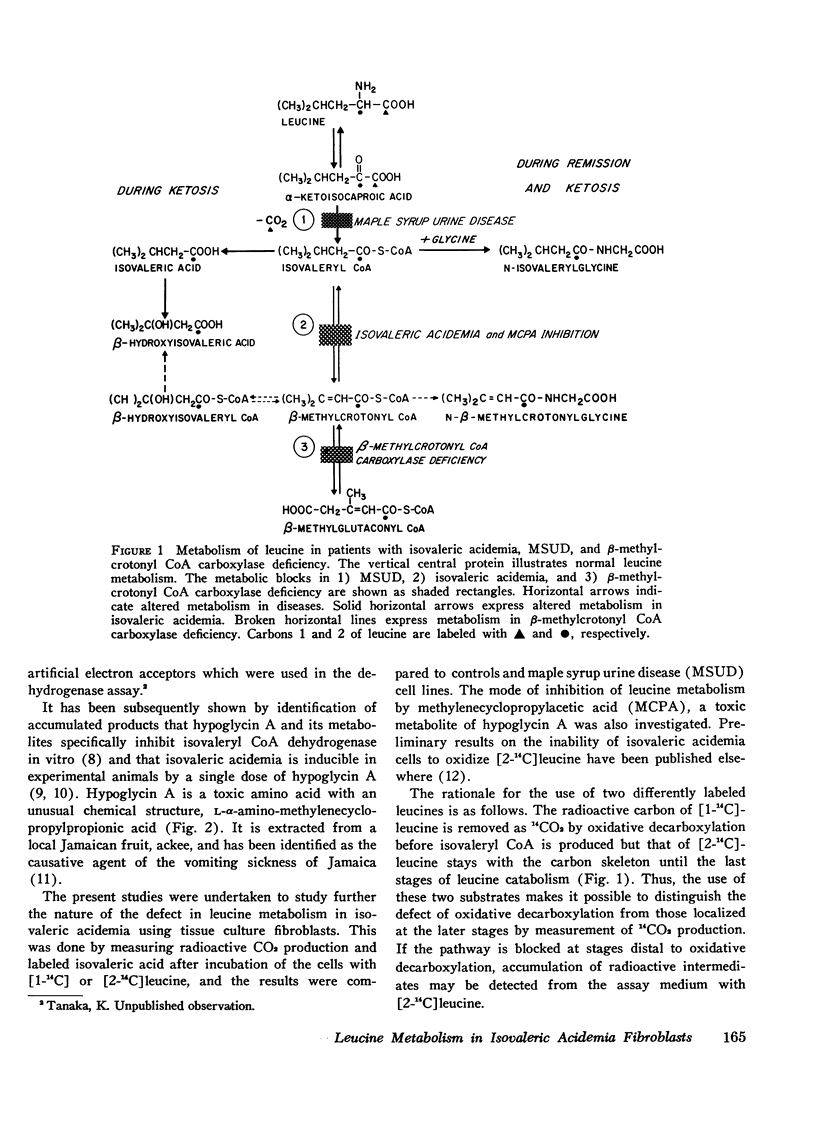
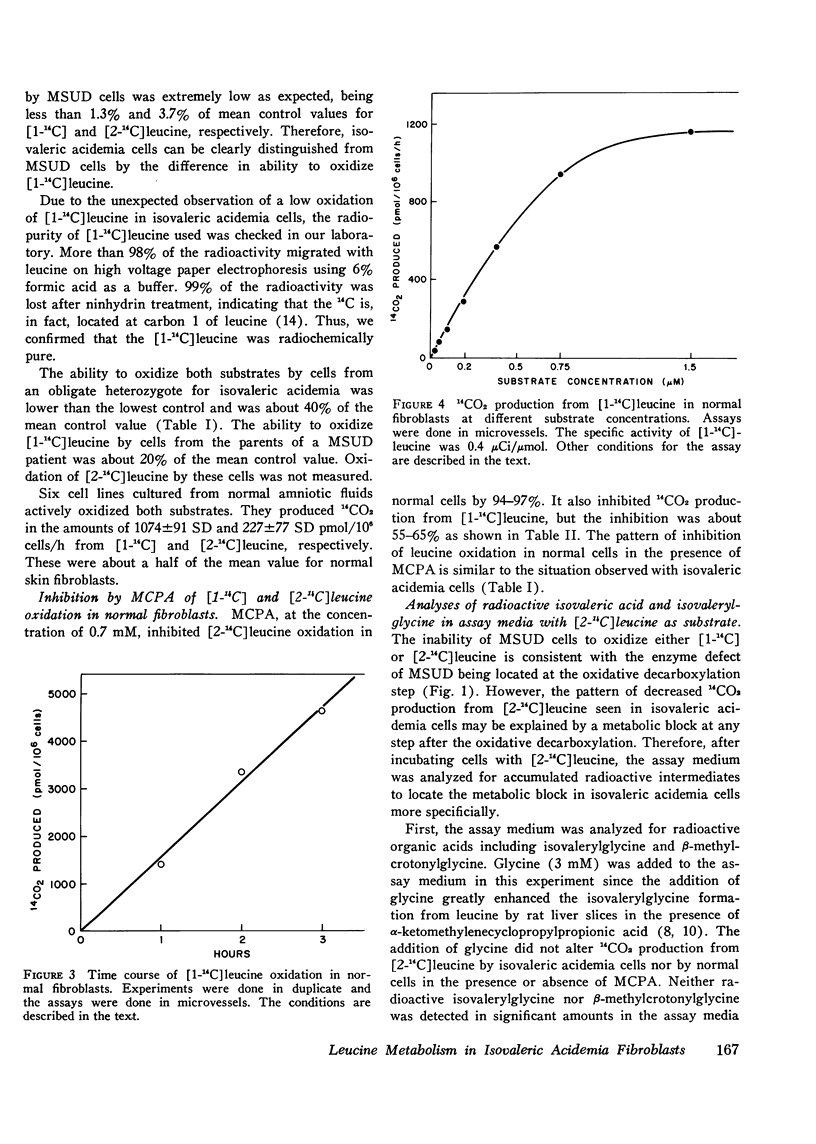
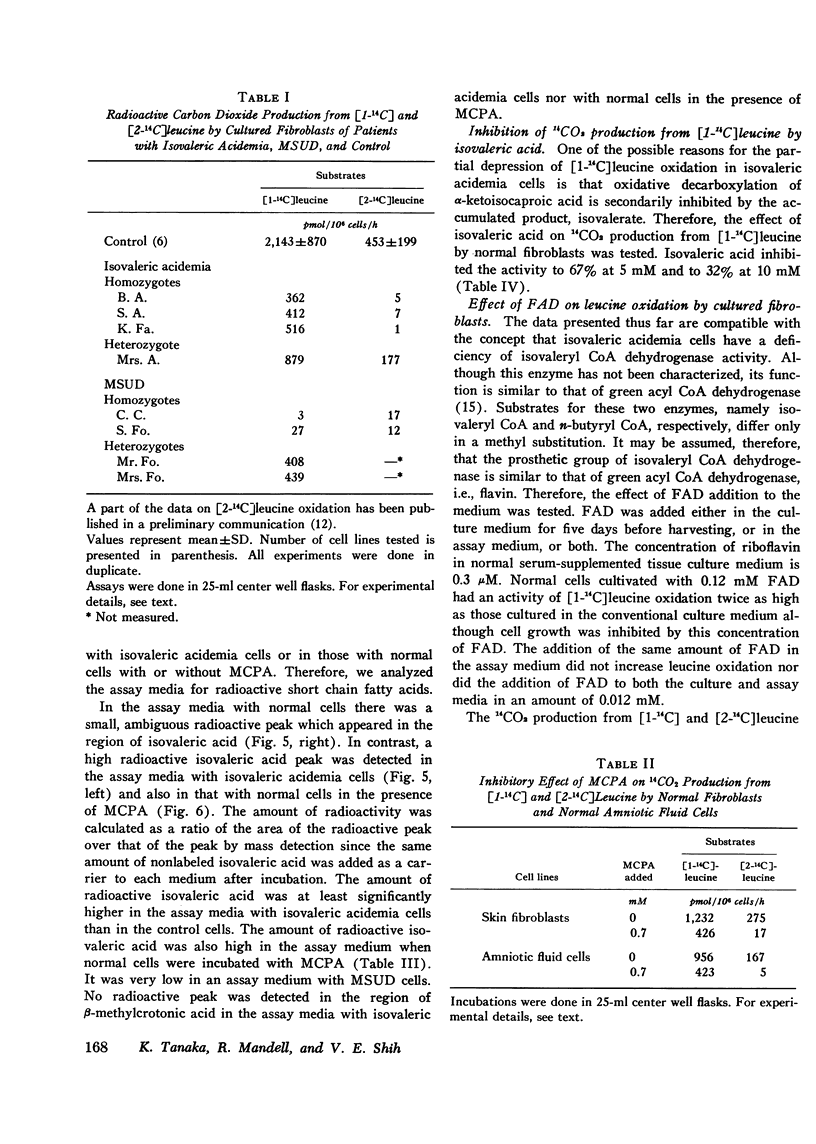
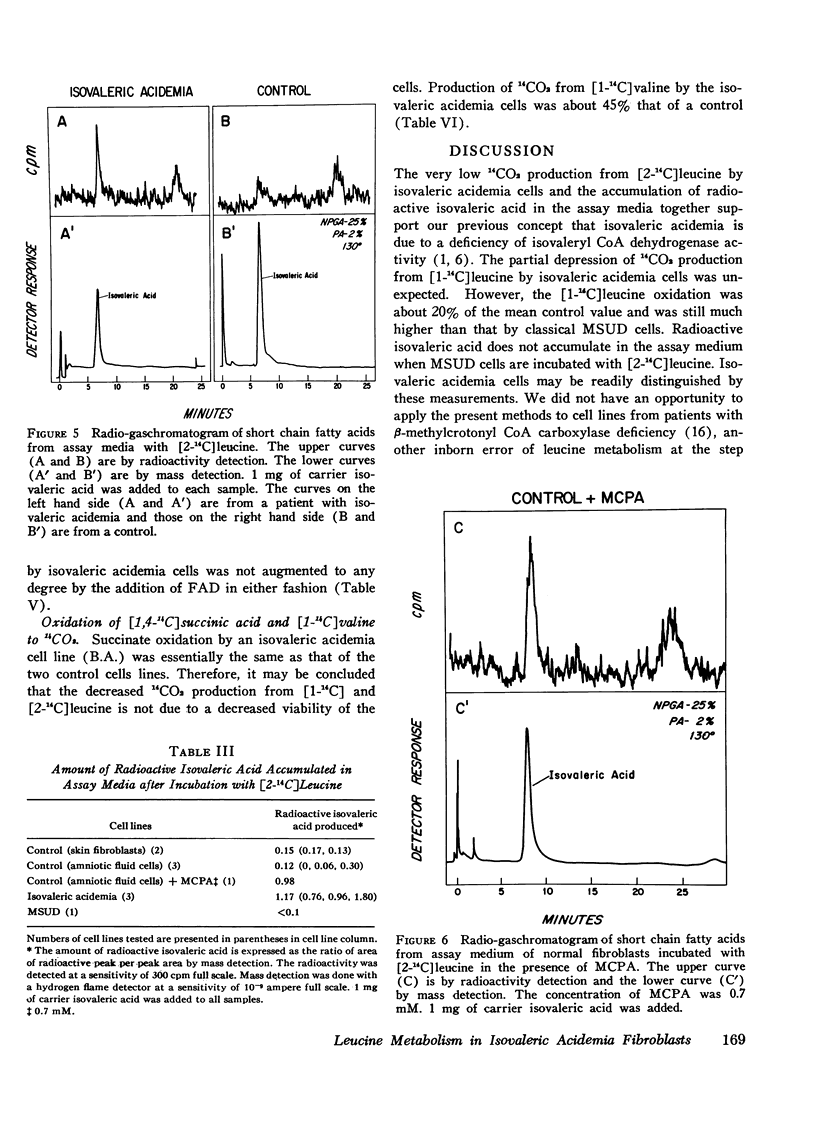
Leucine metabolism in cultured skin fibroblasts from patients with isovaleric acidemia was compared with that in normal fibroblasts and in cells from patients with maple syrup urine disease using [1-(14)C] and [2-(14)C] leucine as substrates. Inhibitory effects of methylenecyclopropylacetic acid on leucine metabolism in normal cells were also investigated. Production of 14CO2 from [2-(14)C] leucine was very reduced (96-99%) in both types of mutant cells. Radioactive isovaleric acid accumulated in assay media with isovaleric acidemia cells but not in those with maple syrup urine disease cells. Unexpectedly, 14CO2 production from [1-(14)C] leucine was partially depressed (80%) in isovaleric acidemia cells whereas in maple syrup urine disease cells it was strongly depressed (99%) as expected. These two mutant cells were clearly distinguished by detection of 14C-isovaleric acid accumulation after incubation with [2-(14)C] leucine. A pattern of inhibition of leucine oxidation similar to that seen in isovaleric acidemia cells was induced in normal cells by the addition of 0.7 mM methylenecyclopropylacetic acid to the assay medium. The partial inhibition of [1-(14)C] leucine oxidation seen in isovaleric acidemia cells and also in normal cells in the presence of the inhibitor appears to be, at least in part, due to an accumulation of isovalerate in the cells. Isovaleric acid (5-10) mM) inhibited [1-(14)C] leucine oxidation 32-68% when added to the assay medium with normal cells. Addition of flavin adenine dinucleoside to culture medium or assay medium or both did not restore oxidation of either leucine substrate in isovaleric acidemia cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando T., Nyhan W. L., Bachmann C., Rasmussen K., Scott R., Smith E. K. Isovaleric acidemia: identification of isovalerate, isovalerylglycine, and 3-hydroxyisovalerate in urine of a patient previously reported as having butyric and hexanoic acidemia. J Pediatr. 1973 Feb;82(2):243–248. doi: 10.1016/s0022-3476(73)80161-1. [DOI] [PubMed] [Google Scholar]
- BEINERT H., PAGE E. On the mechanism of dehydrogenation of fatty acyl derivatives of coenzyme A. V. Oxidation-reductions of the flavoproteins. J Biol Chem. 1957 Mar;225(1):479–497. [PubMed] [Google Scholar]
- Bressler R., Corredor C., Brendel K. Hypoglycin and hypoglycin-like compounds. Pharmacol Rev. 1969 Jun;21(2):105–130. [PubMed] [Google Scholar]
- Budd M. A., Tanaka K., Holmes L. B., Efron M. L., Crawford J. D., Isselbacher K. J. Isovaleric acidemia. Clinical features of a new genetic defect of leucine metabolism. N Engl J Med. 1967 Aug 17;277(7):321–327. doi: 10.1056/NEJM196708172770701. [DOI] [PubMed] [Google Scholar]
- Dancis J., Hutzler J., Snyderman S. E., Cox R. P. Enzyme activity in classical and variant forms of maple syrup urine disease. J Pediatr. 1972 Aug;81(2):312–320. doi: 10.1016/s0022-3476(72)80301-9. [DOI] [PubMed] [Google Scholar]
- GREEN D. E., MII S., MAHLER H. R., BOCK R. M. Studies on the fatty acid oxidizing system of animal tissues. III. Butyryl coenzyme A dehydrogenase. J Biol Chem. 1954 Jan;206(1):1–12. [PubMed] [Google Scholar]
- Langenbeck U., Rüdiger H. W., Schulze-Schencking M., Keller W., Brackertz D., Goedde H. W. Evaluation of a heterozygote test for maple syrup urine disease in leucocytes and cultured fibroblasts. Humangenetik. 1971;11(4):304–315. doi: 10.1007/BF00278658. [DOI] [PubMed] [Google Scholar]
- Newman C. G., Wilson B. D., Callaghan P., Young L. Neonatal death associated with isovalericacidaemia. Lancet. 1967 Aug 26;2(7513):439–442. doi: 10.1016/s0140-6736(67)90854-9. [DOI] [PubMed] [Google Scholar]
- SCHACHTER D., TAGGART J. V. Glycine N-acylase: purification and properties. J Biol Chem. 1954 May;208(1):263–275. [PubMed] [Google Scholar]
- Shih V. E., Mandell R., Scholl M. L. Letter: Historical observation in maple syrup urine disease. J Pediatr. 1974 Dec;85(6):868–869. doi: 10.1016/s0022-3476(74)80363-x. [DOI] [PubMed] [Google Scholar]
- Shih V. E., Mandell R., Tanaka K. Diagnosis of isovaleric acidemia in cultured fibroblasts. Clin Chim Acta. 1973 Nov 15;48(4):437–439. doi: 10.1016/0009-8981(73)90425-7. [DOI] [PubMed] [Google Scholar]
- Stokke O., Eldjarn L., Jellum E., Pande H., Waaler P. E. Beta-methylcrotonyl-CoA carboxylase deficiency: a new metabolic error in leucine degradation. Pediatrics. 1972 May;49(5):726–735. [PubMed] [Google Scholar]
- Tanaka K., Budd M. A., Efron M. L., Isselbacher K. J. Isovaleric acidemia: a new genetic defect of leucine metabolism. Proc Natl Acad Sci U S A. 1966 Jul;56(1):236–242. doi: 10.1073/pnas.56.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Isselbacher K. J., Shih V. Isovaleric and -methylbutyric acidemias induced by hypoglycin A: mechanism of Jamaican vomiting sickness. Science. 1972 Jan 7;175(4017):69–71. doi: 10.1126/science.175.4017.69. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Isselbacher K. J. The isolation and identification of N-isovalerylglycine from urine of patients with isovaleric acidemia. J Biol Chem. 1967 Jun 25;242(12):2966–2972. [PubMed] [Google Scholar]
- Tanaka K., Miller E. M., Isselbacher K. J. Hypoglycin A: a specific inhibitor of isovaleryl CoA dehydrogenase. Proc Natl Acad Sci U S A. 1971 Jan;68(1):20–24. doi: 10.1073/pnas.68.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K. On the mode of action of hypoglycin A. 3. Isolation and identification of cis-4-decene-1,10-dioic, cis, cis-4,7-decadiene-1,10-dioic, cis-4-octene-1,8-dioic, glutaric, and adipic acids, N-(methylenecyclopropyl)acetylglycine, and N-isovalerylglycine from urine of hypoglycin A-treated rats. J Biol Chem. 1972 Dec 10;247(23):7465–7478. [PubMed] [Google Scholar]
- Tanaka K., Orr J. C., Isselbacher K. J. Identification of beta-hydroxyisovaleric acid in the urine of a patient with isovaleric acidemia. Biochim Biophys Acta. 1968 May 1;152(3):638–641. doi: 10.1016/0005-2760(68)90107-0. [DOI] [PubMed] [Google Scholar]



