Abstract
While a distinct minicolumnar phenotype seems to be an underlying factor in a significant portion of cases of autism, great attention is being paid not only to genetics but to epigenetic factors which may lead to development of the conditions. Here we discuss the indivisible role the molecular environment plays in cellular function, particularly the pivotal position which the transcription factor and adhesion molecule, β-catenin, occupies in cellular growth. In addition, the learning environment is not only integral to postnatal plasticity, but the prenatal environment plays a vital role during corticogenesis, neuritogenesis, and synaptogenesis as well. To illustrate these points in the case of autism, we review important findings in genetics studies (e.g., PTEN, TSC1/2, FMRP, MeCP2, Neurexin-Neuroligin) and known epigenetic factors (e.g., valproic acid, estrogen, immune system, ultrasound) which may predispose towards the minicolumnar and connectivity patterns seen in the conditions, showing how one-gene mutational syndromes and exposure to certain CNS teratogens may ultimately lead to comparable phenotypes. This in turn may shed greater light on how environment and complex genetics combinatorially give rise to a heterogenetic group of conditions such as autism.
Keywords: beta catenin, minicolumns, neural stem cells, Rett syndrome, fragile X syndrome, tuberous sclerosis, valproic acid, PTEN phosphohydrolase, ultrasonography, cell adhesion molecules, neuronal
Introduction
There is continuing debate over what defines autism at the various levels of comparison. While specificity of data improves as one approaches the molecular level, it is now very apparent that at the genetic level of comparison autism is highly heterogeneous [1, 2]. We have therefore proposed that the “Least Common Denominator” of autism lies in the next level beyond, within the neuroanatomy of the conditions [3]. An increase in number of neocortical minicolumns seems to be a common underlying element to a significant number of cases, a phenotype which complements many fMRI studies which have reported differences in coordinated processing in autism during various cognitive tasks ([4] for review). It also complements well the molecular work thus far performed and which will be reviewed here in subsequent sections. As Casanova et al. [5] have proposed, the increase in minicolumnar number in autism may be due to a rise in germinal cell numbers within the paraventricular area underlying the developing neocortex, which may predispose towards a particular phenotype of neuronal connectivity, one which preferences short-range arcuate fibers over longer-range white matter tracts. An increase in neural stem cells will subsequently lead to an increase in neocortical tissue, i.e., because cortical tissue adheres to a general radial configuration, more minicolumns will be generated provided there are no considerable aberrations in processes such as migration or differentiation (for example, see [6]). In addition, increased neuronal cell numbers and density have been noted in other areas of the telencephalon in autism aside from neocortex, including the hippocampus, the amygdala, and other portions of the limbic system ([7], for review). Ultimately, the overall number of minicolumns within a local tissue may impact neuropil width, brainwide connectivity patterns, and local patterns of neuritogenesis, synaptogenesis, and plasticity [5, 8–11].
Autism as a larger group is heterogenetic and while this group of conditions is highly heritable, they are not strictly “inherited”, as evidenced by the discordance even amongst some monozygotic twins [12]. The monozygote concordance rates in the idiopathic conditions range from approximately 60%–92% depending upon behavioral measurements used, and therefore even at measures most lenient concordance falls shy of 100% ([13] for review). Thus, environmental factors, as with many such developmental conditions, are suggested to play their role in autism [14]. Whether these factors act as though flipping a genetic on/off switch or they simply exacerbate an already present condition is still to be determined, although the presence of a broader autism phenotype existing amongst family of the affected may help to answer this question in future.
In early infancy, autistic babies usually present with average cranial size, in contrast to the larger cranial sizes seen in early childhood [15]. Many studies have therefore focused their efforts on investigating postnatal development during the time when head circumference in the conditions seems to increase so dramatically. In addition, cases of autism in which regression occurs during this time period has lead investigators to focus on development of the first two years of life [16]. And therefore many scientists have placed their efforts into the study of topics such as neuritogenesis, postnatal synaptogenesis, or pruning, but have given less energy to studying embryonic development based on the assumption that early embryogenesis could not set in motion the regressive events later seen in some cases of autism. While we do not criticize much of the work, both genetic and molecular, that has been performed to date investigating such topics, instead we would propose that: 1) embryogenic events may affect aspects of later arborization, synaptogenesis, and pruning to the point of determining phenotypic expression patterns of molecules such as the NMDA receptor, cadherins, neuroligins, and other synaptic adhesion or adaptor molecules, and 2) the molecular pathways which underlie many of the events during neuritogenesis and synaptogenesis (e.g., growth pathways, cytoskeletal rearrangement, etc.) are the same as those seen during mitotic division of neural stem cells, albeit used in a re-routed capacity (for example, see [17]). Therefore, if a given molecule along one of these pathways is genetically or environmentally targeted, particularly if these pathways are subject to positive feedback loops or epigenetic alterations, then effects may be seen both in cellular division and in later connectivity patterns. There is also the possibility that alterations in neural proliferation may have downstream effects on arborization in general due of the altered demands that an increase in total neuronal number places on the maintenance of interconnectivity. While the abnormalities of connectivity in autism may become grossly apparent after the first year of life, their roots may still lie in embryogenesis. So while we do not strive to tear down current blossoming theory of autism as a neuritopathic or synaptopathic condition while proffering our own theories of autistic minicolumnopathy as all-inclusive, instead we propose to view autism from a broader molecular framework in which neural proliferation elegantly complements later neuritic and synaptic development.
Big brains: megalencephaly and co-occurring autism
Humans have had a long-standing fascination with big brains, therefore given the larger cranial sizes in autism and some of the intelligences associated with the conditions, it has not only peaked our sympathy but our curiosity. While a weak positive correlation exists across ages and sexes between brain size and intelligence [18], such a simplistic view of “bigger is better” fails to acknowledge that it isn’t simply how much gray matter one has but how it is connected together and therefore how it functions. Hence, there have been notable geniuses throughout history who have had average or even small total brain volume (TBV) but are nevertheless remembered for their incredible advances in the sciences and humanities [19]. Therefore, increased TBV (suggestive of increased gray and white matter volumes) may play a key role in the varied human intelligences but is still a limited determinant variable. As has been discussed elsewhere, autism is simultaneously known for islands of genius yet clear deficits in generalized realms of social and communicative intelligences [20, 21]. However, while autism is illustrative of some of the extreme localized intelligences humanity can achieve, it also shows us that bigger is not always better.
As one might expect, if a defining element of many, if not all, cases of autism lies in the increase in minicolumnar number, then larger brain volumes may seem unsurprising. And in fact, larger TBVs are consistently noted in the conditions, at least during the early childhood years [22]. Macrocephaly is also more common in autism, although some of these cases may instead be indicative of the unique molecular underpinnings of select cases. For instance, PTEN germline mutations have been associated with a number of hamartomatic conditions such as Cowden’s disease, Bannayan-Riley-Ruvalcaba syndrome, Proteus syndrome, Lhermitte-Duclos disease, and has also been found in autism with co-occurring macrocephaly ([23] for review). Each condition, while often targeting different tissues, ultimately triggers overproliferation of cells, leading to increases in overall tissue size, various tumors, and even cancers. While PTEN germline mutations have been identified in as much as 7% of the autistic population, it is almost always associated with extreme macrocephaly [24]. In part, this is likely due to the upstream position at which PTEN sits, regulating a number of different processes integral to mitotic division ([25] for review). If PTEN’s action is downregulated or inhibited, these mitotic processes are upregulated, thereby increasing risk of aberrant proliferation and tumorogenesis. In fact, many late stage tumors present with PTEN mutations [25]. In the case of autism, if a PTEN mutation targets neural stem cells, these cells will be maintained for a longer period in a state of symmetric division, leading to greater neuronal numbers and ultimately increased cerebral volume.
However, there is more than one molecular target vulnerable to dysregulation with the potential to create such a phenotype. While PTEN normally inhibits inappropriate cellular proliferation, the MeCP2 gene codes for a protein product which is heavily involved in both methylation and deacetylation of DNA [26]. Normally, MeCP2 binds with molecules such as mSin3, Sox2, and histone deacetylases (HDAC), forming complexes which prevent DNA transcription [26, 27]. While its various complexes are thought to act genome-wide within neurons, MeCP2 frequently acts to inhibit promoter genes, so if the complex is downregulated or inactivated the promoter is freed and DNA transcription is more likely to occur [28]. Amongst many others, MeCP2 complexes target LEF/TCF transcription. LEF/TCF is a transcription factor which binds and is activated by the armadillo-repeat protein, β-catenin [29]. As will be discussed in subsequent sections, β-catenin is a bifunctional molecule involved in both cellular adhesion (for instance, at the soma in epithelial cells and at the synapse in neurons) and in transcription. In particular, it is seated in such a key position as to be involved in both maintaining homeostasis of a given state or promoting growth. In addition, its cytosolic accumulation is not only indirectly inhibited by MeCP2 but is regulated by PTEN as well, linking both PTEN-associated macrocephalic autism and Rett’s disorder to the action of specific growth pathways [30].
MeCP2 in past years has garnered considerable attention from those who have an interest in autism. It has been shown that MeCP2 mutations occur in approximately 80% of Rett’s disorder cases, a condition currently listed under Pervasive Developmental Disorders in the DSM-IV-TR [31]. While the majority of Rett’s cases are no longer considered idiopathic but as single-gene conditions, some of the behavioral similarities between Rett’s and the more common idiopathic autism are striking enough that the etiology of one may proffer clues to the other. And therefore scientists who focus on the broader autism have taken an interest in the MeCP2 gene [32]. If the behavioral similarities are more than coincidence and these conditions share some phenotypic commonalities at the neuronatomic level, then this may be good indication that autism, as a whole, is rooted in the overactivation of various growth pathways. And due to some of the positive feedback loops between these pathways, when one pathway is activated, others are more likely to follow suit [33].
As per example, mTOR is a serine/threonine protein kinase that integrates aspects of the various pathways involved in growth and proliferation. It sits downstream of the PI3K/Akt and ERK pathways and is a detector of growth factors, insulin, and even oxidative stress [34]. The mTOR complex is able to indicate to the cell whether resources are available for mitosis. The molecules which indirectly inhibit mTOR by preventing Rheb cycling, Tuberous Sclerosis Complex (TSC) 1/2, are so named for the condition in which their irregularity was discovered. Tuberous sclerosis is a single-gene condition in which approximately 25%–50% of the affected also exhibit autistic symptomatology ([35] for review). The condition itself is caused by heterozygous mutations in either the TSC1 or TSC2 gene, whose protein products, hamartin and tuberin, respectively, act to regulate mTOR [36]. Subsequently, dysregulation of growth pathways within the neural germinal population creates global or localized forms of megalencephaly, tubers, subependymal nodules, and various other cortical malformations indicative of excessive neural stem cell growth and abnormal neuronal differentiation [37, 38, 39]. Interestingly, similar heterotopias and dysplasias have been noted in the broader autism, albeit to a lesser degree, as seen in Tuberous sclerosis [40, 41]. As is perhaps not surprising given the focus of this review, the loss or inhibition of tuberin affects the canonical Wnt pathway, upregulating the production of Wnt target genes such as cyclin D1 and MYC [42, 43].
Cyclin D1 plays an important role in the progression from G1 to S phase during mitosis. And while this protein has numerous regulators, one kwy regulator seems to be Fragile X Mental Retardation Protein (FMRP), the namesake of the condition in which its mutation was discovered. FMRP normally acts as a suppressant to translation by binding mRNA and preventing the production of a given protein, in this case, cyclin D1 [44, 45]. Fragile X syndrome (FXS) is a single-gene condition with a high co-occurrence of autism spectrum conditions: approximately 15%–33% of those with FXS also have autism. While the general population has a CGG trinucleotide repeat sequence on the long arm of the X chromosome ranging from 5–50 repetitions, carriers of the premutation have about 55–200 repeats, and anything more than 230 repeats tends to cause the full syndrome, with greater severity seen in males ([46] for review). In individuals with FXS, the repeat sequences encoding FMRP are usually highly methylated which prevents gene transcription, leaving affected individuals with a deficiency in this protein. In males, there is usually a complete deficiency while females tend to have a partial one due to the availability of FMRP from the second X chromosome [47]. While FMRP mutation has been highly associated with regulation of synapse formation, more recent work has broadened our awareness of the scope of its functions to include that of progenitor proliferation and neurogenesis [48, 49]. In vivo and ex vivo studies have exhibited increased rates of mitosis, due largely to a briefer respite at G1 phase, and increased production of infragranular neurons and intermediate progenitors, possibly indicating prolonged maintenance of radial glial proliferation [49, 50]. Even though there have been reports of microcephaly in FXS particularly in adult females with the condition, overproliferation of certain progenitor and neuronal subsets may ultimately link FXS to neocortical development in idiopathic autism and make some sense of the common behavioral phenotype which exists between the two [51].
When considering the molecular theme which slowly begins to develop upon closer investigation of various forms of single-gene autism, and in viewing some of the microscopic and gross morphometrical commonalities that these conditions share with the idiopathic condition, one might hypothesize that at the molecular level the broader autism can be defined by abnormal activation, specifically overactivation, of the growth pathways which are common to both mitosis and neuronal arborization. However, whether overproliferation during neurogenesis is a requisite for development of the conditions, is a common yet not a mandatory predisposing factor, or it plays a coincident, minor role in ultimate phenotype due to the pathways it shares in common with neuritogenesis and synaptogenesis is still to be determined.
The role of β-catenin in corticogenesis and synaptogenesis
β-catenin is a protein intimately involved in both cell-cell adhesion and transcription. In adhesion of epithelial cells, β-catenin acts as one of a set of adaptor proteins which anchors the actin cytoskeleton to the intracellular portion of E-cadherin [29]. Anchoring of the cytoskeleton in turn inhibits the “treadmilling” of that filament, stabilizing it [52]. Through formation of the cadherin/β-catenin/α-catenin/vinculin complex, p120 is recruited to E-cadherin, linking the adherens junction to several intracellular signaling pathways such as various receptor tyrosine kinases (RTK) [53]. The extracellular portion of E-cadherin, upon activation, binds an identical extracellular portion of another E-cadherin in an adjacent epithelial cell, enabling cells to communicate information to their neighbors regarding the state of their cytoskeletal structures. This ultimately can facilitate the coordinated migration, adhesion, and differentiation of adjacent cells. In the case of transcription within epithelia, cytosolic β-catenin is normally phosophorylated by both CK1 and GSK3β of the Axin/APC complex and thereby marked for degradation; however, upon canonical Wnt activation β-catenin is freed into the cytosol and is transferred into the nucleus via transporters such as Pygopus and BCL9. Incidentally, anything which downregulates, deactivates, or sequesters GSK3β away from the Axin/APC complex will subsequently upregulate cytosolic β-catenin levels. Once within the nucleus, β-catenin binds LEF/TCF to stimulate transcription of Wnt target genes [54]. All forms of β-catenin, despite their different localizations, are transcribed from the same gene; therefore, distinctions in molecular form and function are thought to be achieved through variations in phosphorylation, binding partners, and cleavage along the arm domain of the protein. It is suspected that binding may enhance the negative charge along β-catenin’s positive groove, increasing binding affinity for certain partners dependent upon the precise site or sites phosphorylated. Changes in E-cadherin phosphorylation can also affect β-catenin binding to the adherens complex [43].
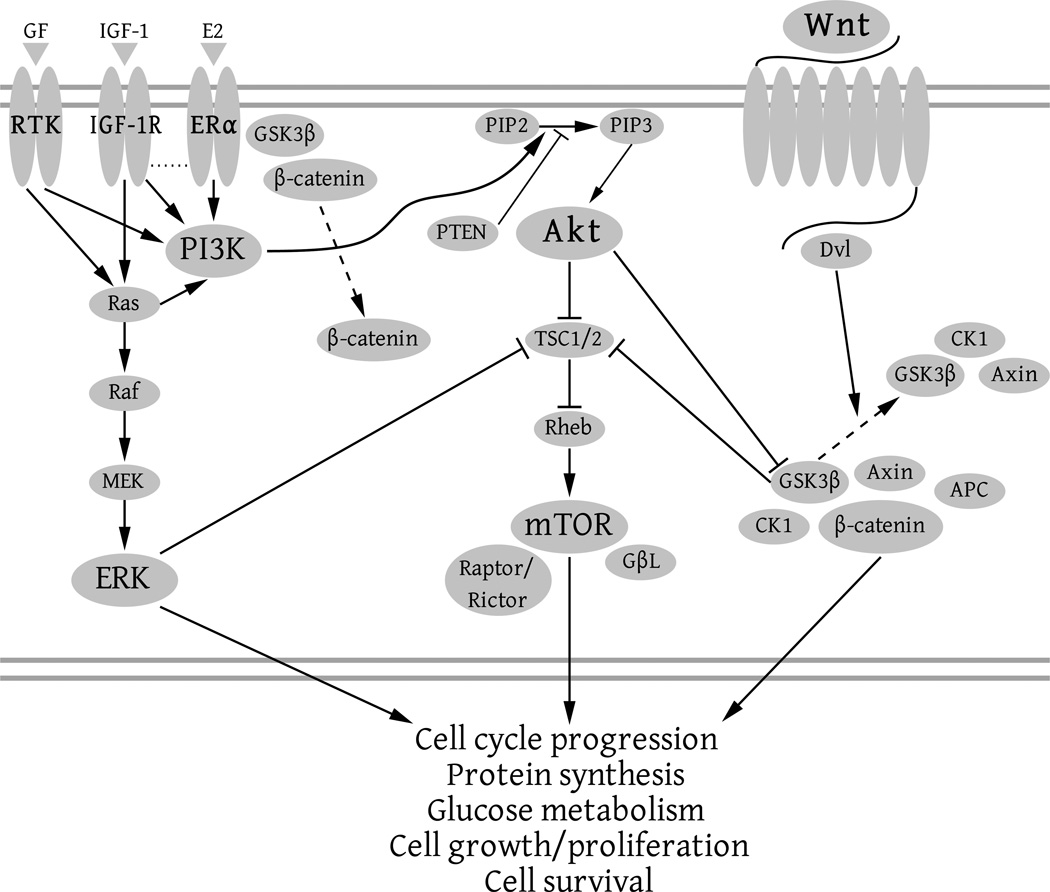
As mentioned, β-catenin plays a vital role in both epithelial adhesion and Wnt-targeted transcription. The Wnt family is a highly conserved set of extracellular signaling molecules which are intimately involved in embryonic development. Canonical Wnt has both direct and indirect gene targets which it activates and which also may cyclically regulate the Wnt pathway. As mentioned earlier, direct targets include genes such as cyclin D1, necessary for G1-to-S phase progression during mitosis, and MYC, a transcription factor which is believed to act as an enhancer for up to 15% of all genes [55, 56, 57]. (For a listing of both direct and indirect targets of Wnt activation, see [58].) However, while β-catenin activation is a prerequisite for transcription of cyclin D1 and MYC and therefore growth and division of totipotent and multipotent cells, canonical Wnt activation is not the only method by which to upregulate cytosolic β-catenin. Direct or indirect regulation, such as via other growth related pathways like Akt and ERK, can influence the β-catenin pathway because all three pathways (Akt, ERK, and Wnt) act in tandem to enable growth and proliferation (Figure 1) [59, 60]. While they each have multiple functions, they serve unique purposes in mitosis and arborization, but do share considerable molecular overlap with the ability to regulate one another. For instance, Akt, among other things, is involved in insulin control: Akt must have some capability to regulate activation downstream of canonical Wnt because a cell with inadequate ATP supply should not be allowed to proceed to S phase (DNA synthesis) due to the high energy demands of such a process.
Figure 1.
This figure illustrates how the different growth-related pathways, ERK, Akt, mTOR, and Wnt, can each regulate one another. As can be visualized with this simple diagram, if one pathway is activated it is easy to see how the other pathways may also be upregulated. Here just a few means of crosstalk are illustrated, although numerous others exist as well.
As one would imagine, expression of Wnt target genes can enhance cellular proliferation. Therefore, targeting of a given molecule upstream or down from β-catenin has the potential to either dramatically upregulate proliferation (in the case of a molecule that ultimately increases β-catenin levels) or promote differentiation or apoptosis (downregulation of cytosolic β-catenin). To illustrate this point within the paraventricular zone, Chenn and Walsh [6] have created a mouse model in which a truncated form of β-catenin specific for radial glia lacks phosphorylation sites for GSK3β, thus preventing ubiquitination of the β-catenin molecule. This leaves β-catenin constitutively active in these neural precursors and able to participate in adhesion and transcription processes. Chenn and Walsh [6] have found that these mice exhibit increased neural progenitor proliferation, larger lateral ventricles, neuritic abnormalities, and on a macroscopic level are macrocephalic and gyrencephalic compared to control mice.
But not only does β-catenin play a role in precursor proliferation, it participates in the adhesion of reserve pools of presynaptic vesicles to presynaptic sites [61]. Rather than link cadherins to the actin cytoskeleton, β-catenin may instead select other binding partners through its PDZ binding domain, linking cadherin to PDZ proteins. Likewise, p120, another adaptor protein which is part of the adherens junction, may connect its associated cadherin to Rac and Rho as well as some receptor tyrosine kinases (RTK). Ultimately, the localization of cadherins to the potential active zone of a newly forming synapse suggests their integral involvement in synaptogenesis [61]. In brief, the β-catenin molecule is intimately involved in the various forms of cellular growth, from mitosis of precursors to the development of growth cones. While a multitude of different effectors come together to coordinate cellular development, β-catenin occupies a key position in which it helps to maintain homeostasis at the adherens junction and at the nascent synapse, yet also becomes a transcription factor once freed into the cytosol, acting as a messenger from membrane to nucleus.
Targeting β-catenin in progenitor proliferation
A number of genetic and teratogenic animal models have been used to study the autism phenotypes. As mentioned, PTEN, TSC1/2, and Fmr1 knockout models have been developed which better illustrate the proliferative aberration reflective of the various conditions [62, 63]. The β-catenin constitutive model designed by Chenn and Walsh [6], while not originally designed to study autism, nevertheless is potentially applicable when considering the downstream position which β-catenin maintains relative to PTEN, TSC1/2, and MeCP2. Yet while we’ve reviewed research pertaining to known genetic causes of autistic phenotypes, we would now like to focus on teratogenic agencies and how they can likewise target mitosis. This includes agents which are foreign to mother and offspring, such as foods or medications, and those which are maternally produced yet are still foreign to the embryo despite sharing an appended closed system with the mother.
Valproic acid (VPA) is a short chained fatty acid which has been used both as an anti-epileptic and a mood stablizer. Concern over VPA’s teratogenicity has existed since the early 1980s, when it was linked with various congenital malformations. These malformations are now grouped as Fetal Valproate Syndrome (FVS), which include hernias, facial deformities, strabismus, microcephaly and smaller-than-average cranial size, and motor and language delays [64, 65, 66]. In 1994, however, increased autism risk was added to the list of developmental disruptions following in utero exposure [66]. A later study by Moore et al. [67] confirmed VPA as a significant risk factor for autism, finding that approximately 60% of those with a diagnosable form of FVS exhibited two or more autistic features, and of this group, six children (~11%) had a fullblown form of autism. Another study performed by Rasalam et al. [68] replicated a similar rate of approximately 9%, although this study looked at cases based on exposure rather than confirmed FVS presentation. Based upon these earlier studies, Markram et al. [69] developed the VPA Rat Model of Autism and a behavioral theory called “The Intense World Theory of Autism”, utilizing the rat model to explore some of the neuroanatomy and neurophysiology which may underlie the human conditions. From these animal studies, Markram’s group found local hyperconnectivity, hyperplasticity, increased NMDA levels, enhanced postsynaptic longterm potentiation, and single-neuron hypoexcitability within postnatal rat neocortex following prenatal exposure on embryonic day 11.5 (E11.5) [9, 10].
VPA acts both as an inhibitor of myo-inositol-1-phosphate (MIP) synthase, acutely reducing concentrations of myo-inositol, and as a class I and class II histone deacetylase (HDAC) inhibitor, which, amongst their other functions, regulate Wnt-targeted transcription [70]. At the level of DNA, Wnt-targeted transcriptional regulation involves two key steps: 1) HDAC1 is involved in active repression of the LEF/TCF transcription factor and once it is dissociated from this complex, the LEF/TCF promoter is inactive but primed; and 2) following priming of the promoter, β-catenin associates with the complex, activating transcription of Wnt-target genes, such as cyclin D1 and MYC [71]. Following HDAC inhibition by VPA, transcription of Wnt-target genes is upregulated and overall β-catenin levels increase [72, 73]. While VPA may affect arborization by directly targeting the metabolism of neurons themselves in fetal and postnatal tissues, cortical neurons are not produced in rat until E13.5 [74]; therefore, as is suggestive of the studies of Markram et al. ([69] for review), any alterations in neuritogenesis and synaptogenesis of neocortical neurons following E11.5 exposure is due to multigenerational effects, perhaps epigenetic in origin given the inhibition of HDAC involvement in chromatin modeling and methylation, which stems from the original neural stem cell exposure.
As mentioned earlier, anything which downregulates, deactivates, or sequesters GSK3β away from the Axin/APC complex leads to greater pools of cytosolic β-catenin and upregulated transcription. While VPA does not target GSK3β but upregulates transcription via HDAC inhibition further downstream of the degradation complex, lithium, another mood stabilizing drug, achieves similar effects by targeting GSK3β both through direct inhibition and indirectly by preventing dephosphorylation of phospho-GSK-3 [75, 76]. In fact, it has been used in partial recovery of Fmr1 knockout mice, a murine model of FXS [77]. While both VPA and lithium target neurogenesis, upregulating progenitor proliferation, they do so by inhibiting different targets of the same pathways, leading to slightly different bioeffects [78, 79]. As mentioned, VPA targets HDACs which subsequently increases canonical Wnt pathway activity at the level of transcription; this leads to an overall increase in β-catenin levels. Lithium, on the other hand, targets GSK3β, leading not to an increase in overall β-catenin levels via transcription but to an increased ratio of β-catenin freed into the cytosol. For this reason, lithium may prove to be a weaker teratogen than VPA due to differences seen at the level of Wnt pathway activation.
The MeCP2 pathway is another potential target for teratogenicity. While MeCP2 involves an HDAC complex and binds DNA not only through methylation but by deacetylation, it is likewise vulnerable to an agent such as VPA. VPA directly targets HDAC, thereby preventing MeCP2 transcriptional regulation via deacetylation. This prevention is vitally important in the regulation of transcription because methylated DNA alone or deacetylated DNA alone continues to remain transcriptionally active and requires both forms of regulation in tandem for repression to occur [26, 80]. While lithium does not seem to have such direct effects on MeCP2 binding as does VPA, rescue of MeCP2 knockout mice with administration of IGF-1 partial peptide would suggest that MeCP2 is regulated upstream by growth pathways in general and that lithium and other such agents could have an indirect hand in MeCP2 downregulation [81]. An earlier study by Samaco et al. [32] would support this, finding that while most cases of Rett’s syndrome can be traced directly back to an MeCP2 mutation, idiopathic cases of autism nevertheless exhibit abnormalities in MeCP2 levels despite no apparent mutation. Not only does this point towards MeCP2 as an important player in growth and proliferation, but leads one to question whether these growth-related pathways are a requisite to the development of autism.
While these pathways can be regulated via intracellular cytosolic interactions as with VPA and lithium, mitosis can also be activated through receptor-mediated interactions at the cellular and nuclear membranes ([82] for review). Various growth factors, such as the Fibroblast Growth Factor (FGF) family and Brain-Derived Growth Factor (BDNF), have received more recent attention in the autism literature due to their obvious roles in embryonic growth and differentiation [83, 84]. However, in contrast, nuclear receptor-mediated ligands such as steroids have only received a modicum of attention in relation to the important roles these hormones play in neurogenesis.
In 2002, Simon Baron-Cohen published “The Extreme Male Brain Theory of Autism” [85]. His team found that autistic individuals tended to exhibit extreme male-pattern performance on certain cognitive tasks, specifically in systemizing (male) versus empathizing (female), and hypothesized that this difference was due to hyperandrogenization of the brain in autism. This same research group performed another study which focused on in utero androgen levels in relation to early childhood performance on a particular cognitive task which likewise tends to show gender differences according to empathic capabilities. They found that androgen levels positively predicted certain male-pattern performances, especially within the male group alone [86]. Knickmeyer et al. [87] went on to study individuals with Congenital Adrenal Hyperplasia (CAH), a group of conditions which often presents with hyperandrogenism; they found that females with the condition scored higher on the Autism Spectrum Quotient (AQ) than control females. While CAH is due to various autosomal dominant mutations which target steroid synthesis, a number of commonalities, such as polycystic ovaries, are shared between this condition and Polycystic Ovarian Syndrome (PCOS) [88]. In part, this may be due to the hyperandrogenism and hyperinsulinemia common to both conditions. Along these lines, Ingudomnukul et al. [89] found that high-functioning autistic females and their mothers were more likely than controls to present with testosterone-related disorders, including that of PCOS. These studies together support a link of high androgen levels to autistic symptomotology.
PCOS, as a heterogeneous condition, frequently presents with obesity, hyperinsulinemia, hyperandrogenism, poor progesterone sensitivity, abnormal patterns of leutenizing hormone (LH) activity, and extended periods of anovulation with high levels of estrogen [90]. While androgens at the molecular level tend to promote entry of β-catenin into the nucleus [91, 92], there is evidence to suggest that activation of the androgen receptor within the neocortex and hippocampus is in fact antagonistic to Wnt-targeted gene transcription [93]. Therefore, at face value, androgen's effects on canonical Wnt may suggest that hyperandrogenism would not lead to increased autism risk; however, if one delves further into the nature of these hormones, as well as the timing of developmental events, high androgen levels may indirectly promote growth after all.
Estrogen is vitally important in sexual differentiation of the mammalian brain, with higher levels leading to greater masculinization [94]. While prenatal and neonatal androgen levels may be high in autism, it is estrogen which is largely responsible for exacerbation of the sexually dimorphic development of the neocortex and hippocampus [94]. Neural stem cells in these areas exhibit high levels of both aromatase and the estrogen receptor, ERβ, throughout neurogenesis, and ERα during late neurogenesis [95], suggesting that excess androgens may be aromatized into estrogen and used to promote male-pattern growth [96, 97]. Likewise, steroids can easily cross the placental and blood-brain barriers, such that maternal hormone levels may be shared with the developing embryo and ultimately affect development of the neural progenitor population [98]. However, at this point in time it is unknown to what extent maternal steroids play a role in embryonic development in general, and therefore further research is still needed to understand how excessive levels may affect embryogenesis and corticogenesis in particular.
At the molecular level, estrogen and its receptors are located within the nucleus (the classic mode of transmission), within the cytosol, and at the outer membrane. ERs have been associated with RTKs and various growth factor pathways. Of great interest is the synergistic relationship ERα has with Insulin-like Growth Factor-I (IGF-I) receptor and their downstream activations of Akt and Erk [99]. GSK3, of which β-catenin is a substrate, is downstream of both the ER and IGF-IR and appears to be a direct target of these two receptors. Upon estradiol activation of the ER, β-catenin is released from this complex and freed into the cytosol, not unlike lithium's inhibition of GSK3β, freeing β-catenin from the Axin/APC complex [99]. However, not only does the relationship between the ER and IGF-IR seem to be synergistic, at least in some circumstances they are interdependent: Perez-Martin et al. [100] have shown that the pure estrogen antagonist, ICI 182,780, is capable of blocking IGF-I-induced adult neurogenesis in the hippocampus of rats. What this relationship means to the etiology of PCOS is still unclear despite a known association of these hormones with the condition [101], however, both estrogen and IGF-I are capable of targeting the ERK, Akt, and Wnt pathways and therefore sit at important regulatory positions of growth and development. Following the freeing of β-catenin into the cytosol after ER activation, transcription of Wnt target genes is upregulated [102, 103]; however, not only does estradiol upregulate β-catenin activity by inhibiting GSK3β, it interacts with a regulatory subunit of PI3K, upregulating Akt activation as well [99, 104]. And so it is a potentially potent growth factor, targeting multiple pathways necessary for mitosis, neuritogenesis, and synaptogenesis. Therefore, given the high rates of PCOS in the female population today (6%–8%), the incredible promiscuity of the estrogen receptor to large numbers of estrogen mimics, the sheer number of steroidal mimics which exist today, and the significant association between testosterone-related disorders and autism, estrogens may be playing a critical role in the rising diagnostic rates of both of these conditions [90, 105].
Due to the considerable crosstalk between the endocrine and immune systems, sex steroids may also impact other systems aside from the central nervous system. It has been shown that prenatal hormone levels promote sexual dimorphism of the immune system, and therefore numerous immunological abnormalities have been reported in autism [106]. These include evidence of heightened levels of proinflammatory cytokines, autoantibodies to brain such as anti-myelin basic protein (MBP), increased rates of various autoimmune disorders both in autistics and in close family members, heightened antibody titers to viruses such as measles, altered immunoglobulin levels and immunodeficiencies, and an increase in the innate immune response [107–113]. While proinflammatory cytokines and T cell overactivation have been reported in the autism literature, differences have been found in helper T cell profiles between postmortem brain tissue (Th1) and serum samples (Th2) for reasons still unknown [108, 110]. However, the immune system may act more directly upon the central nervous system in reaction to infection. Following the rubella epidemics which hit the United States in the 1960s, it became apparent that prenatal rubella conferred a considerably higher risk for developing autism. Chess et al. [114] found that of those children diagnosed with congenital rubella, approximately 12% developed autism. When considering the stricter criteria for autism used in the 1970s, a similar study performed present day may well find an even higher percentage risk than was found previously.
Due to the crosstalk between the central, endocrine, and immune systems, it is difficult to predict which type of chemical messenger is the direct harbinger of risk. And while we’ve reviewed ways in which sex steroids may increase the risk of developing autism, the immune system can likewise confer direct effects on neural stem cell proliferation. Neural stem cells themselves express immune-related ligands and receptors, such as receptors for IL-6 which, like other receptors mentioned, can activate the JAK/STAT, Akt, and ERK pathways [115]. In addition, these pathways are also regulated by glucocorticoid, an important anti-inflammatory hormone, which plays a vital mediating role between progenitor cells and the immune system [116]. Wolf et al. [117] have found in vitro that when exposing murine hippocampal neural stem cells to high levels of glucocorticoid, neurogenesis halts and a greater proportion of cells undergo apoptosis; yet with exposure to low levels of glucocorticoid, an increase in progenitor proliferation occurs. Aside from local production of glucocorticoid, maternal levels can also have a critical effect on embryonic development, and so maternal infections and antenatal stress have received increasing attention over the years in the investigation of maternal-fetal interactions [118, 119]. In conclusion, a given immune response may not only be shaped by an invading antigen, it can be shaped by past infection, guided by the endocrine system, and even altered by mood and stress levels. This complexity and crosstalk makes it extraordinarily difficult to pinpoint a single effector of pathology, and therefore it is critical to study these systems in relation to one another.
This review has thus far focused on teratogenic agents which have received considerable attention in the literature. And, while we still struggle to understand their complexities, we are gaining ground rapidly. However, we would also like to present the reader with a potential teratogen which has been little studied and, for the most part, assumed safe. While various epidemiological and animal studies have been performed to date investigating the safety of prenatal ultrasound, we would like to present an alternate view of the method which will hopefully lead the reader to the conclusion that further research elucidating its bioeffects and stricter regulations of its application are imperative.
Prenatal ultrasound use has grown in popularity since the 1970s, with some mothers receiving four or more ultrasounds in a single pregnancy. This explosive use, however, does not necessarily reflect the occurrence rate of at-risk pregnancies, and in fact one study by You et al. [120] found that increased use was even more pronounced in those at low-risk. Ultrasound practice has changed within the last several decades as well. In heavier patients with greater amounts of adipose tissue, a higher intensity level is necessary in order to attain adequate image resolution; therefore, regulations were loosened in the 1990s in order to accommodate a greater range of patients [121]. Unfortunately, practitioners, nurses, and technicians tend to have a poor understanding of the thermal and cavitational risks which accompany ultrasound use, and so the safety of the patient is dependent upon the ultrasound “smart machine” to gauge exposure levels and shut down when dangerous levels have been reached [122].
In 2010, we proposed a link to the rising diagnostic rates of autism, increasing ultrasound use, and its poor regulation [123]. In lieu of an earlier report of migrational aberration in mouse neocortex following prenatal exposure, as well as the healing effects ultrasound is known to have on bone fractures and varicose ulcers, we decided to delve further into the molecular biology of ultrasound [124, 125, 126]. While earlier animal studies utilized gross morphometry and evidence of tissue necrosis to establish safety levels, fewer studies have focused on ultrasound effects at the single cell level ([127] for review). Upon review of the literature, we found that ultrasound targets multipotent cell populations in particular. It can trigger tissue regeneration in bone breaks by activating cellular growth pathways, as evidenced by heightened Wnt activation in osteoblasts [128]. And it also has similar growth effects on chondrocytes, activating the PI3K/Akt pathway [129]. Aside from its use in tissue regeneration, ultrasound has been utilized for transdermal delivery of medication due to its capacity for increasing cellular permeability; it therefore has been used with topical drugs which are normally limited by their molecular weights [130, 131]. Ultrasound also has predictable effects on the endothelium: because the shear stress created by ultrasonic pulses is interpreted by the endothelium as an increase in blood pressure, dilation of the vasculature occurs [132]. This effect has been used to the particular benefit of cardiovascular imaging studies, for instance not only allowing surgeons to view signs of coronary artery disease but also to guide the placement of arterial stents with greater accuracy [133, 134]. The endothelium also responds to shear stress, such as ultrasound interpreted as blood pressure, via proliferation of endothelial progenitors and mature endothelial cells and elongation into the direction of the flow [135].
Accompanying growth following ultrasound exposure, the endothelium produces molecules such as nitric oxide (NO), prostaglandin E2, interleukin-8 (IL-8), basic fibroblast growth factor (bFGF or FGF2), and vascular endothelial growth factor (VEGF) [136, 137]. In particular, FGF2 and VEGF are shared by both the endothelium and neural stem cell populations [138]. Neural progenitors produce VEGF which then attracts vascular growth towards the germinal zone in order to support progenitor mitosis; the vasculature can subsequently produce additional growth factors to continue to support the proliferation of the neural progenitor pool ([139] for review) [140]. In this way, the vascular system forms a “niche” for these multipotent cells and is then able to influence their growth and differentiation by releasing growth factors into the local area [141]. Following endothelial growth factor mediation, neural stem cells continue to form epithelial-like sheets, an earlier form of progenitor division, which may be indicative of upregulation of β-catenin. β-catenin activation thus promotes simultaneous maintenance of adherens junctional contacts between neighboring cells and symmetrical mitotic division [139]. Therefore, because it has been shown that ultrasound consistently upregulates growth factor production of the endothelium which would be necessary for promoting the maintenance of continued symmetric division in neural stem cell population, it is vitally important to determine to what extent ultrasound exposure may affect development of the central nervous system. In consideration of the proliferative phenotype which may also underlie a portion of cases of autism, these unanswered questions illustrate the need for further research and stricter regulations on prenatal ultrasound use.
We have attempted to cover a range of teratogenic agencies, reviewing some of the resultant molecular and neuroanatomic phenotypes particular to each effector. However, we hope that the underlying theme, that of growth pathway overactivation in autism, is apparent even to those readers who are less familiar with the molecular biology presented. While numerous autism phenotypes exist, as evidenced even amongst the single-gene conditions, we have given strong evidence to show that proliferative rates of the neural stem cell population are common to the etiology of the conditions. In the following section, however, we would like to suggest that while an increase in neural proliferation may be common to the autistic phenotype, it is in fact the nature of neuronal connectivity itself which determines the behavioral syndrome.
Nature and nurture of the synapse in autism
Recent research has made important strides in understanding how synaptic genes and their products play integral roles in the development of autism. In particular, mutations within genes coding for synaptic adhesion have become associated with the conditions ([142] for review). One receptor-ligand pairing, neurexin-neuroligin, has garnered considerable attention due to certain X-linked mutations which have been found in autism, specifically in neuroligins 3 and 4 and neurexin 1 [143–146]. Neurexin-neuroligins are involved in synaptic adhesion during early stages of neuronal arborization, providing transient contact stabilization independent of NMDA activity [147]. They also help control excitatory and inhibitory synapse formation and while more is known about neuroligin 1 in general, all the neuroligins seem to share some common functions [148, 149]. Neurexin-neuroligin pairings serve as adhesion molecules within synaptic junctions, not only providing mechanical strength to the synapse but also by binding to PSD-95, a scaffolding protein, which subsequently binds NMDA receptors and potassium channels through its PDZ binding domain. This allows a tight association to form between adhesive molecules and channels and receptors integral to conduction, thereby linking structure with function [150, 151]. As one can imagine, the relationship between adhesion and excitation/inhibition has considerable implications for neural plasticity in general.
While we had mentioned cadherin in relation to the adherens junction of epithelial-like cells, cadherin also plays an adhesive role at synaptic sites. With increasing depolarization of the neuron during development, β-catenin is moved into the spines of the neuron where is it able to interact with cadherins once again at the nascent synapse [152]. As with its E-cadherin counterpart, β-catenin similarly associates with N-cadherin. N-cadherin also associates with neuroligins in the developing synapse via interaction through a scaffolding molecule called S-SCAM, thereby helping to control vesicle accumulation. N-cadherin and neuroligins associate within the mature synapse as well, necessary for the increase in probability of vesicular release and for the occurrence of excitatory postsynaptic currents [153]. And so with increasing electrical activity of the new neuron, β-catenin is driven into the dendritic spines and to the nascent synapses where it once again serves as an adaptor protein to cadherin complexes. In a related set of experiments, Yu and Malenka [154] have found that β-catenin, in partnership with N-cadherin and αN-catenin, is necessary for the complexity of dendritic arborization during neuritogenesis. They showed that neurons which overexpress β-catenin exhibit an increase in total dendritic branch number and length, with a slight decrease in average dendritic length due to the rise in number of shorter branches. Therefore, in its roles at the synapse, β-catenin plays a crucial role not only in chemical signaling but in the development and maintenance of the synapse.
Just as with the adherens junction, β-catenin serves a dual purpose in synaptic transmission. Remember that not only does it act as an adaptor protein for E-cadherin but it simultaneously serves as a transcription factor promoting mitosis. And so β-catenin acts as a mediator for neural conduction as well. Due to N-cadherin’s association with neuroligins and the NMDA receptor, β-catenin is in a key position to relay messages back and forth from the synapse to the nucleus. Thus recently it was discovered that NMDA receptor activation of calpain, a proteolytic enzyme, cleaves the N-terminus of β-catenin creating a stabilized form which can then shuttle back to the nucleus, constitutively activating LEF/TCF transcription and production of gene products such as FosL1 which is necessary for the formation of the transcription factor complex, AP-1 [155]. Abe and Takeichi [155] have also found that the same truncation of β-catenin occurs during mouse exploratory behavior, suggestive of the molecule’s important role in learning and adaptation. Therefore, while neurexin-neuroligin and cadherin expression are requisites for the localization of NMDA to the synaptic membrane, NMDA receptor activity (i.e., learning) may in turn dictate expression levels of these adhesion molecules [156].
As mentioned prior, while FMRP regulates progenitor and neural proliferation as mentioned earlier, it also appears to act as a translational suppressor within synapses [48]. Following glutamatergic stimulation of metabotropic glutamate receptors, FMRP expression is upregulated at the synapse. Despite its involvement in progenitor mitosis, in postmitotic neurons FMRP appears to be localized to the spines, dendrities, and cell bodies however not the axons or nuclei [157]. Interestingly, FMRP has also been shown to associate with microtubules, suggesting that this multifunctional molecule is not only incorporated into elongating polyribosomes within the cytoplasm, determining translation, but may be involved in dendritic transport [158]. Accordingly, the layer V pyramidal neurons of Fmr1 knock-out mice exhibit an increase in spinal length and dendritic density during the early postnatal period [159]. While microarray studies have indicated FMRP’s association with over 400 mRNAs in mouse brain, suggesting that many different molecular pathways may disrupted by the deficiency, the resultant neuronal phenotype is reminiscent of both the VPA rat model of autism and the β-catenin overexpression model, which result in pyramidal hyperconnectivity [9, 44].
While it is unclear precisely how precursor proliferation may later influence neurite growth and synaptic development, the considerable overlap of signaling pathways is enough to suggest a means by which this influence may be achieved. As can be seen at both the gross morphometric and molecular levels, evolution is known for the exaptation of one aspect of ontogenesis, utilizing it for another. Just as the articular and quadrate bones of the reptilian jaws have been coopted to form the ossicles of the middle ear in mammals [160], so is the electrochemical nervous system reflected in the paracrine pre-nervous system of phylogenically ancient metazoa ([161, 162] for review). And so while mitosis of epithelial-like progenitor cells and neuritogenesis and synaptogenesis of their neuronal offspring are unique processes, the redundancy in pathway activation suggests that these latter processes may have been exapted from the mitotic cycle.
Discussion
We have attempted to cover a considerable breadth of material within this review. And even though that breadth may seem overwhelming at first, we hope the reader may appreciate that the etiology of autism probably lies in very fundamental processes of central nervous system development. Only processes so fundamental could link such genetic heterogeneity to a common behavioral phenotype. Ultimately, we believe it is the nature of connectivity which defines the autistic syndromes. While overproliferation of the neural stem cell population may also commonly be targeted and may lead to subtler variations in behavioral phenotype, directly targeting neuritic and synaptic growth may be a “shortcut” to the conditions as well, although further research is still needed to determine whether conditions such as Fragile X share the minicolumnar phenotype seen in idiopathic autism as reported by Casanova et al. [5].
In support of our proposal, recent work published by the Autism Genome Project Consortium [163] has found that in comparing 996 autistic individuals of European descent to 1,287 matched controls, copy number variations were found consistently across three functional domains: 1) cellular proliferation, 2) cell projection and motility (e.g., neurite growth and synaptogenesis), and 3) GTPase/Ras signaling. While GTPase/Ras signaling is heavily involved in the former two processes and, therefore, may potentially be subsumed under either category, proliferation and projection are distinct enough to suggest that whatever common ground these domains do share, either molecularly or functionally, it is that which may underlie the development of autism [164, 165].
We have also presented a number of teratogenic agencies which may mimic or exacerbate effects as those seen in the single-gene syndromes and genetic animal models. Hopefully, these examples of teratogenicity may impress upon the reader the nature of the molecular environment and that regardless of whether an acting agent is endogenously or exogenously synthesized, the molecular boundaries between self and non-self are somewhat blurred. If a foreign molecule is capable of interaction with the cell either at the membrane or within the cell itself, that molecule becomes a part of the molecular landscape. Therefore, viewing said agent not as something separate and foreign but as an integrated albeit potentially pathological part of the larger gestalt is a necessary framework for understanding and predicting cellular development. Likewise, it is necessary for our own awareness of the different ways in which genetics and environment, especially the present-day environment, cooperate to produce the autistic phenotype. The estrogen receptor, for example, is highly promiscuous and binds a huge number of ligands [105], including phytoestrogens which are plant produced, mycoestrogens which are fungally produced, and other xenoestrogens which are man-made, such as bisphenol A in plastics and methoxychlor, an insecticide [166]. If the estrogen receptor does in fact recognize such a variety of agents and this receptivity has been conserved across numerous clades, then those agents have most probably taken part in our evolutionary development. However, today, as with examples of many man-made estrogens, we may no longer be subjected to comparable levels as we have been in the past, potentially tilting a longheld balance between genetics and molecular epigenetics. As also may be found with ultrasound, through its unwise use we are potentially altering the molecular environment in which our children are developing prenatally.
Autism may be indicative of how our environment has changed over time: while a considerable portion of increased diagnostic rates are due to changes in diagnostic criteria, inclusion of milder cases, and greater general awareness, a majority of the increase is still unaccounted for [167], and therefore autism may be a barometer for the changing molecular climate. However, while there are potentially more environmental variables we must consider nowadays, now that we are coming to understand embryogenesis and postnatal stages of development more intimately, instead we suggest that the molecular environment has been integral to aspects of our development which we may not have considered before. If, for example, growth pathways within neural stem cells, neurites, and synapses can be so easily regulated by exogenous agents which are not unique to our present-day environment, then neurogenesis may not only be affected by these agents but may require some of them for our development. While any layman is capable of recognizing that oxygen is a necessary for life, the vital roles that maternal hormones may play in embryo and fetal development, for instance, may be less obvious though no less necessary. And therefore with the awareness that such molecular agents may not only be potentially teratogenic but requisites for normal development, we may slowly move away from the long-held notion of nature versus nurture.
References
- 1.Liu J, Nyholt DR, Magnussen P, Parano E, Pavone P, Geschwind D, et al. A genomewide screen for autism susceptibility loci. Am J Hum. Genet. 2001;69:327–340. doi: 10.1086/321980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yonan AL, Alarcón M, Cheng R, Magnusson PK, Spence SJ, Palmer AA, et al. A genomewide screen of 345 families for autism-susceptibility loci. Am. J. Hum. Genet. 2003;73:886–897. doi: 10.1086/378778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams EL, Casanova MF. Autism or autisms? Finding the lowest common denominator. Bol. Asoc. Méd. P.R. 2010 Oct;102(4):17–24. [PubMed] [Google Scholar]
- 4.Minshew NJ, Williams DL. The new neurobiology of autism: Cortex, connectivity, and neuronal organization. Arch. Neurol. 2007;64:945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58:428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- 6.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 7.Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: A review and future directions. Int. J. Dev. Neurosci. 2005;23:183–187. doi: 10.1016/j.ijdevneu.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, et al. Localization of white matter volume increase in autism and developmental language disorder. Ann. Neurol. 2004;55:530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- 9.Rinaldi T, Kulangara K, Antoniello K, Markram H. Elevated NMDA receptor levels and enhanced postsynaptic long-term potentiation induced by prenatal exposure to valproic acid. Proc. Natl. Acad. Sci. U.S.A. 2007;104:13501–13506. doi: 10.1073/pnas.0704391104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rinaldi T, Perrodin C, Markram H. Hyper-connectivity and hyper-plasticity in the medial prefrontal cortex in the valproic acid animal model of autism. Front. Neural Circuits. 2008;2:1–7. doi: 10.3389/neuro.04.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casanova MF, El-Baz A, Mott M, Mannheim G, Hassan H, Fahmi R, et al. Reduced gyral window and corpus callosum size in autism: Possible macroscopic correlates of a minicolumnopathy. J. Autism Dev. Disord. 2009;39:751–764. doi: 10.1007/s10803-008-0681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beaudet AL. Autism: highly heritable but not inherited. Nat. Med. 2007;13:534–536. doi: 10.1038/nm0507-534. [DOI] [PubMed] [Google Scholar]
- 13.Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113:e472–e486. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- 14.Herbert MR. Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Curr. Opin. Neurol. 2010;23:103–110. doi: 10.1097/WCO.0b013e328336a01f. [DOI] [PubMed] [Google Scholar]
- 15.Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. J. Am Med. Assoc. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- 16.Rogers SJ. Developmental regression in autism spectrum disorders. Ment. Retard. Dev. Disabil. Res. Rev. 2004;10:139–143. doi: 10.1002/mrdd.20027. [DOI] [PubMed] [Google Scholar]
- 17.Kumar V, Zhang MX, Swank MW, Kunz J, Wu GY. Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J. Neurosci. 2005;25:11288–11299. doi: 10.1523/JNEUROSCI.2284-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDaniel MA. Big-brained people are smarter: A meta-analysis of the relationship between in vivo brain volume and intelligence. Intelligence. 2005;33:337–346. [Google Scholar]
- 19.Burrell B. Postcards from the brain museum. New York: Broadway Books; 2004. [Google Scholar]
- 20.Happé F, Frith U. The weak central coherence account: Detail-focused cognitive style in autism spectrum disorders. J. Autism Dev. Disord. 2006;36:5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- 21.Treffert DA. Extraordinary people: Understanding savant syndrome. Lincoln: iUniverse; 2006. [Google Scholar]
- 22.Redcay E, Courchesne E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol. Psychiatry. 2005;58:1–9. doi: 10.1016/j.biopsych.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 23.Pilarsky R. Cowden syndrome: A critical review of the clinical literature. J. Genet. Couns. 2009;18:13–27. doi: 10.1007/s10897-008-9187-7. [DOI] [PubMed] [Google Scholar]
- 24.McBride KL, Varga EA, Pastore MT, Prior TW, Manickam K, Atkin JF, et al. Confirmation of study of PTEN mutations among individuals with autism or developmental delays/mental retardation and macrocephaly. Autism Res. 2010;3:137–141. doi: 10.1002/aur.132. [DOI] [PubMed] [Google Scholar]
- 25.Tamguney T, Stokoe D. New insights into PTEN. J. Cell. Sci. 2007;120:4071–4079. doi: 10.1242/jcs.015230. [DOI] [PubMed] [Google Scholar]
- 26.Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 27.Muotri AR, Marchetto MC, Coufal NG, Oefner R, Yeo G, Nakashima K, et al. L1 retrotransposition in neurons is modulated by MeCP2. Nature. 2010;468:443–446. doi: 10.1038/nature09544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skene PJ, Illingworth RS, Webb S, Kerr AR, James KD, Turner DJ, et al. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol. Cell. 2010;37:457–468. doi: 10.1016/j.molcel.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson WJ, Nusse R. Convergence of Wnt, β-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Persad S, Troussard AA, McPhee TR, Mulholland DJ, Dedhar S. Tumor suppressor PTEN inhibits nuclear accumulation of beta-catenin and T cell/lymphoid enhancer factor 1-mediated transcriptional activation. J. Cell Biol. 2001;153:1161–1174. doi: 10.1083/jcb.153.6.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carney RM, Wolpert CM, Ravan SA, Shahbazian M, Ashley-Koch A, Cuccaro ML, et al. Identification of MeCP2 mutations in a series of females with autistic disorder. Pediatr. Neurol. 2003;28:205–211. doi: 10.1016/s0887-8994(02)00624-0. [DOI] [PubMed] [Google Scholar]
- 32.Samaco RC, Nagarajan RP, Braunschweig D, LaSalle JM. Multiple pathways regulate MeCP2 expression in normal brain development and exhibit defects in autism-spectrum disorders. Hum. Mol. Genet. 2004;13:629–639. doi: 10.1093/hmg/ddh063. [DOI] [PubMed] [Google Scholar]
- 33.Steelman LS, Abrams SL, Whelan J, Bertrand FE, Ludwig DE, Bäsecke J, et al. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia. 2008;22:686–707. doi: 10.1038/leu.2008.26. [DOI] [PubMed] [Google Scholar]
- 34.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 35.Wiznitzer M. Autism and tuberous sclerosis. J. Child Neurol. 2004;19:675–679. doi: 10.1177/08830738040190090701. [DOI] [PubMed] [Google Scholar]
- 36.Ehninger D, De Vries PJ, Silva AJ. From mTOR to cognition: Molecular and cellular mechanisms of cognitive impairments in tuberous sclerosis. J. Intellect. Disabil. Res. 2009;53:838–851. doi: 10.1111/j.1365-2788.2009.01208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griffiths PD, Gardner SA, Smith M, Rittey C, Powell T. Hemimegalencephaly and focal megalencephaly in tuberous sclerosis complex. Am. J. Neuroradiol. 1998;19:1935–1938. [PMC free article] [PubMed] [Google Scholar]
- 38.Christophe C, Sékhara T, Rypens F, Ziereisen F, Christiaens F, Dan B. MRI spectrum of cortical malformations in tuberous sclerosis complex. Brain Dev. 2000;22:487–493. doi: 10.1016/s0387-7604(00)00186-8. [DOI] [PubMed] [Google Scholar]
- 39.Way SW, McKenna J, 3rd, Mietzsch U, Reith RM, Wu HC, Gambello MJ. Loss of Tsc2 in radial glia models the brain pathology of tuberous sclerosis complex in the mouse. Hum. Mol. Genet. 2009;18:1252–1265. doi: 10.1093/hmg/ddp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, et al. A clinicopathological study of autism. Brain. 1998;121:889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- 41.Wegiel J, Kuchna I, Nowicki K, Imaki H, Wegiel J, Marchi E, et al. The neuropathology of autism: Defects of neurogenesis and neuronal migration, and dysplastic changes. Acta Neuropathol. 2010;119:755–770. doi: 10.1007/s00401-010-0655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mak BC, Takemaru K, Kenerson HL, Moon RT, Yeung RS. The tuberin-hamartin complex negatively regulates beta-catenin signaling activity. J. Biol. Chem. 2003;278:5947–5951. doi: 10.1074/jbc.C200473200. [DOI] [PubMed] [Google Scholar]
- 43.Daugherty RL, Gottardi CJ. Phospho-regulation of β-catenin adhesion and signaling functions. Physiology. 2007;22:303–309. doi: 10.1152/physiol.00020.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown V, Jin P, Ceman S, Darnell JC, O’Donnell WT, Tenenbaum SA, et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- 45.Luo Y, Shan G, Guo W, Smrt RD, Johnson EB, Li X, et al. Fragile X mental retardation protein regulates proliferation and differentiation of adult neural stem/progenitor cells. PLoS Genet. 2010;6:e1000898. doi: 10.1371/journal.pgen.1000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hagerman RJ. Fragile X syndrome. In: Bauman ML, Kemper TL, editors. The neurobiology of autism. 2nd ed. London: The Johns Hopkins University Press; 2005. pp. 251–264. [Google Scholar]
- 47.Fatemi SH, Folsom TD. The role of fragile X mental retardation protein in major mental disorders. Neuropharmacology. 2011;60:1221–1226. doi: 10.1016/j.neuropharm.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zalfa F, Marcello G, Primerano B, Moro A, Di Penta A, Reis S, et al. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003;112:317–327. doi: 10.1016/s0092-8674(03)00079-5. [DOI] [PubMed] [Google Scholar]
- 49.Castrén M, Tervonen T, Kärkkäinen V, Heinonen S, Castrén E, Larsson K, et al. Altered differentiation of neural stem cells in fragile X syndrome. Proc. Natl. Acad. Sci. U.S.A. 2005;102:17834–17839. doi: 10.1073/pnas.0508995102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tervonen TA, Louhivuori V, Sun X, Hokkanen ME, Kratochwil CF, Zebryk P, et al. Aberrant differentiation of glutamatergic cells in neocortex of mouse model for fragile X syndrome. Neurobiol. Dis. 2009;33:250–259. doi: 10.1016/j.nbd.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 51.De Vries BBA, Mohkamsing S, Van den Ouweland AMW, Mol E, Gelsema K, Van Rijn M, et al. Screening for the fragile X syndrome among the mentally retarded: a clinical study. J. Med. Genet. 1999;36:467–470. [PMC free article] [PubMed] [Google Scholar]
- 52.Chausovsky A, Bershadsky AD, Borisy GG. Cadherin-mediated regulation of microtubule dynamics. Nat. Cell Biol. 2000;2:797–804. doi: 10.1038/35041037. [DOI] [PubMed] [Google Scholar]
- 53.Reynolds AB, Daniel J, McCrea PD, Wheelock MJ, Wu J, Zhang Z. Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol. Cell Biol. 1994;14:8333–8342. doi: 10.1128/mcb.14.12.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bienz M. β-catenin: A pivot between cell adhesion and Wnt signalling. Curr. Biol. 2004;15:R65. doi: 10.1016/j.cub.2004.12.058. [DOI] [PubMed] [Google Scholar]
- 55.Ziegler S, Röhrs S, Tickenbrock L, Möröy T, Klein-Hitpass L, Vetter IR, et al. Novel target genes of the Wnt pathway and statistical insights into Wnt target promoter regulation. FEBS J. 2005;272:1600–1615. doi: 10.1111/j.1742-4658.2005.04581.x. [DOI] [PubMed] [Google Scholar]
- 56.Gearhart J, Pashos EE, Prasad MK. Pluripotency redux—advances in stem-cell research. N. Engl. J. Med. 2007;357:1469–1472. doi: 10.1056/NEJMp078126. [DOI] [PubMed] [Google Scholar]
- 57.Cotterman R, Jin VX, Krig SR, Lemen JM, Wey A, Farnham PJ, et al. N-Myc regulates a widespread euchromatic program in the human genome partially independent of its role as a classic transcription factor. Cancer Res. 2008;68:9654–9662. doi: 10.1158/0008-5472.CAN-08-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nusse R. A list of target genes of Wnt/beta-catenin signaling [online resource] Stanford: Howard Hughes Medical Center; 2009. [accessed 2011 Jan 28]. http://www.stanford.edu/~rnusse/pathways/targets.html. [Google Scholar]
- 59.Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, et al. Erk associates with and primes GSK-3β for its inactivation resulting in upregulation of β-catenin. Mol. Cell. 2005;19:159–170. doi: 10.1016/j.molcel.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 60.Gherzi R, Trabucchi M, Ponassi M, Ruggiero T, Corte G, Moroni C, et al. The RNA-binding protein KSRP promotes decay of beta-catenin mRNA and is inacitvated by PI3K-AKT signaling. PLoS Biol. 2006;5:e5. doi: 10.1371/journal.pbio.0050005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Bamji SX, Shimazu K, Kimes N, Huelsken J, Birchmeier W, Lu B, et al. Role of beta-catenin in synaptic vesicle localization and presynaptic assembly. Neuron. 2003;40:719–731. doi: 10.1016/s0896-6273(03)00718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, et al. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y, Greenwood JS, Calcagnotto ME, Kirsch HE, Barbaro NM, Baraban SC. Neocortical hyperexcitability in a human case of tuberous sclerosis complex and mice lacking neuronal expression of TSC1. Ann. Neurol. 2007;61:139–152. doi: 10.1002/ana.21058. [DOI] [PubMed] [Google Scholar]
- 64.Nau H, Rating D, Koch S, Häuser I, Helge H. Valproic acid and its metabolites: Placental transfer, neonatal pharmacokinetics, transfer via mother’s milk and clinical status in neonates of epileptic mothers. J. Pharmacol. Exp. Ther. 1981;219:768–777. [PubMed] [Google Scholar]
- 65.DiLiberty JH, Farndon PA, Dennis NR, Curry CJ. The fetal valproate syndrome. Am. J. Med. Genet. 1984;19:473–481. doi: 10.1002/ajmg.1320190308. [DOI] [PubMed] [Google Scholar]
- 66.Christianson AL, Chesler N, Kromberg JG. Fetal valproate syndrome: Clinical and neuro-developmental features in two sibling pairs. Dev. Med. Child Neurol. 1994;36:361–369. doi: 10.1111/j.1469-8749.1994.tb11858.x. [DOI] [PubMed] [Google Scholar]
- 67.Moore SJ, Turnpenny P, Quinn A, Glover S, Lloyd DJ, Montgomery T, et al. A clinical study of 57 children with fetal anticonvulsant syndromes. J. Med. Genet. 2000;37:489–497. doi: 10.1136/jmg.37.7.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rasalam AD, Hailey H, Williams JH, Moore SJ, Turnpenny PD, Lloyd DJ, et al. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev. Med. Child Neurol. 2005;47:551–555. doi: 10.1017/s0012162205001076. [DOI] [PubMed] [Google Scholar]
- 69.Markram H, Rinaldi T, Markram K. The intense world syndrome—an alternative hypothesis for autism. Front. Neurosci. 2007;1:77–96. doi: 10.3389/neuro.01.1.1.006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shimshoni JA, Dalton EC, Jenkins A, Eyal S, Ewan K, Williams RS, et al. The effects of central nervous system-active valproic acid constitutional isomers, cyclopropyl analogs, and amide derivatives on neuronal growth cone behavior. Mol. Pharmacol. 2007;71:884–892. doi: 10.1124/mol.106.030601. [DOI] [PubMed] [Google Scholar]
- 71.Billin AN, Thirlwell H, Ayer DE. β-catenin-histone deacetylase interactions regulate the transition of LEF1 from a transcriptional repressor to an activator. Mol. Cell Biol. 2000;20:6882–6890. doi: 10.1128/mcb.20.18.6882-6890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wiltse J. Mode of action: inhibition of histone deacetylase, altering WNT-dependent gene expression, and regulation of beta-catenin—developmental effects of valproic acid. Crit. Rev. Toxicol. 2005;35:727–738. doi: 10.1080/10408440591007403. [DOI] [PubMed] [Google Scholar]
- 73.Wang Z, Xu L, Zhu X, Cui W, Sun Y, Nishijo H, et al. Demethylation of specitic Wnt/β-catenin pathway genes and its upregulation in rat brain induced by prenatal valproate exposure. Anat. Rec. 2010;293:1947–1953. doi: 10.1002/ar.21232. [DOI] [PubMed] [Google Scholar]
- 74.Raballo R, Rhee J, Lyn-Cook R, Leckman JF, Schwartz ML, Vaccarino FM. Basic fibroblast growth factor (Fgf2) is necessary for cell proliferation and neurogenesis in the developing cerebral cortex. J. Neurosci. 2000;20:5012–5023. doi: 10.1523/JNEUROSCI.20-13-05012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ryves JW, Dalton EC, Harwood AJ, Williams RS. GSK-3 activity in neocortical cells is inhibited by lithium but not carbamazepine or valproic acid. Bipolar Disord. 2005;7:260–265. [Google Scholar]
- 76.Jope RS. Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol. Sci. 2003;24:441–443. doi: 10.1016/S0165-6147(03)00206-2. [DOI] [PubMed] [Google Scholar]
- 77.Yuskaitis CJ, Mines MA, King MK, Sweatt JD, Miller CA, Jope RS. Lithium ameliorates altered glycogen synthase kinase-3 and behavior in a mouse model of fragile X syndrome. Biochem. Pharmacol. 2010;79:632–646. doi: 10.1016/j.bcp.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hashimoto R, Senatorov V, Kanai H, Leeds P, Chuang DM. Lithium stimulates progenitor proliferation in cultured brain neurons. Neuroscience. 2003;117:55–61. doi: 10.1016/s0306-4522(02)00577-8. [DOI] [PubMed] [Google Scholar]
- 79.Laeng P, Pitts RL, Pemire AL, Drabik CE, Weiner A, Tang H, et al. The mood stabilizer valproic acid stimulates GABA neurogenesis from rat forebrain stem cells. J. Neurochem. 2004;91:238–251. doi: 10.1111/j.1471-4159.2004.02725.x. [DOI] [PubMed] [Google Scholar]
- 80.Vecsler M, Simon AJ, Amariglio N, Rechavi G, Gak E. MeCP2 deficiency downregulates specific nuclear proteins that could be partially recovered by valproic acid in vitro. Epigenetics. 2010;5:61–67. doi: 10.4161/epi.5.1.10630. [DOI] [PubMed] [Google Scholar]
- 81.Tropea D, Giacometti E, Wilson NR, Beard C, McCurry C, Fu DD, et al. Partial reversal of Rett syndrome-like symptoms in MeCP2 mutant mice. Proc. Natl. Acad. Sci. U.S.A. 2009;106:2029–2034. doi: 10.1073/pnas.0812394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McCaffrey P, Deustch CK. Macrocephaly and the control of brain growth in autistic disorders. Prog. Neurobiol. 2005;77:38–56. doi: 10.1016/j.pneurobio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 83.Croen LA, Goines P, Braunschweig D, Yolkne R, Yoshida CK, Grether JK, et al. Brain-derived neurotrophic factor and autism: Maternal and infant peripheral blood levels in the Early Markers for Autism (EMA) study. Autism Res. 2008;1:130–137. doi: 10.1002/aur.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vaccarino FM, Grigorenko EL, Smith KM, Stevens HE. Regulation of cerebral cortical size and neuron number by fibroblast growth factors: Implications for autism. J. Autism Dev. Disord. 2009;39:511–520. doi: 10.1007/s10803-008-0653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baron-Cohen S. The extreme male brain theory of autism. Trends Cogn. Sci. 2002;6:248–254. doi: 10.1016/s1364-6613(02)01904-6. [DOI] [PubMed] [Google Scholar]
- 86.Knickmeyer R, Baron-Cohen S, Raggatt P, Taylor K, Hackett G. Fetal testosterone and empathy. Horm. Behav. 2006;49:282–292. doi: 10.1016/j.yhbeh.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 87.Knickmeyer R, Baron-Cohen S, Fane BA, Wheelwright S, Mathews GA, Conway GS, et al. Androgens and autistic traits: a study of individuals with congenital adrenal hyperplasia. Horm. Behav. 2006;50:148–153. doi: 10.1016/j.yhbeh.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 88.Hague WM, Adams J, Rodda C, Brook CG, De Bruyn R, Grant DB, et al. The prevalence of polycystic ovaries in patients with congenital adrenal hyperplasia and their close relatives. Clin. Endocrinol. 1990;33:501–510. doi: 10.1111/j.1365-2265.1990.tb03887.x. [DOI] [PubMed] [Google Scholar]
- 89.Ingudomnukul E, Baron-Cohen S, Wheelwright S, Knickmeyer R. Elevated rates of testosterone-related disorders in women with autism spectrum conditions. Horm. Behav. 2007;51:597–604. doi: 10.1016/j.yhbeh.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 90.Shayya R, Chang RJ. Reproductive endocrinology of adolescent polycystic ovary syndrome. BJOG. 2010;117:150–155. doi: 10.1111/j.1471-0528.2009.02421.x. [DOI] [PubMed] [Google Scholar]
- 91.Yang F, Li X, Sharma M, Sasaki CY, Longo DL, Lim B, et al. Linking beta-catenin to androgen-signaling pathway. J. Biol. Chem. 2002;277:11336–11344. doi: 10.1074/jbc.M111962200. [DOI] [PubMed] [Google Scholar]
- 92.Pawlowski JE, Ertel JR, Allen MP, Xu M, Butler C, Wilson EM, et al. Liganded androgen receptor interaction with beta-catenin: Nuclear co-localization and modulation of transcriptional activity in neuronal cells. J. Biol. Chem. 2002;277:20702–20710. doi: 10.1074/jbc.M200545200. [DOI] [PubMed] [Google Scholar]
- 93.Cullen DA, Killick R, Leigh PN, Gallo JM. The effect of polyglutamine expansion in the human androgen receptor on its ability to suppress β-catenin-Tcf/Lef dependent transcription. Neurosci. Lett. 2004;354:54–58. doi: 10.1016/j.neulet.2003.09.074. [DOI] [PubMed] [Google Scholar]
- 94.MacLusky NJ, Clark AS, Naftolin F, Goldman-Rakic PS. Estrogen formation in the mammalian brain: Possible role of aromatase in sexual differentiation of the hippocampus and neocortex. Steroids. 1987;50:459–474. doi: 10.1016/0039-128x(87)90032-8. [DOI] [PubMed] [Google Scholar]
- 95.Lemmen JG, Broekhof JLM, Kuiper GGJM, Gustafsson JÅ, van der Saag PT, van der Burg B. Expression of estrogen receptor alpha and beta during mouse embryogenesis. Mech. Dev. 1999;81:163–167. doi: 10.1016/s0925-4773(98)00223-8. [DOI] [PubMed] [Google Scholar]
- 96.Forlano PM, Deitcher DL, Myers DA, Bass AH. Anatomical distribution and cellular basis for high levels of aromatase activity in the brain of teleost fish: Aromatase enzyme and mRNA expression identify glia as source. J. Neurosci. 2001;21:8943–8955. doi: 10.1523/JNEUROSCI.21-22-08943.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang L, Andersson S, Warner M, Gustafsson JA. Estrogen receptor (ER)beta knockout mice reveal a role for ERbeta in migration of cortical neurons in the developing brain. Proc. Natl. Acad. Sci. U.S.A. 2003;100:703–708. doi: 10.1073/pnas.242735799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pardridge WM, Mietus LJ. Transport of steroid hormones through the rat blood-brain barrier. J. Clin. Invest. 1979;64:145–154. doi: 10.1172/JCI109433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cardona-Gomez P, Perez M, Avila J, Garcia-Segura LM, Wandosell F. Estradiol inhibits GSK3 and regulates interaction of estrogen receptors, GSK3, and beta-catenin in the hippocampus. Mol. Cell. Neurosci. 2004;25:363–373. doi: 10.1016/j.mcn.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 100.Perez-Martin M, Azcoitia I, Trejo JL, Sierra A, Garcia-Segura LM. An antagonist of estrogen receptors blocks the induction of adult neurogenesis by insulin-like growth factor-I in the dentate gyrus of adult female rat. Eur. J. Neurosci. 2003;18:923–930. doi: 10.1046/j.1460-9568.2003.02830.x. [DOI] [PubMed] [Google Scholar]
- 101.Homburg R, Pariente C, Lunenfeld B, Jacobs HS. The role of insulin-like growth factor-1 (IGF-1) and IGF binding protein-1 (IGFBP-1) in the pathogenesis of polycystic ovary syndrome. Hum. Reprod. 1992;7:1379–1383. doi: 10.1093/oxfordjournals.humrep.a137577. [DOI] [PubMed] [Google Scholar]
- 102.Kouzmenko AP, Takeyama K, Ito S, Furatani T, Sawatsubashi S, Maki A, et al. Wnt/β-catenin and estrogen signaling converge in vivo. J. Biol. Chem. 2004;279:40255–40258. doi: 10.1074/jbc.C400331200. [DOI] [PubMed] [Google Scholar]
- 103.Varea O, Garrido JJ, Dopazo A, Mendex P, Garcia-Segura LM, Wandosell F. Estradiol activates beta-catenin dependent transcription in neurons. PLoS ONE. 2009;4:e5153. doi: 10.1371/journal.pone.0005153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Simoncini T, Hafezi-Mghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 106.Martin JT. Sexual dimorphism in immune function: The role of prenatal exposure to androgens and estrogens. Eur. J. Pharmacol. 2000;405:251–261. doi: 10.1016/s0014-2999(00)00557-4. [DOI] [PubMed] [Google Scholar]
- 107.Warren RP, Odell JD, Warren WL, Burger RA, Maciulis A, Daniels WW, et al. Brief report: Immunoglobulin A deficiency in a subset of autistic subjects. J. Autism Dev. Disord. 1997;27:187–192. doi: 10.1023/a:1025895925178. [DOI] [PubMed] [Google Scholar]
- 108.Gupta S, Aggarwal S, Rashanravan B, Lee T. Th1- and Th2-like cytokines in CD4+ and CD8+ T cells in autism. J. Neuroimmunol. 1998;85:106–109. doi: 10.1016/s0165-5728(98)00021-6. [DOI] [PubMed] [Google Scholar]
- 109.Ashwood P, Van de Water J. Is autism an autoimmune disease? Autoimmun. Rev. 2004;3:557–562. doi: 10.1016/j.autrev.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 110.Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, et al. Elevated immune response in the brain of autistic patients. J. Neuroimmunol. 2009;207:111–116. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Singh VK. Phenotypic expression of autoimmune autistic disorder (AAD): A major subset of autism. Ann. Clin. Psychiatry. 2009;21:148–161. [PubMed] [Google Scholar]
- 112.Grether JK, Croen LA, Anderson MC, Nelson KB, Yolken RH. Neonatally measured immunoglobulins and risk of autism. Autism Res. 2010;3:323–332. doi: 10.1002/aur.160. [DOI] [PubMed] [Google Scholar]
- 113.Angelidou A, Alysandratos KD, Asadi S, Zhang B, Francis K, Vasiadi M, et al. Brief report:“Allergic symptoms” in children with autism spectrum disorders. More than meets the eye? J. Autism Dev. Disord. doi: 10.1007/s10803-010-1171-z. (in press) [DOI] [PubMed] [Google Scholar]
- 114.Chess S, Fernandez P, Korn S. Behavioral consequences of congenital rubella. J. Pediatr. 1978;93:699–703. doi: 10.1016/s0022-3476(78)80921-4. [DOI] [PubMed] [Google Scholar]
- 115.Taga T, Fukuda S. Role of IL-6 in the neural stem cell differentiation. Clin. Rev. Allergy Immunol. 2005;28:249–256. doi: 10.1385/CRIAI:28:3:249. [DOI] [PubMed] [Google Scholar]
- 116.Carpentier PA, Palmer TD. Immune influence on adult neural stem cell regulation and function. Neuron. 2009;64:79–92. doi: 10.1016/j.neuron.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wolf SA, Steiner B, Wengner A, Lipp M, Kammertoens T, Kempermann G. Adaptive peripheral immune response increases proliferation of neural precursor cells in the adult hippocampus. FASEB J. 2009;23:3121–3128. doi: 10.1096/fj.08-113944. [DOI] [PubMed] [Google Scholar]
- 118.Sarkar P, Bergman K, O’Connor TG, Glover V. Maternal antenatal anxiety and amniotic fluid cortisol and testosterone: Possible implications for foetal programming. J. Neuroendocrinol. 2008;20:489–496. doi: 10.1111/j.1365-2826.2008.01659.x. [DOI] [PubMed] [Google Scholar]
- 119.Pascual R, Ebner D, Araneda R, Urqueta MJ, Bustamante C. Maternal stress induces long-lasting Purkinje cell developmental impairments in mouse offspring. Eur. J. Pediatr. 2010;169:1517–1522. doi: 10.1007/s00431-010-1258-8. [DOI] [PubMed] [Google Scholar]
- 120.You JJ, Alter DA, Stukel TA, McDonald SD, Laupacis A, Liu Y, et al. Proliferation of prenatal ultrasound. Can. Med. Assoc. J. 2010;182:143–151. doi: 10.1503/cmaj.090979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Miller MW, Brayman AA, Abramowicz JS. Obstetric ultrasonography: a biophysical consideration of patient safety—the “rules” have changed. Am. J. Obstet. Gynecol. 1998;179:241–254. doi: 10.1016/s0002-9378(98)70279-0. [DOI] [PubMed] [Google Scholar]
- 122.Sheiner E, Shoham-Vardi I, Abramowicz JS. What do clinical users know regarding safety of ultrasound during pregnancy? J. Ultrasound Med. 2007;26:319–325. doi: 10.7863/jum.2007.26.3.319. [DOI] [PubMed] [Google Scholar]
- 123.Williams EL, Casanova MF. Potential teratogenic effects of ultrasound on corticogenesis: Implications for autism. Med. Hypotheses. 2010;75:53–58. doi: 10.1016/j.mehy.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 124.Dyson M, Franks C, Suckling J. Stimulation of healing of varicose ulcers by ultrasound. Ultrasonics. 1976;14:232–236. doi: 10.1016/0041-624x(76)90024-x. [DOI] [PubMed] [Google Scholar]
- 125.Duarte LR. The stimulation of bone growth by ultrasound. Arch. Orthop. Trauma Surg. 1983;101:153–159. doi: 10.1007/BF00436764. [DOI] [PubMed] [Google Scholar]
- 126.Ang ES, Jr, Gluncic V, Duque A, Schafer ME, Rakic P. Prenatal exposure to ultrasound waves impacts neuronal migration in mice. Proc. Natl. Acad. Sci. U.S.A. 2006;103:12903–12910. doi: 10.1073/pnas.0605294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sikov MR. Effects of ultrasound on development. Part 2: Studies in mammalian species and overview. J. Ultrasound Med. 1986;5:651–661. doi: 10.7863/jum.1986.5.11.651. [DOI] [PubMed] [Google Scholar]
- 128.Olkku A, Leskinen JJ, Lammi MJ, Hynynen K, Mahonen A. Ultrasound-induced activation of Wnt signaling in human MG-63 osteoblastic cells. Bone. 2010;47:320–330. doi: 10.1016/j.bone.2010.04.604. [DOI] [PubMed] [Google Scholar]
- 129.Takeuchi R, Ryo A, Komitsu N, Mikuni-Takagaki Y, Fukui A, Takagi Y, et al. Low-intensity pulsed ultrasound activates the phosophatidylinositol 3 kinase/Akt pathway and stimulates the growth of chondrocytes in three-dimensional cultures: A basic science study. Arthritis Res. Ther. 2008;10:R77. doi: 10.1186/ar2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mitragotri S, Blankschtein D, Langer R. Ultrasound-mediated transdermal protein delivery. Science. 1995;269:850–853. doi: 10.1126/science.7638603. [DOI] [PubMed] [Google Scholar]
- 131.Van Wamel A, Bouakaz A, Versluis M, De Jong N. Micromanipulation of endothelial cells: Ultrasound-microbubble-cell interaction. Ultrasound Med. Biol. 2004;30:1255–1258. doi: 10.1016/j.ultrasmedbio.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 132.VanBavel E. Effects of shear stress on endothelial cells: Possible relevance for ultrasound applications. Prog. Biophys. Mol. Biol. 2007;93:374–383. doi: 10.1016/j.pbiomolbio.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 133.Colombo A, Hall P, Nakamura S, Almagor Y, Maiello L, Martini G, et al. Intracoronary stenting without anticoagulation accomplished with intravascular ultrasound guidance. Circulation. 1995;91:1676–1688. doi: 10.1161/01.cir.91.6.1676. [DOI] [PubMed] [Google Scholar]
- 134.Rioufol G, Finet G, Ginon I, André-Fouët X, Rossi R, Vialle E, et al. Multiple atherosclerotic plaque rupture in acute coronary syndrome: A three-vessel intravascular ultrasound study. Circulation. 2002;106:804–808. doi: 10.1161/01.cir.0000025609.13806.31. [DOI] [PubMed] [Google Scholar]
- 135.Yamamoto K, Takahashi T, Asahara T, Ohura N, Sokabe T, Kamiya A, et al. Proliferation, differentiation, and tube formation by endothelial progenitor cells in response to shear stress. J. Appl. Physiol. 2003;95:2081–2088. doi: 10.1152/japplphysiol.00232.2003. [DOI] [PubMed] [Google Scholar]
- 136.Reher P, Doan N, Bradnock B, Meghji S, Harris M. Effect of ultrasound on the production of IL-8, basic FGF and VEGF. Cytokine. 1999;11:416–423. doi: 10.1006/cyto.1998.0444. [DOI] [PubMed] [Google Scholar]
- 137.Reher P, Harris M, Whiteman M, Hai HK, Meghji S. Ultrasound stimulates nitric oxide and prostaglandin E2 production by human osteoblasts. Bone. 2002;31:236–241. doi: 10.1016/s8756-3282(02)00789-5. [DOI] [PubMed] [Google Scholar]
- 138.Raab S, Plate KH. Different networks, common growth factors: Shared growth factors and receptors of the vascular and the nervous system. Acta Neuropathol. 2007;113:607–626. doi: 10.1007/s00401-007-0228-3. [DOI] [PubMed] [Google Scholar]
- 139.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 140.Sun J, Zhou W, Ma D, Yang Y. Endothelial cells promote neural stem cell proliferation and differentiation associated with VEGF activated Notch and Pten signaling. Dev. Dyn. 2010;239:2345–2353. doi: 10.1002/dvdy.22377. [DOI] [PubMed] [Google Scholar]
- 141.Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, et al. Adult SVZ stem cells lie in a vascular niche: A quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ye H, Liu J, Wu JY. Cell adhesion molecules and their involvement in autism spectrum disorder. Neurosignals. 2011;18:62–71. doi: 10.1159/000322543. [DOI] [PubMed] [Google Scholar]
- 143.Jamain S, Quach H, Betancur C, Råstam M, Colineaux C, Gillberg IC, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Moizard MP, et al. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am. J. Hum. Genet. 2004;74:552–557. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Feng J, Schroer R, Yan J, Song W, Yang C, Bockholt A, et al. High frequency of neurexin 1beta signal peptide structural variants in patients with autism. Neurosci. Lett. 2006;409:10–13. doi: 10.1016/j.neulet.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 146.Kim HG, Kishikawa S, Higgins AW, Seong IS, Donovan DJ, Shen Y, et al. Disruption of neurexin 1 associated with autism spectrum disorder. Am. J. Hum. Genet. 2008;82:199–207. doi: 10.1016/j.ajhg.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen SX, Tari PK, She K, Haas K. Neurexin-neuroligin cell adhesion complexes contribute to synaptotropic dendritogenesis via growth stabilization mechanisms in vivo. Neuron. 2010;67:967–983. doi: 10.1016/j.neuron.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 148.Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- 149.Budreck EC, Scheiffele P. Neuroligin-3 is a neuronal adhesion protein at GABAergic and glutamatergic synapses. Eur. J. Neurosci. 2007;26:1738–1748. doi: 10.1111/j.1460-9568.2007.05842.x. [DOI] [PubMed] [Google Scholar]
- 150.Hirao K, Hata Y, Ide N, Takeuchi M, Irie M, Yao I, et al. A novel multiple PDZ domain-containing molecule interacting with N-methyl-D-aspartate receptors and neuronal cell adhesion proteins. J. Biol. Chem. 1998;273:21105–21110. doi: 10.1074/jbc.273.33.21105. [DOI] [PubMed] [Google Scholar]
- 151.Barrow SL, Constable JR, Clark E, El-Sabeawy F, McAllister AK, Washbourne P. Neuroligin1: A cell adhesion molecule that recruits PSD-95 and NMDA receptors by distinct mechanisms during synaptogenesis. Neural Dev. 2009;4:17. doi: 10.1186/1749-8104-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Murase S, Mosser E, Schuman EM. Depolarization drives beta-catenin into neuronal spines promoting changes in synaptic structure and function. Neuron. 2002;35:91–105. doi: 10.1016/s0896-6273(02)00764-x. [DOI] [PubMed] [Google Scholar]
- 153.Stan A, Pielarski KN, Brigadski T, Wittenmayer N, Fedorchenko O, Gohla A, et al. Essential cooperation of N-cadherin and neuroligin-1 in the transsynaptic control of vesicle accumulation. Proc. Natl. Acad. Sci. U.S.A. 2010;107:11116–11121. doi: 10.1073/pnas.0914233107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yu X, Malenka RC. β-catenin is critical for dendritic morphogenesis. Nat. Neurosci. 2003;6:1169–1177. doi: 10.1038/nn1132. [DOI] [PubMed] [Google Scholar]
- 155.Abe K, Takeichi M. NMDA-receptor activation induces calpain-mediated β-catenin cleavages for triggering gene expression. Neuron. 2007;53:387–397. doi: 10.1016/j.neuron.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 156.Derksen MJ, Ward NL, Hartle KD, Ivanco TL. MAP2 and synaptophysin protein expression following motor learning suggests dynamic regulation and distinct alterations coinciding with synaptogenesis. Neurobiol. Learn. Mem. 2007;87:404–415. doi: 10.1016/j.nlm.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 157.Antar LN, Afroz R, Dictenberg JB, Carroll RC, Bassell GJ. Metabotropic glutamate receptor activation regulates fragile X mental retardation protein and Fmr1 mRNA localization differentially in dendrites and at synapses. J. Neurosci. 2004;24:2648–2655. doi: 10.1523/JNEUROSCI.0099-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang H, Dictenberg JB, Ku L, Li W, Bassell GJ, Feng Y. Dynamic association of the fragile X mental retardation protein as a messenger ribonucleoprotein between microtubules and polyribosomes. Mol. Biol. Cell. 2008;19:105–114. doi: 10.1091/mbc.E07-06-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nimchinsky EA, Oberlander AM, Svoboda K. Abnormal development of dendritic spines in FMR1 knock-out mice. J. Neurosci. 2001;21:5139–5146. doi: 10.1523/JNEUROSCI.21-14-05139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Allin EF. Evolution of the mammalian middle ear. J. Morphol. 1975;147:403–437. doi: 10.1002/jmor.1051470404. [DOI] [PubMed] [Google Scholar]
- 161.Sakarya O, Armstrong KA, Adamska M, Adamski M, Wang IF, Tidor B, et al. A post-synaptic scaffold at the origin of the animal kingdom. PLoS ONE. 2007;2:e506. doi: 10.1371/journal.pone.0000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nickel M. Evolutionary emergence of synaptic nervous systems: What can we learn from the non-synaptic, nerveless Porifera? Invertebr. Biol. 2010;129:1–16. [Google Scholar]
- 163.Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R, et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466:368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kolkova K, Novitskaya V, Pedersen N, Berezin V, Bock E. Neural cell adhesion molecule-stimulated neurite outgrowth depends on activation of protein kinase C and the Ras-mitogen-activated protein kinase pathway. J. Neurosci. 2000;20:2238–2246. doi: 10.1523/JNEUROSCI.20-06-02238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 166.Laws SC, Carey SA, Ferrell JM, Bodman GJ, Cooper RL. Estrogenic activity of octylphenol, nonylphenol, bisphenol A and methoxychlor in rats. Toxicol. Sci. 2000;54:154–167. doi: 10.1093/toxsci/54.1.154. [DOI] [PubMed] [Google Scholar]
- 167.Hertz-Picciotto I, Delwiche L. The rise in autism and the role of age at diagnosis. Epidemiology. 2009;20:84–90. doi: 10.1097/EDE.0b013e3181902d15. [DOI] [PMC free article] [PubMed] [Google Scholar]