Abstract
Accumulations of insoluble deposits of amyloid β-peptide are major pathological hallmarks of Alzheimer disease. Amyloid β-peptide is derived by sequential proteolytic processing from a large type I trans-membrane protein, the β-amyloid precursor protein. The proteolytic enzymes involved in its processing are named secretases. β- and γ-secretase liberate by sequential cleavage the neurotoxic amyloid β-peptide, whereas α-secretase prevents its generation by cleaving within the middle of the amyloid domain. In this chapter we describe the cell biological and biochemical characteristics of the three secretase activities involved in the proteolytic processing of the precursor protein. In addition we outline how the precursor protein maturates and traffics through the secretory pathway to reach the subcellular locations where the individual secretases are preferentially active. Furthermore, we illuminate how neuronal activity and mutations which cause familial Alzheimer disease affect amyloid β-peptide generation and therefore disease onset and progression.
The neurotoxic amyloid β-peptide protein in Alzheimer disease is produced when γ- and β-secretase cleave the β-amyloid precursor protein (APP). α-Secretase prevents its generation.
PROTEOLYTIC PROCESSING OF APP
APP Processing: The Amyloidogenic and Anti-Amyloidogenic Pathways
The 37–43 amino acid amyloid β-peptide (Aβ) is generated by proteolytic processing from its precursor, the β-amyloid precursor protein (APP) in a physiologically normal pathway (Haass et al. 1992, 1993a; Seubert et al. 1992; Shoji et al. 1992; Busciglio et al. 1993; Haass and Selkoe 1993). APP is a type-I oriented membrane protein with its amino terminus within the lumen/extracellular space and its carboxyl terminus within the cytosol (Kang et al. 1987; Dyrks et al. 1988). Although APP is initially targeted into the secretory pathway (see below), it is proteolytically processed at several different subcellular sites (Weidemann et al. 1989). Three protease activities called α-, β-, and γ-secretase are involved in specific processing steps (Haass 2004). The name “secretases” refers to the secretion of the proteolytically cleaved substrates. All three protease activities have been identified and are described below.
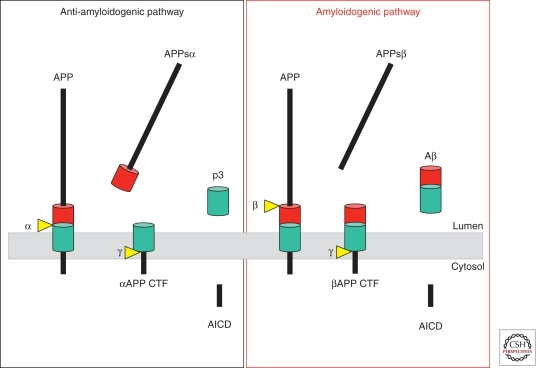
We discriminate two principal processing pathways: the amyloidogenic pathway, which leads to Aβ generation; and the anti-myloidogenic pathway, which prevents Aβ generation (Fig. 1). Aβ is produced in the amyloidogenic pathway by the consecutive action of β- and γ-secretase (Haass 2004). The β-secretase activity initiates Aβ generation by shedding a large part of the ectodomain of APP (APPsβ) and generating an APP carboxy-terminal fragment (βCTF or C99), which is then cleaved by γ-secretase. The latter cleavage occurs within the hydrophobic environment of biological membranes. Consecutive shedding and intramembrane proteolysis is now summarized under the term “regulated intramembrane proteolysis” (Brown et al. 2000; Rawson 2002; Lichtenthaler et al. 2011), a cellular process, which is frequently involved in important signaling pathways (Selkoe and Kopan 2003; see DeStrooper et al. 2011). On γ-secretase cleavage, Aβ is liberated and then found in extracellular fluids such as plasma or cerebrospinal fluid (Seubert et al. 1992). In the anti-amyloidogenic pathway, APP is cleaved approximately in the middle of the Aβ region by the α-secretase activity (Esch et al. 1990; Sisodia et al. 1990). This processing step generates a truncated APP CTF (αCTF or C83), which lacks the amino-terminal portion of the Aβ domain. The subsequent intramembrane cut by γ-secretase liberates a truncated Aβ peptide called p3 (Haass et al. 1993b), which apparently is pathologically irrelevant. γ-Secretase not only liberates Aβ (from C99) and p3 (from C83) but also generates the APP intracellular domain (AICD) (Gu et al. 2001; Sastre et al. 2001; Weidemann et al. 2002), which is released into the cytosol and which may have a function in nuclear signaling (Cao and Sudhof 2001; von Rotz et al. 2004). The amyloidogenic and the anti-amyloidogenic processing pathways compete with each other at least in some subcellular loci, since enhancing α-secretase activity in animal models of Alzheimer disease (AD) or in cultured cells can significantly lower Aβ generation and even amyloid plaque formation (Nitsch et al. 1992; Postina et al. 2004).
Figure 1.
Proteolytic processing of APP within the anti-amyloidogenic (left) and amyloidogenic (right) pathways.
Familial Alzheimer Disease–Associated Mutations within the APP Gene Affect Aβ Generation and Aggregation
A number of familial Alzheimer disease (FAD)-associated mutations have been found within and around the Aβ domain (Chartier-Harlin et al. 1991; Selkoe 2001; discussed in detail in Schenk et al. 2011). These mutations accelerate disease progression via diverse mechanisms. The Swedish mutation at the amino terminus of the Aβ region (Mullan et al. 1992) results in a significant increase of total Aβ production (such as Aβ40 and Aβ42) by providing a better substrate for the β-secretase activity (Citron et al. 1992; Cai et al. 1993). Mutations located just beyond the carboxyl terminus of Aβ (such as the so-called Austrian, Iranian, French, German, London, and Florida mutations) cause the increased production of longer Aβ species (Aβ42), which aggregate more rapidly and are believed to be the major neurotoxic Aβ species (Suzuki et al. 1994). Mutations in the mid region, such as the Arctic (Nilsberth et al. 2001) and Dutch mutations (Levy et al. 1990), affect the primary sequence of Aβ and apparently change the structure of Aβ, resulting in its enhanced aggregation propensity. Some of these intra-Aβ mutations can lead to mixed amyloid pathologies: marked cerebral angiopathy and marked amyloid plaque formation. For the Flemish mutation, an unexpected pathological mechanism was described recently. This mutation is located in an apparent substrate inhibitory domain that negatively regulates γ-secretase activity by binding to an unknown allosteric site within the complex. The Flemish mutation can reduce the activity of this inhibitory domain and consequently increase Aβ generation (Tian et al. 2010).
The Amyloidogenic Proteases: β- and γ-Secretase
β-Secretase
β-Secretase mediates the initial and rate-limiting processing step during Aβ generation (Vassar 2004). Expression cloning or biochemical purification led to the identification of a unique β-secretase enzyme (Sinha et al. 1999; Vassar et al. 1999; Yan et al. 1999; Hussain et al. 2000; Lin et al. 2000). Although many different names were originally used to describe this activity, such as memapsin, aspartyl protease 2, or BACE1 (β-site APP cleaving enzyme-1), BACE1 is now the generally accepted term for the enzyme harboring β-secretase activity. BACE1 is a membrane-bound aspartyl protease with its active site in the lumen/extracellular space and with structural similarities to the pepsin family (Hong et al. 2000). Besides BACE1, a homologous protease called BACE2 was identified (Vassar 2004). However, BACE2 is not involved in amyloidogenesis and may rather exert an anti-amyloidogenic activity in non-neuronal cells somewhat similar to α-secretase (Bennett et al. 2000a; Farzan et al. 2000; Fluhrer et al. 2002; Basi et al. 2003). BACE1 is the sole β-secretase, because its knockout completely blocks Aβ generation (Cai et al. 2001; Roberds et al. 2001; Luo et al. 2003). The protease is ubiquitously expressed, with highest levels in brain and pancreas; the physiological relevance of high pancreatic expression is currently not understood. Because APP is also expressed at very high levels in the brain, the concomitant high levels of BACE1 and APP make the brain the primary tissue for high Aβ generation and help explain why AD is a brain disease even though APP is expressed ubiquitously.
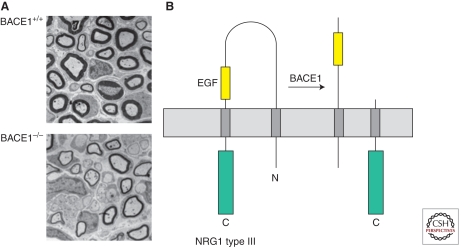
BACE1 is an important therapeutic target (Citron 2004), because its inhibition not only reduces Aβ levels but also prevents the accumulation of βCTFs, which contain the entire Aβ domain and serve as the final substrate for Aβ production (see Fig. 1). This is an important issue, because accumulation of such CTFs may cause additional, poorly understood toxic effects. Progress has been made toward the generation of BACE1 inhibitors, and clinical studies are on the way (Citron 2004; Schenk et al. 2011). However, one must be aware that such an approach also inhibits the physiological function of BACE1. So far, only very few physiological substrates have been validated whose cleavage by BACE1 is associated with a clear biological function. BACE1 knockout mice are viable and fertile and do not show any major behavioral, morphological, or developmental deficits (Cai et al. 2001; Roberds et al. 2001; Luo et al. 2003). However, subtle behavioral phenotypes such as some memory impairment and changes in spontaneous activity (Harrison et al. 2003; Dominguez et al. 2005) indicate that a loss of function of BACE1 can have detrimental consequences. Very high postnatal expression levels of BACE1 (Willem et al. 2006) revealed a function of BACE1 in myelination, a process which occurs after birth. Indeed all available BACE1 knockout mice show a significant hypomyelination phenotype in the peripheral nervous system (Fig. 2A; Hu et al. 2006; Willem et al. 2006). Whether myelination within the CNS is also under the control of BACE1, as described by Hu et al. (2006), is currently under debate. Schwann cell-mediated myelination in the peripheral nervous system is regulated via the Neuregulin-1 (NRG1) signaling pathway (Birchmeier and Nave 2008). Interestingly, proteolytic processing of NRG1 (Fig. 2B) is believed to facilitate its signaling activity. Indeed, in the BACE1 knockout animals, uncleaved NRG1 accumulates. Thus, NRG1 is a physiological substrate for BACE1, and at least one of the physiological functions of BACE1 concerns myelination. BACE1 has also been shown to be involved in the regulation of voltage-dependent sodium channels (Kim et al. 2007). Moreover, other substrates such as Type II α-2,6-sialyltransferase, platelet selectin glycoprotein ligand-1, APP-like proteins, Aβ itself, and the interleukin-like receptor type II have also been shown to be processed by BACE1 (summarized in Willem et al. 2009). However, the physiological consequences of these cleavages are unclear, and one should keep in mind that most substrates were identified on overexpression of BACE1 and/or the substrate, which is likely to generate conditions allowing artificial substrate/protease interactions.
Figure 2.
Biological function of BACE1 in myelination. (A) A BACE1 knockout in mice results in a hypomyelination phenotype within the peripheral nervous system. Cross-sections through the sciatic nerve of wild-type mice and BACE knockout mice are shown. (B) Proteolytic processing of NRG1 type III. NRG1 type III is cleaved by BACE1. This processing step leads to the exposure of EGF-containing domain and facilitates signaling via ErbB4 in Schwann cells.
γ-Secretase
The Aβ-liberating cleavage of APP is mediated by γ-secretase and occurs within the transmembrane domain (TMD). γ-Secretase structure and function is discussed in DeStrooper et al. (2011). Here, we will briefly introduce γ-secretase and then focus on the cellular assembly of γ-secretase and its subcellular sites of activity.
γ-Secretase is a protease complex consisting of four subunits (reviewed in Steiner et al. 2008). Presenilin (PS) 1 or PS2 contain the two critical aspartyl residues within TMDs 6 and 7, which are part of the catalytic domain of the aspartyl protease activity of γ-secretase (Wolfe et al. 1999). Additional complex components are nicastrin (NCT), anterior pharynx defective (APH)-1a or APH-1b, and the PS enhancer (PEN)-2 (Yu et al. 2000; Francis et al. 2002). These four components are necessary and sufficient for full γ-secretase activity (Edbauer et al. 2003). Little is known about the biological function of NCT, APH-1, and PEN-2. NCT is probably required as a size-selecting substrate receptor (Shah et al. 2005; Dries et al. 2009), although recent findings may challenge such a function (Chavez-Gutierrez et al. 2008; Martin et al. 2009). PEN-2 apparently facilitates PS endoproteolysis into its active heterodimeric state and stabilizes PS within the γ-secretase complex (Hasegawa et al. 2004; Prokop et al. 2004). No specific function has so far been assigned to APH-1, although it may act as a scaffold for the initial binding of NCT and assembly of the complex (LaVoie et al. 2003).
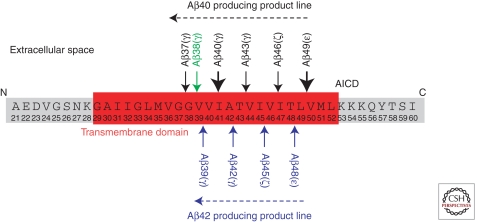
The intramembrane processing of APP by γ-secretase is not restricted to a single site. Rather, it appears that γ-secretase substrates are cleaved several times within their TMDs. Cleavages at the so-called ε-, ζ-, and γ-sites that are separated by approximately three amino acids are postulated (Fig. 3; Sastre et al. 2001; Weidemann et al. 2002; Qi-Takahara et al. 2005; Takami et al. 2009). To make things even more complicated, the final γ-cleavage is also not precise and can occur under physiological conditions at least between amino acids 37 and 43 of the Aβ domain. This difference is of greatest relevance for the understanding of AD pathology, because the longer Aβ42 species is more aggregation prone and believed to be the toxic building block of Aβ oligomers, which affect memory and cell survival (Haass and Selkoe 2007). How can these multiple cleavages be explained? Although it is not yet finally proven, it is likely that this phenomenon is due to a stepwise cleavage mechanism performed by one and the same γ-secretase. Moreover, stepwise endoproteolysis may be a general phenomenon of intramembrane proteolysis mediated by all γ-secretase-like proteases (Fluhrer et al. 2006, 2008, 2009). Apparently, once APP (or another substrate) is bound to the active site of the γ-secretase complex, intramembrane proteolysis begins with the ε-cleavage after amino acids 49 or 48. This is then followed by cleavage after amino acids 46 or 45 (ζ-cleavage) and terminates with the cleavage at the γ-site mostly at amino acids 42 or 40 (but also after amino acids 37, 38, 39, and 43; Fig. 3). Two product lines are discussed (Fig. 3): one leading predominantly to Aβ42 generation (starting with the ε-cleavage after amino acid 48 and followed by cleavages after amino acids 45 and 42); and the other leading predominantly to Aβ40 (starting with the ε-cleavage after amino acid 49 and followed by cleavages after amino acids 46 and 43). As discussed in Schenk et al. (2011), these cleavages may be therapeutically modulated to selectively prevent Aβ42 generation.
Figure 3.
Sequential processing of APP by γ-secretase.
The Anti-Amyloidogenic α-Secretase
As mentioned above, the anti-amyloidogenic processing of APP occurs within the Aβ domain between residues Lys16 and Leu17 (Esch et al. 1990; Sisodia et al. 1990; Wang et al. 1991) and results in the secretion of the large APP amino-terminal domain and the generation of α-CTF (C83). This cleavage is performed by a set of proteases termed α-secretases. Shortly after the identification of the α-secretase cleavage site, it was noted that in cultured cells this cleavage predominantly occurs at the cell surface, suggesting that α-secretases are plasma membrane (PM)-bound proteases (Sisodia 1992). Activation of protein kinase C by phorbol esters stimulates α-secretase processing and secretion of the APP ectodomain (Buxbaum et al. 1990). This protein kinase C-dependent APP processing was dubbed “regulated α-secretase cleavage” of APP. Several zinc metalloproteinases that are members of the “a disintegrin and metalloprotease” family such as ADAM9, ADAM10, TACE/ADAM17 and ADAM19 can function as α-secretase (Allinson et al. 2003). Targeted disruption of individual genes that encode ADAM10, TACE/ADAM17, or ADAM19 has no effect on constitutive α-secretase processing of APP, indicating that α-secretase activity is shared by a set of ADAM proteases (Buxbaum et al. 1998b; Merlos-Suarez et al. 1998; Hartmann et al. 2002; Weskamp et al. 2002). However, recent evidence suggests that, at least in neurons, the principal constitutive α-secretase activity is exerted by ADAM10 (Kuhn et al. 2010). Besides APP, Notch receptors and ligands, tumor necrosis factor α, cadherins and IL-6 receptor, EGF receptor ligands, and several other type I transmembrane proteins are cleaved by α-secretases to release their extracellular domain. Consequently, the process of ectodomain shedding mediated by α-secretases appears to be largely sequence independent. At a minimum, α-secretase cleavage of APP is determined by an α-helical conformation and the distance (12–13 residues) of the hydrolyzed bond from the membrane (Sisodia 1992).
Amyloidogenic processing appears to be the favored pathway of APP metabolism in neurons, largely because of the greater abundance of BACE1, whereas anti-amyloidogenic pathway is predominant in all other cell types. Overexpression of ADAM10 and other putative α-secretases in cultured cells as well as in transgenic mice increases the secretion of the APP ectodomain ending at the α-secretase site. Interestingly, neuronal overexpression of ADAM10 in transgenic mice reduces BACE1 processing of APP and amyloid deposition (Postina et al. 2004). This finding is of physiological relevance because ADAM10 is expressed throughout the cortex and hippocampus in the adult central nervous system, and APP, BACE1, and ADAM10 are co-expressed in human cortical neurons (Marcinkiewicz et al. 2000). Other putative α-secretases such as ADAM9, TACE/ADAM17 and ADAM19 are also expressed in the adult brain. Thus, up-regulation of α-secretase activity to promote anti-amyloidogenic processing of APP is potentially of therapeutic value (Postina et al. 2004).
Commitment of APP to amyloidogenic and anti-amyloidogenic pathways can be differentially modulated by the activation of cell-surface receptors such as the serotonin/5-hydroxytryptamine (5-HT4) receptor, metabotropic glutamate receptors, muscarinic acetylcholine receptors, and platelet-derived growth factor receptor (Allinson et al. 2003). Signaling downstream from these receptors regulates APPsα and Aβ secretion by engaging intermediates including protein kinase C, protein kinase A, phosphatidylinositol-3-kinase, mitogen-activated protein kinase kinase, extracellular signal-regulated kinase, Src tyrosine kinase, small GTPase Rac, inositol 1,4,5-trisphosphate, cAMP, and cytosolic calcium (reviewed in Gandy et al. 1994; Allinson et al. 2003). Lowering cholesterol levels in cultured cells stimulates α-secretase cleavage of APP through mechanisms involving impaired APP endocytosis and increased steady-state levels of ADAM10 (Kojro et al. 2001). The effect of cholesterol depletion is not specific to APP cleavage, because the shedding of the human interleukin-6 receptor by ADAM10 and TACE/ADAM17 is also stimulated under these conditions (Matthews et al. 2003).
CELLULAR TRAFFICKING OF APP
Biosynthesis and Trafficking through the Secretory Pathway
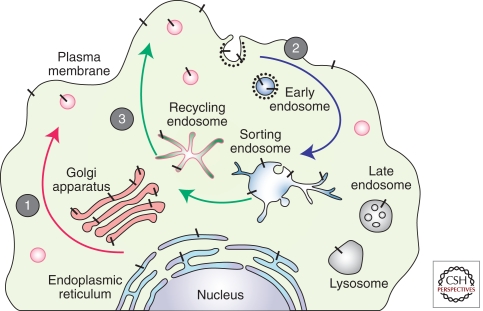
The pathways of APP trafficking in a nonpolarized mammalian cell are depicted in Figure 4. During its transit from the ER to the PM, nascent APP is posttranslationally modified by N- and O-linked glycosylation, ectodomain and cytoplasmic phosphorylation, and tyrosine sulphation. Only a small fraction of nascent APP molecules reach the PM (estimated at ∼10% based on APP overexpression in cultured cells), whereas the majority of APP at steady-state localizes to the Golgi apparatus and trans-Golgi network (TGN). APP which is not shed from the cell surface is internalized within minutes of arrival at the cell surface because of the presence of its “YENPTY” internalization motif near the carboxyl terminus of APP (residues 682–687 of APP695 isoform) (Lai et al. 1995; Marquez-Sterling et al. 1997). Following endocytosis, APP is delivered to endosomes, and a fraction of endocytosed molecules is recycled to the cell surface. Measurable amounts of internalized APP also undergo degradation in lysosomes (Haass et al. 1992).
Figure 4.
Intracellular trafficking of APP. Nascent APP molecules (black bars) mature through the constitutive secretory pathway (1). Once APP reaches the cell surface, it is rapidly internalized (2) and subsequently trafficked through endocytic and recycling organelles to the TGN or the cell surface (3). A small fraction is also degraded in the lysosome. Nonamyloidogenic processing mainly occurs at the cell surface where α-secretases are present. Amyloidogenic processing involves transit through the endocytic organelles where APP encounters β- and γ-secretases.
Endocytic APP Sorting and Aβ Production
Although attempts to characterize the role of endocytic APP trafficking by expression of dominant-negative dynamin mutants resulted in discrepant findings (Chyung et al. 2003; Ehehalt et al. 2003; Carey et al. 2005), mutations within the APP cytosolic YENPTY motif selectively inhibit APP internalization and decrease Aβ generation (Perez et al. 1999). This motif and the flanking region serve as the binding site for many cytosolic adaptors that have phosphotyrosine-binding domains, including Fe65, Fe65L1, Fe65L2, Mint 1 (also called X11α), Mint 2, Mint 3, Dab1, sorting nexin 17, and c-Jun amino-terminal kinase-interacting protein family members. Overexpression of Mint 1, Mint 2, or Fe65 causes reduction in Aβ generation and less deposition in the brains of APP transgenic mice, strongly suggesting a physiological role for these adaptors in regulating amyloidogenic processing of APP in the nervous system (Miller et al. 2006). In addition to binding APP, Mint proteins can directly bind ADP-ribosylation factors, raising the intriguing possibility that Mints may regulate vesicular trafficking of APP by serving as coat proteins (Hill et al. 2003). A conformational change introduced by phosphorylation at Thr-668 (14 amino acids proximal to the YENPTY motif) interferes with Fe65 binding to APP and facilitates BACE1 and γ-secretase cleavage of APP in cultured cells (Ando et al. 2001; Lee et al. 2003). However, analysis of Thr-668-Ala knock-in mice indicates that the phosphorylation status of Thr-668 does not affect physiological processing of APP into Aβ peptides in vivo (Sano et al. 2006). In addition to its potential role in APP endocytosis, Fe65 stabilizes the highly labile AICD, which may serve as a regulatory step in modulating the physiological function of AICD. Fe65 is capable of interacting with APP and LRP (a multifunctional endocytosis receptor containing two NPXY motifs) via distinct protein interaction domains (Trommsdorff et al. 1998). Lack of LRP expression causes reduced APP internalization and Aβ secretion (Ulery et al. 2000; Pietrzik et al. 2002; Cam et al. 2005), leading to the conclusion that endocytosis of LRP is coupled to APP internalization and processing, and Fe65 acts as a functional linker between APP and LRP in modulating endocytic APP trafficking (Pietrzik et al. 2004). In addition, Ran-binding protein 9 promotes APP interaction with APP and facilitates APP internalization in a Fe65-independent manner (Lakshmana et al. 2009). Finally, the type I transmembrane protein sorLA/LR11 (a member of the VPS10p-domain receptor family), which functionally interacts with cytosolic adaptors GGA and PACS-1, regulates Aβ production by acting as a Golgi/TGN retention factor for APP (Andersen et al. 2005; Offe et al. 2006; Schmidt et al. 2007). Aβ levels are reduced on overexpression of sorLA/LR11 in cultured cells and increased in the brains of sorLA/LR11 knockout mice (Andersen et al. 2005; Offe et al. 2006). sorLA/LR11 is also genetically associated with AD and its steady-state levels are markedly reduced in the brains of patients with AD, further implicating this sorting molecule in the physiological regulation of APP metabolism (Andersen et al. 2005; Offe et al. 2006; Rogaeva et al. 2007).
Polarized Trafficking of APP in Non-Neuronal Cells
Obviously, neurons are of pivotal interest for the analysis of APP trafficking and processing. Because of the polarized nature of neurons, a strong interest emerged in understanding how APP is targeted to selected destinations and where during its cellular transport APP is processed into its cleavage products by the three secretase activities described above. Cellular model systems have been developed that allow analysis of polarized sorting in relatively simple peripheral cells. Madin–Darby canine kidney (MDCK) cells are a suitable cell system, as they form polarized monolayers with defined apical and basolateral surfaces on culturing in Transwell chambers. This widely used tool in cell biology not only allows the separate collection of apically and basolaterally secreted proteins but also the detection of membrane-bound proteins on either surface, for example, via biotinylation.
Polarized Trafficking of APP via Two Independent Sorting Mechanisms
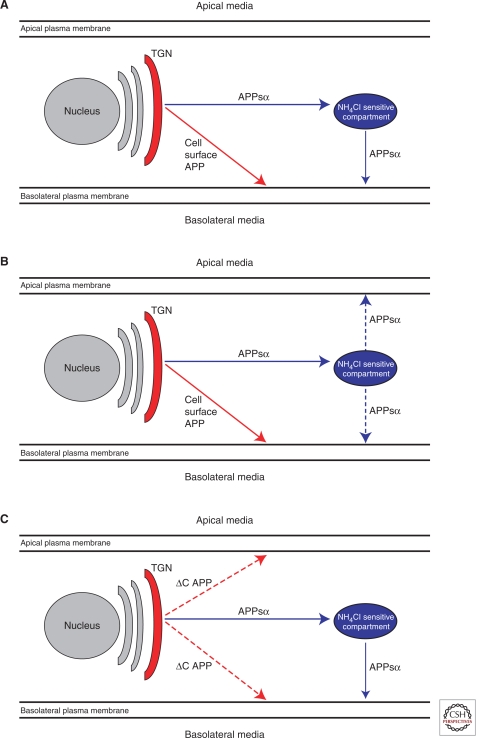
Using MDCK cells, it has been shown that a number of cleavage products of APP including soluble APP (α-secretase generated APP; αAPPs), Aβ, and p3 are selectively targeted to the basolateral compartment (Haass et al. 1994; De Strooper et al. 1995a). In addition, surface APP also accumulates on the basolateral surface, and APPsα shed from surface APP accumulates in the basolateral media (Fig. 5). These findings suggested that APP contains a sorting signal that selectively targets it to the basolateral side of polarized cells. Deletion of the last 42 amino acids of the cytoplasmic domain of APP (aa 654–695 according to the numbering of APP695) causes a random (default) transport of the resultant APP-ΔC to both surfaces (Fig. 5C). Moreover, Tyr 653 was identified as a critical amino acid required for efficient basolateral APP sorting (Haass et al. 1995a). Interestingly, this signal acts independently of a re-internalization signal between aa 684–687 (see above). Surprisingly, when a Tyr653-mutant APP was expressed, the bulk of αAPPs was still sorted efficiently to the basolateral compartment, although the membrane-bound holoprotein was transported equally to both surfaces (De Strooper et al. 1995a; Haass et al. 1995a). This result indicates two independent sorting mechanisms, one for membrane-bound APP and one for soluble APPsα (Fig. 5A–C). Whereas the first sorting pathway is dependent on a cytoplasmic sorting signal similar to other polarized sorted proteins, the soluble ectodomain of APP apparently contains an independent basolateral sorting signal (De Strooper et al. 1995b; Haass et al. 1995a). Indeed, when APPsα was expressed as a recombinant protein, it was still rather efficiently sorted to the basolateral compartment (Haass et al. 1995a). Evidence exists that alternative splicing of APP can affect polarized secretion of soluble APP (Hartmann et al. 1996). APP variants lacking the exon 15 encoded domain are sorted equally into both compartments. It is assumed that the 3D structure by itself, not a linear sequence motif, determines polarized sorting of secreted APPsα. Cellular fractionation studies show that a large amount of APPsα is already generated by α-secretase within the Golgi compartment long before it reaches the cell surface of MDCK cells (Haass et al. 1995a). Based on previous findings it was assumed that sorting of soluble proteins occurs within a pH-sensitive compartment (De Strooper et al. 1995a; Haass et al. 1995a). Indeed NH4Cl treatment randomizes polarized secretion of αAPPs. Thus, two distinct pathways mediate polarized sorting of APP in MDCK cells (Fig. 5A–C).
Figure 5.
Polarized sorting of APP in MDCK cells. (A) Two independent basolateral sorting pathways for αAPPs via a NH4Cl-sensitive compartment (blue) and full-length APP (red). (B) Inhibition of vesicular acidification by NH4Cl leads to random secretion of αAPPs whereas full-length APP is still sorted to the basolateral membrane. (C) APP lacking a cytoplasmic signal for basolateral sorting (ΔC APP) undergoes random surface transport.
Polarized Sorting of Secretases
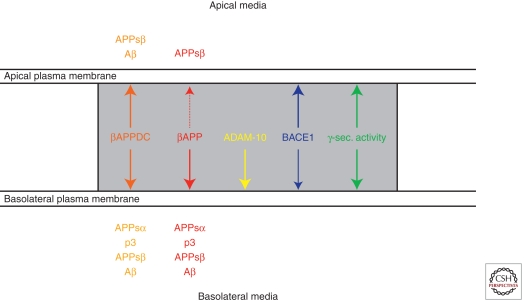
The above findings have major implications for the polarized trafficking of all three secretase activities. As described in the first publication on polarized secretion of Aβ, only small amounts of authentic Aβ beginning with Asp1 of the Aβ sequence are observed in the basolateral media (Haass et al. 1994). This is consistent with the finding that BACE1, which cleaves at Asp1, is predominantly targeted to the apical surface of MDCK, a process which markedly limits Aβ generation in polarized cells (Capell et al. 2002). Indeed, when APP is mis-sorted to the apical surface, it is almost exclusively processed by BACE-1. This also indicates that little α-secretase activity is present in the apical sorting pathway, because apparently there is almost no competition for BACE-1 cleavage of APP on the apical side. Indeed ADAM-10, the major α-secretase activity (see above), is targeted basolaterally (Wild-Bode et al. 2006); here, it competes efficiently with the rather small amount of BACE1 present within the basolateral pathway. ADAM-10, like many other members of the ADAM family, contains two cytoplasmic Src homology 3 (SH3)-binding domains. Sequential deletion revealed critical Pro residues within the juxtamembrane SH3-binding domain. This trafficking signal was required to target ADAM-10 to adherens junctions and to support its function in cell migration and E-cadherin processing (Wild-Bode et al. 2006). Therapeutic strategies aimed at increasing α-secretase-mediated anti-amyloidogenic processing of APP have to take this important physiological function—and numerous other functions which depend on α-secretase-mediated shedding—into consideration.
In contrast to BACE1 and ADAM-10, γ-secretase activity is found on both surfaces (Capell et al. 2002), a finding, which is consistent with the observation that Aβ and p3 can be found in both compartments, dependent on the APP variant expressed (i.e., with or without a basolateral sorting sequence) (Haass et al. 1995a). Thus the two competing proteases, ADAM-10 and BACE1, are sorted differentially to the basolateral or apical surface, whereas γ-secretase is found on both sides (Fig. 6).
Figure 6.
Polarized sorting APP, its processing products, and its secretases.
As described above, several familial autosomal dominant mutations were linked to APP. One of these mutations, the so-called Swedish mutation (Mullan et al. 1992) occurs immediately adjacent to the BACE1 cleavage site (Met-Asp). This mutation strongly facilitates BACE-1-mediated processing of APP and therefore results in enhanced Aβ generation (Citron et al. 1992; Cai et al. 1993). In parallel, a significantly enhanced amount of the shorter variant of sAPP (APPsβ) is secreted (Haass et al. 1995). Interestingly, when Swedish mutant APP was expressed in MDCK cells, APPsβ was found to be secreted apically, while the endogenous APPs (mainly consisting of APPsα) and membrane-bound holoAPP still underwent basolateral sorting (Lo et al. 1994; De Strooper et al. 1995a). Based on the findings described above, this observation may reflect the Swedish mutation-induced processing of the small amounts of APP targeted to the apical surface by the predominantly apically targeted BACE1.
Trafficking of APP in Neurons
Although MDCK cells have yielded important insights into the polarized trafficking and processing of APP, these findings had to be confirmed in neurons, which are presumably the primary source of Aβ production in vivo. Neurons are highly polarized into soma, axons, and dendrites, all of which perform different functions and therefore are equipped with distinct sets of proteins and lipids that regulate protein trafficking. To complicate matters further, axons and dendrites are subdivided into separate compartments, e.g., dendritic shafts, dendritic spines, axonal shaft, axonal presynaptic endings, and others. An elaborate system of tracks (microtubules), trucks (kinesin and dynein motor proteins), and address labels (specific sorting signals) ensures proper delivery of proteins to their respective destinations. Disturbances in this system could affect APP processing and have been linked to AD pathogenesis (De Vos et al. 2008; Morfini et al. 2009). It is therefore essential to understand in detail the trafficking and processing of APP in neurons.
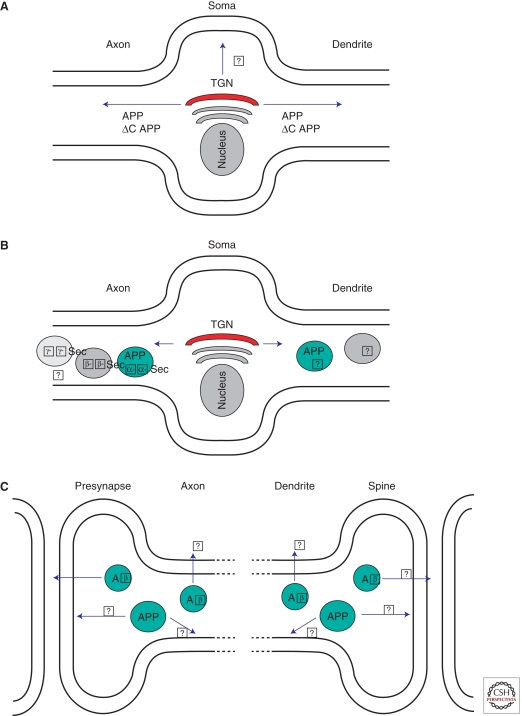
The transport from ER to Golgi and TGN described earlier is thought to be similar in nonpolarized cells and in the neuronal soma. But after leaving the TGN in neurons, APP is transported to axons and dendrites in post-Golgi transport vesicles (reviewed in Kins et al. 2006). APP delivery to the axon makes use of the fast axonal transport system (Koo et al. 1990; Sisodia et al. 1993), with kinesin-1 as the microtubule motor protein (reviewed in Kins et al. 2006). As visualized by GFP-tagged fusion proteins, APP is transported in vesicular and often elongated tubular structures which move with special characteristics along the axon (Kaether et al. 2000; Stamer et al. 2002; Goldsbury et al. 2006; Szodorai et al. 2009). Whereas most other proteins are axonally transported in vesicles that display a saltatory movement, often changing directions, the APP tubules continuously move unidirectionally, with an average speed of 4.5 µm/s, reaching maximal speeds up to 10 µm/s (Kaether et al. 2000). This is among the fastest transport velocities measured in cultured neurons. Significant retrograde transport with slightly slower kinetics was also observed (Kaether et al. 2000; Stamer et al. 2002). Little is known about the fate of the axonal transport carrier vesicles. Where do they fuse with the axonal PM? What are their fusion kinetics? Where do the retrograde carriers go? A small fraction of the axonal APP has been suggested to undergo transcytotic transport to dendrites, but the significance and kinetics of this process need to be determined (Simons et al. 1995; Yamazaki et al. 1995). Likewise a detailed study of dendritic transport kinetics of APP is lacking.
What are the sorting signals mediating axonal and/or dendritic transport? An axonal sorting signal was mapped to a juxtamembrane domain which includes the Aβ-domain (Tienari et al. 1996); however, a recent report showed that APP is transported into axons and dendrites without apparent sorting signals (Back et al. 2007). Along this line, APP lacking the cytoplasmic domain (ΔC) (where the basolateral sorting signal is located; see above) is transported along neurites with characteristics indistinguishable from wild-type APP (Fig. 7a; Back et al. 2007; Szodorai et al. 2009). Therefore, the basolateral sorting signal in the carboxy-terminal region of APP documented in MDCK studies seems to have no function for polarized sorting in neurons. What emerges at this point is that the sorting of APP in neurons is fundamentally different from that of other polarized cells, and much more work has to be done to fully understand polarized trafficking of APP in neurons.
Figure 7.
Neuronal APP transport and processing. (A) Post-Golgi sorting of APP. APP as well as APPΔC is transported by fast axonal transport along the axon and by as yet uncharacterized transport into dendrites. Transport to and fusion with the plasma membrane in the neuronal soma is likely, but has not been characterized. (B) Transport and sorting of APP and secretases. APP and α-secretase are transported in axonal carriers that do not contain β- or γ-secretase. Whether β- and γ-secretase are transported together is not known. Colocalization of APP and secretases in dendritic transport vesicles has not been studied. (C) Release of Aβ occurs at presynaptic sites, but whether it is also released from axon shafts is unknown. Aβ is released from dendrites, but the exact location has not been determined. Where APP fuses with the axonal and dendritic plasma membrane and where processing by secretases occurs remains elusive.
SUBCELLULAR SITES OF APP PROCESSING
Subcellular Sites of α-Secretase-Mediated APP Processing
As described above, α-secretase-mediated shedding occurs predominantly on the cell surface. However, substantial cleavage by α-secretase has also been reported to occur within TGN, for example in MDCK cells (see above).
Subcellular Sites of β-Secretase-Mediated APP Processing
As described above, BACE1 is a type-1 membrane protein that is co-translationally translocated into the ER as an immature pro-enzyme. During maturation, BACE1 undergoes a number of post/co-translational modifications, including N-glycosylation, disulfide bridge formation, and palmitoylation (Bennett et al. 2000b; Capell et al. 2000; Huse et al. 2000; Benjannet et al. 2001). The propeptide of immature BACE1 is removed by Furin and related proteases during maturation (Capell et al. 2000; Creemers et al. 2001). Surprisingly, the propeptide seems not to significantly affect BACE1 proteolytic function (Creemers et al. 2001). BACE1 reaches the PM and becomes enriched in lipid rafts (Riddell et al. 2001; Cordy et al. 2003). Rafts are discussed as potential sites for efficient Aβ generation, as APP and BACE1 apparently come into immediate contact within this compartment (Ehehalt et al. 2003; Abad-Rodriguez et al. 2004). This would be consistent with the finding that BACE1 undergoes palmitoylation. However, inhibition of palmitoylation and raft localization still permits relatively normal Aβ production (Vetrivel et al. 2009). Thus, the role of rafts in Aβ generation is still under debate. On reaching the PM, BACE1 undergoes internalization. Internalization and targeting to endosomes is dependent on a di-leucine motif located within the short cytoplasmic domain of BACE1 (Huse et al. 2000; Pastorino et al. 2002). From endosomes, BACE1 can recycle to the TGN (Walter et al. 2001). A single phosphorylation at serine 498 within the carboxy-terminal domain of BACE1 is sufficient for its transport from endosomes to the trans-Golgi. Recycling requires the Golgi-localized, gamma-ear containing ADP (GGA) ribosylation-binding factor (He et al. 2005; Wahle et al. 2005, 2006), and two such GGA proteins are involved in BACE1 trafficking, GGA1 and GGA2. GGA1 is involved in endosomal retrieval of BACE1 and its recycling to the PM, whereas GGA2 targets BACE1 to lysosomes for its final degradation.
As an aspartyl protease, BACE1 has an acidic pH optimum (around pH 4.5) (Vassar et al. 1999), which is consistent with a major β-secretase activity within endosomes. Indeed, βCTFs accumulate in this compartment on inhibition of endosomal/lysosomal protein degradation (Golde et al. 1992; Haass et al. 1992). Selective processing of APP by BACE1 within endosomes has been used to design and generate highly effective BACE1 inhibitors. To ensure their accumulation within endosomes, a membrane-anchored BACE1 transition-state inhibitor was linked to a sterol moiety. Because of its selective accumulation within endosomes, the membrane-bound inhibitor reduced BACE1 activity much more efficiently than a nonmembrane anchored version (Rajendran et al. 2008). However, BACE1 activity is not exclusively restricted to endosomes. For example, Swedish mutant APP which, because of its missense mutation at the Met-Asp cleavage site is more efficiently processed by BACE1, can already be cleaved within late Golgi compartments (see above).
Subcellular Sites of γ-Secretase-Mediated APP Processing
Early studies on APP processing indicated that γ-secretase cleaves APP to generate Aβ at or near the PM. However, initial characterization of PS subcellular localization—before it was clear that it is not only implicated in γ-secretase processing but is its catalytic subunit—suggested that PS is mainly localized to the ER. This apparent contradiction was coined the spatial paradox by Annaert and De Strooper (1999). Although still not resolved completely, the work of many labs now indicates that mature, proteolytically active PS/γ-secretase is principally localized not in ER, Golgi, or post-Golgi transport vesicles but rather at the PM and in the endosomal/lysosomal system, including phagosomes and autophagosomes (reviewed in Pasternak et al. 2004; Kaether et al. 2006a; Nixon 2007; Dries and Yu 2008). The γ-secretase subunits found in the ER/early secretory pathway most likely represent unassembled or partially assembled subcomplexes, but not the active enzyme. The evidence supporting such a model is manifold and, because of space constraints, only key arguments will be highlighted: (1) APP/C99/C83 are not cleaved by γ-secretase in the ER (Cupers et al. 2001; Maltese et al. 2001; Grimm et al. 2003; Kaether et al. 2006b); (2) by immuno-electron microscopy, PS1 is not detected in Golgi or TGN but in ER and PM (Rechards et al. 2003); whereas the ER pool most likely reflects unassembled PS1, the PM pool has assembled, active complex; (3) only the mature, glycosylated NCT, not immature unglycosylated NCT, is present in the fully assembled, active γ-secretase complex (Edbauer et al. 2002; Kaether et al. 2002), and therefore γ-secretase-associated NCT must have passed the Golgi; (4) γ-secretase has been shown by various methods, including cell surface biotinylation, binding of biotinylated inhibitors specific for the active complex, and microscopic techniques, to be present at the PM (Kaether et al. 2002; Tarassishin et al. 2004; Chyung et al. 2005). It has been shown that the ε-cleavage of APP differs in endosomes and PM (Fukumori et al. 2006), suggesting that γ-secretase has different properties depending on its subcellular localization, maybe because of differences in pH or lipid composition. One can speculate that changing the ratio of γ-secretase present in the different subcellular compartments might have an impact on AD pathology. Taken together, γ-secretase cleaves APP on the surface and in endosomes/lysosomes, but the relative contribution of the two remains to be determined and may vary among cell types.
Where in neurons do secretases process APP? Given the complex morphology of neurons, it is not surprising that even less is known about the precise subcellular site of γ-secretase processing in neurons (axonal or somal or dendritic endosomes/lysosomes? dendritic or axonal autophagosomes? other organelles?) than in non-neuronal cells (Fig. 7B). A major secretion site of Aβ seems to be distal axons/synapses (Lazarov et al. 2002; Sheng et al. 2002), but recently it was reported that Aβ can be secreted from axons and dendrites and can elicit local effects on neighboring neurons (Fig. 7C; Wei et al. 2010). A detailed analysis aimed at determining the ratio of axonally versus dendritically secreted Aβ, using for example cultured neurons in compartmentalized chambers, has not been performed. It has been suggested that APP is transported in axonal vesicles which harbor β- and γ-secretase and that consequently Aβ is produced in these vesicles (Kamal et al. 2001). This view has been challenged, and several experiments indicate that APP is not processed in axonal transport vesicles, with the exception of limited processing by α-secretase. Ligation experiments in sciatic nerve indicated that APP, but not PS as component of the γ-secretase, accumulated at the ligation site, indicating completely different transport kinetics of these proteins (Lazarov et al. 2005). In addition, using video microscopy, it was shown that APP and β-secretase are not transported in the same vesicles (Goldsbury et al. 2006). Also, by analyzing immuno-isolated APP-carrying transport vesicles, only α- but not the other secretases were found to colocalize in these vesicles (Szodorai et al. 2009). γ-secretase is present in synapses/distal axons (Beher et al. 1999; Ribaut-Barassin et al. 2003; Inoue et al. 2009; Frykman et al. 2010); therefore Aβ could be produced there, but the precise subcellular site(s) of APP processing by β- and γ-secretase in neurons remains to be determined.
Degradation of APP
It should be noted that alternative, secretase-independent processing of APP exists. Early studies showed that the half-life of APP is very short and that not all APP is secreted as APPs, suggesting secretase-independent processing pathways (Weidemann et al. 1989). Later it was shown that APP is degraded in lysosomes to amyloidogenic and nonamyloidogenic fragments (Golde et al. 1992; Haass et al. 1992). In addition, APP was shown to be a caspase substrate (Weidemann et al. 1999; Lu et al. 2003), but the impact of this processing on Aβ generation and/or AD pathology is probably minor (Harris et al. 2010).
Activity-Dependent APP Processing
Emerging evidence from a variety of human studies has suggested that Aβ levels and metabolism might be regulated by neuronal activity. In this regard, some patients with temporal lobe epilepsy, who experience elevated neuronal activity, develop Aβ-containing plaques as early as 30 years of age (Mackenzie and Miller 1994; Gouras et al. 1997). Moreover, regions of the brain that develop the highest levels of Aβ plaques, including the frontal and parietal lobes and posterior cingulate cortex, exhibit the highest baseline metabolic activity in the so-called “default network” (Gusnard et al. 2001; Raichle et al. 2001; Buckner et al. 2005). This high metabolic activity is a reflection of elevated neuronal and synaptic activity. Parallel studies in animal models and cell culture have linked APP transport, neuronal activity, and Aβ metabolism. APP is axonally transported from the entorhinal cortex to the hippocampal formation via the perforant pathway (Buxbaum et al. 1998a), and lesions of this pathway in transgenic mice overexpressing FAD-linked mutant APP and PS1 transgenes results in substantially less Aβ deposition within the hippocampus (Lazarov et al. 2002). Early studies showed that electrical depolarization increases the release of soluble APP-α in rat hippocampal brain slices (Nitsch et al. 1993), and it appears that these effects are mediated by activation of muscarinic M1 acetylcholine receptors which leads to parallel decreases in Aβ levels (Beach et al. 2001; Hock et al. 2003). On the other hand, specific stimulation of NMDA receptors up-regulates APP, inhibits α-secretase activity, and promotes Aβ production (Lesne et al. 2005).
To determine the effects of neuronal activity on Aβ secretion, Kamenetz et al. (2003) prepared hippocampal slices from transgenic mice overexpressing APP harboring the FAD-linked Swedish mutation (APPSWE) and maintained these preparations in the presence of pharmacological agents that either decrease neuronal activity [tetrodotoxin (TTX), high magnesium, or flunitrazepam than in non-neuronal cells (Fig. 7B). A major secretion site of Aβ seems to be distal axons/synapses a GABA-A receptor potentiator)] or increase it (picrotoxin [a GABA-A channel blocker]). Agents that decreased or increased activity resulted in significant reductions or elevations, respectively, in levels of Aβ (both Aβ40 and Aβ42) detected in the slice medium, indicating that the secretion of Aβ from neuronal cells that overexpress APP can be controlled by neuronal activity. Western blot analyses revealed that increasing neuronal activity significantly enhanced the levels of β-CTF, the penultimate precursor of Aβ peptides, and elevated levels of secreted APPsβ, findings which suggested that the level of BACE cleavage can be controlled by neuronal activity. To address whether APP or its products can control synaptic function, wild-type rat hippocampal slice neurons were transduced with viruses expressing APP or APP mutants, and synaptic responses were evoked onto side-by-side pairs of simultaneously recorded postsynaptic neurons where only one neuron expresses the exogenous protein. Excitatory AMPA and NMDA responses onto neurons expressing recombinant APP were significantly depressed, whereas inhibitory (GABA) currents were unaffected. Neurons expressing APP showed a significant decrease in the frequency of miniature EPSCs, with no change in their amplitude nor in paired-pulse facilitation, suggesting that the depressive effects of APP overexpression are due to a decrease in the number of functional synapses. Indeed, expression of an APP variant harboring an experimental mutation (APPMV) just before Aβ Asp1 that blocks β-secretase cleavage produced no significant depression of transmission. Furthermore, transduced slices treated with a highly potent and selective γ-secretase inhibitor (L-685,458) failed to show synaptic depression onto nearby control cells. These results indicate that γ-secretase processing of APP is required for the depressive effects of APP and that this phenotype is independent of the formation of the large ectodomain of APP following either α or β cleavage events. The results indicate that processing of APP into Aβ is dependent on neuronal activity and that formation of Aβ results in synaptic depression. Treatment of transduced slices with NBQX (an AMPA receptor antagonist) or d,l-AP5, an agent that blocks NMDA-Rs, prevented the synaptic depression caused by APP overexpression. Finally, Kamenetz et al. (2003) tested whether Aβ produced from overexpressing neurons can affect neighboring neurons in a cell nonautonomous manner. Here, synaptic function in two uninfected cells, one from a region containing many infected cells and another from a region with no infected cells, was examined. Uninfected neurons surrounded by APPSWE-infected neurons had significantly depressed transmission when compared to distant control neurons, suggesting that uninfected neurons in infected regions were responding to the local high concentrations of Aβ. These results are consistent with the notion that high Aβ levels may disrupt synaptic function and, more importantly, that Aβ may also have a normal negative feedback function: increased neuronal activity produces more Aβ; the enhanced Aβ production depresses synaptic function; the depressed synaptic function will decrease neuronal activity.
How do high levels of released Aβ mediate synaptic depression? To address this issue, Hsieh and colleagues (2006) examined the effects of increased Aβ levels on the structure of dendritic spines of CA1 pyramidal dendrites in organotypic slices that acutely overexpress APP. In this setting, spine density in APP-expressing cells was decreased compared to cells expressing the APP(MV) variant that undergoes much less processing by β-secretase. Similar to earlier studies showing that application of synthetic Aβ reduces the levels of surface NMDA and GluR1 receptors in dissociated cultured hippocampal neurons (Almeida et al. 2005; Snyder et al. 2005), Hsieh et al. (2006) confirmed that Aβ generated in situ also leads to decreases in surface and synaptic AMPA receptors on both spines and dendrites. Using an AMPA-R subunit tagged on its amino terminus with a pH-sensitive GFP variant, Hsieh revealed that APP overexpression led to a significant decrease of spine and dendritic surface AMPA receptors compared to cells expressing the APP(MV) variant.
Although these studies made clear that APP overexpression and resultant Aβ secretion can lead to synaptic dysfunction, the subcellular sites from which Aβ acts remained uncertain. To examine this issue, Wei and colleagues (2010) isolated the sites of increased Aβ production by selectively expressing APP in pre- or postsynaptic neurons. Using two-photon laser-scanning imaging to monitor the synaptic deficits caused by such dendritic or axonal Aβ, Wei and colleagues found that either dendritic or axonal Aβ overproduction was sufficient to cause local spine loss and compromise synaptic plasticity in the nearby dendrites of neurons that did not overexpress APP. The Aβ-mediated synaptic dysfunction could be pharmacologically ameliorated by blockade of either neural activity, NMDA receptors, or nicotinic acetylcholine receptors.
Extending these latter studies to an in vivo setting, Cirrito et al. (2005) asked whether synaptic activity influences the levels of Aβ in brain interstitial fluid (ISF). A microdialysis probe with concurrent hippocampal electrophysiological recording was used to assess whether there are dynamic changes in ISF Aβ levels in conjunction with differing levels of neuronal activity in awake, behaving mice. Here, a recording electrode was attached to a microdialysis probe to monitor electroencephalographic (EEG) activity (extracellular field potentials) and simultaneously collect ISF Aβ in the hippocampus of Tg2576 mice at 3–5 months of age. Neuronal and synaptic activity within the hippocampus was enhanced by electrically stimulating the perforant pathway, the major afferent projection from the entorhinal cortex to the hippocampus, conditions that created transient epileptiform discharges within the hippocampus noted on hippocampal EEGs. During these electrical seizures, ISF Aβ levels increased, showing a direct relationship between increased neuronal activity and increases in ISF Aβ in vivo. To determine if endogenous neural activity of the perforant pathway modulates ISF Aβ levels within the hippocampus, a selective metabotropic glutamate receptor 2/3 agonist, LY354740, was infused into the hippocampus via reverse microdialysis, and ISF Aβ levels were monitored. This treatment caused a decrease in ISF Aβ within the hippocampus, consistent with the hypothesis that modulation of endogenous neuronal activity in this pathway can alter hippocampal Aβ production. Indeed, strong depression of local activity using the sodium channel blocker TTX revealed that a decline in EEG amplitude was paralleled by a concomitant decrease in Aβ levels. Taken together with the studies of Kamenetz and colleagues, these studies by Cirrito et al. establish that neuronal or synaptic activity can directly influence Aβ levels.
The question remained as to the mechanism by which increased synaptic activity elevates Aβ secretion. One possibility is that the half-life of extracellular Aβ is extended; alternatively, this effect may involve increased APP processing. Using a γ-secretase inhibitor in TTX or vehicle-treated mice, Cirrito et al. (2005) showed that Aβ half-life was unaltered by the depression of neuronal activity. Additionally, expression of the Aβ-degrading enzyme neprilysin was unchanged in TTX-treated mice.
In further attempts to delineate the mechanism(s) responsible for activity-dependent elevations of ISF Aβ, Cirritto and colleagues (2008) hypothesized that Aβ released into the brain ISF following synaptic transmission requires an intermediate event involving APP endocytosis, analogous to that shown by Koo and Squazzo (1994) in cultured immortalized mammalian cells. To assess the influence of endocytosis on ISF Aβ levels under normal conditions, a myristylated, cell-permeable peptide (dynamin-DN) which inhibits clathrin-mediated endocytosis was infused into hippocampus by reverse microdialysis. As an in vivo control, the dynamin-DN reduced biotinylated transferrin uptake into cells within the dentate gyrus. Importantly, ISF Aβ levels were significantly decreased. Because inhibition of synaptic activity (Cirrito et al. 2005) and inhibition of endocytosis (Cirrito et al. 2008) each reduce ISF Aβ levels, there remained the question of the extent as to which (or both) contributes to ISF Aβ levels. Cirritto et al (2008) co-administered TTX and dynamin-DN. Administration of dynamin-DN first led to a decrease in ISF Aβ levels by 70%, but if the microdialysis perfusion buffer was then switched to contain dynamin-DN and TTX, no additional change in ISF Aβ levels were observed. These findings suggested that all of the ISF Aβ that is produced by synaptic activity requires endocytosis. On the other hand, if animals were pretreated with TTX followed by dynamin-DN, ISF Aβ levels were further reduced when endocytosis was inhibited, a finding that strongly suggested that a “releasable” stored pool of ISF Aβ that is generated via endocytosis is distinct from an Aβ pool that is acutely released by synaptic activity.
Although these studies using hippocampal slices and in vivo microdialysis provide compelling support for activity-dependent Aβ production, most such studies have been performed in acute settings. Studies by Tampellini and colleagues (2010) chronically reduced synaptic activity in vivo via unilateral ablation of whiskers or chronic diazepam treatment. For the whisker ablation experiments, bulbs were unilaterally removed in 2- to 3-month-old Tg19959 mice that express the FAD-linked Swedish (KM-NL) and London (V717F) APP mutations and develop plaques by age 3 months. At 6 months, cytochrome oxidase (COX) staining was reduced in the deafferented versus the synaptically active (control) barrel cortices, indicating that the lesion reduced synaptic activity. The barrel cortices with reduced activity showed a striking decrease in both the number of Aβ plaques and the area covered by plaques compared to the control side. Parallel studies in which the transgenic mice were chronically treated with diazepam for 1 month beginning at age 3 months also led to reduced plaque burden in cortex and hippocampus.
Despite evidence from these elegant in vitro, ex vivo and in vivo studies for an important role for synaptic activity in modulating Aβ production and subsequent synaptotoxicity, there remain gaps in our understanding of the underlying neurobiology. For example, does synaptic activity alter the trafficking of APP and/or the secretases to axonal and/or dendritic compartments? How does synaptic activity elevate the steady-state levels of βCTF, the precursor of Aβ? What are the pre- and postsynaptic receptor(s) involved in binding Aβ and/or its oligomeric forms (see Mucke and Selkoe 2011)? What are the signaling mechanisms by which activated postsynaptic Aβ receptor(s) lead to removal of AMPA and NMDA receptors and how do these changes alter structural plasticity? Finally, is there selectivity in specific neuronal circuits that underlies selectivity vulnerability of certain neuronal populations early in the disease process (i.e., preclinically)?
CONCLUSIONS
APP is the central protein involved in AD pathology as it serves as the precursor for Aβ generation. Intensive analysis of the cell biology of this protein and its proteolytic processing led not only to a detailed understanding of Aβ generation but also allowed the generation of therapeutically relevant secretase inhibitors. Moreover, understanding the biology of secretases allowed major progress in other fields of cell biological research. For example, research on secretases paved the road toward the understanding of RIP and the signaling pathways triggered by this process.
Footnotes
Editors: Dennis J. Selkoe, Eckhard Mandelkow, and David M. Holtzman
Additional Perspectives on The Biology of Alzheimer Disease available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Abad-Rodriguez J, Ledesma MD, Craessaerts K, Perga S, Medina M, Delacourte A, Dingwall C, De Strooper B, Dotti CG 2004. Neuronal membrane cholesterol loss enhances amyloid peptide generation. J Cell Biol 167: 953–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allinson TM, Parkin ET, Turner AJ, Hooper NM 2003. ADAMs family members as amyloid precursor protein α-secretases. J Neurosci Res 74: 342–352 [DOI] [PubMed] [Google Scholar]
- Almeida CG, Tampellini D, Takahashi RH, Greengard P, Lin MT, Snyder EM, Gouras GK 2005. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis 20: 187–198 [DOI] [PubMed] [Google Scholar]
- Andersen OM, Reiche J, Schmidt V, Gotthardt M, Spoelgen R, Behlke J, von Arnim CA, Breiderhoff T, Jansen P, Wu X, et al. 2005. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc Natl Acad Sci 102: 13461–13466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando K, Iijima KI, Elliott JI, Kirino Y, Suzuki T 2001. Phosphorylation-dependent regulation of the interaction of amyloid precursor protein with Fe65 affects the production of beta-amyloid. J Biol Chem 276: 40353–40361 [DOI] [PubMed] [Google Scholar]
- Annaert W, De Strooper B 1999. Presenilins: Molecular switches between proteolysis and signal transduction. Trends Neurosci 22: 439–443 [DOI] [PubMed] [Google Scholar]
- Back S, Haas P, Tschape JA, Gruebl T, Kirsch J, Muller U, Beyreuther K, Kins S 2007. Beta-amyloid precursor protein can be transported independent of any sorting signal to the axonal and dendritic compartment. J Neurosci Res 85: 2580–2590 [DOI] [PubMed] [Google Scholar]
- Basi G, Frigon N, Barbour R, Doan T, Gordon G, McConlogue L, Sinha S, Zeller M 2003. Antagonistic effects of beta-site amyloid precursor protein-cleaving enzymes 1 and 2 on beta-amyloid peptide production in cells. J Biol Chem 278: 31512–31520 [DOI] [PubMed] [Google Scholar]
- Beach TG, Kuo YM, Schwab C, Walker DG, Roher AE 2001. Reduction of cortical amyloid beta levels in guinea pig brain after systemic administration of physostigmine. Neurosci Lett 310: 21–24 [DOI] [PubMed] [Google Scholar]
- Beher D, Elle C, Underwood J, Davis JB, Ward R, Karran E, Masters CL, Beyreuther K, Multhaup G 1999. Proteolytic fragments of Alzheimer’s disease-associated presenilin 1 are present in synaptic organelles and growth cone membranes of rat brain. J Neurochem 72: 1564–1573 [DOI] [PubMed] [Google Scholar]
- Benjannet S, Elagoz A, Wickham L, Mamarbachi M, Munzer JS, Basak A, Lazure C, Cromlish JA, Sisodia S, Checler F, et al. 2001. Post-translational processing of beta-secretase (beta-amyloid-converting enzyme) and its ectodomain shedding. The pro- and transmembrane/cytosolic domains affect its cellular activity and amyloid-beta production. J Biol Chem 276: 10879–10887 [DOI] [PubMed] [Google Scholar]
- Bennett BD, Babu-Khan S, Loeloff R, Louis JC, Curran E, Citron M, Vassar R 2000a. Expression analysis of BACE2 in brain and peripheral tissues. J Biol Chem 275: 20647–20651 [DOI] [PubMed] [Google Scholar]
- Bennett BD, Denis P, Haniu M, Teplow DB, Kahn S, Louis JC, Citron M, Vassar R 2000b. A furin-like convertase mediates propeptide cleavage of BACE, the Alzheimer’s beta-secretase. J Biol Chem 275: 37712–37717 [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Nave KA 2008. Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation. Glia 56: 1491–1497 [DOI] [PubMed] [Google Scholar]
- Brown MS, Ye J, Rawson RB, Goldstein JL 2000. Regulated intramembrane proteolysis: A control mechanism conserved from bacteria to humans. Cell 100: 391–398 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, et al. 2005. Molecular, structural, and functional characterization of Alzheimer’s disease: Evidence for a relationship between default activity, amyloid, and memory. J Neurosci 25: 7709–7717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busciglio J, Gabuzda DH, Matsudaira P, Yankner BA 1993. Generation of beta-amyloid in the secretory pathway in neuronal and nonneuronal cells. Proc Natl Acad Sci 90: 2092–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum JD, Gandy SE, Cicchetti P, Ehrlich ME, Czernik AJ, Fracasso RP, Ramabhadran TV, Unterbeck AJ, Greengard P 1990. Processing of Alzheimer beta/A4 amyloid precursor protein: Modulation by agents that regulate protein phosphorylation. Proc Natl Acad Sci 87: 6003–6006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum JD, Thinakaran G, Koliatsos V, O’Callahan J, Slunt HH, Price DL, Sisodia SS 1998a. Alzheimer amyloid protein precursor in the rat hippocampus: Transport and processing through the perforant path. J Neurosci 18: 9629–9637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum JD, Liu KN, Luo Y, Slack JL, Stocking KL, Peschon JJ, Johnson RS, Castner BJ, Cerretti DP, Black RA 1998b. Evidence that tumor necrosis factor alpha converting enzyme is involved in regulated alpha-secretase cleavage of the Alzheimer amyloid protein precursor. J Biol Chem 273: 27765–27767 [DOI] [PubMed] [Google Scholar]
- Cai XD, Golde TE, Younkin SG 1993. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science 259: 514–516 [DOI] [PubMed] [Google Scholar]
- Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC 2001. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat Neurosci 4: 233–234 [DOI] [PubMed] [Google Scholar]
- Cam JA, Zerbinatti CV, Li Y, Bu G 2005. Rapid endocytosis of the LDL receptor-related protein modulates cell surface distribution and processing of the beta amyloid precursors protein. J Biol Chem 280: 15464–15470 [DOI] [PubMed] [Google Scholar]
- Cao X, Sudhof TC 2001. A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science 293: 115–120 [DOI] [PubMed] [Google Scholar]
- Capell A, Steiner H, Willem M, Kaiser H, Meyer C, Walter J, Lammich S, Multhaup G, Haass C 2000. Maturation and pro-peptide cleavage of beta-secretase. J Biol Chem 275: 30849–30854 [DOI] [PubMed] [Google Scholar]
- Capell A, Meyn L, Fluhrer R, Teplow DB, Walter J, Haass C 2002. Apical sorting of beta-secretase limits amyloid beta-peptide production. J Biol Chem 277: 5637–5643 [DOI] [PubMed] [Google Scholar]
- Carey RM, Balcz BA, Lopez-Coviella I, Slack BE 2005. Inhibition of dynamin-dependent endocytosis increases shedding of the amyloid precursor protein ectodomain and reduces generation of amyloid beta protein. BMC Cell Biol 6: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Crawford F, Houlden H, Warren A, Hughes D, Fidani L, Goate A, Rossor M, Roques P, Hardy J, et al. 1991. Early-onset Alzheimer’s disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene. Nature 353: 844–846 [DOI] [PubMed] [Google Scholar]
- Chavez-Gutierrez L, Tolia A, Maes E, Li T, Wong PC, de Strooper B 2008. Glu(332) in the Nicastrin ectodomain is essential for gamma-secretase complex maturation but not for its activity. J Biol Chem 283: 20096–20105 [DOI] [PubMed] [Google Scholar]
- Chyung JH, Selkoe DJ 2003. Inhibition of receptor-mediated endocytosis demonstrates generation of amyloid beta-protein at the cell surface. J Biol Chem 278: 51035–51043 [DOI] [PubMed] [Google Scholar]
- Chyung JH, Raper DM, Selkoe DJ 2005. Gamma-secretase exists on the plasma membrane as an intact complex that accepts substrates and effects intramembrane cleavage. J Biol Chem 280: 4383–4392 [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM 2005. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron 48: 913–922 [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM 2008. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron 58: 42–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron M 2004. Beta-secretase inhibition for the treatment of Alzheimer’s disease—promise and challenge. Trends Pharmacol Sci 25: 92–97 [DOI] [PubMed] [Google Scholar]
- Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ 1992. Mutation of the beta-amyloid precursor protein in familial Alzheimer’s disease increases beta-protein production. Nature 360: 672–674 [DOI] [PubMed] [Google Scholar]
- Cordy JM, Hussain I, Dingwall C, Hooper NM, Turner AJ 2003. Exclusively targeting beta-secretase to lipid rafts by GPI-anchor addition up-regulates beta-site processing of the amyloid precursor protein. Proc Natl Acad Sci 100: 11735–11740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creemers JW, Ines Dominguez D, Plets E, Serneels L, Taylor NA, Multhaup G, Craessaerts K, Annaert W, De Strooper B 2001. Processing of beta-secretase by furin and other members of the proprotein convertase family. J Biol Chem 276: 4211–4217 [DOI] [PubMed] [Google Scholar]
- Cupers P, Bentahir M, Craessaerts K, Orlans I, Vanderstichele H, Saftig P, De Strooper B, Annaert W 2001. The discrepancy between presenilin subcellular localization and gamma-secretase processing of amyloid precursor protein. J Cell Biol 154: 731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B, Craessaerts K, Dewachter I, Moechars D, Greenberg B, Van Leuven F, Van den Berghe H 1995a. Basolateral secretion of amyloid precursor protein in Madin–Darby canine kidney cells is disturbed by alterations of intracellular pH and by introducing a mutation associated with familial Alzheimer’s disease. J Biol Chem 270: 4058–4065 [DOI] [PubMed] [Google Scholar]
- De Strooper B, Craessaerts K, Van Leuven F, Van Den Berghe H 1995b. Exchanging the extracellular domain of amyloid precursor protein for horseradish peroxidase does not interfere with alpha-secretase cleavage of the beta-amyloid region, but randomizes secretion in Madin–Darby canine kidney cells. J Biol Chem 270: 30310–30314 [DOI] [PubMed] [Google Scholar]
- *.De Strooper B, Iwatsubo T, Wolfe MS 2011. Presenilins and γ-secretase: Structure, function, and role in Alzheimer disease. Cold Spring Harb Perspect Med 10.1101/cshperspect.a006304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos KJ, Grierson AJ, Ackerley S, Miller CC 2008. Role of axonal transport in neurodegenerative diseases. Annu Rev Neurosci 31: 151–173 [DOI] [PubMed] [Google Scholar]
- Dominguez D, Tournoy J, Hartmann D, Huth T, Cryns K, Deforce S, Serneels L, Camacho IE, Marjaux E, Craessaerts K, et al. 2005. Phenotypic and biochemical analyses of BACE1- and BACE2-deficient mice. J Biol Chem 280: 30797–30806 [DOI] [PubMed] [Google Scholar]
- Dries DR, Yu G 2008. Assembly, maturation, and trafficking of the gamma-secretase complex in Alzheimer’s disease. Curr Alzheimer Res 5: 132–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dries DR, Shah S, Han YH, Yu C, Yu S, Shearman MS, Yu G 2009. GLU333 of nicastrin directly participates in gamma-secretase activity. J Biol Chem 284: 29714–29724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyrks T, Weidemann A, Multhaup G, Salbaum JM, Lemaire HG, Kang J, Muller-Hill B, Masters CL, Beyreuther K 1988. Identification, transmembrane orientation and biogenesis of the amyloid A4 precursor of Alzheimer’s disease. EMBO J 7: 949–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edbauer D, Winkler E, Haass C, Steiner H 2002. Presenilin and nicastrin regulate each other and determine amyloid beta-peptide production via complex formation. Proc Natl Acad Sci 99: 8666–8671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C 2003. Reconstitution of gamma-secretase activity. Nat Cell Biol 5: 486–488 [DOI] [PubMed] [Google Scholar]
- Ehehalt R, Keller P, Haass C, Thiele C, Simons K 2003. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol 160: 113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch FS, Keim PS, Beattie EC, Blacher RW, Culwell AR, Oltersdorf T, McClure D, Ward PJ 1990. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science 248: 1122–1124 [DOI] [PubMed] [Google Scholar]
- Farzan M, Schnitzler CE, Vasilieva N, Leung D, Choe H 2000. BACE2, a beta-secretase homolog, cleaves at the beta site and within the amyloid-beta region of the amyloid-beta precursor protein. Proc Natl Acad Sci 97: 9712–9717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhrer R, Capell A, Westmeyer G, Willem M, Hartung B, Condron MM, Teplow DB, Haass C, Walter J 2002. A non-amyloidogenic function of BACE-2 in the secretory pathway. J Neurochem 81: 1011–1020 [DOI] [PubMed] [Google Scholar]
- Fluhrer R, Grammer G, Israel L, Condron MM, Haffner C, Friedmann E, Bohland C, Imhof A, Martoglio B, Teplow DB, et al. 2006. A gamma-secretase-like intramembrane cleavage of TNFalpha by the GxGD aspartyl protease SPPL2b. Nat Cell Biol 8: 894–896 [DOI] [PubMed] [Google Scholar]
- Fluhrer R, Fukumori A, Martin L, Grammer G, Haug-Kroper M, Klier B, Winkler E, Kremmer E, Condron MM, Teplow DB, et al. 2008. Intramembrane proteolysis of GXGD-type aspartyl proteases is slowed by a familial Alzheimer disease-like mutation. J Biol Chem 283: 30121–30128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhrer R, Steiner H, Haass C 2009. Intramembrane proteolysis by signal peptide peptidases: A comparative discussion of GXGD-type aspartyl proteases. J Biol Chem 284: 13975–13979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis R, McGrath G, Zhang J, Ruddy DA, Sym M, Apfeld J, Nicoll M, Maxwell M, Hai B, Ellis MC, et al. 2002. aph-1 and pen-2 are required for Notch pathway signaling, gamma-secretase cleavage of betaAPP, and presenilin protein accumulation. Dev Cell 3: 85–97 [DOI] [PubMed] [Google Scholar]
- Frykman S, Hur JY, Franberg J, Aoki M, Winblad B, Nahalkova J, Behbahani H, Tjernberg LO 2010. Synaptic and endosomal localization of active gamma-secretase in rat brain. PloS One 5: e8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumori A, Okochi M, Tagami S, Jiang J, Itoh N, Nakayama T, Yanagida K, Ishizuka-Katsura Y, Morihara T, Kamino K, et al. 2006. Presenilin-dependent gamma-secretase on plasma membrane and endosomes is functionally distinct. Biochemistry 45: 4907–4914 [DOI] [PubMed] [Google Scholar]
- Gandy S, Greengard P 1994. Regulated cleavage of the Alzheimer amyloid precursor protein: Molecular and cellular basis. Biochimie 76: 300–303 [DOI] [PubMed] [Google Scholar]
- Golde TE, Estus S, Younkin LH, Selkoe DJ, Younkin SG 1992. Processing of the amyloid protein precursor to potentially amyloidogenic derivatives. Science 255: 728–730 [DOI] [PubMed] [Google Scholar]
- Goldsbury C, Mocanu MM, Thies E, Kaether C, Haass C, Keller P, Biernat J, Mandelkow E, Mandelkow EM 2006. Inhibition of APP trafficking by tau protein does not increase the generation of amyloid-beta peptides. Traffic 7: 873–888 [DOI] [PubMed] [Google Scholar]
- Gouras GK, Relkin NR, Sweeney D, Munoz DG, Mackenzie IR, Gandy S 1997. Increased apolipoprotein E epsilon 4 in epilepsy with senile plaques. Ann Neurol 41: 402–404 [DOI] [PubMed] [Google Scholar]
- Grimm HS, Beher D, Lichtenthaler SF, Shearman MS, Beyreuther K, Hartmann T 2003. Gamma-secretase cleavage site specificity differs for intracellular and secretory amyloid beta. J Biol Chem 278: 13077–13085 [DOI] [PubMed] [Google Scholar]
- Gu Y, Misonou H, Sato T, Dohmae N, Takio K, Ihara Y 2001. Distinct intramembrane cleavage of the beta-amyloid precursor protein family resembling gamma-secretase-like cleavage of Notch. J Biol Chem 276: 35235–35238 [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME, Raichle ME 2001. Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci 2: 685–694 [DOI] [PubMed] [Google Scholar]
- Haass C 2004. Take five—BACE and the gamma-secretase quartet conduct Alzheimer’s amyloid beta-peptide generation. EMBO J 23: 483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ 1993. Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell 75: 1039–1042 [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ 2007. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol 8: 101–112 [DOI] [PubMed] [Google Scholar]
- Haass C, Koo EH, Mellon A, Hung AY, Selkoe DJ 1992a. Targeting of cell-surface beta-amyloid precursor protein to lysosomes: Alternative processing into amyloid-bearing fragments. Nature 357: 500–503 [DOI] [PubMed] [Google Scholar]
- Haass C, Scholssmacher M, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski B, Liederburg I, Koo F, Schenk D, Teplow D, et al. 1992b. Amyloid β-peptide is produced by cultured cells during normal metabolism. Nature 359: 322–325 [DOI] [PubMed] [Google Scholar]
- Haass C, Hung AY, Schlossmacher MG, Oltersdorf T, Teplow DB, Selkoe DJ 1993a. Normal cellular processing of the beta-amyloid precursor protein results in the secretion of the amyloid beta peptide and related molecules. Ann NY Acad Sci 695: 109–116 [DOI] [PubMed] [Google Scholar]
- Haass C, Hung AY, Schlossmacher MG, Teplow DB, Selkoe DJ 1993b. Beta-amyloid peptide and a 3-kDa fragment are derived by distinct cellular mechanisms. J Biol Chem 268: 3021–3024 [PubMed] [Google Scholar]
- Haass C, Koo EH, Teplow DB, Selkoe DJ 1994. Polarized secretion of beta-amyloid precursor protein and amyloid beta-peptide in MDCK cells. Proc Natl Acad Sci 91: 1564–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Koo EH, Capell A, Teplow DB, Selkoe DJ 1995a. Polarized sorting of beta-amyloid precursor protein and its proteolytic products in MDCK cells is regulated by two independent signals. J Cell Biol 128: 537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Lemere CA, Capell A, Citron M, Seubert P, Schenk D, Lannfelt L, Selkoe DJ 1995b. The Swedish mutation causes early-onset Alzheimer’s disease by beta-secretase cleavage within the secretory pathway. Nat Med 1: 1291–1296 [DOI] [PubMed] [Google Scholar]
- Harris JA, Devidze N, Halabisky B, Lo I, Thwin MT, Yu GQ, Bredesen DE, Masliah E, Mucke L 2010. Many neuronal and behavioral impairments in transgenic mouse models of Alzheimer’s disease are independent of caspase cleavage of the amyloid precursor protein. J Neurosci 30: 372–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SM, Harper AJ, Hawkins J, Duddy G, Grau E, Pugh PL, Winter PH, Shilliam CS, Hughes ZA, Dawson LA, et al. 2003. BACE1 (beta-secretase) transgenic and knockout mice: Identification of neurochemical deficits and behavioral changes. Mol Cell Neurosci 24: 646–655 [DOI] [PubMed] [Google Scholar]
- Hartmann T, Bergsdorf C, Sandbrink R, Tienari PJ, Multhaup G, Ida N, Bieger S, Dyrks T, Weidemann A, Masters CL, et al. 1996. Alzheimer’s disease betaA4 protein release and amyloid precursor protein sorting are regulated by alternative splicing. J Biol Chem 271: 13208–13214 [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Sanjo N, Chen F, Gu YJ, Shier C, Petit A, Kawarai T, Katayama T, Schmidt SD, Mathews PM, et al. 2004. Both the sequence and length of the C terminus of PEN-2 are critical for intermolecular interactions and function of presenilin complexes. J Biol Chem 279: 46455–46463 [DOI] [PubMed] [Google Scholar]
- Hartmann D, de Strooper B, Serneels L, Craessaerts K, Herreman A, Annaert W, Umans L, Lubke T, Lena Illert A, von Figura K, et al. 2002. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alpha-secretase activity in fibroblasts. Hum Mol Genet 11: 2615–2624 [DOI] [PubMed] [Google Scholar]
- He X, Li F, Chang WP, Tang J 2005. GGA proteins mediate the recycling pathway of memapsin 2 (BACE). J Biol Chem 280: 11696–11703 [DOI] [PubMed] [Google Scholar]
- Hill K, Li Y, Bennett M, McKay M, Zhu X, Shern J, Torre E, Lah JJ, Levey AI, Kahn RA 2003. Munc18 interacting proteins: ADP-ribosylation factor-dependent coat proteins that regulate the traffic of beta-Alzheimer's precursor protein. J Biol Chem 278: 36032–36040 [DOI] [PubMed] [Google Scholar]
- Hock C, Maddalena A, Raschig A, Muller-Spahn F, Eschweiler G, Hager K, Heuser I, Hampel H, Muller-Thomsen T, Oertel W, et al. 2003. Treatment with the selective muscarinic m1 agonist talsaclidine decreases cerebrospinal fluid levels of A beta 42 in patients with Alzheimer’s disease. Amyloid 10: 1–6 [DOI] [PubMed] [Google Scholar]
- Hong L, Koelsch G, Lin X, Wu S, Terzyan S, Ghosh AK, Zhang XC, Tang J 2000. Structure of the protease domain of memapsin 2 (beta-secretase) complexed with inhibitor. Science 290: 150–153 [DOI] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R 2006. AMPA-R removal underlies Aβ-induced synaptic depression and dendritic spine loss. Neuron 52: 831–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Hicks CW, He W, Wong P, Macklin WB, Trapp BD, Yan R 2006. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci 9: 1520–1525 [DOI] [PubMed] [Google Scholar]
- Huse JT, Pijak DS, Leslie GJ, Lee VM, Doms RW 2000. Maturation and endosomal targeting of beta-site amyloid precursor protein-cleaving enzyme. The Alzheimer’s disease beta-secretase. J Biol Chem 275: 33729–33737 [DOI] [PubMed] [Google Scholar]
- Hussain I, Powell DJ, Howlett DR, Chapman GA, Gilmour L, Murdock PR, Tew DG, Meek TD, Chapman C, Schneider K, et al. 2000. ASP1 (BACE2) cleaves the amyloid precursor protein at the beta-secretase site. Mol Cell Neurosci 16: 609–619 [DOI] [PubMed] [Google Scholar]
- Inoue E, Deguchi-Tawarada M, Togawa A, Matsui C, Arita K, Katahira-Tayama S, Sato T, Yamauchi E, Oda Y, Takai Y 2009. Synaptic activity prompts gamma-secretase-mediated cleavage of EphA4 and dendritic spine formation. J Cell Biol 185: 551–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaether C, Skehel P, Dotti CG 2000. Axonal membrane proteins are transported in distinct carriers: A two-color video microscopy study in cultured hippocampal neurons. Mol Biol Cell 11: 1213–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaether C, Lammich S, Edbauer D, Ertl M, Rietdorf J, Capell A, Steiner H, Haass C 2002. Presenilin-1 affects trafficking and processing of betaAPP and is targeted in a complex with nicastrin to the plasma membrane. J Cell Biol 158: 551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaether C, Haass C, Steiner H 2006a. Assembly, trafficking and function of gamma-secretase. Neurodegener Dis 3: 275–283 [DOI] [PubMed] [Google Scholar]
- Kaether C, Schmitt S, Willem M, Haass C 2006b. Amyloid precursor protein and notch intracellular domains are generated after transport of their precursors to the cell surface. Traffic 7: 408–415 [DOI] [PubMed] [Google Scholar]
- Kamal A, Almenar-Queralt A, LeBlanc JF, Roberts EA, Goldstein LS 2001. Kinesin-mediated axonal transport of a membrane compartment containing beta-secretase and presenilin-1 requires APP. Nature 414: 643–648 [DOI] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Seabrook G, Borchelt D, Iwatsubo T, Sisodia SS, Malinow R 2003. APP processing and synaptic function. Neuron 37: 925–937 [DOI] [PubMed] [Google Scholar]
- Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Muller-Hill B 1987. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature 325: 733–736 [DOI] [PubMed] [Google Scholar]
- Kim DY, Carey BW, Wang H, Ingano LA, Binshtok AM, Wertz MH, Pettingell WH, He P, Lee VM, Woolf CJ, et al. 2007. BACE1 regulates voltage-gated sodium channels and neuronal activity. Nat Cell Biol 9: 755–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kins S, Lauther N, Szodorai A, Beyreuther K 2006. Subcellular trafficking of the amyloid precursor protein gene family and its pathogenic role in Alzheimer’s disease. Neurodegener Dis 3: 218–226 [DOI] [PubMed] [Google Scholar]
- Kojro E, Gimpl G, Lammich S, Marz W, Fahrenholz F 2001. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the alpha-secretase ADAM 10. Proc Natl Acad Sci 98: 5815–5820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo EH, Squazzo SL 1994. Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem 269: 17386–17389 [PubMed] [Google Scholar]
- Koo EH, Sisodia SS, Archer DR, Martin LJ, Weidemann A, Beyreuther K, Fischer P, Masters CL, Price DL 1990. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci 87: 1561–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn PH, Wang H, Dislich B, Colombo A, Zeitschel U, Ellwart JW, Kremmer E, Rossner S, Lichtenthaler SF 2010. ADAM10 is the physiologically relevant, constitutive alpha-secretase of the amyloid precursor protein in primary neurons. EMBO J 29: 3020–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai A, Sisodia SS, Trowbridge IS 1995. Characterization of sorting signals in the beta-amyloid precursor protein cytoplasmic domain. J Biol Chem 270: 3565–3573 [PubMed] [Google Scholar]
- Lakshmana MK, Yoon IS, Chen E, Bianchi E, Koo EH, Kang DE 2009. Novel role of RanBP9 in BACE1 processing of amyloid precursor protein and amyloid beta peptide generation. J Biol Chem 284: 11863–11872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVoie MJ, Fraering PC, Ostaszewski BL, Ye W, Kimberly WT, Wolfe MS, Selkoe DJ 2003. Assembly of the gamma-secretase complex involves early formation of an intermediate subcomplex of Aph-1 and nicastrin. J Biol Chem 278: 37213–37222 [DOI] [PubMed] [Google Scholar]
- Lazarov O, Lee M, Peterson DA, Sisodia SS 2002. Evidence that synaptically released beta-amyloid accumulates as extracellular deposits in the hippocampus of transgenic mice. J Neurosci 22: 9785–9793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O, Morfini GA, Lee EB, Farah MH, Szodorai A, DeBoer SR, Koliatsos VE, Kins S, Lee VM, Wong PC, et al. 2005. Axonal transport, amyloid precursor protein, kinesin-1, and the processing apparatus: Revisited. J Neurosci 25: 2386–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Kao SC, Lemere CA, Xia W, Tseng HC, Zhou Y, Neve R, Ahlijanian MK, Tsai LH 2003. APP processing is regulated by cytoplasmic phosphorylation. J Cell Biol 163: 83–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesne S, Ali C, Gabriel C, Croci N, MacKenzie ET, Glabe CG, Plotkine M, Marchand-Verrecchia C, Vivien D, Buisson A 2005. NMDA receptor activation inhibits alpha-secretase and promotes neuronal amyloid-beta production. J Neurosci 25: 9367–9377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy E, Carman MD, Fernandez-Madrid IJ, Power MD, Lieberburg I, van Duinen SG, Bots GT, Luyendijk W, Frangione B 1990. Mutation of the Alzheimer’s disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science 248: 1124–1126 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler SF, Haass C, Steiner H 2011. Regulated intramembrane proteolysis—lessons from amyloid precursor protein processing. J Neurochem 117: 779–796 [DOI] [PubMed] [Google Scholar]
- Lin X, Koelsch G, Wu S, Downs D, Dashti A, Tang J 2000. Human aspartic protease memapsin 2 cleaves the beta-secretase site of beta-amyloid precursor protein. Proc Natl Acad Sci 97: 1456–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo AC, Haass C, Wagner SL, Teplow DB, Sisodia SS 1994. Metabolism of the “Swedish” amyloid precursor protein variant in Madin–Darby canine kidney cells. J Biol Chem 269: 30966–30973 [PubMed] [Google Scholar]
- Lu DC, Soriano S, Bredesen DE, Koo EH 2003. Caspase cleavage of the amyloid precursor protein modulates amyloid beta-protein toxicity. J Neurochem 87: 733–741 [DOI] [PubMed] [Google Scholar]
- Luo Y, Bolon B, Damore MA, Fitzpatrick D, Liu H, Zhang J, Yan Q, Vassar R, Citron M 2003. BACE1 (beta-secretase) knockout mice do not acquire compensatory gene expression changes or develop neural lesions over time. Neurobiol Dis 14: 81–88 [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Miller LA 1994. Senile plaques in temporal lobe epilepsy. Acta Neuropathol 87: 504–510 [DOI] [PubMed] [Google Scholar]
- Maltese WA, Wilson S, Tan Y, Suomensaari S, Sinha S, Barbour R, McConlogue L 2001. Retention of the Alzheimer’s amyloid precursor fragment C99 in the endoplasmic reticulum prevents formation of amyloid beta-peptide. J Biol Chem 276: 20267–20279 [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz M, Seidah NG 2000. Coordinated expression of beta-amyloid precursor protein and the putative beta-secretase BACE and alpha-secretase ADAM10 in mouse and human brain. J Neurochem 75: 2133–2143 [DOI] [PubMed] [Google Scholar]
- Marquez-Sterling NR, Lo ACY, Sisodia SS, Koo EH 1997. Trafficking of cell-surface beta-amyloid precursor protein: Evidence that a sorting intermediate participates in synaptic vesicle recycling. J Neurosci 17: 140–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L, Fluhrer R, Haass C 2009. Substrate requirements for SPPL2b-dependent regulated intramembrane proteolysis. J Biol Chem 284: 5662–5670 [DOI] [PubMed] [Google Scholar]
- Matthews V, Schuster B, Schutze S, Bussmeyer I, Ludwig A, Hundhausen C, Sadowski T, Saftig P, Hartmann D, Kallen KJ, et al. 2003. Cellular cholesterol depletion triggers shedding of the human interleukin-6 receptor by ADAM10 and ADAM17 (TACE). J Biol Chem 278: 38829–38839 [DOI] [PubMed] [Google Scholar]
- Merlos-Suarez A, Fernandez-Larrea J, Reddy P, Baselga J, Arribas J 1998. Pro-tumor necrosis factor-alpha processing activity is tightly controlled by a component that does not affect notch processing. J Biol Chem 273: 24955–24962 [DOI] [PubMed] [Google Scholar]
- Miller CC, McLoughlin DM, Lau KF, Tennant ME, Rogelj B 2006. The X11 proteins, Abeta production and Alzheimer's disease. Trends Neurosci 29: 280–285 [DOI] [PubMed] [Google Scholar]
- Morfini GA, Burns M, Binder LI, Kanaan NM, LaPointe N, Bosco DA, Brown RH Jr, Brown H, Tiwari A, Hayward L, et al. 2009. Axonal transport defects in neurodegenerative diseases. J Neurosci 29: 12776–12786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Mucke L, Selkoe DJ 2011. Neurotoxicity of amyloid β-protein: Synaptic and network dysfunction. Cold Harbor Perspect Med 10.1101/cshperspect.a006338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullan M, Crawford F, Axelman K, Houlden H, Lilius L, Winblad B, Lannfelt L 1992. A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet 1: 345–347 [DOI] [PubMed] [Google Scholar]
- Nilsberth C, Westlind-Danielsson A, Eckman CB, Condron MM, Axelman K, Forsell C, Stenh C, Luthman J, Teplow DB, Younkin SG, et al. 2001. The “Arctic” APP mutation (E693G) causes Alzheimer’s disease by enhanced A-beta protofibril formation. Nat Neurosci 4: 887–893 [DOI] [PubMed] [Google Scholar]
- Nitsch RM, Slack BE, Wurtman RJ, Growdon JH 1992. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science 258: 304–307 [DOI] [PubMed] [Google Scholar]
- Nitsch RM, Farber SA, Growdon JH, Wurtman RJ 1993. Release of amyloid beta-protein precursor derivatives by electrical depolarization of rat hippocampal slices. Proc Natl Acad Sci 90: 5191–5193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA 2007. Autophagy, amyloidogenesis and Alzheimer disease. J Cell Sci 120: 4081–4091 [DOI] [PubMed] [Google Scholar]
- Offe K, Dodson SE, Shoemaker JT, Fritz JJ, Gearing M, Levey AI, Lah JJ 2006. The lipoprotein receptor LR11 regulates amyloid beta production and amyloid precursor protein traffic in endosomal compartments. J Neurosci 26: 1596–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak SH, Callahan JW, Mahuran DJ 2004. The role of the endosomal/lysosomal system in amyloid-beta production and the pathophysiology of Alzheimer’s disease: Reexamining the spatial paradox from a lysosomal perspective. J Alzheimers Dis 6: 53–65 [DOI] [PubMed] [Google Scholar]
- Pastorino L, Ikin AF, Nairn AC, Pursnani A, Buxbaum JD 2002. The carboxyl-terminus of BACE contains a sorting signal that regulates BACE trafficking but not the formation of total A(beta). Mol Cell Neurosci 19: 175–185 [DOI] [PubMed] [Google Scholar]
- Perez RG, Soriano S, Hayes JD, Ostaszewski B, Xia W, Selkoe DJ, Chen X, Stokin GB, Koo EH 1999. Mutagenesis identifies new signals for beta-amyloid precursor protein endocytosis, turnover, and the generation of secreted fragments, including Abeta42. J Biol Chem 274: 18851–18856 [DOI] [PubMed] [Google Scholar]
- Pietrzik CU, Busse T, Merriam DE, Weggen S, Koo EH 2002. The cytoplasmic domain of the LDL receptor-related protein regulates multiple steps in APP processing. EMBO J 21: 5691–5700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzik CU, Yoon IS, Jaeger S, Busse T, Weggen S, Koo EH 2004. FE65 constitutes the functional link between the low-density lipoprotein receptor-related protein and the amyloid precursor protein. J Neurosci 24: 4259–4265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postina R, Schroeder A, Dewachter I, Bohl J, Schmitt U, Kojro E, Prinzen C, Endres K, Hiemke C, Blessing M, et al. 2004. A disintegrin-metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. J Clin Invest 113: 1456–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop S, Shirotani K, Edbauer D, Haass C, Steiner H 2004. Requirement of PEN-2 for stabilization of the presenilin N-/C-terminal fragment heterodimer within the gamma-secretase complex. J Biol Chem 279: 23255–23261 [DOI] [PubMed] [Google Scholar]
- Qi-Takahara Y, Morishima-Kawashima M, Tanimura Y, Dolios G, Hirotani N, Horikoshi Y, Kametani F, Maeda M, Saido TC, Wang R, et al. 2005. Longer forms of amyloid beta protein: Implications for the mechanism of intramembrane cleavage by gamma-secretase. J Neurosci 25: 436–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL 2001. A default mode of brain function. Proc Natl Acad Sci 98: 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran L, Schneider A, Schlechtingen G, Weidlich S, Ries J, Braxmeier T, Schwille P, Schulz JB, Schroeder C, Simons M, et al. 2008. Efficient inhibition of the Alzheimer’s disease beta-secretase by membrane targeting. Science 320: 520–523 [DOI] [PubMed] [Google Scholar]
- Rawson RB 2002. Regulated intramembrane proteolysis: From the endoplasmic reticulum to the nucleus. Essays Biochem 38: 155–168 [DOI] [PubMed] [Google Scholar]
- Rechards M, Xia W, Oorschot VM, Selkoe DJ, Klumperman J 2003. Presenilin-1 exists in both pre- and post-Golgi compartments and recycles via COPI-coated membranes. Traffic 4: 553–565 [DOI] [PubMed] [Google Scholar]
- Ribaut-Barassin C, Dupont JL, Haeberle AM, Bombarde G, Huber G, Moussaoui S, Mariani J, Bailly Y 2003. Alzheimer’s disease proteins in cerebellar and hippocampal synapses during postnatal development and aging of the rat. Neuroscience 120: 405–423 [DOI] [PubMed] [Google Scholar]
- Riddell DR, Christie G, Hussain I, Dingwall C 2001. Compartmentalization of beta-secretase (Asp2) into low-buoyant density, noncaveolar lipid rafts. Curr Biol 11: 1288–1293 [DOI] [PubMed] [Google Scholar]
- Roberds SL, Anderson J, Basi G, Bienkowski MJ, Branstetter DG, Chen KS, Freedman SB, Frigon NL, Games D, Hu K, et al. 2001. BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: Implications for Alzheimer’s disease therapeutics. Hum Mol Genet 10: 1317–1324 [DOI] [PubMed] [Google Scholar]
- Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, Katayama T, Baldwin CT, Cheng R, Hasegawa H, et al. 2007. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nature Genet 39: 168–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano Y, Nakaya T, Pedrini S, Takeda S, Iijima-Ando K, Iijima K, Mathews PM, Itohara S, Gandy S, Suzuki T 2006. Physiological mouse brain Abeta levels are not related to the phosphorylation state of threonine-668 of Alzheimer's APP. PLoS One 1: e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre M, Steiner H, Fuchs K, Capell A, Multhaup G, Condron MM, Teplow DB, Haass C 2001. Presenilin-dependent gamma-secretase processing of beta-amyloid precursor protein at a site corresponding to the S3 cleavage of Notch. EMBO Rep 2: 835–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Schenk D, Basi GS, Pangalos MN 2011. Treatment strategies targeting amyloid beta-protein. Cold Spring Harb Perspect Med 10.1101/cshperspect.a006387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt V, Sporbert A, Rohe M, Reimer T, Rehm A, Andersen OM, Willnow TE 2007. SorLA/LR11 regulates processing of amyloid precursor protein via interaction with adaptors GGA and PACS-1. J Biol Chem 282: 32956–32964 [DOI] [PubMed] [Google Scholar]
- Selkoe DJ 2001. Alzheimer’s disease: Genes, proteins, and therapy. Physiol Rev 81: 741–766 [DOI] [PubMed] [Google Scholar]
- Selkoe D, Kopan R 2003. Notch and presenilin: Regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci 26: 565–597 [DOI] [PubMed] [Google Scholar]
- Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C, et al. 1992. Isolation and quantification of soluble Alzheimer’s beta-peptide from biological fluids. Nature 359: 325–327 [DOI] [PubMed] [Google Scholar]
- Shah S, Lee SF, Tabuchi K, Hao YH, Yu C, LaPlant Q, Ball H, Dann CE 3rd, Sudhof T, Yu G 2005. Nicastrin functions as a gamma-secretase-substrate receptor. Cell 122: 435–447 [DOI] [PubMed] [Google Scholar]
- Sheng JG, Price DL, Koliatsos VE 2002. Disruption of corticocortical connections ameliorates amyloid burden in terminal fields in a transgenic model of Abeta amyloidosis. J Neurosci 22: 9794–9799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji M, Golde TE, Ghiso J, Cheung TT, Estus S, Shaffer LM, Cai XD, McKay DM, Tintner R, Frangione B, et al. 1992. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science 258: 126–129 [DOI] [PubMed] [Google Scholar]
- Simons M, Ikonen E, Tienari PJ, Cid AA, Monning U, Beyreuther K, Dotti CG 1995. Intracellular routing of human amyloid protein precursor: Axonal delivery followed by transport to the dendrites. J Neurosci Res 41: 121–128 [DOI] [PubMed] [Google Scholar]
- Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Davis D, Doan M, Dovey HF, Frigon N, Hong J, et al. 1999. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature 402: 537–540 [DOI] [PubMed] [Google Scholar]
- Sisodia SS 1992. Beta-amyloid precursor protein cleavage by a membrane-bound protease. Proc Natl Acad Sci 89: 6075–6079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisodia SS, Koo EH, Beyreuther K, Unterbeck A, Price DL 1990. Evidence that β-amyloid protein in Alzheimer's disease is not derived by normal processing. Science 248: 492–495 [DOI] [PubMed] [Google Scholar]
- Sisodia SS, Koo EH, Hoffman PN, Perry G, Price DL 1993. Identification and transport of full-length amyloid precursor proteins in rat peripheral nervous system. J Neurosci 13: 3136–3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P 2005. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci 8: 1051–1058 [DOI] [PubMed] [Google Scholar]
- Stamer K, Vogel R, Thies E, Mandelkow E, Mandelkow EM 2002. Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. J Cell Biol 156: 1051–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Fluhrer R, Haass C 2008. Intramembrane proteolysis by gamma-secretase. J Biol Chem 283: 29627–29631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Cheung TT, Cai XD, Odaka A, Otvos L Jr, Eckman C, Golde TE, Younkin SG 1994. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science 264: 1336–1340 [DOI] [PubMed] [Google Scholar]
- Szodorai A, Kuan YH, Hunzelmann S, Engel U, Sakane A, Sasaki T, Takai Y, Kirsch J, Muller U, Beyreuther K, et al. 2009. APP anterograde transport requires Rab3A GTPase activity for assembly of the transport vesicle. J Neurosci 29: 14534–14544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takami M, Nagashima Y, Sano Y, Ishihara S, Morishima-Kawashima M, Funamoto S, Ihara Y 2009. Gamma-secretase: Successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment. J Neurosci 29: 13042–13052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampellini D, Capetillo-Zarate E, Dumont M, Huang Z, Yu F, Lin MT, Gouras GK 2010. Effects of synaptic modulation on beta-amyloid, synaptophysin, and memory performance in Alzheimer’s disease transgenic mice. J Neurosci 30: 14299–14304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarassishin L, Yin YI, Bassit B, Li YM 2004. Processing of Notch and amyloid precursor protein by gamma-secretase is spatially distinct. Proc Natl Acad Sci 101: 17050–17055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Bassit B, Chau D, Li YM 2010. An APP inhibitory domain containing the Flemish mutation residue modulates gamma-secretase activity for Abeta production. Nat Struct Mol Biol 17: 151–158 [DOI] [PubMed] [Google Scholar]
- Tienari PJ, De SB, Ikonen E, Simons M, Weidemann A, Czech C, Hartmann T, Ida N, Multhaup G, Masters CL, et al. 1996. The beta-amyloid domain is essential for axonal sorting of amyloid precursor protein. Embo J 15: 5218–5229 [PMC free article] [PubMed] [Google Scholar]
- Trommsdorff M, Borg JP, Margolis B, Herz J 1998. Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J Biol Chem 273: 33556–33560 [DOI] [PubMed] [Google Scholar]
- Ulery PG, Beers J, Mikhailenko I, Tanzi RE, Rebeck GW, Hyman BT, Strickland DK 2000. Modulation of beta-amyloid precursor protein processing by the low density lipoprotein receptor-related protein (LRP). Evidence that LRP contributes to the pathogenesis of Alzheimer's disease. J Biol Chem 275: 7410–7415 [DOI] [PubMed] [Google Scholar]
- Vassar R 2004. BACE1: The beta-secretase enzyme in Alzheimer’s disease. J Mol Neurosci 23: 105–114 [DOI] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, et al. 1999. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286: 735–741 [DOI] [PubMed] [Google Scholar]
- Vetrivel KS, Meckler X, Chen Y, Nguyen PD, Seidah NG, Vassar R, Wong PC, Fukata M, Kounnas MZ, Thinakaran G 2009. Alzheimer disease Abeta production in the absence of S-palmitoylation-dependent targeting of BACE1 to lipid rafts. J Biol Chem 284: 3793–3803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Rotz RC, Kohli BM, Bosset J, Meier M, Suzuki T, Nitsch RM, Konietzko U 2004. The APP intracellular domain forms nuclear multiprotein complexes and regulates the transcription of its own precursor. J Cell Sci 117: 4435–4448 [DOI] [PubMed] [Google Scholar]
- Wahle T, Prager K, Raffler N, Haass C, Famulok M, Walter J 2005. GGA proteins regulate retrograde transport of BACE1 from endosomes to the trans-Golgi network. Mol Cell Neurosci 29: 453–461 [DOI] [PubMed] [Google Scholar]
- Wahle T, Thal DR, Sastre M, Rentmeister A, Bogdanovic N, Famulok M, Heneka MT, Walter J 2006. GGA1 is expressed in the human brain and affects the generation of amyloid beta-peptide. J Neurosci 26: 12838–12846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J, Fluhrer R, Hartung B, Willem M, Kaether C, Capell A, Lammich S, Multhaup G, Haass C 2001. Phosphorylation regulates intracellular trafficking of beta-secretase. J Biol Chem 276: 14634–14641 [DOI] [PubMed] [Google Scholar]
- Wang R, Meschia JF, Cotter RJ, Sisodia SS 1991. Secretion of the β/A4 amyloid precursor protein. Identification of a cleavage site in cultured mammalian cells. J Biol Chem 266: 16960–16964 [PubMed] [Google Scholar]
- Wei W, Nguyen LN, Kessels HW, Hagiwara H, Sisodia S, Malinow R 2010. Amyloid beta from axons and dendrites reduces local spine number and plasticity. Nat Neurosci 13: 190–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann A, Konig G, Bunke D, Fischer P, Salbaum JM, Masters CL, Beyreuther K 1989. Identification, biogenesis, and localization of precursors of Alzheimer’s disease A4 amyloid protein. Cell 57: 115–126 [DOI] [PubMed] [Google Scholar]
- Weidemann A, Paliga K, U, D., Reinhard FB, Schuckert O, Evin G, Masters CL 1999. Proteolytic processing of the Alzheimer’s disease amyloid precursor protein within its cytoplasmic domain by caspase-like proteases. J Biol Chem 274: 5823–5829 [DOI] [PubMed] [Google Scholar]
- Weidemann A, Eggert S, Reinhard FB, Vogel M, Paliga K, Baier G, Masters CL, Beyreuther K, Evin G 2002. A novel epsilon-cleavage within the transmembrane domain of the Alzheimer amyloid precursor protein demonstrates homology with Notch processing. Biochemistry 41: 2825–2835 [DOI] [PubMed] [Google Scholar]
- Weskamp G, Cai H, Brodie TA, Higashyama S, Manova K, Ludwig T, Blobel CP 2002. Mice lacking the metalloprotease-disintegrin MDC9 (ADAM9) have no evident major abnormalities during development or adult life. Mol Cell Biol 22: 1537–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild-Bode C, Fellerer K, Kugler J, Haass C, Capell A 2006. A basolateral sorting signal directs ADAM10 to adherens junctions and is required for its function in cell migration. J Biol Chem 281: 23824–23829 [DOI] [PubMed] [Google Scholar]
- Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, Rittger A, DeStrooper B, Saftig P, Birchmeier C, Haass C 2006. Control of peripheral nerve myelination by the beta-secretase BACE1. Science 314: 664–666 [DOI] [PubMed] [Google Scholar]
- Willem M, Lammich S, Haass C 2009. Function, regulation and therapeutic properties of beta-secretase (BACE1). Semin Cell Dev Biol 20: 175–182 [DOI] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ 1999. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature 398: 513–517 [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Selkoe DJ, Koo EH 1995. Trafficking of cell surface beta-amyloid precursor protein: Retrograde and transcytotic transport in cultured neurons. J Cell Biol 129: 431–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Bienkowski MJ, Shuck ME, Miao H, Tory MC, Pauley AM, Brashier JR, Stratman NC, Mathews WR, Buhl AE, et al. 1999. Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity. Nature 402: 533–537 [DOI] [PubMed] [Google Scholar]
- Yu G, Nishimura M, Arawaka S, Levitan D, Zhang L, Tandon A, Song YQ, Rogaeva E, Chen F, Kawarai T, et al. 2000. Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and betaAPP processing. Nature 407: 48–54 [DOI] [PubMed] [Google Scholar]