Abstract
The ability to recognize oneself in a mirror is an exceedingly rare capacity in the animal kingdom. To date, only humans and great apes have shown convincing evidence of mirror self-recognition. Two dolphins were exposed to reflective surfaces, and both demonstrated responses consistent with the use of the mirror to investigate marked parts of the body. This ability to use a mirror to inspect parts of the body is a striking example of evolutionary convergence with great apes and humans.
The capacity for mirror self-recognition (MSR) has been found only in humans and great apes (1–8). In humans, MSR does not emerge reliably until 18–24 months of age (9) and marks the beginning of a developmental process of achieving increasingly abstract psychological levels of self-awareness, including introspection and mental state attribution (10, 11). The first evidence for MSR in a nonhuman species was experimentally demonstrated in the common chimpanzee (1), but numerous subsequent attempts showed no convincing evidence of self-recognition in a variety of other primates and nonprimates, including monkeys, lesser apes, and elephants (12–18). All of these species, including African gray parrots (19) demonstrate the ability to use a mirror to mediate or guide their behavior. A provocative debate continues to rage about whether self-recognition in great apes implies that they are also capable of more abstract levels of self-awareness (20). Therefore, research on self-recognition in other species will have profound implications for the idea that humans are the only species to conceive of their own identity.
The apparent confinement of self-recognition to great apes and humans has stimulated scientific interest in the evolutionary significance of MSR based on common aspects of the social ecology, cognition, and neurobiology of these species (21–25). Dolphins have a high level of encephalization and behavioral and cognitive complexity (26–29), but previous attempts to demonstrate MSR in dolphins have been suggestive yet inconclusive because of difficulties in implementing adequate controls necessary to obtain robust evidence of MSR in an animal unable to display self-recognition by touching a marked part of the body with a hand (30, 31).
Conclusive evidence of self-recognition in a species as phylogenetically distant from primates as dolphins would play a pivotal role in determining whether this capacity is a byproduct of factors specific to great apes and humans or whether more general characteristics, such as a high level of encephalization, could help to explain the evolution of this capacity.
General Methods and Procedures
This study relies on sampling sufficient combinations of control and test situations to produce specificity effectively equivalent to hand use. We conducted a two-phase experiment to independently test whether two dolphins would use a mirror to view themselves after being marked, sham-marked, or not marked (untouched) in the presence or absence of reflective surfaces. During Phase 1, one subject was marked and sham-marked in a pool with walls of varying degrees of reflectivity (Fig. 1A). We conducted separate subsequent tests on both subjects during Phase 2 in a pool with nonreflective walls in which we affixed a mirror in a subset of sessions (Fig. 1B).
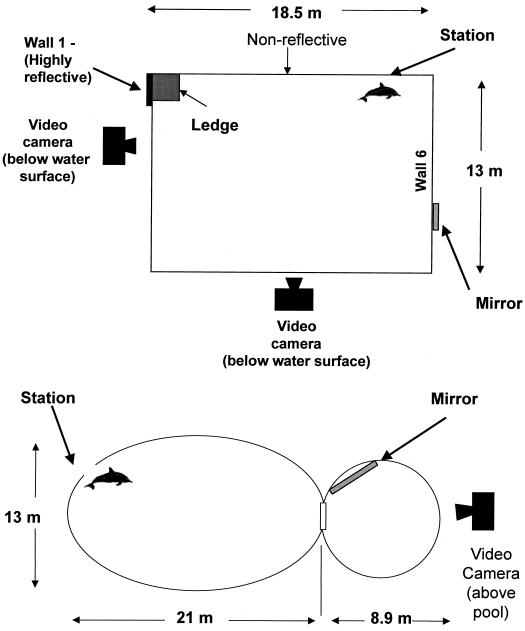
Figure 1.
(A) The pool in Phase 1. Phase 1 sessions were conducted in a rectangular pool 13 m × 18.5 m × 3.05 m with three reflective glass walls. During a subset of test sessions (n = 5), a narrow Plexiglas mirror, 41.9 cm × 101.6 cm × 0.32 cm, was affixed in a vertical orientation to the exterior of one of the reflective walls (Wall 6). The demarcated area (Wall 1) was more highly reflective than the other glass walls because of a black surface behind it. Thus, the Plexiglas mirror was the optimal mirror when present. In the absence of the Plexiglas mirror, Wall 1 had the most reflective properties. (B) The pool in Phase 2. In Phase 2, by using the same procedures as in Phase 1, testing was conducted in two connected pools, a large oval pool (21 m long × 13 mm × 3.66 m deep) connected to a smaller round pool (8.9 m in diameter × 3.05 m deep) with nonreflective walls. During experimental sessions, the subject was stationed at the far end of the larger pool and a Plexiglas mirror (88.9 cm × 119.4 cm × 0.32 cm) was affixed vertically to an open gate just inside the mouth of the connected smaller pool in 16 out of 30 sessions.
The first and primary subject of the study was a 13-year-old captive-born male bottlenose dolphin (Tursiops truncatus) housed with the other subject, a 17-year-old captive-born male bottlenose dolphin at the New York Aquarium in Brooklyn, NY. In none of the trials in which one animal was marked was there any discernable reaction on the part of the companion dolphin to the mark. Furthermore, all instances of mark-directed behaviors occurred when the companion dolphin was not near the same reflective surface as the subject. For 4 years before, and for a period during, the study, the two dolphins were housed for a part of each year in a pool with three reflective glass walls. Therefore, we did not expect our subjects to exhibit behaviors typical of animals who are initially exposed to a mirror (31). The dolphins had prior training on behaviors for public demonstrations but no training in relation to reflective surfaces or on cognitive tasks.
During an experiment, the dolphin was videotaped in the presence or absence of mirrors for 30 min before and 30 min after a feeding context at the end of which the animal was marked, sham-marked, or not touched at all. Both animals were then given the standard signal releasing them from their stations. The synchronized stepping away of the trainers from the pool's edge was the normal release stimulus signifying that the feed was over. The dolphins were routinely trained to go to and stay (or station) at a particular location in the pool, and during the experiments, they were stationed at two separate locations for the marking, sham-marking, or nonmarking procedures. A nontoxic temporary black ink Entré marker (Entré, Westborough, MA) was used to mark the dolphin on different parts of its body that were not visible to it without the use of a mirror (Fig. 2A). Marks were either cross-hatched and triangular or circular, and were ≈6.4 cm in diameter (Fig. 2B). Marks were placed on different body areas so that the subject would not habituate to the marking location and to enable us to test whether the dolphin would orient its body differentially to the mirror to view the marked areas. In sham-mark sessions, the protocol was identical to that in the actual mark sessions except that a water-filled marker was used to control for the possibility that the animal's behavior was attributable to the tactile sensation of marking rather than the mark itself. The real mark and the sham-mark were applied to the dolphin's skin in as close to the same manner as possible (e.g., duration of marking, amount of pressure to skin, and type of mark). Postfeeding mark, sham-mark, and nonmarked sessions were videotaped for 30 min in the presence or absence of specific mirror conditions (mirror, no mirror, or covered mirror in Phase 2 only). In Phase 2, the covered mirror condition was achieved by turning the nonreflective mirror backing toward the pool.
Figure 2.

(A) Locations of the nontoxic, temporary mark and the number of times the dolphins were marked in each location in mark and sham-mark sessions. Marks were applied to either side of the body. Subject 1: b, above eye (right, n = 1); c, above and posterior to ear (right, n = 3; left, n = 4); d, between ear and pectoral fin (right, n = 2; left, n = 2); e, above pectoral fin (right, n = 2; left, n = 1); f, posterior to pectoral fin (left, n = 1); g, below dorsal fin (right, n = 3; left, n = 7); h, between pectoral fin (n = 2); i, umbilical (n = 1); j, underside and tip of pectoral fin (right, n = 1). Subject 2: a, on melon (right, n = 1; left, n = 2); e, above pectoral fin (right, n = 5; left, n = 2); g, below dorsal fin (right, n = 2; left, n = 1; umbilical, n = 2); h, between pectoral fin (n = 1). (B) The dolphin marked above the right eye.
Procedures for the First Subject
Sessions conducted and taped before the feeding sessions served as baseline control sessions in which the subject was not touched, marked, or sham-marked. All experimental sessions were videotaped. A series of 63 15-min taped samples were prepared from the 30 min prefeeding and postfeeding sessions, 33 in Phase 1 and 30 in Phase 2. These samples consisted of 15-min random segments of prefeeding control sessions and the initial 15 min immediately after the release from a feeding session after the mark, sham-mark, or no-mark treatment.
Four observers (two experienced dolphin researchers and two highly trained assistants) were randomly assigned to score videotape segments. Each segment was scored by two independent raters who were blind to experimental condition and treatment. The videotape segments began immediately after the dolphin's release from station after the actual mark, sham-mark, or non-mark procedure. Marks were not visible to the raters because of the distance and viewing angle of the video camera. The independent observers conducted a second-by-second video analysis and scored the duration, location, and time of occurrence of specific behaviors. Behaviors were coded as Self-directed (mark-directed, sham-directed, or exploratory), Nondirected, Ambiguous, or Social. Mark- and sham-directed behaviors were those in which the animal positioned himself at the reflective surface and then produced orienting or repetitive body movements so that the mark, or sham-marked area, was visible to the animal in the reflective surface. Exploratory behaviors are self-directed behaviors, other than mark- or sham-directed behaviors, that occurred at the reflective surface and included behaviors such as repetitious head circling and close viewing of the eye or genital region reflected in the mirror. Finally, Social behaviors were defined as those behaviors typically observed when these and other dolphins confront a familiar or unfamiliar dolphin, including aggressive responses, e.g., jaw-clapping and charging, and affiliative responses, e.g., solicitous sexual postures. Table 1 displays behavioral categories, descriptions, and examples.
Table 1.
Descriptions and examples of behavioral categories
| Behavioral category | Description | Example |
|---|---|---|
| Self-directed | ||
| Mark-directed or sham-directed | Specific orienting or repetitive behaviors while positioned that, when the dolphin is marked, expose the marked body part to the reflective surface or, when the dolphin is sham-marked, expose the sham-marked part of the body to the reflective surface | Repetitive upward neck stretches when the dolphin is marked ventrally, e.g., under the chin, or sustained left-side orientation when the dolphin is marked behind the left pectoral fin |
| Exploratory | Specific orienting or repetitive behaviors while positioned that occur when the dolphin is either unmarked or marked but do not expose the marked body part to the mirror | Repetitive vertical head movements when the dolphin is marked in the horizontal plane, e.g., behind the right pectoral fin, or sustained right-side orientation when the dolphin is marked on the ventral surface, e.g., near genitals |
| Non-directed | Does not occur while positioned at a particular location | Swimming, surfacing |
| Ambiguous | Positioned behavior at a reflective surface that does not indicate a specific orientation or exposure of one particular part of the body to the mirror | Head-on position without other specific body movements |
| Social | Behaviors typically observed when subject, or other dolphins in general, confront another unfamiliar dolphin | Threats, such as squawks or jaw claps, or invitations to interact, such as underwater tail slaps |
Only behaviors for which there was 100% agreement between the two raters were analyzed. A strict agreement criterion required identical ratings on behavior, location, and timing. There was 100% agreement for 81.1% of the behaviors in Phase 1 and for 85.1% of the behaviors (including 100% agreement on initial behaviors at mirror) in Phase 2. The reason for the small proportion of nonagreement was the observers' inability to see the subject clearly through the water.
During Phase 1, the dolphin was first exposed to sham-marking and marking while in the pool with three reflective glass walls (Fig. 1A). In a subset of 5 out of 33 sessions, an actual mirror was affixed to the exterior of one of the glass walls. A corner area (Wall 1) of one of the glass walls was more highly reflective than the other glass walls because of a black surface behind it. Thus, the Plexiglas mirror was the optimal reflective surface when present. In the absence of the Plexiglas mirror, Wall 1 had the most reflective properties.
Thirty-three sessions were conducted during Phase 1, including eight prefeeding control sessions, eight postfeeding control sessions, one postfeeding control session with additional mirror affixed to exterior of wall, three early sham-mark sessions, six mark sessions, four mark sessions with additional mirror affixed to exterior of wall, and three late sham-mark sessions. The term “early sham-mark session” refers to those sham-mark sessions that occurred before the first actual marking and “late sham-mark session” refers to those sham-mark sessions that occurred after the dolphin experienced the first real mark session. These were distinguished from one another because the dolphin treated the two kinds of marks differently, as described below.
Results for First Subject
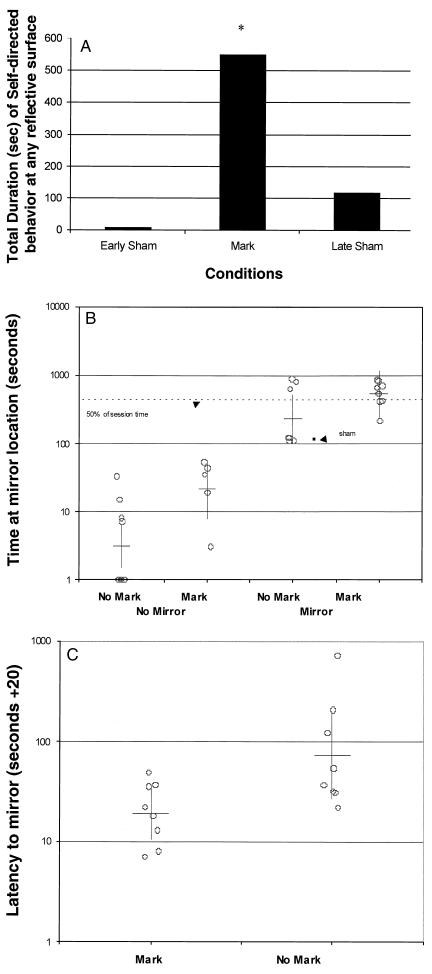
The results of Phase 1 (Fig. 3A) are presented as histograms giving the total amount of time the dolphin engaged in mark- or sham-directed behaviors during early sham-markings, genuine markings, and late sham-markings. The animal spent a significantly greater cumulative amount of time engaged in Self-directed behaviors at reflective surfaces when marked than when early sham-marked and a substantially greater cumulative amount of time when marked than when late sham-marked. The dolphin also spent a significantly greater cumulative amount of time engaged in mark-directed behaviors at reflective surfaces when marked than engaged in sham-directed behaviors when early sham-marked (6.1% vs. 0.3% of total time, respectively) (X2df=4 = 155.85, P < 0.001), and substantially more time engaged in mark-directed behaviors when marked than when late sham-marked (6.1% vs. 4.4% of total time, respectively).
Figure 3.
(A) Total amount of time (sec) engaged in mark- or sham-directed behaviors during early sham-marking, genuine markings, and late sham-marking. The subject spent a significantly greater cumulative amount of time engaged in Self-directed behaviors at reflective surfaces when marked than when early sham-marked or late sham-marked. (B) In Phase 2, time (sec) at mirror location under different experimental conditions: No mirror–no mark, No mirror–mark, Mirror–no mark, and Mirror–mark. The mean time spent at the mirror location when the subject was marked and the mirror present and uncovered was significantly greater than under any other condition. In the one session in which the subject was sham-marked, he spent a comparatively brief time at the mirror location. (C) Latency from departure by the subject from stationing to the first mirror orientation when marked, sham-marked, or unmarked. The criterion for mirror orientation was that the dolphin came to a stop at the mirror.
In a total of three 15-min early sham-marking sessions conducted before the first marking session, the dolphin displayed only one 8-sec bout (0.3% of total time for this condition) of sham-directed behavior to a reflective surface. The data from these early sham control trials showed that sham marking, conducted before the dolphin actually being marked, by itself did not elicit investigation at a reflective surface or any pattern of behaviors that could be interpreted as Self-directed.
In the series of late sham-markings that occurred after the first genuine marking, the dolphin began to respond differently by engaging in substantially more sham-directed behaviors at reflective surfaces than during the early sham-markings (4.4% vs. 0.3%, respectively). These late sham-markings were thus differentiated from the early sham-markings. Only after repeated exposure to being marked did the animal begin to use the mirror to investigate its body after being sham-marked. In the late sham-marking sessions, the dolphin oriented to make the appropriate area visible substantially more often than when early sham-marking had occurred. These results, therefore, suggest that, in the late sham-marking trials, the dolphin used the mirror to investigate the touched area to determine whether it was marked and, upon discovering no mark, abandoned further self-directed behaviors. This pattern of behavior made it difficult to interpret the subjects' responses to late sham-markings as “true shams” and, as a result, only one sham-marking was conducted in Phase 2.
Furthermore, if the dolphin was using reflective surfaces to investigate its body, one would expect him to be more likely to use those surfaces with the best reflective properties. In the trials when there was no mirror present, the dolphin spent more time engaged in mark- or sham-directed behaviors at the best reflective surface available (Wall 1 in Fig. 1A) than at other reflective surfaces (87.5% vs. 12.5% of total time, respectively). When the mirror was present, the dolphin spent considerably more time engaged in self-directed behaviors either at the mirror or at Wall 1 than at any other reflective surfaces (16.1% vs. 82.2% vs. 1.7%. of total time, respectively).
In Phase 2, by using the Phase 1 procedures, testing was conducted in two connected pools, a large oval pool connected to a smaller round pool, both with nonreflective concrete walls. Analysis was conducted by independent raters blind to condition. The distance of the above-water video camera prevented raters from determining whether the animal was marked or not. We conducted 30 postfeeding sessions. The dolphin was videotaped during 16 nonmarked prefeeding control sessions and during the postfeeding sessions consisting of 8 marked condition with the mirror sessions, 5 marked condition with a covered mirror or no mirror sessions, 7 unmarked condition with the mirror sessions, 9 unmarked condition with no mirror sessions, and 1 sham-mark condition with mirror session.
We statistically tested three predictions regarding behavior indicative of self-recognition, adopting the conservative position that a convincing demonstration of self-recognition required all three of these predictions be confirmed.
Prediction 1.
The dolphin should display no social behavior at the mirror. A typical response pattern in monkeys and other non-self-recognizing species to a mirror is initially social behavior, then disinterest, followed by a reinstatement of social responses upon relocation or reintroduction of the mirror (16). Our subject showed no social behaviors to reflective walls or mirrors throughout the entire test period in either pool. Although habituated to reflective surfaces, the dolphin, unlike non-self-recognizing species, did not show social responses to the mirrors in either pool when they were introduced.
Prediction 2.
The dolphin should spend more time at the mirror when marked than under any other conditions. This prediction was confirmed. An analysis of variance (ANOVA) showed that he spent significantly more time at the mirror location when marked than when unmarked [F(1, 26) = 11.75, P = 0.002] (Fig. 3B). The dolphin also spent more time at the mirror location when the mirror was present and uncovered than otherwise (covered or not present) [F(1, 26) = 88.28, P < 0.001]. The effects of mirror presence and marking were independent based on the absence of a significant interaction term in the ANOVA [F(1, 26) = 1.91, P = 0.179]. Accordingly, the mean time spent at the mirror location when the subject was marked and the mirror present and uncovered was significantly greater than under any other condition. In the one trial in which the dolphin was sham-marked, he spent a comparatively brief time at the mirror location (Fig. 3B). This result is consistent with the subject's typical response to late sham-markings.
Prediction 3.
The dolphin should exhibit shorter latencies in the temporal interval from the release signal from its stationed position to mirror use after being marked or sham-marked than after sessions when not handled. That is, if the dolphin was using the mirror to view the marked parts of its body after being marked or sham-marked, upon release he should swim to the mirror earlier than when not marked. Furthermore, the first behavior exhibited at the mirror under the marked or late sham condition should be more likely to be one that exposes a marked (or sham-marked) part of the body to the mirror surface. This was a risky prediction because if the subject were capable of self-recognition but was not more motivated (an important factor in mark-tests) to view itself when marked than when unmarked, this prediction would be disconfirmed. Latency data were transformed to their common logarithms after adding a constant value of 20 to eliminate negative values. Negative values occurred because in half of the sessions in the mark or sham-mark condition, the dolphin departed from the station before the release time began. A t test showed that the mean latency to the mirror from release was significantly lower when the dolphin was marked (or sham-marked) than when he was unmarked (t = 2.7df=14, P = 0.017) (Fig. 3C). Moreover, the subject's initial behavior was oriented to the mark as opposed to other body areas in six out of eight mark sessions (P < 0.05 using a binomial test). In the remaining two mark sessions, the dolphin's behavior was ambiguous. Therefore, when the animal was marked or late sham-marked, he quickly swam to the mirror upon release (and in some cases before the release signal) and immediately exposed the marked (or sham-marked) part of his body to the mirror (Fig. 4A and see Movies 1 and 2, which are published as supplemental data on the PNAS web site, www.pnas.org).
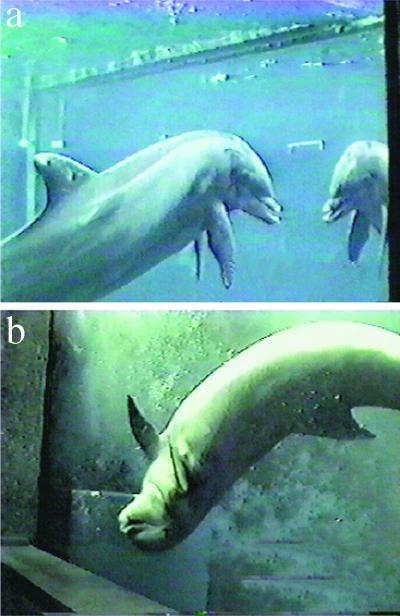
Figure 4.
(A) Mark-directed behavior by subject to a real mirror immediately after release from being marked. A narrow Plexiglas mirror, 41.9 cm × 101.6 cm × 0.32 cm is affixed in a vertical orientation to the exterior of one of the reflective walls (Wall 6). During this session, the mirror was the best reflective surface in the subject's environment. The faint white line on the wall indicates the location of mirror. (B) The dolphin at Wall 1, the best reflective surface in the session, exhibiting late sham-directed behavior: a continuous and repetitive sequence of 12 dorsal-to-lateral-ventral flips exposing the location of the sham-marked area of his body, the underside and tip of the right pectoral fin, to the reflective surface. This unusual behavioral sequence continued for 32 sec.
Moreover, numerous qualitative observations corroborate our more formal quantitative analyses. For example, a striking example occurred after a late sham-marking to the dolphin's right pectoral fin. He exhibited a continuous sequence of 12 dorsal-to-ventral body flips in front of Wall 1 that brought the right pectoral fin into close viewing range at this highly reflective surface (Fig. 4B and see Movie 3, which is published as supplemental data on the PNAS web site). Another notable example occurred during a series of additional sessions that were conducted after the formal experimental sessions included in the data set were completed. The dolphin, upon being marked for the first and only time on the tongue, immediately swam to the Plexiglas mirror and engaged in a mouth opening and closing sequence never before observed by him during the study.
Procedures for Second Subject
To conduct an efficient and clear-cut assessment of whether this capacity is replicable in a second subject, the same three predictions for Phase 2 were made and tested on the second animal (who was still housed in the Phase 2 pool). We used an assessment technique whereby only the most definitive aspects of our tests on the first subject were repeated on the second.
The dolphin was videotaped during 20 nonmarked prefeeding control sessions and during 11 postfeeding sessions consisting of the marked (or sham-marked) condition with the mirror, 5 marked (or sham-marked) conditions with a covered mirror or no mirror, and 4 unmarked (or no sham-mark) conditions with the mirror. Thus, 16 mark or sham-mark sessions were conducted in this phase. Fig. 2A displays the locations of marks on the second subject. Mark and sham conditions, i.e., late-shams, were combined because the second subject, like the first, was treating these conditions as equivalent in this setting. Randomized videotaped segments were scored by two independent raters blind to experimental condition and treatment. They conducted a second-by-second video analysis and scored the duration, location, and time of occurrence of specific behaviors exhibited by the dolphin during the initial 10-sec period at the mirror after the release signal. Only the initial 10-sec period of mirror orientation by the dolphin was used for testing predictions 2 and 3 in the second subject because we found this to be the most diagnostic time period for analysis with the first subject. No social behaviors were exhibited by the dolphin for the remainder of time spent at the mirror during these sessions. As with the first subject, only behaviors for which there was 100% agreement between the two raters were included in the analyses. For this analysis, there was 100% agreement on all of the behaviors that occurred in the first 10 sec.
Results for Second Subject
We tested the same three predictions from Phase 2 testing of the first subject, again adopting the stance that if any of these predictions were falsified, we would conclude that we did not demonstrate self-recognition in the subject.
Prediction 1.
For this analysis, the entire videotaped segments were reviewed, but no social behaviors at the mirror occurred during these segments or when the mirror was reintroduced into the pool after being absent for some time.
Prediction 2.
This prediction was confirmed. An ANOVA of the time spent at the mirror locations showed that the dolphin spent significantly more time at the mirror location when marked than when unmarked [F(1, 15) = 9.38, P = 0.008] and spent more time at the mirror location when the mirror was uncovered than when covered [F(1, 15) = 15.26, P = 0.001]. Therefore, the mean time spent at the mirror location when the animal was marked and the mirror present and uncovered was significantly greater than under any other condition.
Prediction 3.
Latency data were transformed as with the first subject to account for negative values, which occurred in four out of nine of the sessions in the mark or sham condition because the dolphin departed from the station before the release time began. A t test showed that the mean latency to the mirror from release was significantly lower when the dolphin was marked (or sham-marked) than when he was unmarked (t = 2.6df=10, P = 0.028). Moreover, the animal's initial behavior was oriented to the mark or sham-mark as opposed to other body areas in 9 out of 11 mark or sham-mark sessions (P < 0.05 binomial test) and, of the actual mark sessions, the initial behavior was mark-oriented in all 9 sessions. Therefore, when the dolphin was marked or late sham-marked, he quickly swam to the mirror upon release (and in some cases before the release signal) and immediately exposed the marked (or sham-marked) part of its body to the mirror.
General Discussion
Collectively, these findings provide definitive evidence that the two dolphins in this study used the mirror (and other reflective surfaces) to investigate parts of their bodies that were marked. These findings, therefore, offer the first convincing evidence that a nonprimate species, the bottlenose dolphin, is capable of MSR.
Our study revealed interesting similarities and differences between the way dolphins and chimpanzees respond to mirrors and body marks. Chimpanzees have been reported to habituate rapidly to body marks in the context of mark tests (2). Likewise, although our subjects displayed clear self-orienting mark-directed behavior after being marked or after late-sham marking, neither one maintained a continuous orientation to the mirror throughout the entire session. This may indicate habituation to the mark after inspection of it. Also, unlike chimpanzees, dolphins do not attend to marks on companions. Dolphins may pay less attention to marks on the bodies of companions because, unlike primates, they do not groom each other. This difference makes our findings even more interesting because dolphins clearly are interested in marks on their own body despite the fact that they do not have a natural tendency toward social grooming.
Bottlenose dolphins share several behavioral and social ecological features with great apes and humans, including sophisticated memory and classification of relationships among events (27), the ability to learn rudimentary symbol-based artificial codes (27, 32), and complex social behavior (28). Bottlenose dolphins, great apes, and humans all possess high degrees of encephalization and neocortical expansion (26, 33, 34). Yet the brains of dolphins are markedly different from those of primates on many levels, including cortical cytoarchitecture and organization (33, 34), reflecting the fact that the cetacean (dolphin, whale, and porpoise) and primate ancestral lines diverged at least 65–70 million years ago. The present findings imply that the emergence of self-recognition is not a byproduct of factors specific to great apes and humans but instead may be attributable to more general characteristics such as a high degree of encephalization and cognitive ability. Hypotheses about the evolution of self-recognition have, to date, focused on primate characteristics. Our findings show that self-recognition may be based on a different neurological substrate in dolphins.
More generally, these results represent a striking case of cognitive convergence in the face of profound differences in neuroanatomical characteristics and evolutionary history. The question of whether dolphins are capable of more complex forms of self-awareness, such as introspection and mental state attribution, remains unanswered. The present findings should motivate further investigation of other indicators of self-awareness.
Supplementary Material
Acknowledgments
We thank the marine mammal training staff of the New York Aquarium for their time and effort. Special thanks to Kevin M. Walsh, Director of Training, and Martha Hiatt, Kristin Tillis, and Guenter Skammel. We also wish to acknowledge the assistance of our students Diana Praschnik-Buchman, Reshma Patel, Debbie Lepsinger, Shinko Lin, Sheila Abichandani, Evan Sloan, and Julie Mintz. Special thanks to Paul Boyle, Lou Garibaldi, Cynthia Reich, and William Conway for their encouragement and support. We thank Fred Marsteller for his statistical assistance. Finally, we wish to thank Irene Pepperberg, Frans de Waal, Duane Rumbaugh, Brenda McCowan, Herb Terrace, Scott Lilienfeld, and Stuart Firestein for their valuable advice and support. This research was partly supported by funds from the City Council of the City of New York to the New York Aquarium (D.R.) and by a Cetacean Society International Research Grant (L.M.).
Abbreviation
- MSR
mirror self-recognition
References
- 1.Gallup G G. Science. 1970;167:86–87. doi: 10.1126/science.167.3914.86. [DOI] [PubMed] [Google Scholar]
- 2.Povinelli D J, Gallup G G, Eddy T J, Bierschwale D T, Engstrom M C, Perilloux H K, Toxopeus I B. Anim Behav. 1997;53:1083–1088. [Google Scholar]
- 3.Povinelli D J, Rulf A B, Landau K, Bierschwale D T. J Comp Psychol. 1993;107:347–372. doi: 10.1037/0735-7036.107.4.347. [DOI] [PubMed] [Google Scholar]
- 4.Suarez S, Gallup G G. J Hum Evol. 1981;11:175–188. [Google Scholar]
- 5.Walraven V, van Elsacker L, Verheyen R. Primates. 1995;36:145–150. [Google Scholar]
- 6.Miles H L. In: Self-Awareness in Animals and Humans: Developmental Perspectives. Parker S T, Mitchell R W, Boccia M L, editors. Cambridge, U.K.: Cambridge Univ. Press; 1994. pp. 254–272. [Google Scholar]
- 7.Lethmate J, Ducker G. Z Tierpsychol. 1973;33:248–269. [PubMed] [Google Scholar]
- 8.Patterson F G, Cohn R H. In: Self-Awareness in Animals and Humans: Developmental Perspectives. Parker S T, Mitchell R W, Boccia M L, editors. Cambridge, U.K.: Cambridge Univ. Press; 1994. pp. 273–290. [Google Scholar]
- 9.Amsterdam B. Dev Psychobiol. 1972;5:297–305. doi: 10.1002/dev.420050403. [DOI] [PubMed] [Google Scholar]
- 10.Lewis M. In: Psychological Perspectives on the Self. Greenwald A G, Suls J, editors. Hillsdale, NJ: Erlbaum; 1986. pp. 55–78. [Google Scholar]
- 11.Piaget J. The Origins of Intelligence in Children. New York: Norton; 1952. [Google Scholar]
- 12.Hyatt C W. Am J Primatol. 1998;45:30–311. doi: 10.1002/(SICI)1098-2345(1998)45:3<307::AID-AJP7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 13.Anderson J R. Q J Exp Psychol. 1983;35B:201–212. doi: 10.1080/14640748308400905. [DOI] [PubMed] [Google Scholar]
- 14.Bayart F, Anderson J R. Behav Processes. 1985;10:219–227. doi: 10.1016/0376-6357(85)90069-5. [DOI] [PubMed] [Google Scholar]
- 15.Gallup G G, Wallnau L, Suarez S D. Folia Primatol. 1980;33:210–219. doi: 10.1159/000155935. [DOI] [PubMed] [Google Scholar]
- 16.Suarez S D, Gallup G G. Am J Primatol. 1986;11:239–244. doi: 10.1002/ajp.1350110305. [DOI] [PubMed] [Google Scholar]
- 17.Anderson J R, Roeder J J. Primates. 1989;30:581–587. [Google Scholar]
- 18.Povinelli D J. J Comp Psychol. 1989;103:122–131. [Google Scholar]
- 19.Pepperberg I M, Garcia S E, Jackson E C, Marconi S. J Comp Psychol. 1995;109:182–195. [Google Scholar]
- 20.Gallup G G, Povinelli D J. Sci Am. 1998;9:66–76. [Google Scholar]
- 21.Gallup G G. Ann NY Acad Sci. 1997;818:73–84. doi: 10.1111/j.1749-6632.1997.tb48247.x. [DOI] [PubMed] [Google Scholar]
- 22.Povinelli D J. Am Psychol. 1993;48:493–509. doi: 10.1037//0003-066x.48.5.493. [DOI] [PubMed] [Google Scholar]
- 23.Povinelli D J, Cant J G. Q Rev Biol. 1995;70:393–421. doi: 10.1086/419170. [DOI] [PubMed] [Google Scholar]
- 24.Byrne R W, Whiten A. Machiavellian Intelligence: Social Expertise and the Evolution of Intellect in Monkeys, Apes, and Humans. Oxford: Clarendon; 1998. [Google Scholar]
- 25.Parker S T, Mitchell R W, Boccia M L. Self-Awareness in Animals and Humans: Developmental Perspectives. Cambridge, U.K.: Cambridge Univ. Press; 1994. [Google Scholar]
- 26.Marino L. Brain Behav Evol. 1998;51:230–238. doi: 10.1159/000006540. [DOI] [PubMed] [Google Scholar]
- 27.Reiss D, McCowan B, Marino L. Trends Cognit Sci. 1997;1(4):140–145. doi: 10.1016/S1364-6613(97)01046-2. [DOI] [PubMed] [Google Scholar]
- 28.Connor R C, Smolker R A, Richards A F. In: Coalitions and Alliances in Humans and Other Animals. Harcourt A H, DeWaal F B M, editors. Oxford: Oxford Univ. Press; 1992. pp. 415–443. [Google Scholar]
- 29.Mitchell R W. New Ideas Psychol. 1993;11:351–377. [Google Scholar]
- 30.Marino L, Reiss D, Gallup G G. In: Self-Awareness in Animals and Humans: Developmental Perspectives. Parker S T, Mitchell R W, Boccia M L, editors. Cambridge, U.K.: Cambridge Univ. Press; 1994. pp. 380–391. [Google Scholar]
- 31.Marten K, Psarakos S. In: Self-Awareness in Animals and Humans: Developmental Perspectives. Parker S T, Mitchell R W, Boccia M L, editors. Cambridge, U.K.: Cambridge Univ. Press; 1994. pp. 361–379. [Google Scholar]
- 32.Herman L M. In: Cetacean Behavior: Mechanisms and Functions. Herman L M, editor. Malabar, FL: Robert E. Krieger Publishing Company; 1988. pp. 363–429. [Google Scholar]
- 33.Morgane P J, Jacobs M S, MacFarland W L. Brain Res Bull. 1980;5:1–107. [Google Scholar]
- 34.Glezer I I, Jacobs M S, Morgane P J. Behav Brain Sci. 1988;11:75–116. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.