Abstract
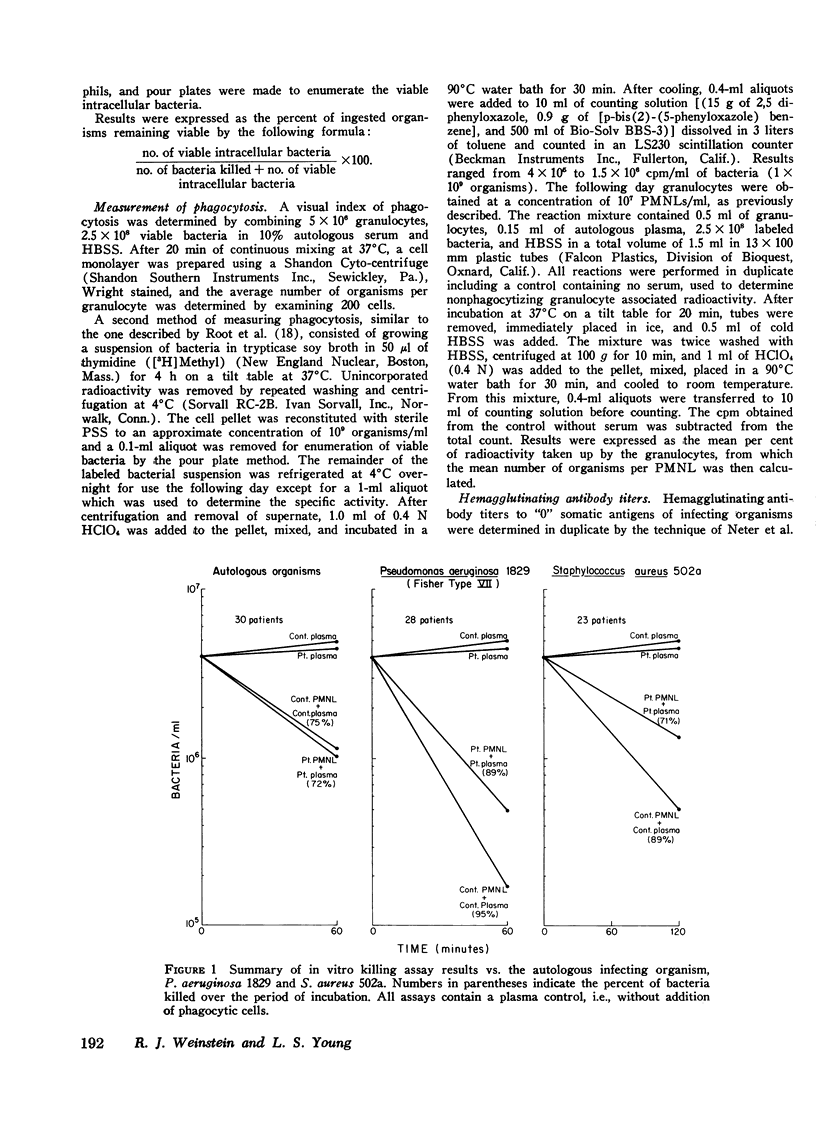
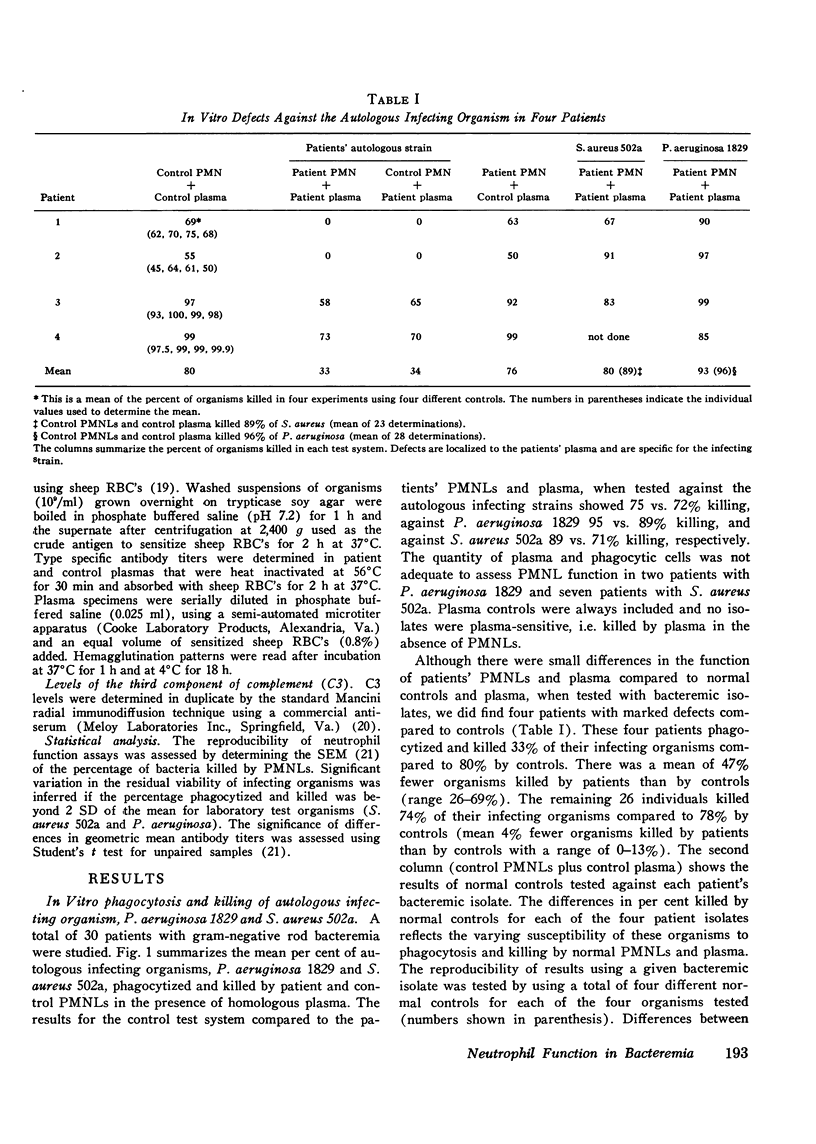
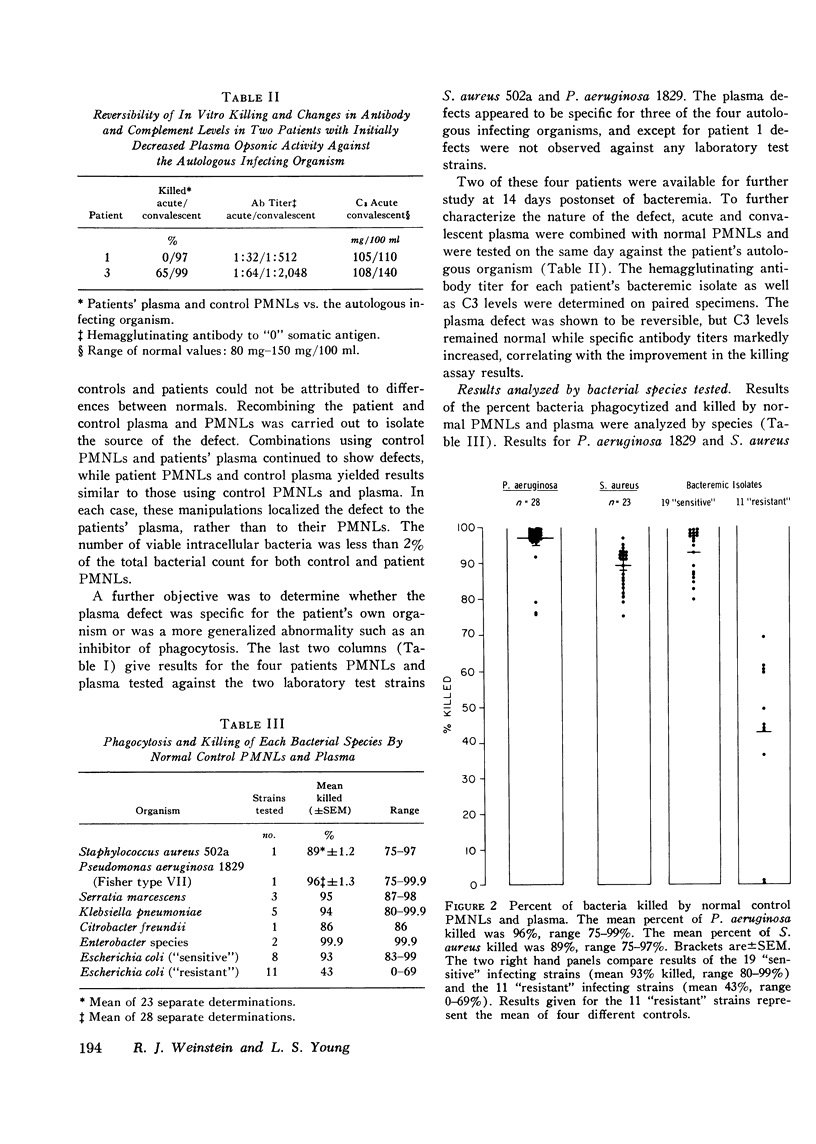
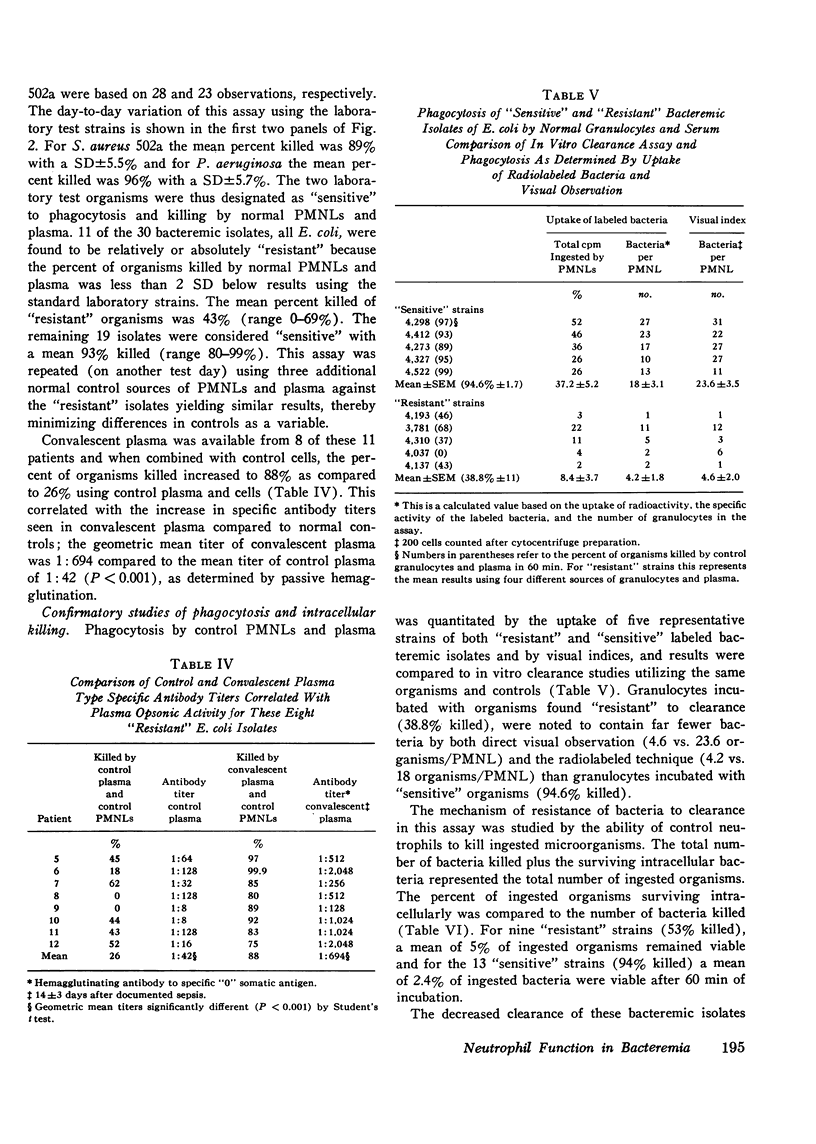
To assess the phagocytic and bactericidal function of neutrophils in the acute stages of gram-negative rod bacteremia, cells from 30 nonleukopenic patients were studied in a test system utilizing plasma obtained simultaneously with culture-positive blood, the autologous infecting strain, and two laboratory test strains of Staphylococcus aureus and Pseudomonas aeruginosa. Results were compared to those obtained with normal neutrophils and plasma. Patient and control plasma were simultaneously tested with each source of phagocytic cells to localize any abnormalities. Four patients had a defect against their infecting strain, 33% of the inoculum phagocytized and killed versus 80% by controls. In these cases differences were localized to the patients' plasma, as normal plasma tested with patients' cells reversed the defect. Thus, four patients had impaired opsonization when compared to normal controls, but we also observed that 11 of 30 bacteremic isolates, all Escherichia coli, showed absolute or relative resistance to phagocytosis in the patient and control assay system. No intrinsic granulocyte killing abnormalities were noted. There was poor correlation between results obtained with infecting strains compared to laboratory test organisms. We conclude that in patients without evidence of an inherited neutrophil bactericidal disorder, recurrent infection, or treatment with cytotoxic drugs, intrinsic bactericidal defects are uncommon at the onset of gram-negative bacteremia, and impaired opsonization is the most commonly encountered cause of neutrophil dysfunction.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. W., Hegg M., Altemeier W. A. Neutrophil function in selected surgical disorders. Ann Surg. 1968 Sep;168(3):447–458. doi: 10.1097/00000658-196809000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J. W., Wixson D. Neutrophil dysfunction and sepsis in burn injury. Surg Gynecol Obstet. 1970 Mar;130(3):431–438. [PubMed] [Google Scholar]
- BYBEE J. D., ROGERS D. E. THE PHAGOCYTIC ACTIVITY OF POLYMORPHONUCLEAR LEUKOCYTES OBTAINED FROM PATIENTS WITH DIABETES MELLITUS. J Lab Clin Med. 1964 Jul;64:1–13. [PubMed] [Google Scholar]
- Bodey G. P., Buckley M., Sathe Y. S., Freireich E. J. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966 Feb;64(2):328–340. doi: 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- Brandt L., Hedberg H. Impaired phagocytosis by peripheral blood granulocytes in systemic lupus erythematosus. Scand J Haematol. 1969;6(5):348–353. doi: 10.1111/j.1600-0609.1969.tb02420.x. [DOI] [PubMed] [Google Scholar]
- Fine D. P. Pneumococcal type-associated variability in alternate complement pathway activation. Infect Immun. 1975 Oct;12(4):772–778. doi: 10.1128/iai.12.4.772-778.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. W., Devlin H. B., Gnabasik F. J. New immunotype schema for Pseudomonas aeruginosa based on protective antigens. J Bacteriol. 1969 May;98(2):835–836. doi: 10.1128/jb.98.2.835-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren A., Quie P. G. Influence of the alternate complement pathway in opsonization of several bacterial species. Infect Immun. 1974 Aug;10(2):402–404. doi: 10.1128/iai.10.2.402-404.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J. G., STRAUSS B. STUDIES ON HEAT-LABILE OPSONIN IN RABBIT SERUM. J Immunol. 1964 Jan;92:145–154. [PubMed] [Google Scholar]
- Hill H. R., Gerrard J. M., Hogan N. A., Quie P. G. Hyperactivity of neutrophil leukotactic responses during active bacterial infection. J Clin Invest. 1974 Apr;53(4):996–1002. doi: 10.1172/JCI107666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C. J., Glynn A. A. The virulence for mice of strains of Escherichia coli related to the effects of K antigens on their resistance to phagocytosis and killing by complement. Immunology. 1971 May;20(5):767–777. [PMC free article] [PubMed] [Google Scholar]
- LANDING B. H., SHIRKEY H. S. A syndrome of recurrent infection and infiltration of viscera by pigmented lipid histiocytes. Pediatrics. 1957 Sep;20(3):431–438. [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte candidacidal activity and resistance to systemic candidiasis in patients with cancer. Cancer. 1971 May;27(5):1211–1217. doi: 10.1002/1097-0142(197105)27:5<1211::aid-cncr2820270528>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall C. E., Caves J., Cooper R., DeChatlet L. Functional characteristics of human toxic neutrophils. J Infect Dis. 1971 Jul;124(1):68–75. doi: 10.1093/infdis/124.1.68. [DOI] [PubMed] [Google Scholar]
- Messner R. P., Reed W. P., Palmer D. L., Bolin R. B., Davis A. T., Quie P. G. A transient defect in leukocytic bactericidal capacity. Clin Immunol Immunopathol. 1973 Jul;1(4):523–532. doi: 10.1016/0090-1229(73)90008-1. [DOI] [PubMed] [Google Scholar]
- Mowat A. G., Baum J. Polymorphonuclear leucocyte chemotaxis in patients with bacterial infections. Br Med J. 1971 Sep 11;3(5775):617–619. doi: 10.1136/bmj.3.5775.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NETER E., BERTRAM L. F., ZAK D. A., MURDOCK M. R., ARBESMAN C. E. Studies on hemagglutination and hemolysis by escherichia coli antisera. J Exp Med. 1952 Jul;96(1):1–15. doi: 10.1084/jem.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K. Effects of cytochalasin B on the intrcellular bactericidal activity of human neutrophils. Antimicrob Agents Chemother. 1975 Jun;7(6):736–741. doi: 10.1128/aac.7.6.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roantree R. J. Salmonella O antigens and virulence. Annu Rev Microbiol. 1967;21:443–466. doi: 10.1146/annurev.mi.21.100167.002303. [DOI] [PubMed] [Google Scholar]
- Root R. K., Rosenthal A. S., Balestra D. J. Abnormal bactericidal, metabolic, and lysosomal functions of Chediak-Higashi Syndrome leukocytes. J Clin Invest. 1972 Mar;51(3):649–665. doi: 10.1172/JCI106854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner F., Valmont I., Kozinn P. J., Caroline L. Leukocyte function in patients with leukemia. Cancer. 1970 Apr;25(4):835–842. doi: 10.1002/1097-0142(197004)25:4<835::aid-cncr2820250412>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- SBARRA A. J., SHIRLEY W., BAUMSTARK J. S. Effect of osmolarity on phagocytosis. J Bacteriol. 1963 Feb;85:306–313. doi: 10.1128/jb.85.2.306-313.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg C. O., Hellum K. B. Neutrophil granulocyte function in bacterial infections. Lancet. 1972 Oct 7;2(7780):727–730. doi: 10.1016/s0140-6736(72)92022-3. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. Phagocytosis (third of three parts). N Engl J Med. 1974 Apr 11;290(15):833–839. doi: 10.1056/NEJM197404112901506. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Root R. K., Vaughan M. Phagocytosis in chronic granulomatous disease and the Chediak-Higashi syndrome. N Engl J Med. 1972 Jan 20;286(3):120–123. doi: 10.1056/NEJM197201202860302. [DOI] [PubMed] [Google Scholar]
- Tan J. S., Watanakunakorn C., Phair J. P. A modified assay of neutrophil function: use of lysostaphin to differentiate defective phagocytosis from impaired intracellular killing. J Lab Clin Med. 1971 Aug;78(2):316–322. [PubMed] [Google Scholar]
- Wenger M. E., Bole G. G. Nitroblue tetrazolium dye reduction by peripheral leukocytes from rheumatoid arthritis and systemic lupus erythematosus patients measured by a histochemical and spectrophotometric method. J Lab Clin Med. 1973 Sep;82(3):513–521. [PubMed] [Google Scholar]
- Young L. S., Meyer R. D., Armstrong D. Pseudomonas aeruginosa vaccine in cancer patients. Ann Intern Med. 1973 Oct;79(4):518–527. doi: 10.7326/0003-4819-79-4-518. [DOI] [PubMed] [Google Scholar]



