Abstract
Objective
In a recent study, we showed that the combination of aspirin plus the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) synergistically inhibited platelet function. As aspirin, EPA, and DHA have demonstrated anti-inflammatory properties, we hypothesized that the ingestion of EPA and DHA, with and without aspirin, would reduce plasma levels of inflammatory cytokines and angiogenesis factors more than aspirin alone and before aspirin was ingested.
Methods
Using multiplex technology, we investigated the effects of aspirin (single-dose 650 mg on day 1), EPA+DHA (3.4 g/d for days 2-29), and aspirin with EPA+DHA (day 30) on plasma levels of inflammatory cytokines and angiogenesis factors in healthy adults.
Results
Aspirin alone had no effect on any factor versus baseline, but EPA+DHA, with and without aspirin, significantly reduced concentrations of 8 of 9 factors. Although EPA+DHA plus aspirin reduced concentrations of a subset of the factors compared to baseline, neither aspirin alone nor the combination significantly reduced the level of any analyte more robustly than EPA+DHA alone.
Conclusions
These data suggest that EPA+DHA has more pronounced down-regulatory effects on inflammation and angiogenesis than aspirin. The implications of these findings for the use of combined therapy for cardiovascular disease remain to be clarified.
Keywords: eicosapentaenoic acid, docosahexaenoic acid, lipid mediators, fatty acids, angiogenesis, hemostasis, platelet function, cytokines, aspirin
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death, disability, and healthcare expense in the United States, and also the leading cause of death worldwide[1]. Angiogenesis is the generation of new blood vessels and a process involved in the pathophysiology of CVD[2]. Several cytokines/chemokines and angiogenesis factors have been associated with the progression of cardiovascular disease (CVD)[2]. These include most prominently tumor necrosis factor-α, interleukin (IL)-6, IL-8, IL-1ß, macrophage chemoattractant protein (MCP), platelet-derived growth factor (PDGF), basic fibroblast growth factor (b-FGF), and vascular endothelial growth factor (VEGF), whereas IL-10 tends to act as an atheroprotective mediator[3].
Aspirin exerts anti-inflammatory effects by downregulating the activity of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which regulates the production of many inflammatory cytokines including TNF and leukotrienes[4]. The fish-derived omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) also have cardioprotective effects[5]. We have previously shown that EPA+DHA does not modulate platelet aggregation but the combination of EPA+DHA with aspirin synergistically reduces it[6]. Using plasma in this current study, we investigated the effects of aspirin and EPA+DHA, alone and in combination, on plasma levels of inflammatory cytokines and angiogenesis factors in healthy adults.
METHODS
Protocol
The original study from which blood samples were obtained was designed to assess the effects of aspirin and EPA+DHA on platelet function[6]. It was an open-label, 4-week, sequential therapy trial in which each subject served as his/her own control. The study involved 4 visits: Day 1 (baseline); Day 2 (1 day after an oral dose of 650 mg of a regular aspirin tablet); Day 29 (after 28 days of daily treatment with 3.4 g/day of prescription EPA+DHA [Lovaza®, GlaxoSmithKline, Philadelphia, PA, USA]); and Day 30 (after 1 day of combined treatment with 3.4 g/day of prescription EPA+DHA and 650 mg aspirin). One day of aspirin treatment was selected because the effects of aspirin on platelet aggregation are complete 1 day after ingestion, and are similar at 1 and 7 days after ingestion in healthy volunteers[7]. Blood was drawn into sodium citrate for all tests after a 10-hour overnight fast and stored immediately at -80°C.
Subjects
Fifteen healthy volunteers not taking medications, vitamin pills, nutritional supplements, or herbal preparations were recruited. Other exclusions included drinking more than 3 alcoholic beverages a day, or having any of the following conditions: ulcer or bleeding in the stomach, liver or kidney disease, a bleeding or blood clotting disorder (e.g. hemophilia), fluid retention, heart disease, hypertension, gout, asthma, arthritis, or nasal polyps. Subjects had no history of allergic reactions to aspirin, fish or fish oils, or to non-steroidal anti-inflammatory drugs. The study was approved by the University of South Dakota Institutional Review Board, and written informed consent was obtained from each subject.
Laboratory Methods
The effects of aspirin and EPA+DHA, alone or in combination, on plasma levels of cytokines and angiogenesis factors were examined using the BioPlex suspension array system (BioRad Life Sciences, Hercules, CA, USA), according to the manufacturer’s instructions. Briefly, beads coated with specific antibodies [anti-tumor necrosis factor alpha (TNF-α), -platelet-derived growth factor (PDGF), interleukins (IL-6, -IL-8, -IL-10, -IL-1β), macrophage chemoattractant protein (MCP), basic fibroblast growth factor (b-FGF), and vascular endothelial growth factor (VEGF)] were immobilized on a 96-well plate and allowed to react with sample (50μL) containing unknown amounts of cytokine. After a series of washes to remove unbound protein, a biotinylated detection antibody specific for a different epitope on the cytokine was added to the plate. Streptavidin-PE was added to the wells, and the reaction was quantified based on bead color and fluorescence intensity. Cytokine concentrations were calculated using BioPlex Manager software (Bio-Rad).
Statistical Analyses
Concentrations of all cytokines and growth factors were tested for normality using the Shapiro-Wilk statistic, and many were not normally distributed based on this metric (p<.05). Therefore, the Wilcoxon signed-rank test was used to determine differences in cytokine and angiogenesis factor levels between the study time points. P-values <0.05 or <0.1 were considered statistically significant due to the small sample-size and were not adjusted for multiple comparisons for any analysis due to the exploratory nature of this study and a priori hypotheses for each analyte. Analysis was conducted using SAS software (version 9.2; SAS Institute Inc., Cary, NC, USA).
RESULTS
The study participants were relatively young (mean age 33.3 years), had a body mass index in the overweight range, consumed very little fish and alcohol, and 60% were female{Table}. The effects of EPA+DHA and/or aspirin ingestion on inflammatory cytokines and angiogenesis factors are presented in Figures 1 and 2, respectively. EPA+DHA alone significantly reduced the concentrations of all factors, as compared with baseline or aspirin alone. EPA+DHA combined with aspirin reduced the concentrations of a subset of all of the factors compared with baseline. Aspirin alone did not significantly reduce the level of any cytokine or factor compared to baseline, and aspirin combined with EPA+DHA did not reduce the concentrations of factors more robustly compared to EPA+DHA alone (p>0.1).
TABLE.
Baseline Characteristics of Study Subjects
| Variable | Mean (n=15) | SD |
|---|---|---|
| Age | 33.3 | 11 |
| Male (%) | 40 | |
| Servings of non-fried fish per month | 1.2 | 1 |
| Alcoholic drinks/wk | 1.3 | 1.1 |
| Body mass index at enrollment | 27.6 | 3.4 |
Figure 1.
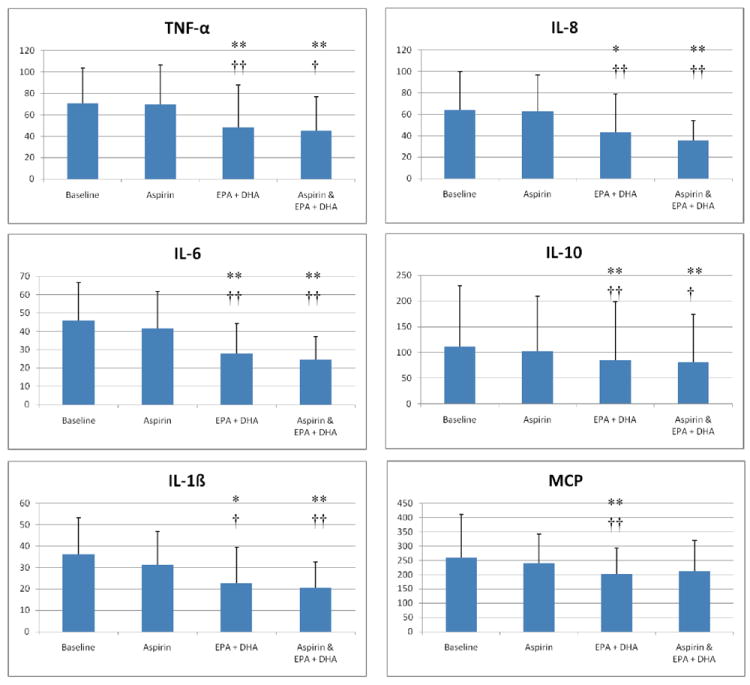
The effects of aspirin and EPA+DHA on plasma cytokine levels. Plasma concentrations of the indicated cytokines were evaluated at baseline, 1 day after an oral dose of 650 mg of regular aspirin (aspirin), after 28 days of treatment with 3.4 mg/day of prescription EPA+DHA (EPA+DHA), and after 1 day of combined treatment with EPA+DHA and aspirin (aspirin & EPA+DHA). Units are in picogram/mL and error bars represent 1 standard deviation. Single asterisks indicate a p-value of <0.1 and double asterisks indicate a p-value of <.05 as compared to baseline. Single crosses indicate a p-value <0.1 and double crosses indicate a p-value <0.05 as compared to the effects of aspirin alone. Aspirin did not significantly alter concentrations of any cytokine when ingested alone, compared to baseline, or in combination with EPA+DHA when compared to EPA+DHA alone.
Figure 2.
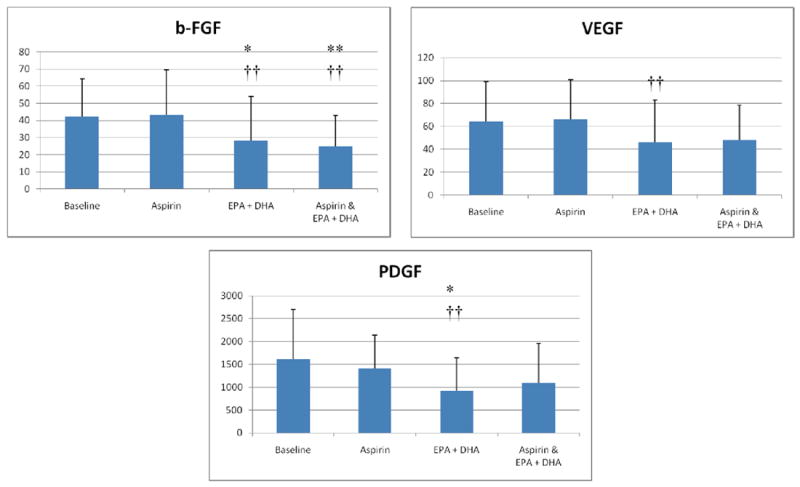
The effects of aspirin and EPA+DHA on plasma angiogenesis factor levels. Plasma concentrations of b-FGF and VEGF were evaluated as described for Figure 1. Single asterisks indicate a p-value of <0.1 and double asterisks indicate a p-value of <.05 as compared to baseline. Single crosses indicate a p-value <0.1 and double crosses indicate a p-value <0.05 as compared to the effects of aspirin alone. Aspirin did not significantly alter the concentrations of any angiogenesis factor when ingested alone, compared to baseline, or in combination with EPA+DHA, when compared to EPA+DHA alone.
DISCUSSION
The key finding of this study is that a brief 28-day supplementation with EPA+DHA led to a significant reduction in plasma levels of cytokines and angiogenesis factors implicated in atherosclerosis. To the best of our knowledge, no prior studies have compared the effects of aspirin and EPA+DHA, alone, and in combination, on all of these analytes simultaneously. EPA and DHA have been shown to exert potent anti-angiogenic effects by inhibiting the production of important inflammatory and angiogenic mediators, namely VEGF, PDGF, platelet-derived endothelial cell growth factor (PD-ECGF), COX-2, prostaglandin-E2 (PGE2), nitric oxide, NF-κB, matrix metalloproteinases and beta-catenin[8]. PDGF concentrations in plasma in our study were reduced by consumption of 3.4g/d of EPA+DHA. This is in contrast to the results of an earlier study of 63 healthy participants in which the consumption of 0.3, 0.6 or 0.9 g/day of EPA+DHA for 8 weeks had no effect on serum PDGF levels[9]. A dose of 0.6 mg/day is consistent with slightly more than 2 portions of fatty fish per week, and 1 g/day is consistent with about 4 fatty fish meals per week. These differences in the effects of EPA+DHA on PDGF suggest that doses of EPA+DHA higher than those typically achieved through diet may be necessary to achieve downregulation of PDGF. In support of this, a dose of 7 g/day for 4 weeks has been shown to suppress adherence-activated and non-activated mononuclear cell-mediated production of PDGF and MCP in humans[10]. To date, the modulation of blood concentrations of angiogenesis activator b-FGF in humans by EPA and DHA has not been reported.
It is known from in vitro studies that EPA and DHA downregulate the production of TNF-α, IL-6, IL-8, and IL-1ß via modulation of nuclear factor (NF)-B[11], while also downregulating monocyte[12] and MCP-1[13] activity. However, the doses used and duration of administration vary considerably from study-to-study in humans. While some of the studies provided <2 g EPA+DHA per day[11], others have examined the effects of higher doses[14]. Perhaps because of these differences, the amount of EPA+DHA required to exert beneficial effects is not clear. Most of these previous studies used ex vivo techniques to examine immune cell function, unlike the current study in which we directly measured plasma levels in human subjects taking approved doses of EPA+DHA.
The formation of vasa vasorum through angiogenesis has been associated with plaque instability and rupture, as micro-vessel formation has a predilection for the shoulder regions of atherosclerotic plaques[15]. Stimulators of angiogenesis that would induce the growth of new blood vessels and thus potentially reduce ischemic burden in the heart and limbs have been considered promising[16], however, despite promising results in preclinical models, data from clinical trials have been inconclusive, and evidence suggests that angiogenic factors actually promote atherosclerosis and potentially destabilize coronary plaques[2]. EPA and DHA have been shown to inhibit pathologic angiogenesis[17].
In contrast to our expectations, IL-10 concentrations decreased with EPA+DHA treatment. In an observational study involving 1123 subjects[18], lower DHA plasma levels were strongly associated with lower IL-10 concentrations. To our knowledge, there are no published data on the effects of pharmaceutical-grade EPA+DHA given at FDA-approved doses on IL-10. Interestingly, the inhibitory effect of EPA+DHA on plasma IL-10 levels was more modest in our study than for most of the other cytokines. Although we cannot know if oral doses of DHA alone would alter IL-10 levels, nor whether, in our study, it was the high dose of omega-3 fatty acids or the provision of EPA that lowered this marker, it seems possible that EPA and/or DHA on IL-10 may affect this cytokine differently depending on the dose and preparation used.
Our study found no effect of a 650mg dose of aspirin alone on plasma cytokines and pro-angiogenesis factors. This dose of aspirin was chosen because we originally wanted to study the potential effects of aspirin and EPA+DHA on platelet function[19]. It remains possible that other doses of aspirin, or ingestion of aspirin for longer time points, might affect some of the inflammatory cytokines or proangiogenesis factors measured. Alternatively, while the clearance of many cytokines and angiogenesis factors occurs quickly with half-lives of < 3 hours, it is possible that the effects of aspirin on molecules with longer half-lives could be detected in studies with longer periods of aspirin use. In addition, the data in this study should be cautiously interpreted as the number of participants was relatively small. Their age was also quite low and they were quite healthy, limiting the ability to predict the process of atherosclerosis and clinical events. Thus, different results could be found in a much larger cohort with older, or diseased, participants. In addition, we were not able to determine if the angiogenesis stimulating factors of cigarette smoking[20] and endothelial progenitor cells[21] influence the effects that EPA+DHA or aspirin had on the pro-angiogenesis molecules that we measured. Future studies will be needed to investigate these possibilities.
In conclusion, our findings support the idea that the omega-3 fatty acids EPA+DHA have anti-inflammatory and anti-angiogenesis effects in vivo, which may contribute to the beneficial effects of fish oil supplementation in susceptible human subjects who take or do not take aspirin.
Acknowledgments
We are indebted to Mary Rose Burnham, PhD, and Kelly Keating, PhD, of the Albany College of Pharmacy, for their assistance with improving the quality and grammar of the text. We are indebted to Kelly Thevenet-Morrison, MD, of the University of Rochester for her assistance with improving the quality of the figures.
Financial Support:
This publication was made possible by Grant Number KL2 RR 024136 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp. LK is supported through a Canadian Institutes of Health Research (CIHR) grant number: MOP-67121.
Footnotes
Disclosures:
Dr. Harris & Dr. Shearer have received speakership honoraria from Glaxo SmithKline. Drs. Block, Shearer, and Larson have received research support from Glaxo SmithKline for investigator-initiated clinical trials.
References
- 1.Bonow RO, Smaha LA, Smith SC, Jr, Mensah GA, Lenfant C. World Heart Day 2002: the international burden of cardiovascular disease: responding to the emerging global epidemic. Circulation. 2002;106:1602–5. doi: 10.1161/01.cir.0000035036.22612.2b. [DOI] [PubMed] [Google Scholar]
- 2.Celletti FL, Waugh JM, Amabile PG, Brendolan A, Hilfiker PR, Dake MD. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat Med. 2001;7:425–9. doi: 10.1038/86490. [DOI] [PubMed] [Google Scholar]
- 3.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kereiakes DJ. Adjunctive pharmacotherapy before percutaneous coronary intervention in non-ST-elevation acute coronary syndromes: the role of modulating inflammation. Circulation. 2003;108:III22–7. doi: 10.1161/01.CIR.0000086951.09881.51. [DOI] [PubMed] [Google Scholar]
- 5.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–57. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 6.Larson MK, Ashmore JH, Harris KA, Vogelaar JL, Pottala JV, Sprehe M, et al. Effects of omega-3 acid ethyl esters and aspirin, alone and in combination, on platelet function in healthy subjects. Thromb Haemost. 2008;100:634–41. [PubMed] [Google Scholar]
- 7.Coleman JL, Alberts MJ. Effect of aspirin dose, preparation, and withdrawal on platelet response in normal volunteers. Am J Cardiol. 2006;98:838–41. doi: 10.1016/j.amjcard.2006.03.071. [DOI] [PubMed] [Google Scholar]
- 8.Spencer L, Mann C, Metcalfe M, Webb M, Pollard C, Spencer D, et al. The effect of omega-3 FAs on tumour angiogenesis and their therapeutic potential. Eur J Cancer. 2009;45(12):2077–86. doi: 10.1016/j.ejca.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 9.Wallace JM, McCabe AJ, Roche HM, Higgins S, Robson PJ, Gilmore WS, et al. The effect of low-dose fish oil supplementation on serum growth factors in healthy humans. Eur J Clin Nutr. 2000;54:690–4. doi: 10.1038/sj.ejcn.1601076. [DOI] [PubMed] [Google Scholar]
- 10.Baumann KH, Hessel F, Larass I, Muller T, Angerer P, Kiefl R, et al. Dietary omega-3, omega-6, and omega-9 unsaturated fatty acids and growth factor and cytokine gene expression in unstimulated and stimulated monocytes. A randomized volunteer study. Arterioscler Thromb Vasc Biol. 1999;19:59–66. doi: 10.1161/01.atv.19.1.59. [DOI] [PubMed] [Google Scholar]
- 11.Calder PC. N-3 Polyunsaturated Fatty Acids, Inflammation, and Inflammatory Diseases. Am J Clin Nutr. 2006;83:1505S–19S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 12.Novak TE, Babcock TA, Jho DH, Helton WS, Espat NJ. NF-kappa B inhibition by omega -3 fatty acids modulates LPS-stimulated macrophage TNF-alpha transcription. Am J Physiol Lung Cell Mol Physiol. 2003;284:L84–9. doi: 10.1152/ajplung.00077.2002. [DOI] [PubMed] [Google Scholar]
- 13.Yin R, Huang H, Zhang J, Zhu J, Jing H, Li Z. Dietary n-3 fatty acids attenuate cardiac allograft vasculopathy via activating peroxisome proliferator-activated receptor-gamma. Pediatr Transplant. 2008;12:550–6. doi: 10.1111/j.1399-3046.2007.00849.x. [DOI] [PubMed] [Google Scholar]
- 14.Kew S, Mesa MD, Tricon S, Buckley R, Minihane AM, Yaqoob P. Effects of oils rich in eicosapentaenoic and docosahexaenoic acids on immune cell composition and function in healthy humans. Am J Clin Nutr. 2004;79:674–81. doi: 10.1093/ajcn/79.4.674. [DOI] [PubMed] [Google Scholar]
- 15.Khurana R, Simons M, Martin JF, Zachary IC. Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation. 2005;112:1813–24. doi: 10.1161/CIRCULATIONAHA.105.535294. [DOI] [PubMed] [Google Scholar]
- 16.Simons M, Ware JA. Therapeutic angiogenesis in cardiovascular disease. Nat Rev Drug Discov. 2003;2:863–71. doi: 10.1038/nrd1226. [DOI] [PubMed] [Google Scholar]
- 17.Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–73. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, et al. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006;91:439–46. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- 19.Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005;353:2373–83. doi: 10.1056/NEJMra052717. [DOI] [PubMed] [Google Scholar]
- 20.Dasgupta P, Chellappan SP. Nicotine-mediated cell proliferation and angiogenesis: new twists to an old story. Cell Cycle. 2006;5:2324–8. doi: 10.4161/cc.5.20.3366. [DOI] [PubMed] [Google Scholar]
- 21.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: part II: cell-based therapies. Circulation. 2004;109:2692–7. doi: 10.1161/01.CIR.0000128596.49339.05. [DOI] [PubMed] [Google Scholar]


