Abstract
Interactions between invasive species can have important consequences for the speed and impact of biological invasions. Containers occupied by the invasive mosquito, Aedes albopictus Skuse, may be sensitive to invasive plants whose leaves fall into this larval habitat. To examine the potential for interactions between invasive leaf species and larval A. albopictus, we conducted a field survey of leaf material found with A. albopictus in containers in Palm Beach County, Florida and measured density dependent responses of A. albopictus larvae to two invasive and one native leaf species in laboratory experiments. We found increased diversity of leaf species, particularly invasive species, in areas further from the urbanized coast, and a significant positive association between the presence of Schinus terebinthifolious (Brazilian pepper) and the abundance of A. albopictus. In laboratory experiments, we determined that larval growth and survivorship were significantly affected by both larval density and leaf species which, in turn, resulted in higher population performance on the most abundant invasive species (Brazilian pepper) relative to the most abundant native species, Quercus virginiana (live oak). These results suggest invasive leaf species can alleviate density dependent reductions in population performance in A. albopictus, and may contribute to its invasion success and potential to spread infectious disease.
Keywords: Vector, Florida, Live oak, Brazilian pepper, Australian pine, Asian tiger mosquito
Introduction
Invasive species can impact resident species, ecosystems, and even human health, often negatively (Juliano and Lounibos 2005; Lounibos 2002; Pimentel 2002; Sax et al. 2005). As co-existence of multiple invasive species becomes more common, their interactions, as opposed to interactions between invasive and resident species, will take on greater importance. Reinforcement effects of invasive species on each other suggest the potential for an “invasional melt-down,” in which the process of invasion is accelerated by positive interactions between invasive species (Simberloff 2006; Simberloff and von Holle 1999).
Plants are the fundamental source of energy in most terrestrial ecosystems. As such, invasive plants have the potential to make drastic changes in the functioning of ecosystems (Sax et al. 2005; Vitousek 1990). Small bodies of water isolated from large light inputs, such as streams, treeholes, and other natural and artificial container habitats are aquatic ecosystems especially sensitive to changes in plant species composition. The identity of native plant parts that fall into aquatic container habitats have significant effects on the invertebrate community, and on the productivity of an important taxon utilizing these habitats, mosquitoes (Diptera: Culicidae) (Barrera et al. 2006; Reiskind et al. 2009; Yanoviak 1999), but invasive plant species have not been examined in container habitats. Invasive plant species do affect invertebrates in other aquatic systems (Brown et al. 2006; Going and Dudley 2008; Maerz et al. 2005). The rapid spread and numerical dominance of invasive plants suggests they could have a large impact on container habitats.
Aedes albopictus Skuse is an invasive, container-inhabiting mosquito which relies on autochthonous plant material as a resource base for larval development. Leaf species can determine growth and survival of A. albopictus larvae (Dieng et al. 2002; Murrell and Juliano 2008; Reiskind et al. 2009; Sota 1993). For example, Reiskind et al. (2009) examined the effects of native leaf species common in intact coastal hammocks in South Florida on A. albopictus larval growth and survival. With the exception of live oak, an abundant and widespread native tree, the four species of native leaves examined in that study are probably not common in artificial container habitats as they are generally restricted to intact coastal hammocks, an increasingly rare habitat in Florida (Myers and Ewel 1990). On the other hand, invasive plant species are very common in human-dominated southern Florida landscapes and may be an important input into container habitats (Ferriter et al. 2008).
Two common invasive plants in southern Florida are Brazilian pepper (Anacardiaceae: Schinus terebinthifolious Raddi) and Australian pine (Casuarinaceae: Casuarina equisetifolia L.) (Ferriter et al. 2008). These species spread rapidly, displace native plant species, alter plant-animal interactions, and may affect whole ecosystem properties (Donnelly et al. 2008; Ferriter et al. 2008). Both plant species have qualities that make them successful invaders and may be important in provisioning container habitats. Australian pine produces copious leaf litter, and Brazilian pepper produces abundant fruits and its leaf litter may have alleleopathic properties (Donnelly et al. 2008; Ferriter et al. 2008; Morton 1978, 1980).
Invasive plants may have important implications for mosquito populations and disease transmission by influencing the distribution of A. albopictus. Aedes albopictus is the most broadly distributed invasive mosquito (Benedict et al. 2007), and a vector of dengue virus, chikungunya virus, and West Nile virus (Cupp et al. 2007; Effler et al. 2005; Gratz 2004; Hawley 1988). A recent outbreak of chikungunya virus in Italy, attributable to invasion by A. albopic tus, demonstrates the importance of this mosquito in increasing the risk of disease emergence (Bonilauri et al. 2008). Areas invaded by A. albopictus are at greater risk for disease, both by the presence of A. albopictus and by interactions of A. albopictus with native mosquitoes (Bevins 2008a, b). Therefore, if invasive plant species affect the spread of A. albopictus, plant invasions may alter the potential for pathogen transmission.
Invasive leaf species may also affect pathogen transmission by altering the survival and growth of larval A. albopictus. Previous studies have suggested that larval environment can impact the susceptibility of adult mosquitoes to infection with dengue fever virus, with larger mosquitoes more resistant to infection and dissemination of virus into peripheral tissues, a precursor to transmission (Alto et al. 2008). Furthermore, larval competition, which can be a function of resource quality, can impact adult longevity, an important determinant of disease transmission (Hawley 1985; Reiskind and Lounibos 2009). Therefore, resources that alleviate density dependent reductions in growth or survival of mosquito larvae may have an effect on disease transmission by changing the susceptibility of mosquitoes to infection, increasing the longevity of adult mosquitoes, or supporting larger populations of adult mosquitoes.
To examine the importance of invasive leaves for A. albopictus, we conducted a month-long survey of leaf material in artificial containers along an urban–rural gradient in Palm Beach County, FL as part of a longer-term survey of container inhabitating mosquitoes. From these survey data, we compared the distribution of three plant species (Australian pine [Casuarina equisetifolia L.] Brazilian pepper [Schinus terebinthifolius Raddi], and live oak [Fagaceae: Quercus virginiana Mill.]) to the abundance of A. albopictus. Then, we tested the hypothesis that A. albopictus larvae benefit from the presence of common invasive species (Australian pine and Brazilian pepper) compared to a common, native leaf species (live oak). Based upon our hypothesis, we predicted that there will be higher growth and survival in microcosms containing invasive leaf species in a laboratory experiment over three larval densities.
Materials and methods
Leaf and mosquito survey
We examined 90 containers in 30 sites (three per site) for the abundance and diversity of container dwelling mosquitoes from June 2006 to July 2008 and for the presence of vegetative parts over 4 weeks during March, 2008 in urban to rural transects in Palm Beach County, FL. Containers were black, plastic cups (9.2 cm diam × 11.11 cm height, total volume 473 ml; Bagwell Promotions, Richardson, TX). At each site, containers were attached to existing plant or man-made (e.g., fences) structures between 0.12 and 1 m (avg. = 0.45 m) above the ground, and at least three meters apart (avg. = 5.7 m). Sites were arranged in six transects with sampling sites at five distances from the coastal/urban fringe to the rural/suburban interior (0, 1, 3, 8, and 15 km from the Intracoastal Waterway). Sites at 0 km from the coast were as close to the Florida Intracoastal Waterway as possible (between 1 and 100 m). We sampled container breeding mosquitoes every 4 weeks, with a 2-week period of sampling and 2 weeks between sampling periods over the same transects described above. At the beginning of each sampling period, any water accumulated in any containers was collected with all material in the habitat. At the same time, cups were baited with 200 ml of 3-day-old oak leaf infusion (5 g/l) and a strip (7 cm × 15 cm) of seed germination paper was placed in each cup for oviposition (Seedburo, Des Plaines, IL). After a week in the field, egg papers and the aquatic fraction (water remaining from the 200 ml of bait water plus any rainfall accumulated) were collected. The cup was again baited with 200 ml of 3-day-old oak leaf infusion (5 g/l) and a strip (7 cm × 15 cm) of seed germination paper, and collected after a week in the field. All eggs were hatched and larvae were reared to fourth instar or pupae for identification. All larvae and pupae were identified to species and counted. Unhatched eggs were counted, but were not identifiable and were removed from the final mosquito counts.
We recorded the presence/absence of each plant species (or the lowest taxonomic classification possible) and type of vegetative part (leaf, fruit, or flower) from each container in each site over a 4-week sampling period in March, 2008. If any identifiable plant material in any of the three containers in a site was present, the site was considered positive for that plant. Status (native, non-native and non-invasive, or invasive) was determined by reference to the Florida Exotic Pest Plant Council list of invasive plants (Anonymous 2008) and flora of Florida (Wunderlin 1998). Some plant parts could only be identified to a higher taxonomic level than species (e.g., Graminaceae), which may contain native and non-native species. These groups are noted in the results.
Experimental leaves
To examine the effects of two common invasive plants and one native, we collected leaf material during the summer of 2008 by species-specific methods. Australian pine leaves were collected by placing tarps (1.7 m × 2.5 m) under stands of Australian pine every other day. We removed any non-Australian pine leaves. We collected Brazilian pepper by hand, only taking senesced leaves that were ready to fall or recently fallen leaves. We collected live oak leaves by hand under large live oaks during the same collection period. We air dried the collected leaf material in a low humidity environment for 3 weeks prior to use. Air drying (as opposed to oven drying) avoids potential alteration of the chemical structure of the leaves due to intense oven heat.
Mosquitoes
All mosquitoes used in this study were F1 larvae derived from A. albopictus collected as immatures during May–July 2008 from transects in Palm Beach County, FL. We allowed eggs to hatch in tap water for 24 h before use.
Microcosm experiments
All microcosms had 1 g of leaf material and 250 ml of tap water in 500 ml plastic food grade containers (Instawares Inc., Wilmington, DE). This amount of leaf material was used based upon field observations (mean = 0.38 g/container, range: 0.00–4.91 g, unpublished data) and preliminary experiments. There was a leaf treatment with three species (Causarinia equisetafolia [Australian pine], Schinus terebinthifolius [Brazilian pepper], Quercus virginiana [live oak]) and a density treatment with three levels (10 [low], 20 [medium], and 30 [high] first instar larvae), for a balanced three by three factorial design of nine individual leaf by density combinations. Combinations of leaf species were not tested. There were five replicates of each combination, for a total of 45 replicates. We added the appropriate number of first-instar larvae to microcosms within 2 h of adding water to the leaf material. We kept microcosms covered at 28° C with a 14:10 light:dark cycle in a single incubator (Percival Co., Perry, IA). We checked microcosms for pupation daily. We recorded pupation and placed all pupae from the same microcosm pupating on the same day into 50 ml conical tubes with a small amount of water. We checked tubes for emergence daily. Upon emergence, the remaining water was decanted, and we allowed emerged adults to live for 48 h before killing them by placement into a drying oven at 45°C for at least 24 h. We then sexed and weighed each adult using a micro-balance (Sartorius Micro M3P, Sartorious Balances, Data Weighing Systems, Elk Grove, IL). After weighing, we measured the left wing of each mosquito using imaging software (iSolution lite, IMT Technologies, Vancouver, BC, Canada) and a dissecting microscope.
Statistical analysis: leaf survey
We examined leaf survey data for relationships between distance from coast and species diversity. For this analysis, any plant part (fruit, flower, and leaf) was interpreted as presence of that species in that site to avoid overestimating species richness by counting leaf, fruit and flower as separate species occurrences. As we could not reject the null hypothesis of normality for these data, we used Pearson’s correlation coefficient (r) to examine correlations between distance from the coast and species richness (PROC CORR, SAS 9.1, SAS Inc., Cary, NC). We then broke species richness into three categories: native species, non-native and non-invasive species, and invasive species, as described previously. We repeated the correlation analysis for each category. Because these data sets were non-normal, we used Spearman rank correlation instead of Pearson correlation as a conservative method to assess significant correlations between distance from the coast and species richness (PROC CORR, SAS 9.1). We then compared the log-transformed total abundance of mosquitoes collected from July 2006 to July 2008 in sites with or without live oak, Brazilian pepper, and Australian pine by t-test (PROC TTEST, SAS 9.1).
Statistical analysis: leaf type and larval density
In the interest of brevity, only data from female mosquitoes are reported in this study. We measured growth rate (time to emergence) and total growth (dry weight) for each female mosquito in each replicate. As the replicate is the independent experimental unit, we calculated a mean female weight and time to emergence for each replicate to compare treatment effects. We also determined percent female survivor-ship for each replicate, assuming a 50:50 sex ratio, as is necessary for the calculation of population growth rate. We used weight as our measure of growth instead of wing length as a more inclusive measurement of total growth. As there were multiple outcomes for each replicate (weight, days to emergence and percent survival), we used multivariate analysis of variance (MANOVA) to analyze these data (Scheiner 2001) (PROC GLM, SAS 9.1). In addition to examining these outcomes, a synthetic measurement of population performance, the finite rate of population increase, λ′, was also calculated. We used the method for calculating λ′ presented in Braks et al. (2004), including the same regression equations for relating wing length to fecundity (Lounibos et al. 2002). As λ′ could not be transformed to achieve normality, and we were interested in examining interactions between leaf species and density, we performed randomization ANOVA (RT 2.1, Manly 1991) to detect differences between leaf types, densities, and the interaction between leaf type and density. Post hoc, pair wise comparisons of leaf species were performed with randomization ANOVA controlling for density, with an adjusted alpha (α = 0.0167).
Results
Leaf survey
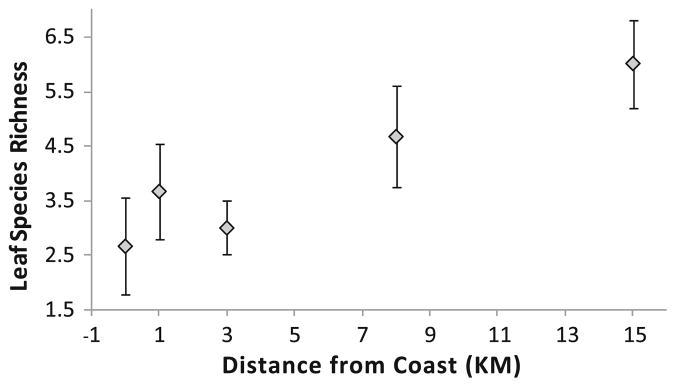
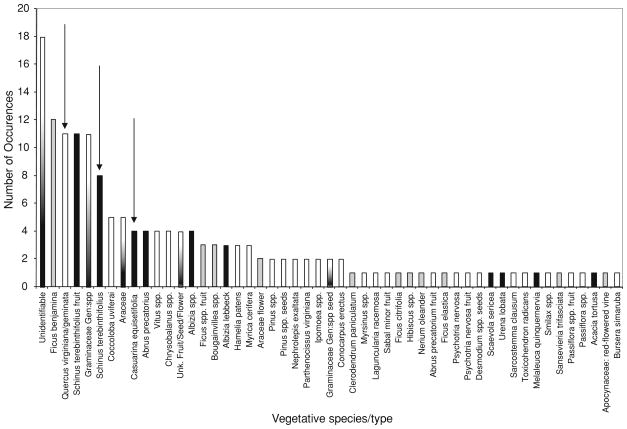
Leaf diversity was positively correlated with distance from the coast, with higher plant diversity further inland (Fig. 1). Subsequent examination of species invasive status demonstrated that this pattern was driven by increases in invasive species richness further inland (native species: Spearman’s rho = 0.2565, df = 29, P = 0.1743; non-native, non-invasive species: Spearman’s rho = −0.1066, df = 29, P = 0.5749; invasive species: Spearman’s rho = 0.5922, df = 29, P = 0.0006). The most common leaf type was “unidentifiable” (Fig. 2), demonstrating the difficulty in identifying partially rotted vegetative material of varying degrees of intactness. The three leaf species used in laboratory experiments were relatively common, being the third, sixth, and ninth most common plant species/type encountered of the 50 species/types of vegetative plant material found (Fig. 2). Sites with Brazilian pepper had more A. albopictus than sites without this plant (t = −3.36, df = 28, P = 0.0023), while there were no differences in number of A. albopictus in sites with live oak versus without live oak (t = −0.53, df = 28, P = 0.6014) nor with or without Australian pine (t = −1.72, df = 28, P = 0.0972).
Fig. 1.
Leaf species richness significantly increases with distance from the coast (Pearson correlation, r = 0.4485, df = 29, P = 0.0129). Each data point is the average of a sample from six sites. Error bars are ±1 SEM
Fig. 2.
Histogram of vegetative parts by plant species/part during March, 2008 in Palm Beach County. White bars are native species, grey bars are non-native, non-invasive species, black bars are non-native, invasive species, and bars with white–grey–black gradient are taxonomic groups that contain species of different categories. Arrows show the three leaf species considered in the laboratory experiments
Microcosm experiments
Outcomes varied significantly as a function of leaf species (Pillai’s trace = 0.809, F6,48 = 5.44, P = 0.0002) and larval density (Pillai’s trace = 0.873, F6,48 = 6.20, P < 0.0001), with no significant interaction term (Pillai’s trace = 0.456, F9,75 = 1.50, P = 0.1653).
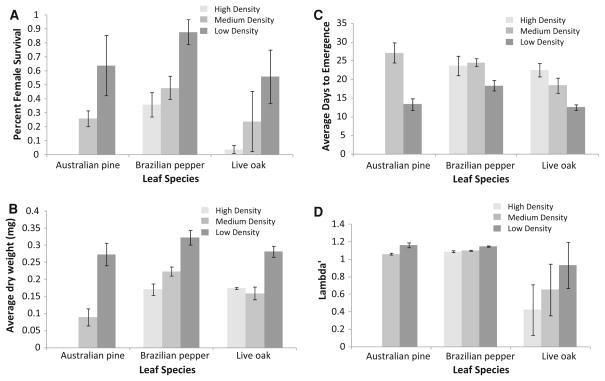
Only the first canonical coefficient was significant for the effect of leaf species (% explained variation = 84.51, F6,46 = 5.17, P < 0.0005). An examination of the standardized canonical coefficients for leaf species in the first canonical shows all three larval outcomes varying in the same direction. This means that Brazilian pepper replicates had higher percent of larvae surviving to adulthood, those that survived were larger and took longer to develop than mosquitoes from Australian pine or live oak replicates (Fig. 3a–c; Table 1). Only the first canonical was significant for density effects on larval outcomes for females (Fig. 3a–c, differently shaded bars, % explained variation = 98.82, F6,46 = 11.08, P <0.0001). Standardized canonical coefficients have weight and survival varying in the same direction, while development time varies in the opposite direction, such that low density replicates produced more mosquitoes, which grew larger and developed faster (Table 1). Estimated population performance (λ′) was affected by density, leaf species, and the interaction between these factors (Fig. 3d; Table 2). Post hoc comparisons demonstrated that, overall, Brazilian pepper microcosms had significantly higher λ′ than live oak or Australian pine microcosms when controlling for density, although Australian pine had higher λ′ than live oak or Brazilian pepper at low density (α = 0.0167).
Fig. 3.
Average female survival (a), weight in mg (b), days to emergence (c), and estimated population performance, λ′ (d). Light grey bars are high larval density (30 first instar), medium grey bars are medium larval density (20 first instar), and dark grey bars are low larval density (10 first instars). No females were produced in the high density, Australian pine treatments. Error bars are ±1 SEM
Table 1.
First canonicals from MANOVA for leaf species and larval density treatment effects for female mosquitoes and standardized canonical coefficients
| Sex | F-value (num df, den df) | P | Standardized canonical coefficients
|
||
|---|---|---|---|---|---|
| Survival | Weight | Days to emerge | |||
| Leaf | 5.17 (6,46) | < 0.0005 | 0.629 | 1.885 | 1.432 |
| Density | 10.28 (6,46) | < 0.0001 | 0.625 | 1.482 | −0.275 |
| Leaf × density | 1.39 (9,56.13) | 0.2141 | |||
The interaction term (leaf × density) was not significant so standardized canonical coefficients are not shown
Table 2.
Results of the randomization ANOVA of density and leaf species effects on estimated finite rate of increase (λ′)
| Source | df | F-value | P |
|---|---|---|---|
| Leaf | 2 | 7.21 | 0.0034 |
| Density | 2 | 8.08 | 0.0028 |
| Leaf × density | 4 | 3.28 | 0.024 |
| Error | 36 |
Discussion
Leaf litter species richness varied across the coastal-inland, urban–rural gradient, with higher diversity of leaf species in more rural, inland sites. When we examined different categories of species, we found this pattern to be significant in invasive species richness, but not native or non-native, non-invasive species. Because inland areas are transitional between rural and suburban areas, they were possibly more disturbed than coastal sites, providing a better environment for invasive plants. One of the coastal sites was an outlier for plant species richness (with six species of leaf material present, four of which were native), and was on the edge of an intact mangrove swamp. Our sampling method targets a very specific type of plant: those with leaves or parts small enough to fit into a container with an opening of 9.2 cm. Therefore, we excluded all plants with large leaves. The methodology used is pertinent to our specific system as it reflects the habitats of our focal mosquito species but does not capture all plant species. In previous studies, A. albopictus abundance has been associated with rural settings, mirroring the patterns in leaf species richness observed in this study (Braks et al. 2003; Rey et al. 2006). This suggests that A. albopictus abundance may be related to degree of urbanization or plant type, or an interaction between these two factors.
As the leaf survey was conducted over a short time frame, it may not capture the full diversity of species in the region over a full year. A previous study examining litter fall in southern Florida in live oaks noted seasonal differences in abundance of both flower and leaf litter, with respective peaks in February/March and October (Lounibos et al. 1992). We did not quantify the amount of each species of litter, and it is possible that widespread species may be sparse within each site, making the contribution to each artificial container rare. We can conclude that the three leaf species considered in the laboratory part of this study were widespread and are often encountered as a component of the nutritional base of larval A. albopictus in southern Florida.
Native live oak was the most common native leaf material found. The two invasive species considered (Brazilian pepper and Australian pine) were the two most common invasive species in the leaf survey. The most common identifiable leaf material encountered was the non-native Ficus benjamina, a commonly planted ornamental in southern Florida. We chose not to examine this species because, while common, it is not considered an invasive species (Anonymous 2008). The Brazilian pepper fruits were more common than Brazilian pepper leaf material, suggesting that plant fruit may be an important and rarely considered input into mosquito larval habitats. Previous studies have demonstrated native tree flowers can be important resources in mosquito dominated tree-holes (Lounibos et al. 1993), although there is no literature on the importance of fruits in larval mosquito habitats. As the large production of highly dispersive fruits is a characteristic of successful invasive plants (Colautti et al. 2006; Sax et al. 2005), this observation may point to an important component of an invasive plant-mosquito interaction complex.
We found sites with Brazilian pepper had more mosquitoes, as sampled over a 2- year period, than sites without Brazilian pepper. The presence of the other two leaf species examined in this study did not have any significant association with mosquito abundance. These data are correlative and we cannot conclude that the reason for the higher abundance of mosquitoes in those sites is due to the presence of Brazilian pepper. Both Brazilian pepper and A. albopictus may be responding to similar conditions and are thus found in similar habitats. The ubiquity of Brazilian pepper in the southern Florida landscape may make it difficult to disentangle the urban–rural pattern of A. albopictus abundance from its association with Brazilian pepper. There may be areas in Central Florida that have little Brazilian pepper which may provide a way of disentangling this correlation. Other options might be the removal of Brazilian pepper from sites and then monitoring the response of A. albopictus populations, although this experimental approach would be labor intensive, and difficult to control for other environmental variables that would be influenced by the removal of a large amount of plant material. Nevertheless, the spatial association of Brazilian pepper and A. albopictus coupled with the superior performance of larvae on Brazilian pepper in laboratory settings is suggestive of a possible relationship.
Mosquito survival and growth were superior on Brazilian pepper relative to larval survival and growth on live oak or Australian pine at the same larval densities. Different leaf species were associated with larger adult weight, higher survival to adulthood, and longer development time. This can be attributed to high survival, increased growth, and slower development on the invasive Brazilian pepper relative to the other two leaf resources. A previous study that examined these same variables for A. albopictus, albeit with different leaf types and a single density of larvae, found a different pattern (Reiskind et al. 2009). In that study, different leaf species were associated with time to emergence and adult size varying in the same direction, while survival varied in the opposite direction. The variation in the direction and magnitude of effects of mosquito outcomes as a function of leaf type provides further evidence that leaf species affects larval growth in an idiosyncratic manner, possibly depending on the specific microorganisms that make up the mosquito larval diet and decay leaf material (Kaufman et al. 2008; Reiskind et al. 2009). Regrettably, other studies examining A. albopictus response to leaf species have not examined relationships among outcome variables (by using MANOVA), so we cannot compare their results (e.g., Dieng et al. 2002; Sota 1993).
Density also has a significant effect on mosquito growth and survival in all leaf types. The relationship among outcomes was different than that observed for leaf species effects. Higher densities were associated with poorer survival, longer development time, and lower adult weight. This same pattern is seen in numerous previous studies showing density dependence in a diverse array of container-dwelling mosquitoes (Juliano 2009).
Why the relationships among outcome variables are altered when comparing different leaf environments to different density environments remains unclear, but does generate several hypotheses. One possibility is that mosquito larvae adjust their development rate based upon habitat quality. Another is that there are stage-specific effects of leaf environment such that early instar larvae are vulnerable to toxicity, retarding their development or increasing early development mortality. Consequently, those that manage to survive the vulnerable period find themselves in an environment with less competition or with less toxic resources and achieve high levels of growth and survival. This hypothesis could be tested by examining stage specific survival/growth rates with the different leaf resources. It is also possible that the relationships among outcome variables may be idiosyncratic for each leaf species. This suggests a more complex process of toxicity, nutrient availability, and larval response.
We found support for our hypothesis that leaf species affects the intensity of density dependent interactions for larval A. albopictus. Although there was no significant leaf by density interaction for female mosquitoes, there was a significant interaction term in the analysis of λ’. Population performance was higher in microcosms provisioned with leaves from the invasive Brazilian pepper, relative to native live oak and invasive Australian pine at high and medium larval densities, but was slightly lower than Australian pine at low densities. In general, this suggests that habitats containing the leaves of Brazilian pepper would support larger mosquito populations relative to the native live oak. Previous studies examining different resources have noted that resource type, broadly defined, may alleviate density dependence in A. albopictus when resource types are very different (e.g., animal versus plants; fresh-cut grass versus senesced oak leaves) (Murrell and Juliano 2008; Yee et al. 2007). This study shows that similar resources, senesced tree leaves, may be sufficiently different in quality to alter density dependence of population growth. As there is no positive feedback between mosquito larvae and invasive plants, they may not truly be participating in an “invasional meltdown” (Simberloff and von Holle 1999), but the spread of Brazilian pepper may contribute to the invasion success of A. albopictus. Therefore, in addition to direct concerns for human health associated with these invasive plants (due to allergic reactions; see Morton 1978), invasive plants, by changing the container ecosystems responsible for disease vector production, may also change the risk of human disease (Alto et al. 2008; Bevins 2008a, b).
Several questions remain unanswered in this system. How do other species of mosquitoes respond to these leaves and does this affect interspecific competition? How do mosquitoes respond to combinations of these leaves? Do female mosquitoes make oviposition decisions based upon the quality of leaf species, and how might this affect invasion success? Finally, is there any direct connection between leaf environment and disease transmission similar to the situation described by Alto et al. (2008)?
Acknowledgments
The authors wish to thank Naoya Nishimura and Krystle Greene for measuring the wing lengths of the mosquitoes in the microcosm experiments and for assisting with identifying leaf material from the field. We thank Talan Klein for assisting with the microcosm experiments and mounting wings. We also wish to thank Dr. George O’Meara, Dr. Eric Rebek and Dr. George Opit for comments on earlier versions of this manuscript. We thank Dr. Kris Giles for the use of his microbalance. This research was funded by NIH grant 5R01AI-044793 to LPL, and by the Oklahoma Agricultural Experiment Station (MSS “NE-507” Hatch Project # 2712 and OAES Hatch Project #2702 to MHR). The authors also wish to thank an anonymous referee for their helpful comments.
Contributor Information
Michael H. Reiskind, Email: michael.h.reiskind@okstate.edu, Department of Entomology and Plant Pathology, Oklahoma State University, 127 Noble Research Center, Stillwater, OK 74078, USA
Ali A. Zarrabi, Department of Entomology and Plant Pathology, Oklahoma State University, 127 Noble Research Center, Stillwater, OK 74078, USA
L. Philip Lounibos, Florida Medical Entomology Laboratory, Department of Entomology and Nematology, University of Florida, 200 9th Street S.E., Vero Beach, FL 32962, USA.
References
- Alto BW, Lounibos LP, Mores CN, Reiskind MH. Larval competition alters susceptibility of adult Aedes mosquitoes to dengue infection. Proc Roy Soc B. 2008;275:463–471. doi: 10.1098/rspb.2007.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous. Florida EPPC’s 2007 Invasive plant species list. [Accessed June 15, 2008];2008 Available from the Florida exotic pest plant council webpage: http://www.fleppc.org/list/07list.htm.
- Barrera R, Amador M, Clark GG. Ecological factors influencing Aedes aegypti (Diptera: Culicidae) productivity in artificial containers in Salinas, Puerto Rico. J Med Entomol. 2006;43:484–492. doi: 10.1603/0022-2585(2006)43[484:efiaad]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Benedict MQ, Levine RS, Hawley WA, Lounibos LP. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector-Borne Zoonotic Dis. 2007;7:76–85. doi: 10.1089/vbz.2006.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins SN. Effects of expanded mosquito range. Science. 2008a;321:1634. doi: 10.1126/science.321.5896.1634. [DOI] [PubMed] [Google Scholar]
- Bevins SN. Invasive mosquitoes, larval competition, and indirect effects on the vector competence of native mosquito species (Diptera: Culicidae) Biol Invasions. 2008b;10:1109–1117. [Google Scholar]
- Bonilauri P, Bellini R, Calzolari M, Angeflni R, Venturi L, Fallacara F, Cordioli P, Angelini P, Venturolli C, Merialdi G, Dottori M. Chikungunya virus in Aedes albopictus, Italy. Emerg Inf Dis. 2008;14:852–854. doi: 10.3201/eid1405.071144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braks MAH, Honorio NA, Lourenco-De-Oliveira R, Juliano SA, Lounibos LP. Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in southeastern Brazil and Florida. J Med Entomol. 2003;40:785–794. doi: 10.1603/0022-2585-40.6.785. [DOI] [PubMed] [Google Scholar]
- Braks MAH, Honorio N, Lounibos LP, Lourenco-De-Oliveira R, Juliano S. Interspecific competition between two invasive species of container dwelling mosquitoes, Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Brazil. Ann Entomol Soc Am. 2004;97:130–139. [Google Scholar]
- Brown CJ, Blossey B, Maerz JC, Joule SJ. Invasive plant and experimental venue affect tadpole performance. Biol Invasions. 2006;8:327–338. [Google Scholar]
- Colautti RI, Grigorovich IA, MacIsaac HJ. Propagule pressure: a null model for biological invasions. Biol Invasions. 2006;8:1023–1037. [Google Scholar]
- Cupp EW, Hassan HK, Yue X, Oldland WK, Lilley BM, Unnasch TR. West Nile virus infection in mosquitoes in the Mid-South USA, 2002–2005. J Med Entomol. 2007;44:117–125. doi: 10.1603/0022-2585(2007)44[117:wnviim]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieng H, Mwandawiro C, Boots M, Morales R, Satho T, Tuno N, Tsuda Y, Takagi M. Leaf litter decay process and the growth performance of Aedes albopictus larvae (Diptera: Culicidae) J Vector Ecol. 2002;27:31–38. [PubMed] [Google Scholar]
- Donnelly MJ, Green DM, Walters LJ. Allelopathic effects of fruits of the Brazilian pepper Schinus terebinthifolius on growth, leaf production and biomass of seedlings of the red mangrove Rhizophora mangle and the black mangrove Avicennia germinans. J Exp Mar Biol Ecol. 2008;357:149–156. [Google Scholar]
- Effler PV, Pang L, Kitsutani P, Vorndam V, Nakata M, Ayers T, Elm J, Tom T, Reiter P, Rigau-Perez JG, Hayes JM, Mills K, Napier M, Clark GG, Gubler DJ Hawaii Dengue Outbreak Investigation Team . Dengue fever, Hawaii, 2001–2002. Emerg Infect Dis. 2005;11:742–749. doi: 10.3201/eid1105.041063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferriter A, Doren B, Winston R, Thayer D, Miller B, Thomas B, Barrett M, Pernas T, Hardin S, Lane J, Kobza M, Schmitz D, Bodle M, Toth L, Rodger L, Pratt P, Snow S, Goodyear C. Protection FDoE. Chapter 9: The Status of Nonindigenous Species in the South Florida Environment. South Florida Water Management District; West Palm Beach: 2008. pp. 9.1–9.101. [Google Scholar]
- Going BM, Dudley TL. Invasive riparian plant litter alters aquatic insect growth. Biol Invasions. 2008;10:1041–1051. [Google Scholar]
- Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004;18:215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- Hawley WA. The effect of larval density on adult longevity of a mosquito, Aedes sierrensis—epidemiological consequences. J Anim Ecol. 1985;54:955–964. [Google Scholar]
- Hawley W. The biology of Aedes albopictus. J Am Mosquito Control Suppl. 1988;1:1–40. [PubMed] [Google Scholar]
- Juliano S. Species interactions among larval mosquitoes: context dependence across habitat gradients. Ann Rev Entomol. 2009;54:37–56. doi: 10.1146/annurev.ento.54.110807.090611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett. 2005;8:558–574. doi: 10.1111/j.1461-0248.2005.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MG, Chen S, Walker ED. Leaf-associated bacterial and fungal taxa shifts in response to larvae of the tree hole mosquito, Ochlerotatus triseriatus. Micro Ecol. 2008;55:673–684. doi: 10.1007/s00248-007-9310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos LP. Invasions by insect vectors of human disease. Ann Rev Entomol. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, Nishimura N, Escher RL. Seasonality and components of Oak litterfall in southeastern Florida. Fla Sci. 1992;55:92–98. [Google Scholar]
- Lounibos LP, Nishimura N, Escher RL. Fitness of a treehole mosquito—influences of food type and predation. Oikos. 1993;66:114–118. [Google Scholar]
- Lounibos LP, Suarez S, Menendez Z, Nishimura N, Escher RL, O’Connell SM, Rey JR. Does temperature affect the outcome of larval competition between Aedes aegypti and Aedes albopictus? J Vector Ecol. 2002;27:86–95. [PubMed] [Google Scholar]
- Maerz JC, Brown CJ, Chapin CT, Blossey B. Can secondary compounds of an invasive plant affect larval amphibians? Funct Ecol. 2005;19:970–975. [Google Scholar]
- Morton JF. Brazilian pepper—its impact on people, animals and the environment. Econ Bot. 1978;32:353–359. [Google Scholar]
- Morton J. The Australian pine or beefwood (Causarina equisetifolia L.), an invasive “Weed” tree in Florida. Proc Fla State Hortic Soc. 1980;93:87–95. [Google Scholar]
- Murrell EG, Juliano SA. Detritus type alters the outcome of interspecific competition between Aedes aegypti and Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2008;45:375–383. doi: 10.1603/0022-2585(2008)45[375:dtatoo]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RL, Ewel JJ. Ecosystems of Florida. xviii. University of Central Florida Press; Orlando: 1990. p. 765. [Google Scholar]
- Pimentel D. Biological invasions: economic and environmental costs of alien plant, animal, and microbe species. CRC Press; Boca Raton: 2002. p. 369. [Google Scholar]
- Reiskind M, Lounibos L. Effects of intraspecific larval competition on adult longevity in the mosquitoes Aedes aegypti and Aedes albopictus. Med Vet Entomol. 2009;23:62–68. doi: 10.1111/j.1365-2915.2008.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiskind M, Greene K, Lounibos L. Leaf species identity and combination affect performance and oviposition choice of two container mosquito species. Ecol Entomol. 2009;34(4):447–456. doi: 10.1111/j.1365-2311.2008.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey JR, Nishimura N, Wagner B, Braks MAH, O’Connell SM, Lounibos LP. Habitat segregation of mosquito arbovirus vectors in south Florida. J Med Entomol. 2006;43:1134–1141. doi: 10.1603/0022-2585(2006)43[1134:hsomav]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sax DF, Stachowicz JJ, Gaines SD. Species invasions: insights into ecology, evolution, and biogeography. xiii. Sinauer Associates; Sunderland: 2005. p. 495. [Google Scholar]
- Scheiner SM. MANOVA. In: Gurevitch J, Schiener SM, editors. Design and analysis of ecological experiments. Oxford University Press; New York: 2001. p. 415. [Google Scholar]
- Simberloff D. Invasional meltdown 6 years later: important phenomenon, unfortunate metaphor, or both? Ecol Lett. 2006;9:912–919. doi: 10.1111/j.1461-0248.2006.00939.x. [DOI] [PubMed] [Google Scholar]
- Simberloff D, von Holle B. Positive interactions of non-indigenous species: invasional meltdown? Biol Invasions. 1999;1:21–32. [Google Scholar]
- Sota T. Performance of Aedes albopictus and A. riversi larvae (diptera, culicidae) in waters that contain tannic-acid and decaying leaves—is the treehole species better adapted to treehole water? Ann Entomol Soc Am. 1993;86:450–457. [Google Scholar]
- Vitousek PM. Biological invasions and ecosystem processes—towards an integration of population biology and ecosystem studies. Oikos. 1990;57:7–13. [Google Scholar]
- Wunderlin RP. Guide to the vascular plants of Florida. x. University Press of Florida; Gainesville: 1998. p. 806. [Google Scholar]
- Yanoviak SP. Effects of leaf litter species on macroin-vertebrate community properties and mosquito yield in neotropical tree hole microcosms. Oecologia. 1999;120:147–155. doi: 10.1007/s004420050843. [DOI] [PubMed] [Google Scholar]
- Yee DA, Kaufman MG, Juliano SA. The significance of ratios of detritus types and micro-organism productivity to competitive interactions between aquatic insect detritivores. J Anim Ecol. 2007;76:1105–1115. doi: 10.1111/j.1365-2656.2007.01297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]