Abstract
Objective
To examine the cellular and molecular mechanisms underlying alcoholic cardiomyopathy.
Background
The mechanism for alcoholic cardiomyopathy remains largely unknown.
Results
Mice were fed alcohol or isocaloric control diet for 2 months. As compared with control, hearts from alcohol-fed mice exhibited increased apoptosis, along with significant nitrative damage, demonstrated by 3-nitrotyrosine (3-NT) abundance. Alcohol exposure to H9c2 cells induced apoptosis, accompanied by 3-NT accumulation and nicotinamide adenine dinucleotide phosphate oxidase (NOX) activation. Pre-incubation of H9c2 cells with urate (peroxynitrite scavenger), L-NAME (nitric oxide synthase inhibitor), MnTMPyP (SOD mimetic) and apocynin (NOX inhibitor) abrogated alcohol-induced apoptosis. Furthermore, alcohol exposure significantly increased the expression of angiotensin II (Ang II) and its type 1 receptor (AT1). A protein kinase C (PKC)-α/β1 inhibitor or PKC-β1 siRNA and an AT1 blocker prevented alcohol-induced activation of NOX, and AT1 blocker losartan significantly inhibited the expression of PKC-β1, indicating that alcohol-induced activation of NOX is mediated by PKC-β1 via AT1. To define the role of AT1-mediated PKC/NOX-derived superoxide generation in alcohol-induced cardiotoxicity, mice with knockout of AT1 gene (AT1-KO) and wide-type mice were simultaneously treated with alcohol for 2 months. Knockout AT1 gene completely prevented cardiac nitrative damage, cell death, remodeling and dysfunction. More importantly, pharmacological treatment of alcoholic mice with superoxide dismutase mimetic also significantly prevented cardiac nitrative damage, cell death and remodeling.
Conclusions
Alcohol-induced nitrative stress and apoptosis, which is mediated by Ang II interaction with AT1 and subsequent activation of a PKCβ1-dependent NOX pathway, is a causal factor in the development of alcoholic cardiomyopathy.
Keywords: Alcoholic cardiomyopathy, cardiac cell death, oxidative and nitrative stress, angiotensin II, PKC, losartan
Introduction
Regular heavy consumption of alcohol is associated with a non-ischemic cardiomyopathy, termed alcoholic cardiomyopathy that contributes to about one-fifth of all sudden cardiac death (1). Oxidative stress is considered responsible for alcoholic cardiomyopathy (2). However, how alcohol generates oxidative stress and how oxidative stress triggers the development of alcoholic cardiomyopathy has not been well defined.
Cell death, resulting from either necrosis or apoptosis, is an important component of the cardiomyopathic phenotype (3). Capasso et al. found significant myocyte loss in the left ventricle of rats fed ethanol in drinking water for 8 months (4). Hearts from alcoholic patients with structural heart disease exhibited apoptotic indices similar to those from hypertensive donors (5), with greater Bax and Bcl-2 expression compared to hearts from control subjects. Moreover, alcoholic patients without structural heart damage only displayed higher Bax and Bcl-2 without apoptosis (5). Therefore, myocardial apoptosis occurs to a similar extent in heavy drinkers and longstanding hypertensive subjects and is related to structural damage. However, how alcohol induces cardiac cell death requires further investigation.
Reactive oxygen and nitrogen species (ROS and RNS) can be generated endogenously by specific enzymes (6). Nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX) generate superoxide through electron transfer from NADPH to molecular oxygen. Seven NOX family members (i.e., NOX1–5 and Duox1 and 2) have been identified (7), of which NOX1, NOX2 and NOX4 are main isoforms expressed in cardiovascular cells. To date, both NOX2 and NOX4 were defined in cardiac myocytes (7). Each of these isoforms exists as a heterodimer with a lower molecular weight p22phox subunit and are predicted to be membrane-bound but two isoforms are also distinct each other. NOX2 is normally quiescent and acutely activated by stimuli such as G-protein-coupled receptor agonists [e.g., angiotensin II (Ang II), endothelin-1], growth factors, and cytokines in a tightly regulated process (7). NOX2 activation requires stimulus-induced membrane translocation of p47phox (i.e. formation of the active oxidase complex at the membrane) (8). We and others have demonstrated that activation of p47phox by diabetes and Ang II lead to significantly cardiac oxidative damage and cell death, and consequently results in cardiomyopathy (9–10). Unlike NOX2, NOX4 does not require additional regulatory subunits with a constitutive low-level activity and is regulated largely by changes in abundance (7). In addition to generating ROS (11), NOX4 also protects the heart against oxidative damage under certain conditions (12).
A previous study has implicated the possible involvement of Ang II in the development of alcoholic cardiomyopathy since simultaneous application of the Ang II type 1 receptor (AT1) blocker irbesartan significantly attenuated alcoholic inhibition of cardiac function (13); however, in this study plasma Ang II levels and cardiac AT1 expression were significantly increased only in alcohol-treated dogs, but not in alcohol/irbesartan-treated dogs. Accordingly this study resulted in several critical questions: [1] why did the dogs of alcohol group show significant increases in both plasma Ang II level and cardiac AT1 expression, but the dogs of the alcohol/irbesartan group did not? [2] Is alcoholic increase of the plasma Ang II level and cardiac AT1 expression in the dogs of alcoholic group really causative of alcoholic cardiomyopathy? [3] How did irbesartan prevent alcohol-induced cardiomyopathy if the dogs did not show increases in plasma Ang II level and cardiac AT1 expression? Therefore, this study actually did not support the involvement of Ang II/AT1 in the development of alcoholic cardiomyopathy.
Considering that NOX-mediated generation of superoxide and associated oxidative and nitrative stress play important roles in the development of various cardiomyopathies, we first investigated whether NOX activation and peroxynitrite formation are involved in alcohol-mediated cardiac cell death; Then we further investigated whether the nitrative stress and damage in the alcoholic heart is associated with an increase in systemic and cardiac Ang II and AT1. To these ends, mechanistic studies were done with chronic alcohol-fed mice and in vitro cultured H9c2 cardiomyocytes. We found that alcohol-induced cardiac cell death is triggered by nitrative stress generated from an Ang II-activated PKCβ1- and NOX-dependent pathway in vitro and in vivo. To define the causative role of Ang II in the alcoholic induction of cardiac cell death, transgenic mice with knockout of Ang II type 1 receptor gene (AT1-KO) were used, and to define the causative role of NOX-derived superoxide in the induction of cardiac nitrative damage and cell death, chronic alcohol-fed mice were simultaneously treated with superoxide dismutase mimetic to scavenge superoxide. Both animal models were completely resistant to alcoholic induction of cardiac nitrative damage and cell death, and the development of cardiomyopathy. Therefore, this study provides, for the first time, direct evidence that Ang II plays a pivotal role in chronic alcohol consumption-induced cardiac nitrative damage, cell death, remodeling, and cardiomyopathy via a PKC/NOX-dependent pathway.
Methods
Detail methods are provided in the Online Appendix. Briefly, 4-month old male AT1-KO mice and the wild-type (WT) C57BL/6 mice were used and treated according to experimental procedures approved by the Institutional Animal Care and Use Committee. Mice were pair-fed a modified Lieber-DeCarli alcohol or isocaloric maltose dextrin control liquid diet for 2 months with a stepwise feeding procedure. Some alcohol-fed mice were simultaneously treated with superoxide dismutase mimetic manganese(III) tetrakis(1-methyl-4-pyridyl)porphyrin (MnTMPyP). At the end of experiments, blood pressure was measured by tail-cuff monitoring system and heart function was evaluated by echocardiography. Then mice were sacrificed to harvest the heart for protein, mRNA and histopathological examination.
H9c2 rat cardiac cell line were exposed to different doses of alcohol (100–400 mmol/L) for 24 h. Effects of alcohol on apoptotic cell, nitrative damage [3-nitrotyrosine (3-NT) accumulation], and NOX expression and translocation (subunit p47phox) were examined. Generation of superoxide (O2·−) and peroxynitrite (ONOO−) was measured using fluorescent probes dihydroethidium (DHE) and dihydrorhodamine-123 (DHR-123), respectively according to previous report (14). The role of superoxide, nitric oxide, peroxynitrite, PKC-β1, and p47phox activation in alcohol-induced caspase-3 cleavage was defined with corresponding inhibitors, scavengers or siRNA, respectively, as described previously (10).
Immunohistochemical staining was conducted as described previously (10,15). AT1 mRNA expression was measured by Real-time quantitative PCR (qPCR). Western blot assay was performed for protein quantification as described previously (10,15). Ang II levels in the plasma, cardiac tissue, H9c2 culture medium, and cell lysate were analyzed with enzyme immunoassay.
Statistical analysis
Data were collected from repeated experiments and presented as mean ± SD. For statistical analysis, one way analysis of variance (ANOVA) was used with overall F-test analysis for ANOVA’s significance. Then multiple comparisons were performed by the Bonferron test with Origin 7.5 Lab data analysis and graphing software (OriginLab Corporation, Northampton, MA). Statistical significance was considered at P <0.05.
Results
Alcohol induces cell death and nitrative damage in the heart
Apoptotic cell death in the heart of alcohol-fed mice, examined by TUNEL staining (Fig. S1A,B) and Western blot of the cleaved caspase-3 (Fig. S1C), was significantly increased along with a significant increase in 3-NT modification of multiple proteins (22 – 98 kDa, Fig. S1D) as nitrative damage.
Alcohol-induced cardiac cell death and nitrative damage are mediated by NOX activation
To define whether the cardiac cell death was a consequence of nitrative damage, H9c2 cells were directly exposed to alcohol in vitro for 24 h. A dose-dependent apoptotic effect of alcohol was found by examining caspase-3 cleavage (Fig. 1A) and DNA fragmentation (Fig. 1B). Alcohol-exposed H9c2 cells also exhibited augmented 3-NT modification of multiple proteins (Fig. 1C).
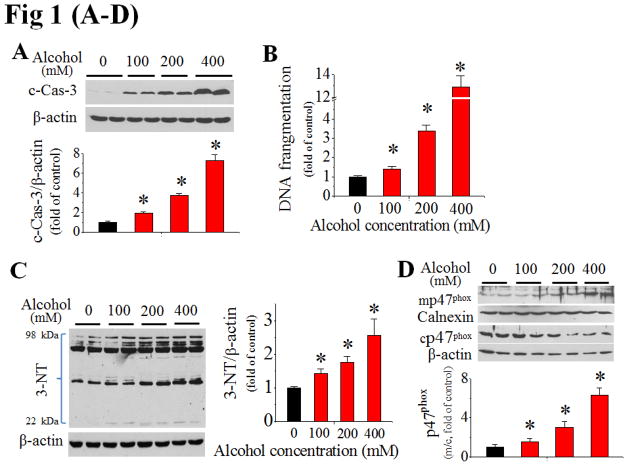
Figure 1. Alcohol-induced cardiac cell death, nitrative damage and NOX activation in vitro.
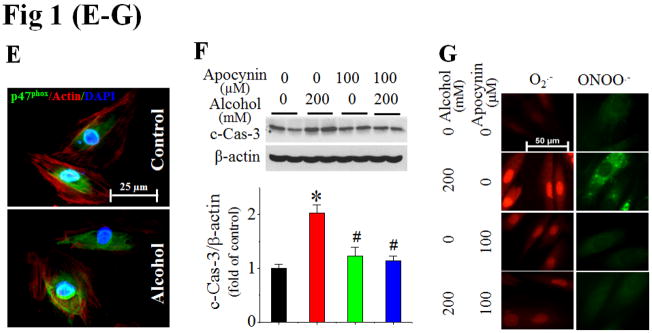
H9c2 cells exposed to alcohol (100–400 mM) for 24 h exhibited increased caspase-3 cleavage [c-Cas-3, (A)], DNA fragmentation (B), and 3-NT accumulation (C). Alcohol also activated p47phox at 2 h, shown by increased ratio of membrane (mp47phox) to cytosol (cp47phox), i.e.: m/c (D), and membrane localization by immunefluorescent staining (E). Pretreatment of H9c2 cells with NOX inhibitor, apocynin at 30 min prior to and during 24 h alcohol (200 mM) exposure prevented alcohol-induced caspase-3 cleavage (F), and superoxide (O2·−) and peroxynitrite (ONOO·−) accumulation, detected by DHE and DHR123 staining, respectively (G). All in vitro data for Fig. 1–3 were from at least three independent experiments. *, p<0.05 vs control; #, p<0.05 vs alcohol treatment.
Next we have defined the role of NOX activation in these effects. First, alcohol exposure of H9c2 cells increased p47phox expression in a dose-dependent manner (Fig. S2). The ratio of p47phox expression in the cell membrane fraction to cytosolic fraction was also significantly increased in a dose-dependent manner by Western blot (Fig. 1D) and fluorescent staining for p47phox (Fig. 1E). Furthermore, H9c2 cells were treated with the NOX inhibitor, apocynin at 100 μmol/L started 30 min prior to alcohol exposure. Apocynin significantly attenuated the caspase-3 activation (Fig. 1F) and the increased accumulation of superoxide and peroxynitrite in alcohol-treated cells (Fig. 1G), suggesting a causative role of NOX activation and nitrative stress in alcohol-mediated cell death.
We further defined the role of nitrative stress in alcohol-induced apoptosis since the peroxynitrite scavenger urate that was applied 30 min prior to 200 mmol/L alcohol exposure could completely attenuate caspase-3 activation (Fig. S3A). Alcohol-induced cell death was also abolished by pre-treatment with NOS inhibitor L-NAME (Fig. S3B) or superoxide dismutase mimetic MnTMPyP (Fig. S3C).
Alcohol-induced NOX activation is mediated by PKC-β1
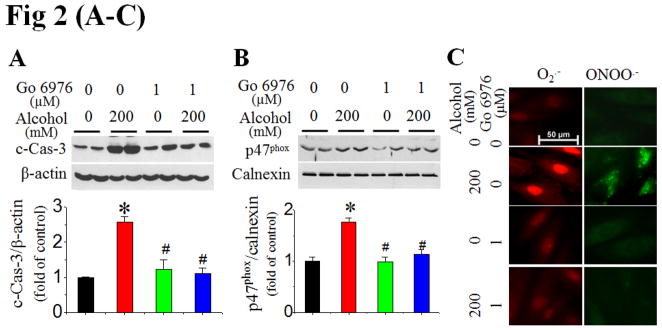
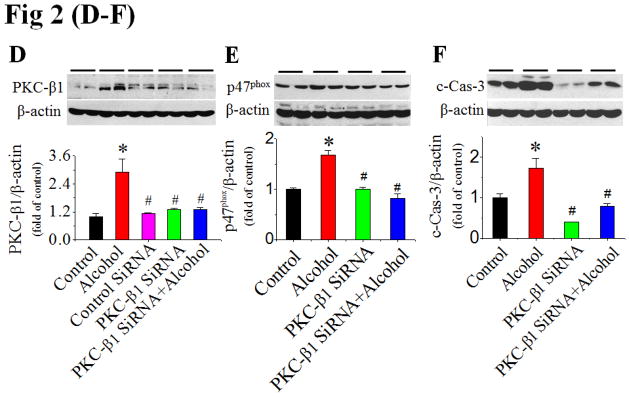
To define the role of PKC in alcohol-induced activation of NOX and subsequent apoptosis, we examined the effect of a specific PKC-α/β1 inhibitor (Go 6976) applied 30 min prior to alcohol exposure in H9c2 cells. Go 6976 significantly attenuated caspase-3 activation (Fig. 2A), NOX2 activation [shown by increased p47phox expression in membrane fraction (Fig. 2B) and scattering distribution of fluorescent staining of p47phox (Fig. S4)], and the accumulation of superoxide and peroxynitrite (Fig. 2C) in cells exposed to 200 mmol/L alcohol. Furthermore, the fact that inhibition of alcohol-induced PKC-β1 expression by its siRNA (Fig. 2D) resulted in a complete abolishment of alcohol-induced p47phox expression (Fig. 2E) and caspase-3 activation (Fig. 2F) established the direct role of PKC-β1 in activation of NOX-mediated apoptosis.
Figure 2. Alcohol activation of NOX is mediated by PKC-β1.
H9c2 cells were treated with PKC-α/β1 inhibitor (Go 6976) at 30 min prior to and during alcohol exposure. Caspase-3 cleavage at 24 h (A), p47phox activation (B) and superoxide and peroxynitrite accumulation at 2 h (C) after alcohol exposure were detected. Knockdown of PKC-β1 expression with PKC-β1 SiRNA in H9c2 cells with and without alcohol (200 mM) was confirmed (D), and completely attenuated alcohol induction of p47phox expression at 2 h (E) and caspase-3 cleavage (F) at 24 h after alcohol exposure. β-actin or calnexin was used as loading control for Western blots, respectively. *, p<0.05 vs control; #, p<0.05 vs alcohol treatment.
Alcohol activation of PKC and NOX is AT1-dependent
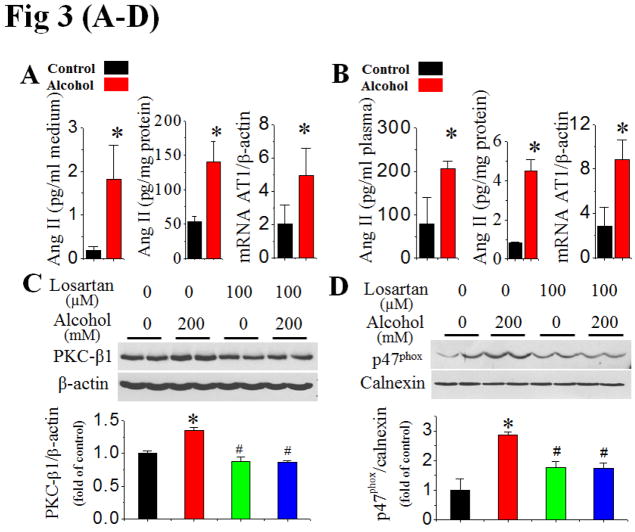
In this study, whether alcohol exposure increases Ang II generation and AT1 receptor expression in cardiac cells was examined. Ang II levels in both cell lysate and the medium of H9c2 cells exposed to alcohol at 200 mmol/L for 24 h significantly increased relative to controls (Fig. 3A). Augmented Ang II levels were accompanied by a significant increase in AT1 gene expression in alcohol-exposed cells (Fig. 3A). Furthermore Ang II levels in the hearts and plasma, and cardiac AT1 gene expression in alcohol-fed mice for 2 months were also significantly increased (Fig. 3B).
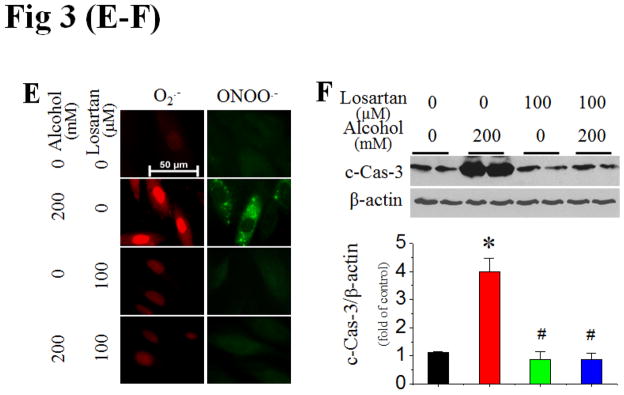
Figure 3. Activation of PKC and NOX is mediated by alcohol-increased Ang II contents and AT1 expression.
Extracellular (in the culture medium) and intracellular (in the cell lysates) Ang II levels at 24 h and AT1 mRNA expression at 2 h after H9c2 cells exposed to alcohol (200 mM) were examined with ELISA and qPCR assays, respectively (A). Ang II contents in the plasma and cardiac tissues, and cardiac AT1 mRNA expression were also detected, respectively, after mice (n 5) were fed with alcohol for 2 months (B). H9c2 cells were treated with AT1 specific blocker losartan at 30 min prior to and during alcohol exposure. PKC-β1 expression (C), p47phox membrane translocation (D), and superoxide and peroxynitrite accumulation (E) were examined at 2 h after alcohol exposure while caspase-3 cleavage (F) was examined at 24 h after alcohol exposure. β-actin or calnexin was used as loading control for Western blots, respectively. *, p<0.05 vs control; #, p<0.05 vs alcohol treatment.
Next study examined the effect of the AT1 blocker losartan on alcohol-induced PKC expression, NOX activation and cell death. Pre-treatment of H9c2 cells with losartan (100 μmol/L) completely prevented alcohol-induced PKC-β1 upregulation (Fig. 3C), p47phox membrane translocation (Fig. 3D, Fig. S4), superoxide and peroxynitrite accumulation (Fig. 3E), and cell death (Fig. 3F). Hence, Ang II interaction with AT1 was required for alcohol-induced nitrative stress and subsequent cell death mediated by PKC-β1and NOX activation.
Knockout AT1 gene prevents the cardiac nitrative stress, cell death, remodeling, and dysfunction in mice fed with chronic alcohol
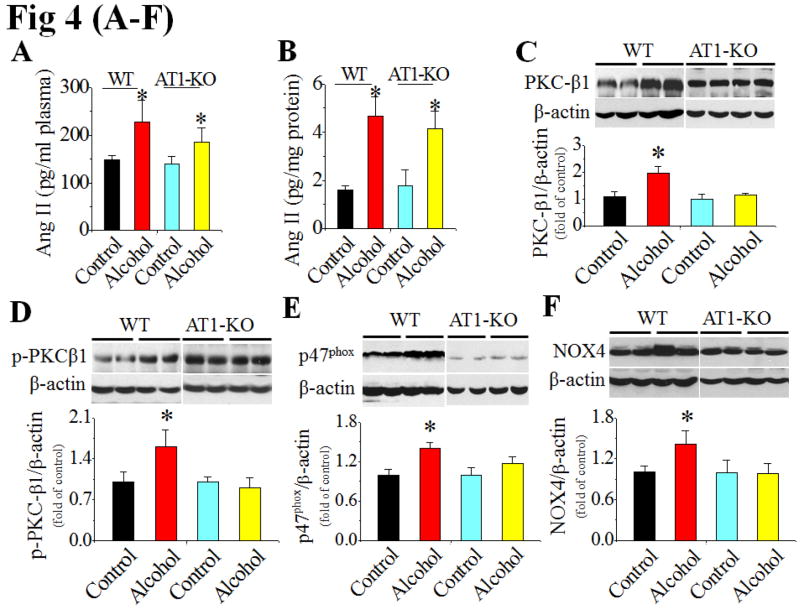
To ensure that the in vitro finding above is applied to in vivo disease, AT1-KO and WT mice were pair-fed alcohol or control liquid diet for 2 months. Analysis of blood pressure showed that AT1-KO mice showed decrease blood pressure compared to WT mice at baseline (Table 1). Chronic alcohol feeding significantly increased plasma and cardiac Ang II levels in both strains of mice (Fig. 4A,B), but only significantly increased blood pressure in the WT mice and not in the AT1-KO mice (Table 1).
Table 1.
Biometric and Echocardiographic Parameters of Mice Fed an Alcohol Diet for 2 months
| Parameter | WT | AT1-KO | ||
|---|---|---|---|---|
| Control (n=5) | Alcohol (n=8) | Control (n=4) | Alcohol (n=4) | |
| Body weight, g | 34.0±1.3 | 30.3±2.1* | 31.1±0.8 | 31.7±1.8 |
| Heart weight, mg | 124±4 | 148±11*,# | 114±3 | 121±8 |
| Tibia length, cm | 1.81±0.05 | 1.82±0.01 | 1.84±0.05 | 1.83±0.04 |
| Heart weight/tibia length, mg/cm | 68.1±2.1 | 81.4±6.0*,# | 65.5±4.2 | 62.6±1.8 |
| Diastolic blood pressure, mmHg | 68±6 | 79±5*,# | 53±4 | 48±2 |
| Systolic blood pressure, mmHg | 95±7 | 111±9*,# | 76±4 | 69±3 |
| Mean blood pressure, mmHg | 76±6 | 89±6*,# | 60±3 | 55±2 |
| LV mass, mg | 76±15 | 103±16 | 91±25 | 80±12 |
| LV mass Normalized by body weight, mg/g | 2.4±0.6 | 3.4±0.5* | 2.9±0.8 | 2.6±0.4 |
| LVLD, mm | 6.3±0.4 | 6.7±0.2* | 6.1±0.5 | 6.1±0.2 |
| LVALD, mm2 | 17.9±1.6 | 20.2±1.7* | 16.7±1.7 | 17.4±1.6 |
| LVLS, mm | 4.9±0.3 | 5.5±0.3* | 4.8±0.5 | 4.8±0.5 |
| LVALS, mm2 | 8.0±0.8 | 10.5±1.4*,# | 8.0±1.6 | 7.9±1.5 |
| Ejection fraction, % | 75±3 | 67±4* | 71±5 | 74±5 |
Notes: WT, wild type mice; AT1-KO, angiotensin II type 1 receptor knock-out mice; LV, left ventricle; LVLD, left ventricle long-axle diameter (end diastolic); LVALD, left ventricle area of long-axle (end diastolic); LVLS, left ventricle long-axle diameter (end systolic); LVALS, left ventricle area of long-axle (end systolic); Values are mean ± SD;
P<0.05 vs WT control;
p<0.05 AT1-KO control.
Figure 4. Response of AT1-KO and WT mice to chronic alcohol feeding.
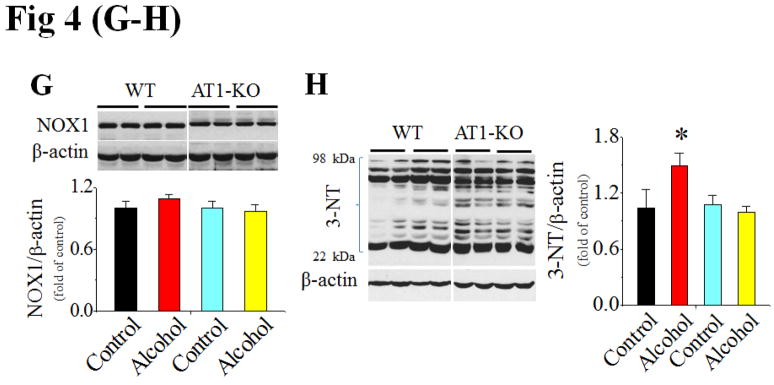
AT1-KO mice and WT mice were alcohol-fed for 2 months (n ≥ 4). Ang II levels in the plasma (A) and heart (B) were detected with ELISA assay. Cardiac PKC-β1 expression (C) and activation (D), NOX2 (p47phox, E), NOX4 (F) and NOX1 (G) expressions, and 3-NT accumulation (H) were examined with Western blots. *, p<0.05 vs control.
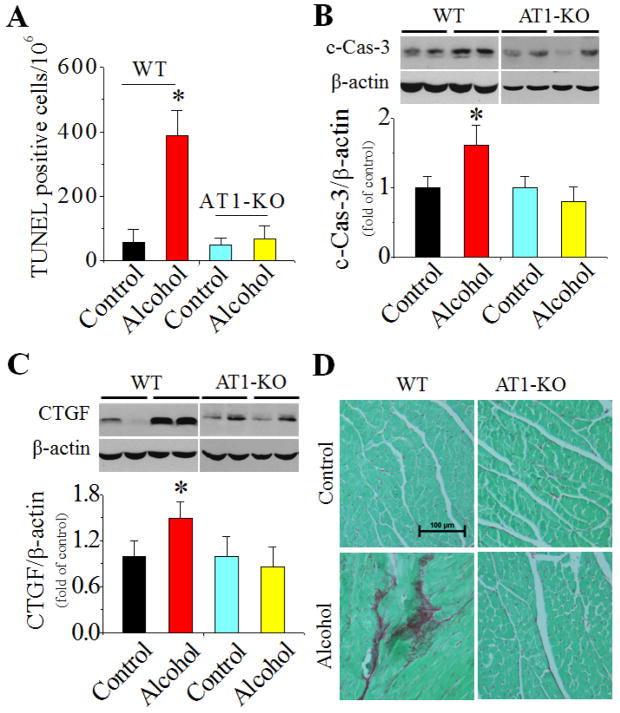
In WT mice chronic alcohol feeding induced cardiac PKC-β1 expression (Fig. 4C) and activation (Fig. 4D), NOX up-regulation, including both p47phox (NOX2, Fig. 4E) and NOX4 (Fig. 4F), and 3-NT accumulation (Fig. 4H). NOX1 expression was not significantly changed among groups in both strains (Fig. 4G). Chronic alcohol feeding also significantly induced cardiac apoptosis [examined by TUNEL staining (Fig. 5A, Fig. S5 for staining images) and caspase-3 activation (Fig. 5B)] and cardiac remodeling [shown by increased fibrosis with connective tissue growth factor (CTGF) expression (Fig. 5C) and Sirius-red staining of collagen (Fig. 5D)]. However, all above pathogenic changes were not observed in alcohol-fed AT1-KO mice (Fig. 4C–H; Fig. 5A–D).
Figure 5. AT1-KO mice are resistant to alcoholic induction of cardiac cell death and remodeling.
Cardiac tissues were collected from mice described in Fig. 4. Cardiac apoptotic cell death was detected with TUNEL staining (A) (representative images of TUNEL staining are provided as Fig. S5) and caspase-3 cleavage (B). Fibrotic response was examined by Western blot of CTGF (C) and Sirius-red staining of collagen (D). *, p<0.05 vs control.
Echocardiographic and gravimetric evaluation (Table 1) revealed that alcohol feeding in WT mice induced left ventricular (LV) chamber enlargement (increased LV long-axis diameter and area at end-diastole and end-systole), mild LV systolic dysfunction (reduced ejection fraction), and chamber hypertrophy (increased heart weight/tibia length ratio and increased LV mass as determined by echocardiography and normalized for body weight). All these changes were observed only in the WT mice and not in AT1-KO mice.
Superoxide dismutase mimetic prevents alcohol-induced cardiac cell death and remodeling in mice
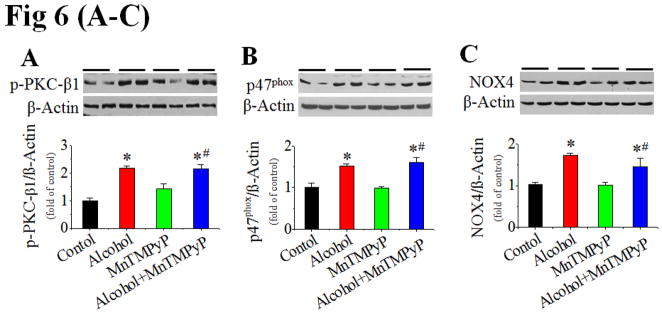
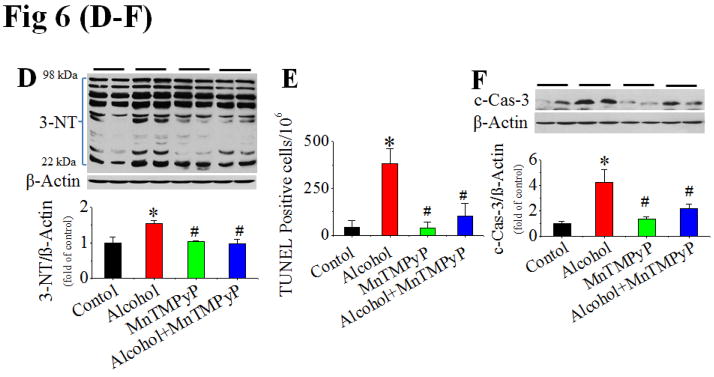
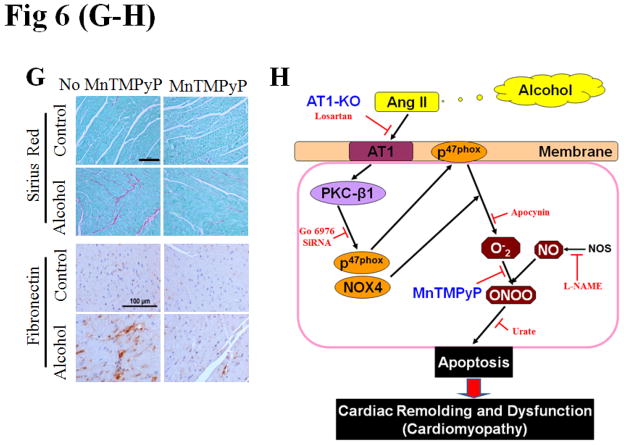
The above study has established the role of Ang II/AT1 in the development of alcoholic cardiomyopathy. To further establish the role of NOX-activation-mediated nitrative stress and damage in alcoholic cardiomyopathy, the pair-fed and alcohol-fed mice were intraperitoneally treated with superoxide dismutase mimetic MnTMPyP at 5 mg/kg or vehicle daily for 2 months. Treatment with MnTMPyP did not change alcohol-induced increases in systolic and diastolic blood pressure (Fig. S6A,B), cardiac PKC expression (Fig. S6C) and activation (Fig. 6A), and p47phox (Fig. 6B) and NOX4 (Fig. 6C) expressions. No change of the above parameters is because they are up-stream mediators of NOX-mediated superoxide generation. However, treatment with MnTMPyP significantly prevented alcohol-induced cardiac nitrative damage (Fig. 6D), cell death [shown by TUNEL staining (Fig. 6E, Fig. S6E for staining images) and caspase-3 cleavage (Fig. 6F)], and remodeling [shown by increased Sirius Red staining for collagen and immunohistochemical staining for fibronectin, respectively (Fig. 6G)].
Figure 6. Prevention of cardiac nitrative damage and cell death in mice by scavenging superoxide with MnTMPyP.
Pair-fed and alcohol-fed mice (n ≥ 8) were treated with and without superoxide dismutase mimetic MnTMPyP at 5 mg/kg body weight daily for 2 months. Cardiac PKC-β1 activation (A), NOX2 (p47phox, B) and NOX4 (C) expressions, and 3-NT accumulation (D) were detected by Western blots. Cell death was examined by TUNEL staining (E) (representative images of TUNEL staining are provided as Fig. S6E) and Western blot of caspase-3 cleavage (F), respectively. Cardiac remodeling was examined by Sirius-red staining of collagen and immunohistochemical staining of fibronectin (G). *, p<0.05 vs control; #, p<0.05 vs alcohol treatment. Panel H is a mechanistic illustration for cardiac nitrative damage and cell death mediated by Ang II/AT1 axis in response to alcohol. The red and black lines are experimentally proved inhibitory and positive effects. The blue fond indicates the in vivo experimental models.
Discussion
Cardiac apoptosis is a pivotal cause of various cardiomyopathies (3). Previous studies have shown that apoptosis increases in the heart of animals and patients with chronic alcohol consumption (4–5). In an analogous manner, in the present study we found the induction of cardiac cell death in hearts of alcohol-fed mice and cardiac cells exposed to alcohol in vitro, and the prevention of cardiac nitrative damage and cell death in AT1-KO mice (Fig. 4, 5). The prevention of the alcohol-induced cell death resulted in a significant prevention of cardiac remodeling (Fig. 5) and dysfunction (Table 1), further confirming the critical role of alcoholic cell death in the development of cardiomyopathy (Fig. 6H).
Oxidative and/or nitrative stress has been thought to play important roles in alcohol-induced cardiotoxicity. In animal models both acute and chronic ingestion of alcohol increases cardiac lipid peroxidation and protein oxidation and reduces mitochondrial glutathione content, suggesting alcohol-induced oxidative stress (2,16). However, how alcohol induces intracellular oxidative stress that leads to cardiac cell death remains largely unknown (17).
A novel finding of the present study is that NOX-mediated superoxide generation and associated peroxynitrite formation play critical roles in alcohol-induced cardiac cell death. Specifically, the alcoholic cell death was prevented by suppression of NOX activation, inhibition of NO formation, and scavenging of either superoxide or peroxynitrite (Fig. S3). Although the role of NOX-associated peroxynitrite accumulation has been implicated in alcohol-induced hepatic cell damages (18), there was no evidence that peroxynitrite formation contributes to alcoholic cardiotoxicity. We directly measured the accumulation of superoxide and peroxynitrite in the cardiac cells exposed to alcohol (Fig. 1G). In vivo MnTMPyP supplementation in conjunction with chronic alcohol feeding for 2 months in mice significantly attenuated alcohol-induced cardiac cell death and nitrative damage without altering blood pressure, PKC and NOX expression and activation (Fig. 6A–C). This study further confirms the pivotal role of NOX-mediated superoxide in alcohol-induced cardiac nitrative damage, cell death and remodeling (Fig. 6H).
The second novel finding is that alcohol activation of NOX and apoptosis is PKC-β1-dependent. In the heart, PKC activation is generally considered to be a protective response (19–20); however, cardioprotection is mainly attributed to the PKCepsilon (19–20), while other isoforms of PKCs may have opposite effects (21). The prevention of alcohol-induced NOX activation and apoptosis by inhibition of PKCα/β1 with its inhibitor (Fig. 2A–C) consists with previously revealed detrimental effects of PKCα/β1 (21). Furthermore, we applied PKC-β1 specific siRNA to clearly define the direct role of PKC-β1 in mediating NOX-activation associated nitrative damage and cell death (Fig. 2E&F).
Although increased expression of AT1 was observed in the heart of the dogs (13) and rats (22) upon chronic alcohol feeding, it remained unclear whether increased cardiac Ang II and AT1 expression are the direct cause for alcoholic cardiomyopathy. In the present study we demonstrate that direct exposure of H9c2 cardiac cells to alcohol augmented intracellular and extracellular Ang II levels and up-regulated Ang II AT1 receptor expression under an in vitro condition (Fig. 3A), clarified the direct role of alcohol on the increases in cardiac Ang II levels and AT1 gene expression. More importantly the present study provides direct evidence for the first time that AT1-KO mice are completely resistant to alcoholic induction of cardiac nitrative damage, cell death and cardiomyopathy development (Fig. 4,5).
In regard of mechanisms responsible for alcoholic induction of cardiac Ang II generation and AT1 expression, we do not have direct experimental evidence now. However, several studies have demonstrated the capability of cardiac cells to generate intracellular Ang II in response to various stresses (23–24). Intracellular increases in Ang II and AT1 expression in cardiac cells challenged by various stresses were related to p53 transcriptional function (23–24). Exposure to alcohol has extensively documented to up-regulate cardiac p53 expression (25). Therefore, whether alcohol increases cardiac intracellular Ang II and AT1 expression via p53 activation needs further investigation. In addition, alcoholic tissue damage may be evident in the liver of these mice; therefore, whether alcohol-induced increase in plasma Ang II is due to increased production of angiotensinogen in the liver also is an interesting topic in the future studies.
In summary, we have investigated the cellular and molecular mechanisms underlying alcohol-mediated cardiac cell apoptosis using a combination of in vivo and in vitro approaches with the following key findings: [1] cardiac cell death induced by alcohol is dependent on NOX-activation and subsequent nitrative damage; [2] alcoholic activation of NOX is PKC-β1-dependent; [3] alcoholic exposure of cardiac cells increases intra- and extra-cellular Ang II levels and AT1 expression, which is required for alcohol activation of PKC- β1 and NOX; [4] AT1-KO mice or superoxide dismutase mimetic-treated mice are completely resistant to alcoholic induction of cardiac nitrative stress, cell death, remodeling and dysfunction. These results indicate that alcohol-induced cardiac cell death, which is mediated by the interaction of Ang II with AT1 to activate PKC-β1-dependent activation of NOX with subsequent superoxide generation and nitrative damage, is a critical cause for alcoholic cardiomyopathy (Fig. 6H).
Supplementary Material
Acknowledgments
Supported, in part, by grants from American Diabetes Association (1-11-BS-17, to Dr. Cai), Zhejiang Province (Extremely Key Subject Building Project ‘Pharmacology and Biochemical Pharmaceutics 2009) and Wenzhou Medical College (Starting-Up Fund for Chinese-American Research Institute for Diabetic Complications, to Dr. Cai & Dr. Li), NIH (R01AA014623 to Dr. Zhou; HL099014 and HL078825 to Dr. Prabhu; R37AA010762 and P01AA017103 to Dr. McClain), and the VA (to Dr. Prabhu and Dr. McClain).
Abbreviations
- Ang II
Angiotensin II
- PKC-β1
Protein kinase C-β1
- NADPH
Nicotinamide adenine dinucleotide phosphate
- TUNEL
Transferase mediated dUTP nick-end labeling
- 3-NT
3-Nitrotyrosine
- NOX
Nicotinamide adenine dinucleotide phosphate oxidase
- L-NAME
NG-Nitro-L-arginine Methyl Ester, Hydrochloride
- MnTMPyP
Manganese(III) tetrakis(1-methyl-4-pyridyl)porphyrin
- AT1
Angiotensin II type 1 receptor
- SiRNA
Small interfering RNA
- AT1-KO
Mice with knockout of angiotensin II type 1 receptor gene
- ROS & RNS
Reactive oxygen and nitrogen species
Footnotes
All authors have no relationships to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hookana E, Junttila MJ, Puurunen VP, et al. Causes of nonischemic sudden cardiac death in the current era. Heart rhythm: the official journal of the Heart Rhythm Society. 2011;8:1570–5. doi: 10.1016/j.hrthm.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 2.Seiva FR, Amauchi JF, Rocha KK, et al. Alcoholism and alcohol abstinence: N-acetylcysteine to improve energy expenditure, myocardial oxidative stress, and energy metabolism in alcoholic heart disease. Alcohol. 2009;43:649–56. doi: 10.1016/j.alcohol.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 3.Gurtl B, Kratky D, Guelly C, et al. Apoptosis and fibrosis are early features of heart failure in an animal model of metabolic cardiomyopathy. Int J Exp Pathol. 2009;90:338–46. doi: 10.1111/j.1365-2613.2009.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capasso JM, Li P, Guideri G, et al. Myocardial mechanical, biochemical, and structural alterations induced by chronic ethanol ingestion in rats. Circ Res. 1992;71:346–56. doi: 10.1161/01.res.71.2.346. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Sola J, Fatjo F, Sacanella E, et al. Evidence of apoptosis in alcoholic cardiomyopathy. Hum Pathol. 2006;37:1100–10. doi: 10.1016/j.humpath.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos CX, Anilkumar N, Zhang M, Brewer AC, Shah AM. Redox signaling in cardiac myocytes. Free Radical Bio Med. 2011;50:777–93. doi: 10.1016/j.freeradbiomed.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. Febs J. 2008;275:3249–77. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- 9.Cai L, Wang J, Li Y, et al. Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes. 2005;54:1829–37. doi: 10.2337/diabetes.54.6.1829. [DOI] [PubMed] [Google Scholar]
- 10.Zhou G, Li X, Hein DW, et al. Metallothionein suppresses angiotensin II-induced nicotinamide adenine dinucleotide phosphate oxidase activation, nitrosative stress, apoptosis, and pathological remodeling in the diabetic heart. J Am Coll Cardiol. 2008;52:655–66. doi: 10.1016/j.jacc.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 11.Kuroda J, Ago T, Matsushima S, et al. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. P Natl Acad Sci USA. 2010;107:15565–70. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang M, Brewer AC, Schroder K, et al. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. P Natl Acad Sci USA. 2010;107:18121–6. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng CP, Cheng HJ, Cunningham C, et al. Angiotensin II type 1 receptor blockade prevents alcoholic cardiomyopathy. Circulation. 2006;114:226–36. doi: 10.1161/CIRCULATIONAHA.105.596494. [DOI] [PubMed] [Google Scholar]
- 14.Ichikawa T, Li J, Meyer CJ, et al. Dihydro-CDDO-trifluoroethyl amide (dh404), a novel Nrf2 activator, suppresses oxidative stress in cardiomyocytes. PLoS One. 2009;4:e8391. doi: 10.1371/journal.pone.0008391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Song Y, Elsherif L, et al. Cardiac metallothionein induction plays the major role in the prevention of diabetic cardiomyopathy by zinc supplementation. Circulation. 2006;113:544–54. doi: 10.1161/CIRCULATIONAHA.105.537894. [DOI] [PubMed] [Google Scholar]
- 16.Jing L, Jin CM, Li SS, et al. Chronic alcohol intake-induced oxidative stress and apoptosis: role of CYP2E1 and calpain-1 in alcoholic cardiomyopathy. Mol Cell Biochem. 2012;359:283–92. doi: 10.1007/s11010-011-1022-z. [DOI] [PubMed] [Google Scholar]
- 17.Guan Z, Lui CY, Morkin E, Bahl JJ. Oxidative stress and apoptosis in cardiomyocyte induced by high-dose alcohol. J Cardiovasc Pharmacol. 2004;44:696–702. doi: 10.1097/00005344-200412000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Yang ES, Lee JH, Park JW. Ethanol induces peroxynitrite-mediated toxicity through inactivation of NADP+-dependent isocitrate dehydrogenase and superoxide dismutase. Biochimie. 2008;90:1316–24. doi: 10.1016/j.biochi.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Cross HR, Murphy E, Bolli R, Ping P, Steenbergen C. Expression of activated PKC epsilon (PKC epsilon) protects the ischemic heart, without attenuating ischemic H(+) production. J Mol Cell Cardiol. 2002;34:361–7. doi: 10.1006/jmcc.2001.1518. [DOI] [PubMed] [Google Scholar]
- 20.Zhou HZ, Karliner JS, Gray MO. Moderate alcohol consumption induces sustained cardiac protection by activating PKC-epsilon and Akt. Am J Physiol Heart Circ Physiol. 2002;283:H165–74. doi: 10.1152/ajpheart.00408.2001. [DOI] [PubMed] [Google Scholar]
- 21.Grossoni VC, Todaro LB, Kazanietz MG, de Kier Joffe ED, Urtreger AJ. Opposite effects of protein kinase C beta1 (PKCbeta1) and PKCepsilon in the metastatic potential of a breast cancer murine model. Breast Cancer Res Treat. 2009;118:469–80. doi: 10.1007/s10549-008-0299-4. [DOI] [PubMed] [Google Scholar]
- 22.Jing L, Li WM, Zhou LJ, et al. Expression of renin-angiotensin system and peroxisome proliferator-activated receptors in alcoholic cardiomyopathy. Alcohol Clin Exp Res. 2008;32:1999–2007. doi: 10.1111/j.1530-0277.2008.00781.x. [DOI] [PubMed] [Google Scholar]
- 23.Wanka H, Kessler N, Ellmer J, et al. Cytosolic renin is targeted to mitochondria and induces apoptosis in H9c2 rat cardiomyoblasts. J Cell Mol Med. 2009;13:2926–37. doi: 10.1111/j.1582-4934.2008.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leri A, Claudio PP, Li Q, et al. Stretch-mediated release of angiotensin II induces myocyte apoptosis by activating p53 that enhances the local renin-angiotensin system and decreases the Bcl-2-to-Bax protein ratio in the cell. J Clin Invest. 1998;101:1326–42. doi: 10.1172/JCI316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jankala H, Eriksson PC, Eklund K, et al. Effect of chronic ethanol ingestion and gender on heart left ventricular p53 gene expression. Alcohol Clin Exp Res. 2005;29:1368–73. doi: 10.1097/01.alc.0000175043.67463.e5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.