Abstract
Background:
This analysis was carried out to evaluate the cost-effectiveness of adjuvant radiation therapy (ART) versus observation, using a decision analysis model based primarily upon the published results of the Southwest Oncology Group prospective trial (SWOG 8794).
Patients and methods:
A decision analysis model was designed to compare ART versus observation over a 10-year time horizon. Probabilities of treatment success, utilization of salvage treatments, and rates of adverse events were taken from published results of SWOG 8794. Cost inputs were based on 2010 Medicare reimbursement rates. Primary outcome measure was incremental cost per prostate-specific antigen (PSA) success (i.e. serum PSA level <0.4 ng/ml).
Results:
ART results in a higher PSA success rate than observation with probability of 0.43 versus 0.22. The mean incremental cost per patient for ART versus observation was $6023. The mean incremental cost-effectiveness ratio was $26 983 over the 10-year period.
Conclusions:
ART appears cost effective compared with observation based upon this decision analysis model. Future research should consider more costly radiation therapy (RT) approaches, such as intensity-modulated RT, and should evaluate the cost-effectiveness of ART versus early salvage RT.
Keywords: adjuvant radiation therapy, cost-effectiveness, post, prostatectomy, prostate cancer
introduction
Prostate cancer (PC) is the most common nonskin cancer to affect males in the United States with an estimated 217 730 new cases in 2010 [1]. Ninety percent of these patients will choose to receive definitive treatment [2], resulting in a projected $12 billion in medical costs in the United States in 2010 [3]. The Institute for Clinical and Economic Review recently completed an analysis of the comparative effectiveness and value of the management options for low-risk PC, and the final report highlighted the substantial and variable lifetime of PC treatment, ranging from $25 000 to $30 000 for active surveillance, radical prostatectomy (RP), or brachytherapy, up to $40 000–$55 000 for high-technology radiation therapy (RT) [4]. Therefore, it is essential to critically consider the value of RT for PC; this report enriches our understanding of the treatment costs of PC by evaluating the cost-effectiveness of postoperative RT for selected PC patients.
An estimated one-third of newly diagnosed men with PC will undergo RP [5, 6]. Approximately one in five PC patients who undergo RP will recur [7] with higher rates of recurrence, 40%–60%, among patients with adverse pathological risk factors [8]. For patients with adverse pathologic features (APFs), adjuvant radiation therapy (ART), given before a rise in prostate-specific antigen (PSA) level, may be an appropriate course of treatment. Three key randomized trials have shown that ART improves biochemical progression-free survival [9–11] and overall survival [12]. In these trials, patients were eligible for study enrollment if one or more APFs were found in the RP surgical specimen: extracapsular extension (ECE) [9–11], seminal vesicle invasion (SVI) [9–11], or positive surgical margin (PSM) [10, 11].
Although prospective trials support the use of ART for select patients after RT, a recent treatment patterns analysis found that <20% of qualifying patients receive ART [13–15]. Instead of using ART, many physicians choose to observe these patients closely with serial PSA tests and offer RT selectively, only as a salvage treatment of a rise in PSA after RP. From the physician perspective, the predominant reasons for favoring close observation with selective salvage RT (SRT) over ART for patients with APF after RP include perceived toxicity of RT, the risk of overtreatment with ART (i.e. a proportion of patients who may not have developed biochemical failure after RP despite adverse pathologic factors), and the perceived equivalent effectiveness of SRT and ART [16], despite evidence suggesting otherwise [17, 18]. From the payers' perspective, the added financial costs associated with ART are a consideration. Therefore, we aimed to evaluate the cost-effectiveness of ART after RP for appropriately selected patients.
Although similar analyses have been conducted for other topics in PC [19–22], the cost-effectiveness of ART versus observation has not been previously described. We utilized the peer-reviewed, published data [11, 23, 24] from the Southwest Oncology Group (SWOG) prospective, randomized trial of ART versus observation (SWOG 8794) to estimate the effectiveness of ART. The SWOG 8794 study showed that ART, compared with observation, improves biochemical control, overall survival, and distant metastasis-free survival [12]. The objective of this study was to construct a decision analytic model to estimate the real world cost of ART versus observation from the payers' perspective. Cost per PSA success was used as the primary measure of effectiveness, which is a relevant consideration since PSA failure is associated with increased mortality and exposure to salvage PC treatments after RP [25–27].
methods
primary data source
This cost-effectiveness model is based primarily upon published data from the SWOG 8794 trial and estimated costs from calendar year 2010 Medicare reimbursement rates. The SWOG 8794 trial evaluated the effect of ART on outcomes, including the primary end point of metastasis-free survival, after RP for selected patients with APFs: ECE, SVI, or PSM [11, 12, 23].
The population that we included in the decision analysis model consisted of 242 patients, out of a total 431 patients enrolled in the SWOG study, with postoperative PSA data available and documented pre-RT PSA ≤0.2 ng/ml, as reported by Swanson et al. [23], representing a pure ART cohort. The analysis was restricted to those patients with PSA ≤0.2 ng/ml in order to evaluate only ‘adjuvant’ therapy, excluding those patients who, by definition [28], received ‘salvage’ therapy with persistently detectable PSA after RP.
model design
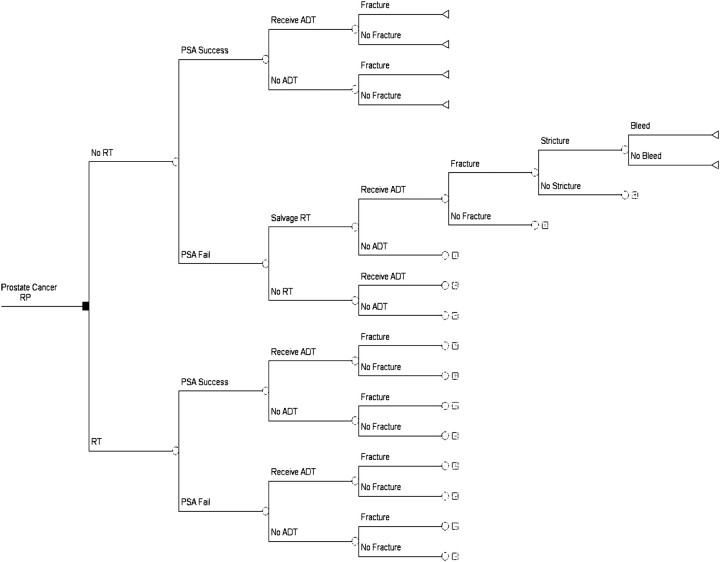
We designed a decision analysis model (Figure 1) from a payer's perspective, using the TreeAge Pro Suite 2009 program, to examine the cost-effectiveness of ART versus observation after RP for patients with APF (ECE, SVI, and/or PSM) over 10 years. The model is consistent with the best practices issued by the International Society for Pharmacoeconomics and Outcomes Research task force on good research practices in modeling studies [29]. Branches of the decision tree model (Figure 1) demonstrate the two arms of the SWOG 8794 trial (ART versus observation) with associated probabilities of PSA success, utilization of SRT and androgen deprivation therapy (ADT), and associated risk of adverse events: bone fracture, bladder neck contracture (‘Stricture’), and rectal bleeding (‘Bleed’).
Figure 1.
Decision analysis model of adjuvant radiation therapy versus observation. The initial decision point [radiation therapy (RT) versus observation] and subsequent probabilities of PSA success, salvage RT, and ADT are based upon the design and results of SWOG 8794. Subsequent decision points reflect the probabilities of adverse events: bone fracture, bladder neck contracture, and rectal bleeding. SWOG, Southwest Oncology Group.
The primary objective of the decision analytic model was to determine the cost per PSA success for ART versus observation based upon the conditions and outcomes of the SWOG 8794 trial.
outcome measure
The primary outcome measure used in the model was treatment success, defined as the absence of PSA failure (PSA ≥0.4 ng/ml), consistent with the methods described in SWOG 8794. [23] Probabilities of treatment success were taken from published results of SWOG 8794 [11, 23].
costs
All probability and costs assumptions used to inform the model are shown in Tables 1 and 2. In short, total cost is a function of cost inputs and the probability of occurrence for each input. Costs included the costs of receiving ART, SRT, management of rectal bleeding, and management of bladder neck contracture. All costs were based on the published national average for 2010 Medicare reimbursement rates [32], assuming that four-field conformal RT was delivered to 60–64 Gy (as used in SWOG 8794). Cost estimates for treatment of adverse events assumed two colonoscopies and one laser treatment of rectal bleeding and two cystoscopies and one cystoscopy with dilation for bladder neck contracture, which reflect worst-case scenario, high-utilization rates based upon discussions with physicians. Costs associated with ADT and treatment of bone fractures were taken from a report published by Krupski et al. [31].
Table 1.
Probability values used in the base-case decision analytic model
| Probability Variable | Value | Low | High | Reference |
| No adjuvant radiation therapy | ||||
| PSA success | 0.28 | 0.22 | 0.33 | Swanson et al. [23] |
| Receive ADT | 0.21 | 0.15 | 0.30 | Thompson et al. [11] |
| Receive salvage adjuvant radiation therapy | 0.33 | 0.44 | 0.66 | Thompson et al. [11] |
| Adjuvant radiation therapy | ||||
| PSA success | 0.58 | 0.45 | 0.69 | Swanson et al. [23] |
| Receive ADT | 0.10 | 0.08 | 0.13 | Thompson et al. [11] |
| Radiation therapy adverse events | ||||
| Bladder neck contracture | 0.178 | 0.14 | 0.21 | Thompson et al. [11] |
| Bleed | 0.03 | 0.026 | 0.039 | Feng et al. [30] |
| Fracture rates | ||||
| Fracture with ADT | 0.187 | 0.69 | 1 | Krupski et al. [31] |
| Fracture without ADT | 0.146 | 0.33 | 0.49 | Krupski et al. [31] |
The low and high values shown were used as the range for one-way sensitivity analyses.
Table 2.
Cost values used in the base-case decision analytic model
| Cost Variable | Value | Low | High | Source |
| Adjuvant radiation therapy | $11 295 | $9036 | $13 554 | CY 2010 Medicare reimbursement rates |
| Management of Adverse Events | ||||
| Rectal bleeding treatment (2 colonoscopies + 1 YAG laser coagulation) | $2660 | $2128 | $3192 | CY 2010 Medicare reimbursement rates |
| Bladder neck contracture treatment (2 cystoscopies + cytoscopy with dilation) | $4648 | $3718 | $5577 | CY 2010 Medicare reimbursement rates |
| ADT (lupron) | $8991 | $7192 | $10 788 | Krupski et al. [31] |
| Fracture | $14 000 | $11 200 | $16 800 | Krupski et al. [31] |
The low and high values shown were used as the range for one-way sensitivity analyses.
discounting
Because the model was over a time span of 10 years, we discounted our outcome measure at a rate of 3%. Costs that were incurred in the first year (ART) were not discounted. All other costs were discounted based on the estimated year they would be incurred in; bleeding and stricture were discounted at 2 years, and fracture and ADT were discounted at 5 years.
incremental cost-effectiveness ratio
The incremental cost-effectiveness ratio (ICER) represents the cost per additional PSA success for ART versus observation. The ICER was calculated as the mean difference in costs between ART and observation divided by the mean difference in effectiveness (probability of PSA success) of ART versus observation.
sensitivity analysis
A series of one-way sensitivity analyses were conducted to evaluate the relative importance of each cost and probability assumption in the model. The standard deviation, when not available, was assumed to be 10% of the mean. A range of two standard deviations (80%–120% of the mean) for each variable was used in the one-way sensitivity analyses (Tables 1 and 2). One-way sensitivity analyses only vary one input at a time. To account for overall variability in the model where each variable changes at the same time, we conducted a probabilistic sensitivity analysis using Monte Carlo simulation in TreeAge Pro Suite 2009 [33]. In a Monte Carlo simulation, each model input is assigned a distribution of values primarily based on the standard deviations (Tables 1 and 2). In cases where the calculated distribution was unavailable, information provided by experts (TNS, LGG, CL, EJT) was utilized. Monte Carlo simulation was then applied to the mean incremental cost and effect and the standard error of the means based on the 1000 microsimulations designed to represent 1000 iterations in which the values for all the variables were randomly selected within the designated distribution.
results
ART results in a higher PSA success rate than observation with a probability of 0.44 versus 0.21 over a 10-year period [23]. Therefore, the mean incremental effect was 0.23. Total cost of ART was $15 900 compared with costs in the observation group of $9876. The mean incremental cost for ART versus observation was $6023 (Table 3). Using the mean incremental cost and effectiveness, the ICER was $26 983 over the 10-year period (Table 3). On a per-year basis, over 10 years, the cost per additional PSA success achieved with ART was $2698.
Table 3.
Mean Incremental cost, Incremental effect, and incremental cost-effectiveness ratio (ICER) of adjuvant radiation therapy (ART) versus observation in the decision analysis model
| Strategy | Cost | Incremental cost | Effect | Incremental effect | ICER |
| Observation | $9876 | –– | 0.21 | –– | –– |
| ART | $15 900 | $6023 | 0.43 | 0.22 | $26 983 |
sensitivity analysis
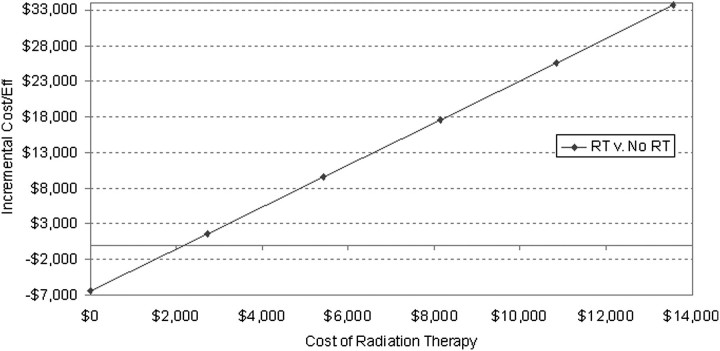
One-way sensitivity analysis revealed that the model is most sensitive to the likelihood of treatment failure, ADT cost and costs of ART. For example, for the ART to cost as much as observation, then the cost of ART has to decrease to $2000 (from the baseline value of $11 295 in current model) (Figure 2).
Figure 2.
Graph of the linear relationship of incremental cost of adjuvant radiation therapy (versus observation) with incremental cost-effectiveness ratio. The solid horizontal line demonstrates the cost at which the ICER is zero, meaning that adjuvant radiation therapy (RT) costs no more than observation.
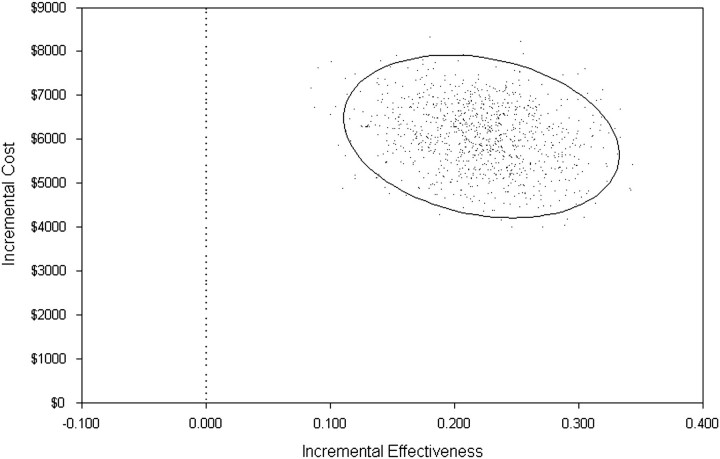
The results from the Monte Carlo simulation (Figure 3) showed that ART continues to have higher incremental cost ($6061) and higher incremental effectiveness (0.22) than observation in all calculated conditions.
Figure 3.
Monte Carlo simulation to evaluate probabilistic sensitivity of the incremental cost-effectiveness ratio. The solid oval denotes the 95% confidence interval around the point estimates.
discussion
Based upon our decision analytic model, ART was both more costly and more effective than observation with each additional PSA success achieved at a cost of $26 983 over a 10-year time horizon. Sensitivity analyses confirmed that ART remained a more costly and more effective treatment than observation.
Standard cost-effectiveness analyses employ a quality-adjusted life year (QALY) as the primary outcome measure. However, since utilities are not available in the medical literature for the specific scenario of ART after RP, it was not possible to calculate QALYs for patients receiving ART (or SRT) after RP. A prior cost-effectiveness analysis of risk-prediction tools in selecting patients for early ART after RP used utilities from a group of 17 patients with intermediate-risk PC who received nonsurgical management with intensity-modulated RT (IMRT) as definitive therapy [22]. Due to significant anatomic changes after RP [34], with different rates of toxicity observed for definitive versus postoperative RT for PC [35, 36], it is not clear that utilities from definitive prostate RT should be applied to ART. Therefore, an alternate end point, cost per PSA success, was chosen for the current analysis. In addition to the data being available in the literature, we believe that PSA is an important outcome measure to payers, clinicians, and patients given that treatment decisions are often based on PSA values in this setting [16, 37]. PSA failure after prostatectomy is associated with higher rates of metastasis and mortality, as well as exposure to salvage treatments [25, 38]. Salvage PC treatments for PSA failure after RP are associated with significant adverse effects [30, 31], so the measure used in the current decision analysis model (cost per PSA success) is a relevant consideration. Although the recently published update of the SWOG 8794 trial showed improved overall survival with ART versus observation, overall survival was not reported by treatment arm for the subset of patients with confirmed undetectable PSA [12]. The other two randomized trials of ART showed improved biochemical progression-free survival but not overall survival [9, 10]. Therefore, the end point cost per PSA success permits the current analysis to reflect the shared conclusions of all three trials.
In our analysis, we used PSA success as our outcome measure rather then QALYS. Consequently, there is no published threshold value to determine cost-effectiveness using PSA success as an outcome measure. However, previous decision analytic modeling reports of IMRT [21], proton beam therapy [20], and a risk-prediction tool for ART decision-making [22] for PC while not using QALYS have compared results to the reference $50 000/QALY willingness-to-pay threshold. We believe that our ICER of $26 983 seems reasonable in consideration of the health gains achieved, though cost per PSA success may not be compared with cost per QALY. Furthermore, we believe that our findings lend further support to ART, which has also been shown to result in higher biochemical failure-free survival [9–11, 23], distant metastasis-free survival [12], and overall survival [12] in randomized trials of ART versus observation for patients with APFs.
The current decision analysis model adheres primarily to the design and outcomes of the SWOG 8794 trial and contains limitations regarding the magnitude and scope of cost inputs, as well as the relationship of the model to contemporary practice, which favors decisions of ART versus early salvage therapy rather than observation [37, 39]. Cost inputs for ART were based upon 2010 Medicare reimbursements rates for 3D-conformal radiation therapy (CRT) to a total dose of 64 Gy, similar to the planning and dose-fractionation schedule permitted on SWOG 8794. It is worth noting that reimbursement rates from private payers may exceed the rates used in the model. Furthermore, a national survey of radiation oncologists conducted by our group suggests that IMRT is used as the standard approach for post-RP RT by ∼80% of respondents with total doses commonly ∼68 Gy (manuscript in preparation). Therefore, the current model may significantly underestimate the costs of ART since IMRT is significantly more costly than 3D-CRT. For instance, based on Medicare calendar year 2010 reimbursement, a 38-fraction course of post-RP IMRT (68.4 Gy in 1.8 Gy per fraction) would cost $22 313, nearly two times higher than the estimated cost of $11 295 for 3D-CRT used in our model. Another limitation of the current analysis relates to the scope of cost inputs since the costs of symptom-management medications, outpatient visits, and hospital charges were not included in the model.
Finally, the current model was designed to test ART versus observation rather than ART versus SRT. When SWOG 8794 was designed, the study question reflected the state of PC at that time, and the investigators evaluated ART versus observation. However, much controversy regarding optimal management for PC after RP now centers instead upon ART versus early SRT [16]. Current practice patterns support the trend toward SRT: Ghia et al. recently reported that <20% of patients, who qualify, based on APFs, receive ART [13]. Although the decision analysis model of ART versus observation does not directly inform decisions regarding ART versus early SRT, this cost-effectiveness analysis nevertheless provides a foundation for considering the value of postprostatectomy RT for PC.
In conclusion, ART is an effective and cost-effective strategy for appropriately selected PC patients, based upon this decision analysis model of ART versus observation using published results of SWOG 8794. This model provides a baseline for considering similar management dilemmas in PC and for evaluating changes in the details of ART planning and delivery that might increase (IMRT and image guidance) or decrease (shorter fractionation schedules) the financial costs of RT. Future research should focus on the comparative effectiveness and cost-effectiveness of ART versus early SRT and should incorporate comprehensive, prospectively collected costs incurred by medication charges and outpatient health care utilization, as well as on quality of life gains.
funding
Kimmel Cancer Center National Institutes of Health Cancer Center Core (P30CA56036).
disclosure
The authors declare no conflict of interest.
Acknowledgments
We thank Eileen Comber, Department of Radiation Oncology, Kimmel Cancer Center, Thomas Jefferson University, for her assistance in identifying and collecting medical cost data for the analysis (she received no compensation for her assistance).
This work was presented in part at the 2010 American Society for Radiation Oncology Annual Meeting in San Diego, CA, 31 October–4 November 2010.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Cooperberg MR, Broering JM, Kantoff PW, Carroll PR. Contemporary trends in low-risk prostate cancer: risk assessment and treatment. J Urol. 2007;178:S14–S19. doi: 10.1016/j.juro.2007.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103:1–12. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ollendorf DA, Hayes J, McMahon P, et al., editors. Management Options for Low-Risk Prostate Cancer: A Report on Comparative Effectiveness and Value. Institute for Clinical and Economic Review, Massachusetts General Hospital: Boston, MA; 2010. pp. 5–76. [Google Scholar]
- 5.Penson DF, Chan JM Urologic Diseases in America Project. Prostate cancer. J Urol. 2007;177:2020–2029. doi: 10.1016/j.juro.2007.01.121. [DOI] [PubMed] [Google Scholar]
- 6.Swanson GP, Riggs MW, Earle JD, Haddock MG. Long-term follow-up of radical retropubic prostatectomy for prostate cancer. Eur Urol. 2002;42:212–216. doi: 10.1016/s0302-2838(02)00276-2. [DOI] [PubMed] [Google Scholar]
- 7.Han M, Partin AW, Pound CR, et al. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28:555–565. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- 8.Swanson GP, Riggs M, Hermans M. Pathologic findings at radical prostatectomy: risk factors for failure and death. Urol Oncol. 2007;25:110–114. doi: 10.1016/j.urolonc.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Wiegel T, Bottke D, Steiner U, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27:2924–2930. doi: 10.1200/JCO.2008.18.9563. [DOI] [PubMed] [Google Scholar]
- 10.Bolla M, van Poppel H, Collette L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911) Lancet. 2005;366:572–578. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 11.Thompson IM, Jr, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA. 2006;296:2329–2335. doi: 10.1001/jama.296.19.2329. [DOI] [PubMed] [Google Scholar]
- 12.Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181:956–962. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghia AJ, Shrieve DC, Tward JD. Adjuvant radiotherapy use and patterns of care analysis for margin-positive prostate adenocarcinoma with extracapsular extension: postprostatectomy adjuvant radiotherapy: a SEER analysis. Urology. 2010;76:1169–1174. doi: 10.1016/j.urology.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman KE, Nguyen PL, Chen MH, et al. Recommendations for post-prostatectomy radiation therapy in the United States before and after the presentation of randomized trial. J Urol. 2011;185(1):116–120. doi: 10.1016/j.juro.2010.08.086. [DOI] [PubMed] [Google Scholar]
- 15.Schreiber D, Rineer J, Yu JB, et al. Analysis of pathologic extent of disease for clinically localized prostate cancer after radical prostatectomy and subsequent use of adjuvant radiation in a national cohort. Cancer. 2010;116:5757–5766. doi: 10.1002/cncr.25561. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen ME, Trock BJ, Walsh PC. Salvage or adjuvant radiation therapy: counseling patients on the benefits. J Natl Compr Canc Netw. 2010;8:228–237. doi: 10.6004/jnccn.2010.0015. [DOI] [PubMed] [Google Scholar]
- 17.Trabulsi EJ, Valicenti RK, Hanlon AL, et al. A multi-institutional matched-control analysis of adjuvant and salvage postoperative radiation therapy for pT3-4N0 prostate cancer. Urology. 2008;72:1298–1302. doi: 10.1016/j.urology.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Budiharto T, Perneel C, Haustermans K, et al. A multi-institutional analysis comparing adjuvant and salvage radiation therapy for high-risk prostate cancer patients with undetectable PSA after prostatectomy. Radiother Oncol. 2010 doi: 10.1016/j.radonc.2010.07.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Fleming C, Wasson JH, Albertsen PC, et al. A decision analysis of alternative treatment strategies for clinically localized prostate cancer. Prostate Patient Outcomes Research Team. JAMA. 1993;269:2650–2658. [PubMed] [Google Scholar]
- 20.Konski A, Speier W, Hanlon AL, et al. Is proton beam therapy cost effective in the treatment of adenocarcinoma of the prostate? J Clin Oncol. 2007;25:3603–3608. doi: 10.1200/JCO.2006.09.0811. [DOI] [PubMed] [Google Scholar]
- 21.Konski A, Watkins-Bruner D, Feigenberg S, et al. Using decision analysis to determine the cost-effectiveness of intensity-modulated radiation therapy in the treatment of intermediate risk prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66:408–415. doi: 10.1016/j.ijrobp.2006.04.049. [DOI] [PubMed] [Google Scholar]
- 22.Zubek VB, Konski A. Cost effectiveness of risk-prediction tools in selecting patients for immediate post-prostatectomy treatment. Mol Diag Ther. 2009;13:31–47. doi: 10.1007/BF03256313. [DOI] [PubMed] [Google Scholar]
- 23.Swanson GP, Hussey MA, Tangen CM, et al. Predominant treatment failure pattern in postprostatectomy patients is local: analysis of patterns of treatment failure in SWOG 8794. J Clin Oncol. 2007;25:2225–2229. doi: 10.1200/JCO.2006.09.6495. [DOI] [PubMed] [Google Scholar]
- 24.Thompson IM, Tangen CM, Klein EA. Is there a standard of care for pathologic stage T3 prostate cancer? J Clin Oncol. 2009;27:2898–2899. doi: 10.1200/JCO.2008.20.9460. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal PK, Sadetsky N, Konety BR, et al. Treatment failure after primary and salvage therapy for prostate cancer: likelihood, patterns of care, and outcomes. Cancer. 2008;112:307–314. doi: 10.1002/cncr.23161. [DOI] [PubMed] [Google Scholar]
- 26.Grossfeld GD, Stier DM, Flanders SC, et al. Use of second treatment following definitive local therapy for prostate cancer: data from the caPSURE database. J Urol. 1998;160:1398–1404. [PubMed] [Google Scholar]
- 27.Mehta SS, Lubeck DP, Sadetsky N, et al. Patterns of secondary cancer treatment for biochemical failure following radical prostatectomy: data from CaPSURE. J Urol. 2004;171:215–219. doi: 10.1097/01.ju.0000100087.83112.23. [DOI] [PubMed] [Google Scholar]
- 28.Hayes SB, Pollack A. Parameters for treatment decisions for salvage radiation therapy. J Clin Oncol. 2005;23:8204–8211. doi: 10.1200/JCO.2005.03.1575. [DOI] [PubMed] [Google Scholar]
- 29.Weinstein MC, O'Brien B, Horberger J, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices–Modeling Studies. Value Health. 2003;64:9–17. doi: 10.1046/j.1524-4733.2003.00234.x. [DOI] [PubMed] [Google Scholar]
- 30.Feng M, Hanlon AL, Pisansky TM, et al. Predictive factors for late genitourinary and gastrointestinal toxicity in patients with prostate cancer treated with adjuvant or salvage radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:1417–1423. doi: 10.1016/j.ijrobp.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 31.Krupski TL, Foley KA, Baser O, et al. Health care cost associated with prostate cancer, androgen deprivation therapy and bone complications. J Urol. 2007;178:1423–1428. doi: 10.1016/j.juro.2007.05.135. [DOI] [PubMed] [Google Scholar]
- 32.Frizzera C, Sebelius K. Medicare Program: changes to the hospital outpatient prospective payment system and CY 2010 payment rates; changes to the ambulatory surgical center payment system and CY 2010 payment rates. Final rule with comment period. Fed Regist. 2009;74:60315–60983. [PubMed] [Google Scholar]
- 33.TreeAge Pro 2009 Suite [Computer Program]. In Edition 1.0.2. Williamstown, MA: TreeAge Software, Inc.; 2009. [Google Scholar]
- 34.Sanguineti G, Castellone P, Foppiano F, et al. Anatomic variations due to radical prostatectomy: impact on target volume definition and dose-volume parameters of rectum and bladder. Strahlenther Onkol. 2004;180:563–572. doi: 10.1007/s00066-004-1245-y. [DOI] [PubMed] [Google Scholar]
- 35.Chen JC, Schultheiss TE, Nguyen KH, Wong JY. Acute toxicity in definitive versus postprostatectomy image-guided radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;71:351–357. doi: 10.1016/j.ijrobp.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 36.Teh BS, Wei-Yuan M, Augspurger ME, et al. Intensity modulated radiation therapy (IMRT) following prostatectomy: more favorable acute genitourinary toxicity profile compared to primary IMRT for prostate cancer. Int J Radiat Oncol Biol Phys. 2001;49:465–472. doi: 10.1016/s0360-3016(00)01474-7. [DOI] [PubMed] [Google Scholar]
- 37.Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299:2760–2769. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 39.Raldow A, Hamstra DA, Kim SN, Yu JB. Adjuvant radiotherapy after radical prostatectomy: evidence and analysis. Cancer Treat Rev. 2011;37:89–96. doi: 10.1016/j.ctrv.2010.07.001. [DOI] [PubMed] [Google Scholar]