Abstract
Background:
A recent study demonstrated that an increased number of CD68+ macrophages were correlated with primary treatment failure, shortened progression-free survival (PFS) and disease-specific survival (DSS) in patients with classical Hodgkin's lymphoma (cHL).
Patients and methods:
The aim of the present study was to verify the relationship between the number of CD68+ and CD163+ macrophages with clinical outcomes in a cohort of 265 well-characterized patients with cHL treated uniformly with the standard doxorubicin, bleomycin, vinblastine and dacarbazine chemotherapy regimen. Two pairs of hematopathologists carried out independent pathological evaluations of tissue microarray slides.
Results:
There were no associations between clinical characteristics and the expression of CD68 or CD163. However, higher levels of CD68 and CD163 expression were correlated with the presence of Epstein–Barr virus-positive Hodgkin tumor cells (P = 0.01 and 0.037, respectively). The expression of CD68 or CD163 was not associated with either the PFS or the DSS.
Conclusion:
CD68 and CD163 expression require further evaluation before their use can be recommended for prognostic stratification of patients with cHL.
Keywords: CD68, Hodgkin's lymphoma, macrophages, prognosis
introduction
With the introduction of combination chemotherapy for Hodgkin's lymphoma (HL) in the 1970s, prospects for prolonged disease-free survival increased markedly, but long-term adverse effects of therapy became a major concern. Recent initiatives have attempted to decrease the late toxic effects of treatment regimens while maintaining or improving their efficacy [1].
In order to tailor treatment to individual risk, robust prognostic indicators are needed. The International Prognostic Score (IPS) is the most widely used prognostic system, but while it is reproducible and consistent, it cannot reliably identify patients in whom treatment is likely to fail [2]. Consequently, the quest for additional reliable prognostic indicators continues.
The tumor microenvironment is an important factor in the development and progression of cancer. Recent evidence suggests that the cellular composition of the tumor microenvironment can significantly modify the clinical outcome in hematologic malignancies, particularly follicular lymphoma and classical Hodgkin's lymphoma (cHL) [3–5].
Recently, data from gene expression studies have identified a gene signature of tumor-associated macrophages that was associated with primary treatment failure in cHL patients. These findings were followed by the observation, made by the same authors, that an increased number of CD68+ macrophages, as determined by immunohistochemistry, were correlated with a shortened progression-free survival (PFS), suggesting that CD68 is a useful biomarker for risk stratification in cHL [6–8].
The aim of the present study was to verify these findings by analyzing the relationship between CD68+ macrophages and clinical outcomes in a large independent cohort of well-characterized patients treated uniformly with the standard doxorubicin, bleomycin, vinblastine and dacarbazine (ABVD) regimen. The tissue microarrays (TMAs) were also stained for CD163, a monocyte/macrophage-specific protein in the scavenger receptor cysteine-rich superfamily [9]. In addition to its receptor function for hemoglobin/haptoglobin complexes, CD163 seems to be involved in anti-inflammatory functions believed to be predominantly associated with M2 macrophages [10].
patients and methods
patient characteristics
This study included 265 consecutive patients with cHL treated on initial diagnosis at the University Hospital, Federal University of Rio de Janeiro and at the Brazilian Instituto Nacional de Câncer from 1997 to 2004. Diagnoses were confirmed on review by three authors (DA, YN and JCM) using morphological and immunohistochemistry criteria defined in the World Health Organisation classification [11]. Patients were selected based on the availability of clinical information and histological material for TMA construction. Expression of CD30 was required for inclusion. Patients with the AIDS were excluded. All patients were staged according to the Ann Arbor system [12]. The following baseline clinical characteristics were recorded: sex, age, stage, presence of bulky disease or B symptoms, performance status and blood counts. The IPS was computed [2]; patients were categorized as low-risk IPS if they presented with up to two risk factors and as high-risk IPS if three or more risk factors were present. Patients were also stratified into early-stage (I–IIA) and advanced (IIB–IV) cHL. We have previously confirmed the prognostic impact of HGAL protein expression in cHL using 232 patients from the same cohort [13]. The study was approved by the institutional ethics committees.
All patients were treated with curative intent. Patients with early-stage disease were treated with two to four cycles of ABVD followed by radiation therapy. Patients with advanced disease were treated with six to eight cycles of ABVD, complemented by radiation therapy in patients with bulky disease. Patients who failed primary therapy received either salvage chemotherapy or high-dose chemotherapy with autologous stem-cell transplantation.
tissue microarray construction and immunohistochemistry
TMAs were constructed using a tissue arrayer (Beecher Instruments, Silver Spring, MD), by a previously described method [14]. Duplicated cores of 2.0 mm were selected from areas of characteristic morphology typical of the case based on examination of the hematoxylin- and eosin-stained original whole-tissue sections, without prior knowledge of immunohistologic stains of individual cases. These areas were circled on the glass slide of the whole section and superimposed on the corresponding paraffin block, which was then punched at the selected location to obtain the desired core. Areas containing fibrosis, necrosis or granulomas were avoided. Sections of 4 mm thickness were cut from the TMA and placed on glass slides, which were then baked for 1 h at 60°C. These slides were then subjected to immunohistochemistry. The number of CD68+ and CD163+ cells was assessed by semiquantitative analyses. The following scale of cut-off values were used to score each core: <5%, 5%–25%, >25%–50% and >50% and represents the percentage of positive cells in relation to overall cellularity as previously described [6]. The values were derived from the visual analysis of three high-power fields (×400), using a Nikon Eclipse E-200 microscope, which correspond to an area of 0.5 mm per field.
CD68 (clone KP1, Dako Corporation, Carpinteria, CA, dilution 1:1600) and CD163 (clone 10D6, Novocastra Laboratories, Newcastle Upon Tyne, UK, dilution 1:100) stainings were carried out at Stanford according to the established protocols for automated immunohistochemistry on the Ventana Benchmark XT (Ventana Medical Systems, Tucson, AZ).
TMAs were jointly evaluated by two hematopathologists in Rio de Janeiro (DA and JCM) and in parallel by two hematopathologists in Stanford (MWA and YN), generating two sets of scores, henceforth, referred as scores A and B, respectively. The two sets of scores were compared for agreement, and both sets were used independently to assess the impact of CD68 and CD163 on clinical outcomes.
Marker expression was stratified according to the percentage of positive cells in relation to the overall cellularity. Results were reported as an average of the duplicate cores and were classified into four groups: <5% positive cells, score 1; 5%–25% positive cells, score 2; >25%–50% positive cells, score 3; and >50% positive cells, score 4 [6].
in situ hybridization for Epstein–Barr viral RNA
In situ hybridization was carried out on 4- to 5-μm thick formalin-fixed paraffin-embedded sections using the INFORM EBER probe (Ventana Medical Systems). Slides were stained on an automated immunostainer (Ventana Benchmark) using the Ventana ISHiVIEW detection kit. Specific nuclear signal was considered positive. An oligo (dT) control to verify intact RNA within the nuclei of the cells composing the tissue and a known positive control were carried out.
statistical analysis
Categorical data were analyzed using Fisher's exact test (two-sided). Survival curves were estimated using the Kaplan–Meier method and compared using the log-rank test. Disease-specific survival (DSS) was calculated as the time from diagnosis to the date of death from lymphoma or its treatment or last contact, with data for patients who died of unrelated causes censored at the time of death. PFS was calculated as the time from diagnosis to the date of disease progression, relapse, death from any cause or last contact. For the CD68 and CD163 analysis, scores A and B were used for survival analysis with 5% and 25% cut-offs.
The kappa test was used to evaluate the agreement between scores A and B. Of note, a kappa between 0.4 and 0.6 was regarded as moderate agreement, a kappa between 0.6 and 0.8 was regarded as substantial agreement and a kappa >0.8 represented excellent agreement [15]. The SPSS version 17.0 software (Chicago, IL) was used for data analysis.
results
The median age of the 265 patients was 29 years (range 15–82), and 128 (48%) were women. The patients’ main characteristics at diagnosis are shown in Table 1.
Table 1.
Clinical characteristics of patients
| Feature | N = 265 (%) |
| Age (years) | |
| <45 | 205 (77) |
| ≥45 | 60 (23) |
| IPS | |
| 0–2 | 160 (72) |
| ≥3 | 63 (28) |
| Performance status | |
| 0–1 | 238 (91) |
| ≥2 | 23 (9) |
| Ann Arbor stage | |
| I–IIA | 114 (43) |
| IIB–IV | 151 (57) |
| Histology | |
| Nodular sclerosis | 180 (68) |
| Mixed cellularity | 52 (20) |
| Lymphocyte rich | 9 (3) |
| Lymphocyte depletion | 9 (3) |
| Nonclassified | 15 (6) |
| Bulky disease | |
| No | 158 (60) |
| Yes | 107 (40) |
| Hemoglobin (g/dl) | |
| ≥10.5 | 199 (78) |
| <10.5 | 57 (22) |
| Albumin (g/dl) | |
| ≥4.0 | 98 (51) |
| <4.0 | 94 (49) |
| Leucocytes | |
| <15 × 109/l | 217 (86) |
| ≥15 × 109/l | 36 (14) |
| Lymphocytes | |
| <0.6 × 109/l | 21 (10) |
| ≥0.6 × 109/l | 181 (90) |
IPS, International Prognostic Score.
All patients were treated with curative intent: 48% received only ABVD chemotherapy, while 52% received a combination of ABVD and radiation therapy. A total of 234 patients (88%) achieved a complete remission after initial treatment; 22 failed primary therapy and 9 died during treatment. An additional 22 patients relapsed after achieving complete remission. Among the 44 patients with primary or secondary failure, 20 received a second-line chemotherapy regimen, while 24 were treated with high-dose chemotherapy with autologous stem-cell support. The median follow-up of the entire cohort of patients was 6 (range 0.06–11.7) years, and the median follow-up of the alive patients was also 6 years (range 1.5–11.7).
interobserver agreement and expression of CD68 and CD163
CD68 and CD163 expression as evaluated in scores A and B, and agreement between the two scores, are shown in Table 2.
Table 2.
CD68 and CD163 expression according to the scores A and B and agreement between scores
| CD68 | CD163 | |
| Score A | ||
| ≥5% (score >1) | 83% | 58% |
| ≥25% | 26% | 27% |
| Score B | ||
| ≥5% (score >1) | 96% | 48% |
| ≥25% | 35% | 20% |
| Kappa between scores A and B | ||
| ≥5% (score >1) | 0.29 | 0.87 |
| ≥25% | 0.58 | 0.70 |
The hematopathologists experienced difficulty in evaluating low levels of CD68 expression, and the interobserver agreement for CD68 using the 5% cut-off was remarkably low. CD163 was considered easier to evaluate than CD68.
CD68 and CD163 expression and clinicopathological features
There were no associations between various clinical characteristics and the expression of CD68 or CD163 (Table 3). However, higher levels of CD68 and CD163 expression were correlated with the presence of Epstein–Barr virus (EBV)-positive Hodgkin's tumor cells (P = 0.01 and 0.037, respectively).
Table 3.
Clinical characteristics according to the CD68 and CD163 expressiona
| Feature | CD68 negative (N = 40) | CD68 positive (N = 201) | P value | CD163 negative (N = 78) | CD163 positive (N = 107) | P value |
| Age (years) | ||||||
| <45 | 30 (75) | 158 (79) | 0.68 | 61 (78) | 78 (73) | 0.49 |
| ≥45 | 10 (25) | 43 (21) | 17 (22) | 29 (27) | ||
| IPS | ||||||
| 0–2 | 20 (59) | 131 (77) | 0.03 | 57 (78) | 57 (69) | 0.20 |
| ≥3 | 14 (41) | 39 (23) | 16 (22) | 26 (31) | ||
| Performance status | ||||||
| 0–1 | 38 (95) | 181 (91) | 72 (94) | 95 (89) | ||
| ≥2 | 2 (5) | 17 (9) | 0.75 | 5 (6) | 12 (11) | 0.31 |
| Ann Arbor stage | ||||||
| I–IIA | 19 (47) | 87 (43) | 0.73 | 34 (44) | 42 (39) | 0.65 |
| IIB–IV | 21 (53) | 114 (57) | 44 (56) | 65 (61) | ||
| Bulky disease | ||||||
| No | 28 (70) | 119 (59) | 0.22 | 52 (67) | 56 (52) | 0.07 |
| Yes | 12 (30) | 82 (41) | 26 (33) | 51 (48) | ||
| Hemoglobin (g/dl) | ||||||
| ≥10.5 | 29 (76) | 153 (79) | 0.83 | 62 (82) | 78 (75) | 0.36 |
| <10.5 | 9 (24) | 41 (21) | 14 (18) | 26 (25) | ||
| Albumin (g/dl) | ||||||
| ≥4.0 | 14 (45) | 78 (54) | 0.43 | 36 (59) | 31 (44) | 0.11 |
| <4.0 | 17 (55) | 66 (46) | 25 (41) | 39 (56) | ||
| Leucocytes | ||||||
| <15 × 109/l | 29 (76) | 170 (88) | 0.07 | 66 (87) | 86 (84) | 0.67 |
| ≥15 × 109/l | 9 (24) | 23 (12) | 10 (13) | 17 (16) | ||
| Lymphocytes | ||||||
| <0.6 × 109 | 4 (13) | 14 (9) | 0.5 | 3 (5) | 10 (13) | 0.14 |
| ≥0.6 × 109 | 26 (87) | 140 (91) | 59 (95) | 65 (87) | ||
| EBV (EBER) | ||||||
| Negative | 29 (83) | 93 (53) | 0.001 | 47 (70) | 54 (54) | 0.037 |
| Positive | 6 (17) | 81 (47) | 20 (30) | 47 (46) |
Data in this table are according to score A using the 5% cut-off.
IPS, International Prognostic Score; EBV, Epstein–Barr virus; EBER, EBV-encoded RNA.
survival analysis
The 5-year PFS and 5-year DSS for the whole cohort were 79% and 91%, respectively. The 5-year PFS in patients classified as low-risk and high-risk IPS were 86% and 66%, respectively (P < 0.0001) (not shown). The 5-year DSS in patients classified as low-risk and high-risk IPS were 96% and 80%, respectively (P < 0.0001) (not shown).
Survival curves for CD68 and CD163 expression were estimated using scores A and B, using both the 5% cut-off proposed in previous publication [6] and the 25% cut-off. The expressions of CD68 and CD163 did not have significant correlations with either the DSS or PFS in any of these analyses (Figures 1–4). In addition, stratified survival analysis according to the IPS-risk group and also according to stage did not identify any impact of CD68 or CD163 on PFS or DSS.
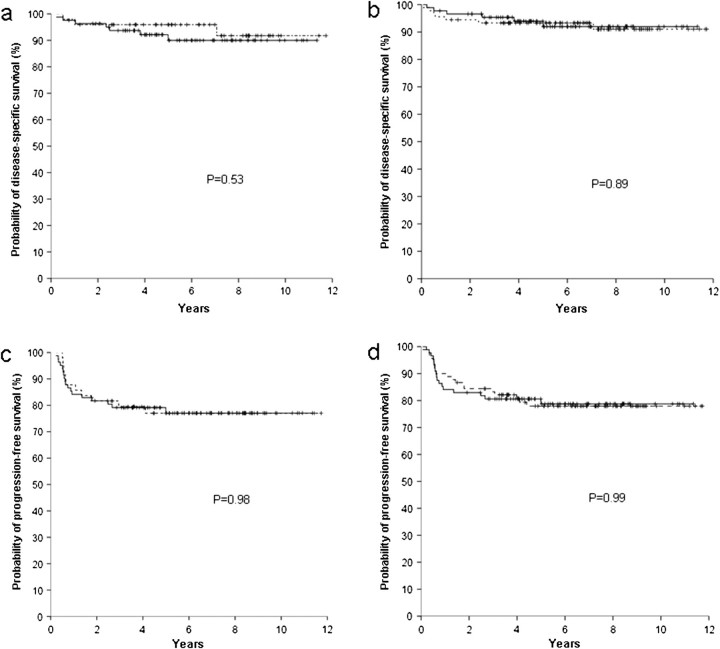
Figure 1.
Survivals according to the CD68 expression (negative - - - , positive —), with a 5% cut-off. Disease-specific survival according to score A (a) and score B (b); progression-free survival according to score A (c) and score B (d).
A subgroup survival analysis, including only nodular sclerosis cases, also did not show any prognostic impact of CD68 or CD163 on PFS or DSS.
impact of EBV positivity on survival
There was a correlation between EBV positivity and DSS. Patients negative for EBV had a 5-year DSS of 96%, while patients with EBV in their tumors had a 5-year DSS of 88% (P = 0.037). On the other hand, no differences in PFS were observed according to the EBV status.
Previous studies have suggested that age may impact association between EBV and survival [16]. Our cohort of cases had only 43 patients over the age of 50 years and was thus underpowered for the specific analysis of the impact of age on survival. There were no differences according to age.
discussion
The number of CD68+ macrophages in the tumor stroma was recently presented as a potential breakthrough in risk stratification for patients with cHL [7]. This impression was based on an elegant study in which, using biocomputational tools, the authors identified a gene signature of tumor-associated macrophages that was significantly associated with clinical outcomes in HL [6]. The number of CD68+ macrophages was then evaluated in an independent cohort of patients and also found to correlate with patient survival.
The attempt to confirm these observations in an independent large cohort of uniformly treated patients presented herein failed to identify any differences in survival according to the CD68 or CD163 expression using either score A or B and either the 5% or the 25% cut-off. The survival curves according to the proportion of CD68 or CD163 expression in macrophages were often overlapping (Figure 1).
The clinical outcomes of the cohort of patients in the present study are in line with expected results for ABVD treatment, with a complete remission rate of 88% and a 5-year DSS of 91% [1]. Good stratification according to the IPS is an additional evidence of the soundness of the clinical data. Furthermore, this cohort of patients was instrumental in confirming the prognostic significance of HGAL protein in cHL [13]. Patient selection for the present study was conventional, with the inclusion of a consecutive cohort of patients. In the previous study of CD68 expression in HL, the cohort was enriched for patients experiencing treatment failure in order to increase the statistical power, so that the studied cohort had similar proportions of patients with treatment success and failure. Although this procedure appears to be methodologically sound, it produces a study cohort that is very different from the reality of clinical practice, and its neutrality regarding different study biases has not been, to our knowledge, fully established.
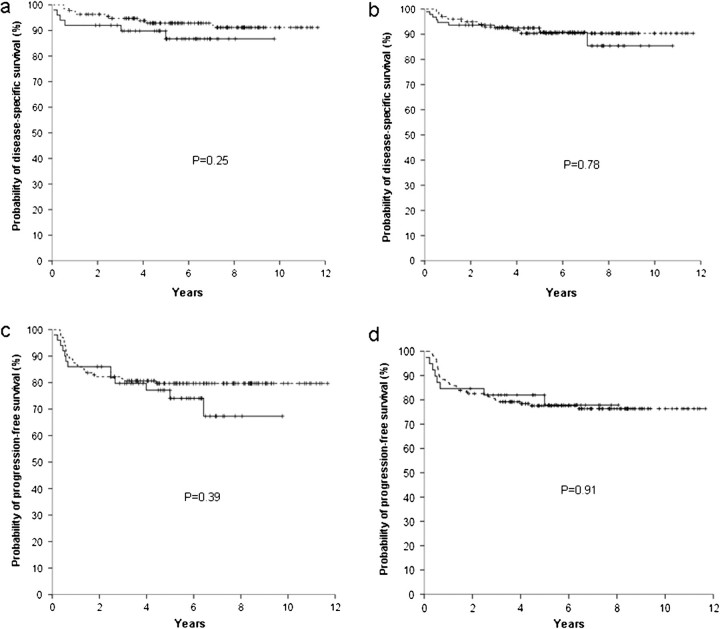
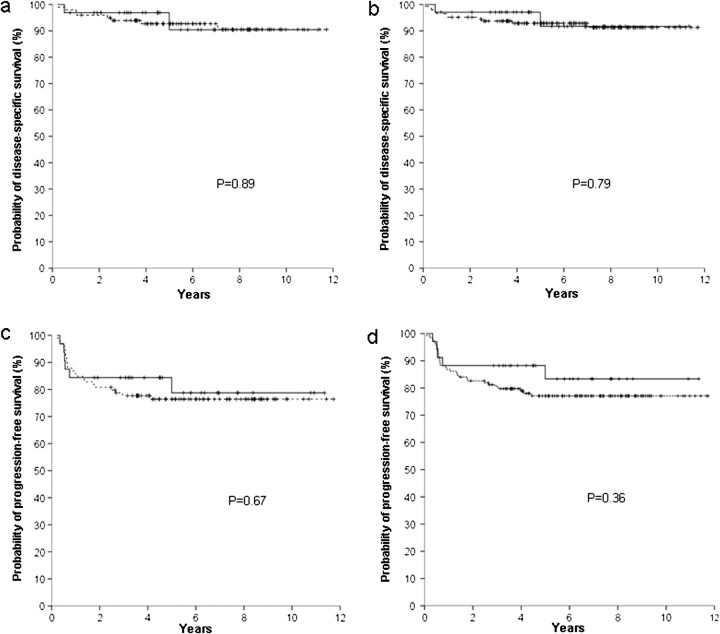
Lack of reproducibility is a major obstacle for the broader clinical application of immunohistochemical prognostic markers in lymphomas [15, 17]. The analysis of interobserver correlation for interpretation of CD68 and CD163 staining in the present study evidenced agreement rates similar to those reported in previous studies of the prognostic role of immunohistochemical markers in lymphomas [15, 18, 19]. As shown in Figures 1–4, clinical significance of CD68 and CD163 expression was uniformly lacking with both scores A and B.
Figure 4.
Survivals according to the CD163 expression (negative - - - , positive —), with a 25% cut-off. Disease-specific survival according to score A (a) and score B (b); progression-free survival according to score A (c) and score B (d).
Figure 2.
Survivals according to the CD68 expression (negative - - - , positive —), with a 25% cut-off. Disease-specific survival according to score A (a) and score B (b); progression-free survival according to score A (c) and score B (d).
Figure 3.
Survivals according to the CD163 expression (negative - - - , positive —), with a 5% cut-off. Disease-specific survival according to score A (a) and score B (b); progression-free survival according to score A (c) and score B (d).
immunohistochemistry for CD163 was considered easier to evaluate than for CD68. The KP1 antigen detected by the CD68 antibody used in the study by Steidl et al. [6] as well as in our study has been well documented to stain nonhematopoietic cells such as fibroblasts and endothelial cells in addition to myelomonocytic cells including neutrophils as well as subsets of lymphocytes [20]. Therefore, in tissue sections, other stromal components besides macrophages may also be responsible for the total CD68 signal on immunohistolochemistry, which complicates interpretation of the stain. Although four experienced hematopathologists scored these slides in our current study, interobserver agreement was low especially at the lower end of the defined range of positivity. In contrast, the restricted staining pattern, as well as the crisp staining quality of CD163 in monocytic/macrophage lineage cells [9] made this immunostain easier to evaluate. These findings raise the important consideration of whether CD68 would serve as a robust marker amenable for wide clinical application.
Kamper et al. [21] recently reported an association between higher levels of CD68 and CD163 expression with the presence of EBV in patients with HL. Our results are in keeping with that observation and show that higher levels of CD68 and CD163 expression were correlated with the presence of EBV-positive Hodgkin's tumor cells and that EBV positivity by itself also shows a correlation with outcome. The association of EBV status with outcome in cHL has been highly controversial, and recent literature points to a possible correlation in older adult patients [16]. It has also been postulated that the EBV-encoded EBNA-1 protein by inducing the up-regulation of the chemokine CCL20 actively recruits regulatory T cells, thereby opposing immune responses against virus-infected tumor cells [22]. This postulated mechanism may indeed be in operation to also recruit other tumor infiltrating cells, such as macrophages, which suggests that the impact of tumor infiltrating cells on outcome may be an epiphenomenon and not due to true causality. Further studies are, however, needed to establish the mechanistic link between EBV infection, macrophage content and outcome in cHL.
In summary, the enumeration of CD68+ cells or CD163+ cells in diagnostic lymph node samples had no correlation with clinical outcomes in a large cohort of patients with cHL. Additional studies are needed to either confirm or refute the previous suggestion that CD68 staining is useful for prognostic purposes in cHL.
funding
DA was supported by a Coordenaçáo de Aperfeiçoamento de Pessoal de Nível Superior/Programa de Doutorado no País com Estágio no Exterior (CAPES/PDEE) grant; ISL is supported by National Institutes of Health (NIH, Bethesda, MD) CA109335 and NIH CA122105, and the Dwoskin Family Foundation (Miami, FL); YN is supported by grant NIH POI CA34233; NS is supported by Faperj grant 102.900/2008, and CNPq grant 302708/2008-1.
disclosure
The authors declare no conflict of interest.
Acknowledgments
Authorship—Conception and design: DA, YN, ISL, IB, JCM, NS. Provision of study materials or patients: DA, YN, IB, JCM, NS. Collection and assembly of data: DA, YN, IB, MWA, JCM. Data analysis and interpretation: DA, YN, IB, ISL, NS. Manuscript writing: DA, YN, ISL, IB, JCM, NS. Final approval of manuscript: DA, YN, IB, ISL, MWA, JCM, NS.
References
- 1.Diehl V, Fuchs M. Early, intermediate and advanced Hodgkin's lymphoma: modern treatment strategies. Ann Oncol. 2007;18(Suppl 9):ix71–ix79. doi: 10.1093/annonc/mdm297. [DOI] [PubMed] [Google Scholar]
- 2.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease. International Prognostic Factors Project on Advanced Hodgkin's Disease. N Engl J Med. 1998;339(21):1506–1514. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 3.Hsi ED. Biologic features of Hodgkin lymphoma and the development of biologic prognostic factors in Hodgkin lymphoma: tumor and microenvironment. Leuk Lymphoma. 2008;49(9):1668–1680. doi: 10.1080/10428190802163339. [DOI] [PubMed] [Google Scholar]
- 4.Dave SS, Wright G, Tan B, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351(21):2159–2169. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- 5.Alvaro T, Lejeune M, Salvado MT, et al. Outcome in Hodgkin's lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res. 2005;11(4):1467–1473. doi: 10.1158/1078-0432.CCR-04-1869. [DOI] [PubMed] [Google Scholar]
- 6.Steidl C, Lee T, Shah SP, et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med. 2010;362(10):875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeVita VT, Jr, Costa J. Toward a personalized treatment of Hodgkin's disease. N Engl J Med. 2010;362(10):942–943. doi: 10.1056/NEJMe0912481. [DOI] [PubMed] [Google Scholar]
- 8.Diehl V. Hematology. Are macrophages the bad guys in Hodgkin lymphoma? Nat Rev Clin Oncol. 2010;7(6):301–302. doi: 10.1038/nrclinonc.2010.71. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen TT, Schwartz EJ, West RB, et al. Expression of CD163 (hemoglobin scavenger receptor) in normal tissues, lymphomas, carcinomas, and sarcomas is largely restricted to the monocyte/macrophage lineage. Am J Surg Pathol. 2005;29(5):617–624. doi: 10.1097/01.pas.0000157940.80538.ec. [DOI] [PubMed] [Google Scholar]
- 10.Moestrup SK, Moller HJ. CD163: a regulated hemoglobin scavenger receptor with a role in the anti-inflammatory response. Ann Med. 2004;36(5):347–354. doi: 10.1080/07853890410033171. [DOI] [PubMed] [Google Scholar]
- 11.Stein H, Delsol G, Pileri SA, et al. Classical Hodgkin lymphoma, Introduction. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues. 4th edition. Lyon, France:: 2008. pp. 326–329. [Google Scholar]
- 12.Carbone PP, Kaplan HS, Musshoff K, et al. Report of the committee on Hodgkin's disease staging classification. Cancer Res. 1971;31(11):1860–1861. [PubMed] [Google Scholar]
- 13.Azambuja D, Lossos IS, Biasoli I, et al. Human germinal center-associated lymphoma protein expression is associated with improved failure-free survival in Brazilian patients with classical Hodgkin lymphoma. Leuk Lymphoma. 2009;50(11):1830–1836. doi: 10.3109/10428190903242628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kallioniemi OP, Wagner U, Kononen J, Sauter G. Tissue microarray technology for high-throughput molecular profiling of cancer. Hum Mol Genet. 2001;10(7):657–662. doi: 10.1093/hmg/10.7.657. [DOI] [PubMed] [Google Scholar]
- 15.de Jong D, Rosenwald A, Chhanabhai M, et al. Immunohistochemical prognostic markers in diffuse large B-cell lymphoma: validation of tissue microarray as a prerequisite for broad clinical applications–a study from the Lunenburg Lymphoma Biomarker Consortium. J Clin Oncol. 2007;25(7):805–812. doi: 10.1200/JCO.2006.09.4490. [DOI] [PubMed] [Google Scholar]
- 16.Diepstra A, van Imhoff GW, Schaapveld M, et al. Latent Epstein-Barr virus infection of tumor cells in classical Hodgkin's lymphoma predicts adverse outcome in older adult patients. J Clin Oncol. 2009;27(23):3815–3821. doi: 10.1200/JCO.2008.20.5138. [DOI] [PubMed] [Google Scholar]
- 17.Spector N, Milito CB, Biasoli I, et al. The prognostic value of the expression of Bcl-2, p53 and LMP-1 in patients with Hodgkin's lymphoma. Leuk Lymphoma. 2005;46(9):1301–1306. doi: 10.1080/10428190500126034. [DOI] [PubMed] [Google Scholar]
- 18.Natkunam Y, Mason DY. Prognostic immunohistologic markers in human tumors: why are so few used in clinical practice? Lab Invest. 2006;86(8):742–747. doi: 10.1038/labinvest.3700447. [DOI] [PubMed] [Google Scholar]
- 19.Zu Y, Steinberg SM, Campo E, et al. Validation of tissue microarray immunohistochemistry staining and interpretation in diffuse large B-cell lymphoma. Leuk Lymphoma. 2005;46(5):693–701. doi: 10.1080/10428190500051844. [DOI] [PubMed] [Google Scholar]
- 20.Pulford KA, Sipos A, Cordell JL, et al. Distribution of the CD68 macrophage/myeloid associated antigen. Int Immunol. 1990;2(10):973–980. doi: 10.1093/intimm/2.10.973. [DOI] [PubMed] [Google Scholar]
- 21.Kamper P, Bendix K, Hamilton-Dutoit S, et al. Tumor-infiltrating macrophages correlate with adverse prognosis and Epstein-Barr virus status in classical Hodgkin's lymphoma. Haematologica. 2011;96(2):269–276. doi: 10.3324/haematol.2010.031542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baumforth KR, Birgersdotter A, Reynolds GM, et al. Expression of the Epstein-Barr virus-encoded Epstein-Barr virus nuclear antigen 1 in Hodgkin's lymphoma cells mediates up-regulation of CCL20 and the migration of regulatory T cells. Am J Pathol. 2008;173(1):195–204. doi: 10.2353/ajpath.2008.070845. [DOI] [PMC free article] [PubMed] [Google Scholar]