Abstract
Liver fructose-1,6-bisphosphatase (FBPase) is a regulatory enzyme in gluconeogenesis that is elevated by obesity and dietary fat intake. Whether FBPase functions only to regulate glucose or has other metabolic consequences is not clear; therefore, the aim of this study was to determine the importance of liver FBPase in body weight regulation. To this end we performed comprehensive physiologic and biochemical assessments of energy balance in liver-specific transgenic FBPase mice and negative control littermates of both sexes. In addition, hepatic branch vagotomies and pharmacologic inhibition studies were performed to confirm the role of FBPase. Compared with negative littermates, liver-specific FBPase transgenic mice had 50% less adiposity and ate 15% less food but did not have altered energy expenditure. The reduced food consumption was associated with increased circulating leptin and cholecystokinin, elevated fatty acid oxidation, and 3-β-hydroxybutyrate ketone levels, and reduced appetite-stimulating neuropeptides, neuropeptide Y and Agouti-related peptide. Hepatic branch vagotomy and direct pharmacologic inhibition of FBPase in transgenic mice both returned food intake and body weight to the negative littermates. This is the first study to identify liver FBPase as a previously unknown regulator of appetite and adiposity and describes a novel process by which the liver participates in body weight regulation.
Over recent years, excessive nutrient intake has been associated with rapidly increasing rates of obesity in both developed and developing societies (1). Despite much effort, the specific biochemical mechanisms involved in body weight regulation are not completely understood.
Body weight is maintained by a fine balance between food intake and energy expenditure. Under normal conditions, energy homeostasis is maintained through a complex interaction between peripheral organs and the central nervous system (CNS). Many peripheral signals from white adipose tissue, the gut, and the pancreas are known to regulate body weight (2). The CNS receives these signals and adjusts food intake and energy expenditure accordingly. Although not normally considered as one of the classic body weight regulatory organs, indirect evidence has accumulated over the years in a variety of models to suggest a role for the liver in controlling food intake (3–7). Russek (7) was the first to propose that a signal to terminate food intake was generated from the liver. This was based on studies demonstrating that direct injection of glucose into the liver of fasted dogs suppressed food intake more effectively than systemic injection of glucose (7). Direct infusion of free fatty acids (FFAs) into the hepatic portal vein of rats has also demonstrated an involvement of the liver in lowering food intake through an increase in liver fatty acid oxidation (FAO) (3,4). Conversely, the fructose analog 2,5-anhydro-d-mannitol (6) and other metabolic and FAO inhibitors have been reported to stimulate appetite when administered into animals (5,8,9).
The gene expressing fructose-1,6-bisphosphatase (FBPase) is one of many genes upregulated in the liver by obesity and fat (10,11). Even though FBPase is known as a regulatory enzyme in gluconeogenesis, a previous study from our laboratory showed that liver-specific FBPase transgenic mice with a physiologic threefold level of overexpression had no change in whole-body glucose tolerance or endogenous glucose production (12). Surprisingly, the mice consistently displayed an approximate 10% reduction in body weight compared with negative littermates (12), leading us to propose that liver FBPase may have a novel role in the control of body weight.
We therefore investigated this potential regulatory role of liver FBPase by using our transgenic mouse model that specifically overexpresses FBPase in the liver. We report that overexpression of this liver enzyme leads to the lean body weight phenotype in the transgenic mice by markedly reducing adiposity levels by ∼50%. Reductions in food intake rather than elevated energy expenditure were found to be the contributing factors. The appetite-stimulating neuropeptides, neuropeptide Y (NPY) and Agouti-related peptide (AgRP), were significantly suppressed, whereas the circulating satiety hormones, cholecystokinin (CCK), and leptin, rose significantly. Elevation of liver FAO via an increased flux through the hexosamine biosynthesis pathway (HBP) appears to be the key linking the increase in liver FBPase to reduced food intake and adiposity in our transgenic mouse.
RESEARCH DESIGN AND METHODS
Animals.
Hemizygous transgenic mice (males and females) overexpressing the human liver FBPase gene (FBP-1) specifically in the liver and their age-matched negative littermate controls, both on a C57Bl6/J background, were used unless otherwise stated. Mice were generated, maintained, and genotyped as described previously (12). All mice were maintained in accordance with guidelines of the Austin Hospital Animal Ethics Committee (AEC#s: A2007/2752 and A2009/03766).
Energy balance studies.
Mice were individually housed and provided a standard laboratory chow diet (3% fat, 77% carbohydrate, 20% protein) and water ad libitum. Body weights were measured at 4, 8, and 12 weeks and subsequently each week until age 22 weeks. Food intake (g/day) was measured each week from age 12 to 22 weeks. Subcutaneous, infrarenal, and gonadal fat pads were collected and weighed in the nonfasted state. Voluntary physical activity, resting energy expenditure (REE), respiratory quotient (RQ), and levels of whole-body fat and glucose oxidation were assessed as previously described (13–16).
Hepatic branch vagotomy.
Common hepatic branch vagotomy was performed on 16-week-old anesthetized mice that specifically targets the vagal nerve branch, the major connection between the liver and brain (17). A laparotomy incision was made on the ventral midline, and the abdominal wall was opened with a second incision. The common hepatic vagal branch was located (under the liver) and transected, stretching the fascia containing the common hepatic branch using fine forceps. A similar incision was also made in the sham-operated mice and the hepatic vagus nerve was located but not transected. The incisions were sutured, mice were administered saline (1 mL) intraperitoneally to aid in postsurgery recovery, and were allowed to recover for 1 week. Food intake and body weight was subsequently measured weekly for 10 weeks.
Pharmacologic inhibition of FBPase.
FBPase transgenic mice, negative littermate controls (∼16 weeks old), and NZO mice (7 weeks old) were gavaged daily a 5 mg/kg dose of benzoxazole benzene sulfonamide, a commercially available human-specific FBPase inhibitor (Calbiochem) (18,19), to specifically target our construct or vehicle (water) 2 h before the dark cycle (feeding period) for 10 days. Body weight and food intake measurements were taken daily. On the 10th day of treatment, plasma was collected from anesthetized mice in the fed state (1 h postdark cycle) via cardiac puncture, the whole brain was collected, and fat depots were collected and weighed.
CCK1 receptor antagonist study.
FBPase transgenic mice and negative littermates (10 weeks old) were injected intraperitoneally with a 300 μg/kg dose of lorglumide, a CCK1 receptor (CCK1R) antagonist (Sigma Aldrich, St. Louis, MO) (20), or saline vehicle at the onset of the dark cycle, and food intake was recorded 24 h later.
3-β-Hydroxybutyrate study.
Male C57BL/6J mice (10 weeks old) were subcutaneously injected with a 10 mmol/kg dose of DL-3-hydroxybutyric acid (sodium salt, 98% purity 3-β-hydroxybutyrate[BHB]; MP Biomedicals) (5) or saline at the onset of the dark cycle. Body weight and food intake were measured 1 and 2 h thereafter. Mice were left to recover for a week, and BHB administration was repeated for plasma collection 1 h after administration only.
Hormone and metabolite assays.
Cardiac punctures were performed on anesthetized transgenic mice and negative littermates (∼16 weeks old) in the fed state (1 h into the dark cycle). Circulating ghrelin was measured using a specific enzyme immunoassay (Phoenix Pharmaceuticals Inc., Belmont, CA), circulating CCK was measured by an in-house radioimmunoassay (21), and circulating leptin was measured by radioimmunoassay (Linco Research, St Charles, MO). BHB was measured from cardiac plasma samples collected after an overnight fast using a 3-hydroxybutyrate II reagent kit (Helena Laboratories Australia Pty Ltd, Melbourne, VIC, Australia). Fructose-1,6-phosphate (F-1,6-P) and fructose-6-phosphate (F6P) assays were performed as previously described (10).
FAO inhibitor study.
FBPase transgenic mice and negative littermates (10 weeks old) were gavaged with one dose (10 mg/kg) of etomoxir, a carnitine palmitoyl-CoA transferase (CPT)-1a inhibitor (Sigma Aldrich, St. Louis, MO) (22) or saline vehicle at the onset of the dark cycle. Food intake was recorded 24 h later.
O-linked N-acetylglucosamine transferase RL2 Western blotting.
Liver protein homogenates were processed and collected as previously described (23), and RL2 protein levels, indicative of O-linked N-acetylglucosamine transferase (OGT) (∼135 kDa) (24) were determined by Western blotting as previously described (25).
Hypothalamic dissection.
Hypothalamic sections (arcuate nucleus [ARC]) and paraventricular nucleus [PVN, as a control]) were collected in hemizygous mice (∼16 weeks old) and in homozygous mice (∼24 weeks old), 1 h into the dark cycle as previously described (13,16,26).
Measurement of mRNA expression levels in hypothalamus and liver.
RNA was extracted from ARC, PVN, and liver samples using TRIzol (Invitrogen, Mount Waverley, VIC, Australia), treated with DNaseI (Ambion, Scoresby, VIC, Australia), and cDNA was synthesized using 1 μg DNase-treated RNA and random primers with the Promega Reverse Transcription kit (Annandale, NSW, Australia). AgRP and proopiomelanocortin (POMC) primers for SYBR-green real-time PCR were designed across an intron-exon boundary to ensure nonamplification of genomic DNA. POMC primers: forward 5′-CTG GCC CTC CTG CTT CAG-3′; reverse 5′-GGA TGC AAG CCA GCA GGT T-3′ (0.3 μmol/L each). AgRP primers: forward 5′-TCC CAG AGT TCC CAG GTC TAA G-3′; reverse 5′-TAG CAC CTC CGC CAA AGC-3′ (0.3 μmol/L each). Primers for α-actin endogenous control were ised as previously described (27). TaqMan gene expression assays (Applied Biosystems, Scoresby, VIC, Australia) were used for OGT (Mm00507300_m1), CPT-1a (Mm00550435_m1), CPT-1c (Mm00463970_m1), peroxisome proliferator–activated receptor (PPAR)α (Mm00440939_m1), peroxisome proliferator–activated receptor-γ coactivator-1(PGC1)-α (Mm01208832_m1), leptin (Mm00434759_m1), and NPY (Mm00445771_m1). 18S primer mix from ABI was used as the endogenous control. ABI sequence detection software and relative quantification using the comparative ΔCt method was used.
Statistical analysis.
All data are presented as mean ± SEM. Comparisons between single parameters were measured using one-way ANOVA analysis (Minitab 15, 2007). The trapezoidal rule was used for area under the curve (AUC). For repeated measures, a general linear model (GLM) ANOVA was used for comparison and a Tukey post hoc t test to determine significance (Minitab 15, 2007). Significance was determined as P < 0.05.
RESULTS
Liver FBPase transgenic mice develop a lean body weight phenotype.
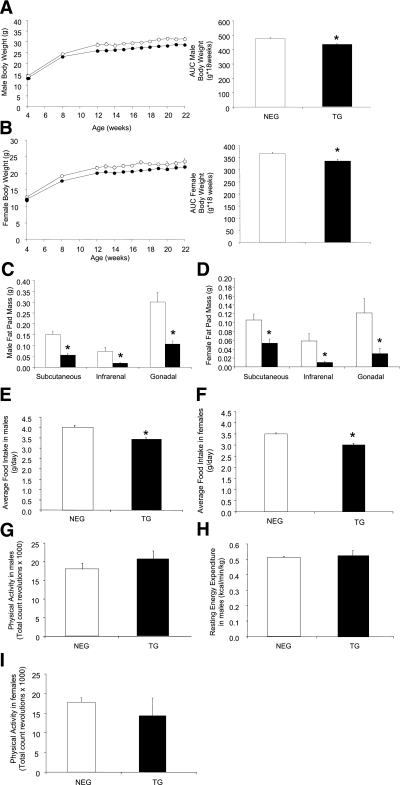
Male (Fig. 1A)and female (Fig. 1B) hemizygous transgenic mice both weighed ∼10% less than their age matched negative littermates, evident from 8 weeks of age. Male homozygous transgenic mice (Supplementary Fig. 1A) and females (data not shown) also weighed significantly less. The reduced body weight in the transgenic mice was associated with a significant reduction in white adipose tissue mass across all three depots (subcutaneous, infrarenal, and gonadal) compared with the negative male (Fig. 1C) and female (Fig. 1D) littermates.
FIG. 1.
Characterization of liver FBPase transgenic (TG) mice. Body weights of male negative (NEG, white circles) and TG (black circles) mice from hemizygous breeding from age 4 weeks to 22 weeks (NEG: n = 11 and TG: n = 14; AUC *P < 0.001 vs. NEG, GLM ANOVA) (A) and female NEG (white circles) and TG (black circles) mice from hemizygous breeding from age 4 weeks to 22 weeks (NEG: n = 13 and TG: n = 10; AUC *P < 0.005 vs. NEG, GLM ANOVA) (B). Fat pad mass of male hemizygous TG mice (black bars) compared with their NEG (white bars) littermates (NEG: n = 14 and TG n = 9; *P < 0.02 vs. NEG, one-way ANOVA) (C) and female hemizygous TG mice (black bars) compared with their NEG (white bars) littermates at age 24 weeks (NEG: n = 11 and TG: n = 6; *P < 0.05 vs. NEG, one-way ANOVA) (D). Average daily food intake of male hemizygous TG mice (black bars) compared with NEG (white bars) littermates (NEG: n = 21 and TG: n = 14; *P < 0.0001 vs. NEG GLM ANOVA) (E) and of female hemizygous TG mice (black bars) compared with NEG (white bars) littermates (NEG: n = 18 and TG; n = 10; *P < 0.05 vs. NEG, GLM ANOVA) (F). Physical activity (NEG: n = 7 and TG: n = 5) (G) and resting energy expenditure (n = 4 for each group) (H) in male hemizygous transgenic mice (black bars) compared with negative (white bars) littermates. I: Physical activity measurements of female hemizygous TG (black bars) compared with NEG (white bars) littermates (NEG: n = 10 and Tg: n = 4). All data expressed as mean ± SEM.
Lean body weight phenotype is associated with reduced food intake.
Daily food intake was reduced by ∼15% in male (Fig. 1E) and female transgenic mice (Fig. 1F) compared with the negative littermates. No differences in physical activity or resting energy expenditure (REE) were seen with either sex (male: Fig. 1G and H, respectively, and female: Fig. 1I [REE data not shown]). Likewise, analysis in another transgenic line overexpressing FBPase in both liver and hypothalamus (27) demonstrated the same lean phenotype, reduction in food intake, and no change in energy expenditure (Supplementary Fig. 2A–E).
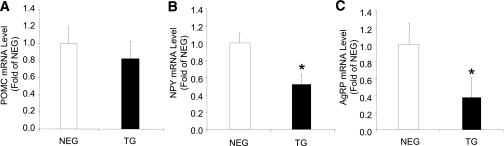
Reduced food intake is associated with suppression of appetite-stimulating neuropeptides.
No differences in ARC mRNA expression level of POMC (the precursor to the appetite-suppressing neuropeptide α-MSH) were detected (Fig. 2A). However, the appetite-stimulating neuropeptides, NPY and AgRP, were significantly reduced by ∼50% in the ARC of transgenic mice (Fig. 2B and C). PVN sections showed no differences in expression in any of the genes measured (data not shown).
FIG. 2.
Neuropeptide levels of liver FBPase transgenic (TG) mice. ARC neuropeptide mRNA levels in hemizygous TG mice (black bars) compared with negative (NEG, white bars) littermates measured by real-time PCR: POMC (A), NPY (B), and AgRP (C) (n = 4 for each group). *P < 0.03 vs. NEG, one-way ANOVA. All data expressed as mean ± SEM.
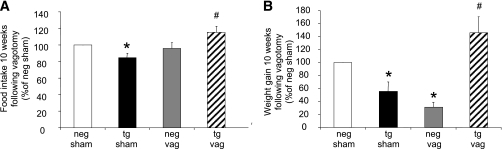
Vagotomized FBPase transgenic mice have a normalized phenotype.
Hepatic branch vagotomies were performed to establish whether the signal to inhibit food intake in the transgenic mice was vagally mediated. As expected, at 10 weeks after vagotomy recovery, sham-operated transgenic mice ate less food compared with sham-operated negative mice, whereas the vagotomized transgenic mice ate more than the sham-operated transgenic mice (Fig. 3A). This reduced food intake effect in the transgenic mice was reflected in the significant weight gain after vagotomy (Fig. 3B).
FIG. 3.
Food intake and body weight in vagotomized (vag) liver FBPase transgenic (tg) mice. Percentage food intake (A) and weight gain (B) from sham-operated and vagotomized hemizygous TG and negative (neg) littermate mice 10 weeks after vagotomy (n = 4 for each group). *P < 0.05 vs. NEG sham; #P < 0.05 vs. TG sham, one-way ANOVA. All data expressed as mean ± SEM.
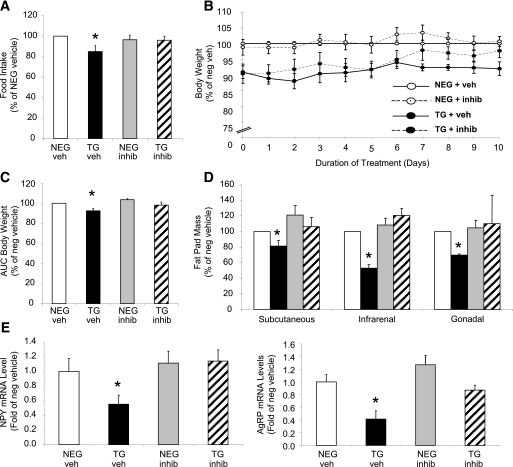
Specific pharmacologic inhibition of FBPase reverses the lean body weight phenotype.
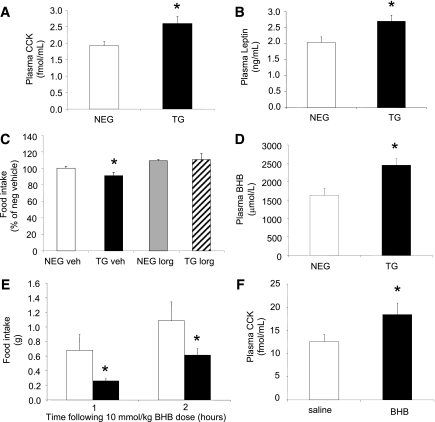
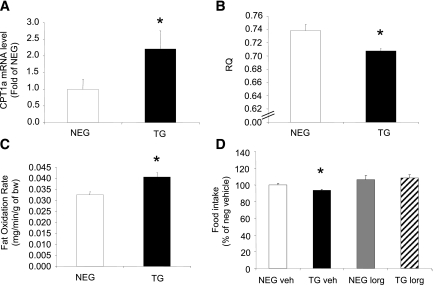
Figure 4A–C illustrates the normalization of food intake in the transgenic mice after 10 days of FBPase inhibitor treatment with a concomitant increase in body weight. Inhibitor treatment had no effect on food intake (Fig. 4A) or body weight (Fig. 4B and C) in the negative mice, implying specificity of the inhibitor to the overexpressed gene. Vehicle-treated transgenic mice, as expected, had smaller fat depots, whereas inhibitor-treated mice had similar fat depot masses to negative littermates (Fig. 4D). When the appetite-stimulating neuropeptides were measured, NPY and AgRP mRNA levels were both decreased in vehicle-treated transgenic mice, whereas inhibitor-treated mice exhibited normalized levels to that of the negative littermates (Fig. 4E).
FIG. 4.
Pharmacologic inhibition of FBPase in transgenic (TG) mice. Percentage daily food intake (A), body weight (B), AUC body weight (C), fat pad mass (D), and ARC neuropeptide mRNA levels (E) of FBPase inhibitor (INHIB)-treated (dashed line) and vehicle (VEH)-treated (bold line) hemizygous TG (black bars/circles) and negative (NEG, white bars/circles) littermate mice after 10 days of inhibitor (5 mg/kg) treatment (n = 4 for each group). *P < 0.05 vs. all groups, one-way ANOVA. All data expressed as mean ± SEM.
Circulating satiety hormones from the gut are increased in transgenic mice.
Levels of the hunger hormone ghrelin remained unchanged (negative: 1300 ± 343 pg/mL vs. transgenic: 1534 ± 562 pg/mL, n = 4), whereas circulating CCK (Fig. 5A) and leptin (Fig. 5B) levels were both elevated ∼30% in the transgenic mice. Duodenal CCK peptide concentrations remained unchanged (negative: 43 ± 16 pmol/g vs. transgenic: 35 ± 12 pmol/g, n = 6), suggesting CCK secretion rather than peptide synthesis was enhanced in the transgenic mice. Administration of the CCK1R antagonist, lorglumide, caused the transgenic mice to consume the same amount of food as the negative littermates 24 h after injection (Fig. 5C), supporting vagal nerve and CCK1R involvement.
FIG. 5.
Increased satiety hormones in livers of FBPase transgenic (TG) mice. Circulating CCK (A) and circulating leptin (B) levels in hemizygous TG (black bars) and negative (NEG, white bars) littermate mice (n = 6). *P < 0.05 vs. NEG, one-way ANOVA. C: Food intake levels after CCK1 receptor antagonist (lorglumide [LORG], 300 μg/kg) treatment (n = 5). *P < 0.05 vs. all groups, one-way ANOVA. D: Circulating BHB concentrations in hemizygous TG (black) and NEG (white) littermate mice (n = 8). *P < 0.05 vs. NEG, one-way ANOVA. Food intake levels at 1 and 2 h (E) and circulating CCK concentration at 1 h (F) after BHB (10 mmol/kg) administration to C57Bl/6J mice (n = 16). *P < 0.05 vs. NEG, one-way ANOVA. All data expressed as mean ± SEM.
The ketone body, BHB, raises circulating CCK levels in vivo.
We have previously shown a positive association between increased levels of the ketone body BHB (a by-product of liver FAO) and increased CCK levels in humans (21). Circulating BHB levels in the transgenic mice were significantly higher than in the negative littermates (Fig. 5D). To determine whether BHB could lower food intake by triggering CCK and/or leptin secretion, studies were conducted in C57Bl/6J wild-type mice. Food intake was significantly reduced 1 h after BHB administration compared with vehicle-treated mice (Fig. 5E). CCK was significantly elevated in the BHB-treated mice (Fig. 5F); however, circulating leptin was lower (negative: 2.18 ± 0.26 µmol/mL vs. transgenic: 1.51 ± 0.14 µmol/mL, n = 4; P = 0.03).
Liver and whole-body FAO is increased in transgenic mice.
Measurement of CPT1-a, the rate-limiting enzyme involved in liver FAO, demonstrated an approximate twofold elevation in mRNA expression levels in the transgenic mice (Fig. 6A), suggestive of increased FAO. To exclude any hypothalamic contribution, we measured CPT1-a and CPT1-c (brain-specific isoform) and found undetectable levels of CPT1-a and no difference in CPT1-c levels (data not shown). Genes regulating fatty acid synthesis (fatty acid synthase [FASN]), stearoyl-CoA-desaturase (SCD1), and sterol regulatory element binding transcription factor-1 (SREBF1) were not significantly different between the transgenic and negative littermate mice (Supplementary Table 1). At a whole-body level, RQ was significantly lower in the transgenic mice (Fig. 6B), suggesting increased fat oxidation, and was verified by the calculated fat oxidation rate (Fig. 6C). No differences could be detected in glucose oxidation rates (negative: 0.007 ± 0.003 mg/min/g vs. transgenic: 0.007 ± 0.0005 mg/min/g, n = 4).
FIG. 6.
Fat oxidation in liver FBPase transgenic (TG) mice. Liver CPT1-a mRNA levels (A), whole-body RQ (B), and whole-body fat oxidation rates (C) in TG and negative (NEG) littermate mice (n = 4). *P < 0.05 vs. NEG, one-way ANOVA. D: Food intake after FAO inhibition with etomoxir (10 mg/kg) treatment (n = 6). *P < 0.05 vs. all groups, one-way ANOVA. All data expressed as mean ± SEM.
Inhibition of FAO in the transgenic mice leads to increased food intake.
Transgenic mice treated acutely with a single dose of the inhibitor etomoxir, a compound specifically inhibiting liver CPT1-a activity (28), increased food intake 24 h after administration compared with vehicle-treated transgenic mice and was normalized to the level of the vehicle and inhibitor-treated negative littermates (Fig. 6D).
Pharmacologic inhibition of FBPase elevates weight gain in the NZO mouse.
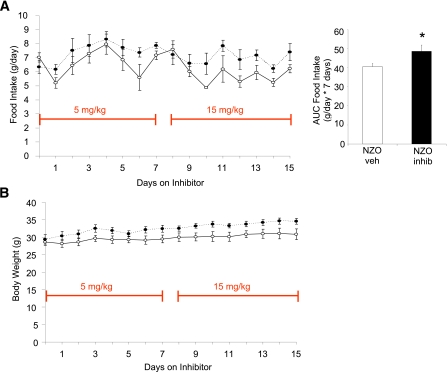
To determine the effect of elevated FBPase in a polygenic animal model of obesity and insulin resistance (29), NZO mice were treated with the allosteric FBPase inhibitor for 15 days, resulting in a sustained increase in food intake (18.6%; Fig. 7A) and body weight (11.5%; Fig. 7B) compared with vehicle-treated mice.
FIG. 7.
Pharmacologic inhibition of FBPase in the NZO mouse. Food intake (absolute g/day) and AUC of food intake on 15 mg/kg inhibitor treatment over 7 days (panel inset) (A) and body weight (B) of NZO mice treated with FBPase inhibitor (black circles) or with vehicle (white circles) for 15 days (n = 5 per group). *P < 0.05 vs. NZO vehicle-treated mice, GLM ANOVA. All data expressed as mean ± SEM. (A high-quality color representation of this figure is available in the online issue.)
Determining the mechanism for increased FAO in the transgenic mice.
We have previously reported lowered glycolysis and ATP levels in β-cell–specific FBPase transgenic mice (30). Reduced ATP leads to increased AMP-activated protein kinase (AMPK) levels and activation of energy-generating pathways such as FAO. Therefore, we postulated that AMPK might be elevated in our transgenic mice; however, neither ATP nor phosphorylated-AMPK levels were increased (Supplementary Table 1) implying an AMPK-independent pathway in action.
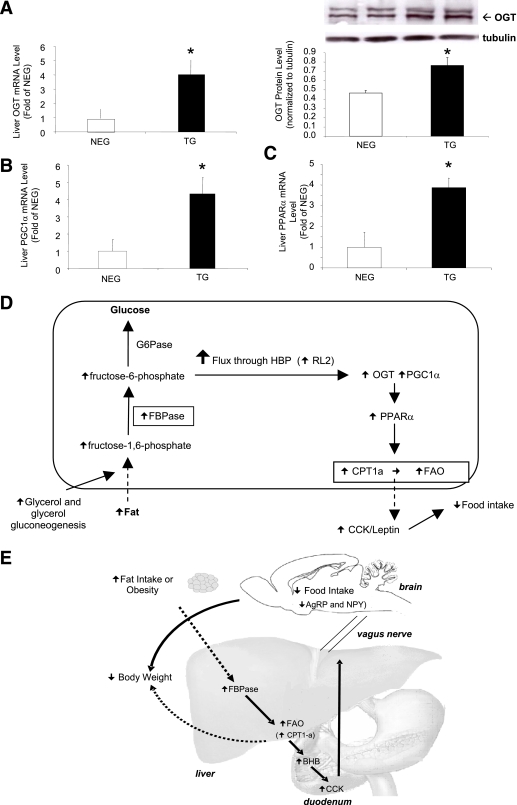
We have shown that F-1,6-P (substrate) and F6P (product) levels are elevated in our transgenic mice (Supplementary Table 2) and that F6P is the entry substrate for the HBP (31). To determine whether this pathway was activated, we measured mRNA expression and protein levels of OGT, the rate-limiting enzyme. Figure 8A demonstrates that mRNA and protein levels of liver OGT were both elevated in the transgenic mice, but there was no increase in the other rate-limiting enzyme glutamine:F-6-P aminotransferase (GFAT; Supplementary Table 1). A recent article suggested that OGT can form a physical complex with one of the regulators of FAO, PGC1-α (32). PGC1-α, has been documented to regulate CPT1-a, via activation of PPARα (33). When we measured PGC1-α and PPARα mRNA in transgenic mouse livers, both were significantly upregulated (Fig. 8B and C respectively). The proposed mechanism linking FBPase to the HBP and FAO is illustrated in Fig. 8D, and Fig. 8E illustrates the final schematic of the proposed working model.
FIG. 8.
Mechanism for FBPase increasing liver FAO and regulating food intake. A: Liver OGT mRNA and OGT Western blot protein levels in transgenic (TG) and negative (NEG) littermate mice using RL2 antibody (n = 4). *P < 0.05 vs. NEG. Liver PGC1-α mRNA levels (B) and PPARα mRNA levels (C) as measured by real-time PCR (n = 4). *P < 0.05 vs. NEG, one-way ANOVA. All data expressed as mean ± SEM. D: Schematic of mechanism by which FBPase increases FAO in the liver. We propose that elevated F6P increases flux through the HBP (as evidenced by an increase in OGT levels), triggering an upregulation of the FAO regulator, PGC1-α that in turn activates PPARα to activate the rate-limiting enzyme in liver FAO, CPT1-a. E: Schematic of mechanism for the overall action of liver FBPase as a regulator of appetite and adiposity. We propose that liver FBPase is upregulated by obesity and/or fat in the diet. This increase in FBPase in the liver appears to be triggering an increase in FAO from the liver, as indicated by a higher concentration of circulating BHB in the TG mice compared with the NEG littermates. BHB then stimulates CCK secretion (which may act synergistically with leptin), which acts on the CCK1R on the vagus nerve to send a signal to the brain to inhibit the appetite stimulating neuropeptides, AgRP and NPY, and in turn reduce food intake and body weight. The bold arrows indicate the proposed mechanism, and the dotted arrows show direct effects. (A high-quality color representation of this figure is available in the online issue.)
DISCUSSION
Obesity has become a major global health issue partly because its major complication is the development of type 2 diabetes. Paradoxically, several antidiabetes medications, including the thiazolidinediones, sulfonylureas, and insulin, have the undesired side effect of promoting weight gain. Because there are data showing inappropriate elevation of gluconeogenesis in type 2 diabetes (34), a recent target for glucose-lowering medications has been the gluconeogenic enzyme FBPase (35,36). Liver FBPase was increased in animal models of obesity and insulin resistance, in patients with type 2 diabetes (30), and after fat-feeding in mice and rats (10,11). The NZO mouse is a model of obesity, glucose intolerance, and liver insulin resistance (37) associated with increased liver FBPase (10,29). After administration of a specific liver FBPase allosteric inhibitor, we were surprised to find significant, sustained increases in food intake and body weight gain compared with vehicle-treated mice. Similarly, administration of another selective FBPase inhibitor to ZDF rats also resulted in a significant increase in body weight after 1 month of treatment (36). Together, the data suggest that upregulation of liver FBPase may not only be important in glucose metabolism but may also play a significant part in a homeostatic mechanism to control body weight in states of excess nutrient intake.
Overexpression of FBPase specifically in the liver of mice resulted in a lean body weight phenotype that was observed consistently throughout adult life, a finding that has not been reported previously. In association with this lean phenotype, there was a dramatic >50% reduction in white adipose tissue mass across the three fat depots measured. We did not directly measure lean body mass so cannot discount the possibility that such a reduction may also be a contributing factor. Nonetheless, the large decrease in adiposity suggests that liver FBPase may be highly important in regulating adiposity. We found that reduced food intake, rather than increases in energy expenditure, was strongly associated with the reduction in adiposity and was specific to liver FBPase expression because we have previously shown no body weight phenotype in mice specifically overexpressing FBPase in the β-cell (30). Reductions in the appetite-stimulating neuropeptides (NPY and AgRP) were the basis of this effect, which we were able to reverse with pharmacologic inhibition of FBPase (along with body weight and food intake). The lack of effect of the inhibitor in the negative littermates is not surprising, given we are proposing that the action of FBPase occurs to limit the amount of weight gained in response to nutrient overconsumption. Body weight is regulated by a plethora of endogenous hormone/peptide combinations, and FBPase alone is not critical in these situations. Studies in humans with a deficiency in endogenous liver FBPase (due to mutations in the FBP1 gene) causes a disruption in gluconeogenesis that leads to recurrent episodes of fasting hypoglycemia and lactic acidosis that is often lethal during the neonatal period and infancy (38–43). These studies show no evidence of increased body weight or appetite in response to reduced FBPase levels and, in fact, show that alternate metabolic consequences occur when FBPase is nonfunctional. This evidence substantiates our hypothesis that increased liver FBPase does not function in the normal physiologic regulation of body weight but acts only when the system is exposed to excess nutrients (i.e., fat). Furthermore, our data demonstrate a strong link between liver and the hypothalamus to suppress appetite-stimulating hormones to lower food intake and reduce adiposity, an observation never before reported.
The vagus nerve serves as the primary line of communication between the liver and the CNS and has both inhibitory and stimulatory effects on food intake (44,45). Indeed, hepatic branch vagotomies in our transgenic mice increased food intake and, consequently, body weight gain, demonstrating a requirement for vagal nerve signaling. In fact, we found a clear 30% increase in circulating CCK and leptin levels in the transgenic mice, hormones known to act via the vagus nerve, supporting a role for this nerve in mediating FBPases’ satiety effects. Furthermore, studies using the specific CCK1R antagonist, lorglumide, clearly showed increased food intake in the transgenic mice to similar levels seen in the negative mice. The paradoxic finding of increased leptin in the transgenic mice is surprising given that leptin is tightly regulated with adiposity. There is a possibility that FBPase may activate a signal from the liver to adipose tissue to stimulate leptin production and secretion, however, this has not been investigated. Nevertheless, our data lend critical support for the important and essential involvement of the vagus nerve in the altered body weight of the FBPase transgenic mice.
Our group has previously shown that increased levels of circulating BHB, a by-product of liver FAO, is linked to elevated CCK levels in humans (21). Our transgenic mice also have elevated circulating BHB levels, and when BHB was administered to C57BL/6J mice, food intake decreased, which was associated with higher circulating CCK concentration. These data not only support a positive link between BHB and CCK levels as seen in humans (21) but also provide direct evidence that ketones are effective in lowering food intake through CCK stimulation. However, the mechanism(s) by which BHB regulates CCK production and secretion is not understood. The increases seen in CPT1-a, its regulators PGC1-α and PPARα (33,46), and the elevated food intake after etomoxir inhibition, support the BHB data and the overall finding of increased FAO, and are consistent with the increase seen in whole-body fat oxidation (RQ and fat oxidation rates). Our data demonstrate for the first time that increased FAO plays a key role in the reduced appetite of our liver-specific FBPase transgenic mice.
In seeking the reason for increased FAO, we considered alterations in ATP levels and induction of AMPK, known to be intimately involved in FAO. Our previous characterization of the β-cell– specific FBPase transgenic mice showed reduced glycolysis and pancreatic ATP levels (30). A reduction in ATP levels may also be occurring in the liver-specific FBPase transgenic mice to increase liver FAO (and generate more ATP) via AMPK activation. However, no differences were found when liver ATP and phospho-AMPK levels were assessed, implying an AMPK-independent pathway. The HBP has been proposed as another cellular sensor of energy availability in muscle and fat (31,47,48), with the main enzyme, OGT, acting as a catalytic subunit for the target of many substrates (32). The transgenic mice have increased glycerol gluconeogenesis and circulating glycerol levels (27) leading to elevated F6P levels, the substrate for the HBP and the product of the reaction that FBPase catalyses, and together with elevated OGT levels, implies increased HBP flux. Recently, PGC1-α has been shown to form a complex with OGT (32) and was significantly elevated in our transgenic mice. PGC1-α works with PPARα to mediate CPT1-a transcriptional activation (33,46) and thus activate FAO, which as our data clearly show, does occur. Thus, the action of liver FBPase that appears to regulate food intake in our transgenic mice is likely due to an increased flux through the HBP because its activation leads to increased CPT1-a expression (via PGC-1α and PPARα upregulation) and an overall increase in FAO.
In conclusion, we show that a specific upregulation of FBPase in the liver leads to increased FAO, overproduction of BHB, stimulation of CCK and leptin release, and the generation of a vagal signal leading to reduction in the appetite stimulating hormones, NPY and AgRP, and a subsequent reduction in food intake. We propose that increased expression of liver FBPase after nutrient excess, such as a high-fat diet (10,11), is a novel negative feedback mechanism developed to limit weight gain in response to an abundance of dietary fat. Furthermore, our finding provides strong evidence that any drug to successfully inhibit FBPase will lead to weight gain. Unlike the insulin-sensitizing thiazolidinediones that increase subcutaneous but decrease visceral fat deposition (49), the use of FBPase inhibitors would also likely increase all fat depots, potentially worsening metabolic control for patients with type 2 diabetes. We have clearly demonstrated that liver FBPase should be viewed not only as a mediator of glucose metabolism but also as an important regulator of appetite and adiposity.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the National Health and Medical Research Council of Australia (APP# 566784).
No potential conflicts of interest relevant to this article were reported.
S.V. performed all of the experiments, collated and analyzed all of the collected data, and wrote and critically reviewed the manuscript. N.F.I.K. coperformed and analyzed the inhibitor and BHB studies. C.N.J. performed and analyzed the CPT1-a and CPT1-c mRNA expression analyses and reviewed the manuscript. A.S. reviewed the manuscript. A.S. and M.Y. performed CCK analysis (circulating and peptide levels). J.W. performed the ATP analysis and reviewed manuscript. T.T. performed the AMPK phosphorylation Western blotting and reviewed manuscript. B.J.L. performed the initial characterization of the mice, provided scientific input, and reviewed manuscript. J.M.F. provided scientific input into mouse generation and critically reviewed the manuscript. J.P. contributed to the study design, intellectual input, and critical revision of the manuscript. S.A. codirected the study, contributed to the study concept and design and interpretation of the data, and provided critical revision of the manuscript for intellectual content. B.C.F. obtained funding for the work, designed and codirected the studies, provided training and supervision for the animal physiology work, collated, interpreted, and statistically analyzed data, and provided critical revision and intellectual input of the manuscript. B.C.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the following individuals from the Department of Medicine (Austin Health), University of Melbourne, for their excellent technical assistance: Zheng Ruan, Rebecca Sgambellone, Christian Rantzau, Amy Blair, Cassie Bush, Kavi Jayatileka, and Therese Boehm. The authors also thank Dr Daniela M. Sartor (Department of Medicine [Austin Health], Clinical Pharmacology and Therapeutics Unit, VIC, Australia) for supplying the lorglumide for the CCK1R antagonist experiment.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1511/-/DC1.
REFERENCES
- 1.Peters JC. Combating obesity: challenges and choices. Obes Res 2003;11(Suppl.):7S–11S [DOI] [PubMed] [Google Scholar]
- 2.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 2000;404:661–671 [DOI] [PubMed] [Google Scholar]
- 3.Jambor de Sousa UL, Arnold M, Langhans W, Geary N, Leonhardt M. Caprylic acid infusion acts in the liver to decrease food intake in rats. Physiol Behav 2006;87:388–395 [DOI] [PubMed] [Google Scholar]
- 4.Jambor de Sousa UL, Benthem L, Arsenijevic D, et al. Hepatic-portal oleic acid inhibits feeding more potently than hepatic-portal caprylic acid in rats. Physiol Behav 2006;89:329–334 [DOI] [PubMed] [Google Scholar]
- 5.Langhans W, Wiesenreiter F, Scharrer E. Different effects of subcutaneous D,L-3-hydroxybutyrate and acetoacetate injections on food intake in rats. Physiol Behav 1983;31:483–486 [DOI] [PubMed] [Google Scholar]
- 6.Rawson NE, Blum H, Osbakken MD, Friedman MI. Hepatic phosphate trapping, decreased ATP, and increased feeding after 2,5-anhydro-D-mannitol. Am J Physiol 1994;266:R112–R117 [DOI] [PubMed] [Google Scholar]
- 7.Russek M. Participation of hepatic glucoreceptors in the control of intake of food. Nature 1963;197:79–80 [DOI] [PubMed] [Google Scholar]
- 8.Horn CC, Friedman MI. Metabolic inhibition increases feeding and brain Fos-like immunoreactivity as a function of diet. Am J Physiol 1998;275:R448–R459 [DOI] [PubMed] [Google Scholar]
- 9.Horn CC, Friedman MI. Methyl palmoxirate increases eating behavior and brain Fos-like immunoreactivity in rats. Brain Res 1998;781:8–14 [DOI] [PubMed] [Google Scholar]
- 10.Andrikopoulos S, Proietto J. The biochemical basis of increased hepatic glucose production in a mouse model of type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1995;38:1389–1396 [DOI] [PubMed] [Google Scholar]
- 11.Song S, Andrikopoulos S, Filippis C, Thorburn AW, Khan D, Proietto J. Mechanism of fat-induced hepatic gluconeogenesis: effect of metformin. Am J Physiol Endocrinol Metab 2001;281:E275–E282 [DOI] [PubMed] [Google Scholar]
- 12.Visinoni S, Fam BC, Blair A, et al. Increased glucose production in mice overexpressing human fructose-1,6-bisphosphatase in the liver. Am J Physiol Endocrinol Metab 2008;295:E1132–E1141 [DOI] [PubMed] [Google Scholar]
- 13.Fam BC, Morris MJ, Hansen MJ, et al. Modulation of central leptin sensitivity and energy balance in a rat model of diet-induced obesity. Diabetes Obes Metab 2007;9:840–852 [DOI] [PubMed] [Google Scholar]
- 14.Mangiafico SP, Lim SH, Neoh S, et al. A primary defect in glucose production alone cannot induce glucose intolerance without defects in insulin secretion. J Endocrinol 2011;210:335–347 [DOI] [PubMed] [Google Scholar]
- 15.Rana K, Fam BC, Clarke MV, Pang TP, Zajac JD, Maclean HE. Increased adiposity in DNA binding-dependent androgen receptor knockout male mice associated with decreased voluntary activity and not insulin resistance. Am J Physiol Endocrinol Metab 2011;301:E767–E778 [DOI] [PubMed] [Google Scholar]
- 16.Wong N, Fam BC, Cempako GR, et al. Deficiency in interferon-gamma results in reduced body weight and better glucose tolerance in mice. Endocrinology 2011;152:3690–3699 [DOI] [PubMed] [Google Scholar]
- 17.Tordoff MG, Rawson N, Friedman MI. 2,5-anhydro-d-mannitol acts in liver to initiate feeding. Am J Physiol 1991;261:R283–R288 [DOI] [PubMed] [Google Scholar]
- 18.Lai C, Gum RJ, Daly M, et al. Benzoxazole benzenesulfonamides as allosteric inhibitors of fructose-1,6-bisphosphatase. Bioorg Med Chem Lett 2006;16:1807–1810 [DOI] [PubMed] [Google Scholar]
- 19.von Geldern TW, Lai C, Gum RJ, et al. Benzoxazole benzenesulfonamides are novel allosteric inhibitors of fructose-1,6-bisphosphatase with a distinct binding mode. Bioorg Med Chem Lett 2006;16:1811–1815 [DOI] [PubMed] [Google Scholar]
- 20.Lo CM, Zhang DM, Pearson K, et al. Interaction of apolipoprotein AIV with cholecystokinin on the control of food intake. Am J Physiol Regul Integr Comp Physiol 2007;293:R1490–R1494 [DOI] [PubMed] [Google Scholar]
- 21.Chearskul S, Delbridge E, Shulkes A, Proietto J, Kriketos A. Effect of weight loss and ketosis on postprandial cholecystokinin and free fatty acid concentrations. Am J Clin Nutr 2008;87:1238–1246 [DOI] [PubMed] [Google Scholar]
- 22.Horn CC, Ji H, Friedman MI. Etomoxir, a fatty acid oxidation inhibitor, increases food intake and reduces hepatic energy status in rats. Physiol Behav 2004;81:157–162 [DOI] [PubMed] [Google Scholar]
- 23.Andrikopoulos S, Rosella G, Kaczmarczyk SJ, Zajac JD, Proietto J. Impaired regulation of hepatic fructose-1,6-biphosphatase in the New Zealand Obese mouse: an acquired defect. Metabolism 1996;45:622–626 [DOI] [PubMed] [Google Scholar]
- 24.Konrad RJ, Tolar JF, Hale JE, Knierman MD, Becker GW, Kudlow JE. Purification of the O-glycosylated protein p135 and identification as O-GlcNAc transferase. Biochem Biophys Res Commun 2001;288:1136–1140 [DOI] [PubMed] [Google Scholar]
- 25.Zraika S, Dunlop M, Proietto J, Andrikopoulos S. The hexosamine biosynthesis pathway regulates insulin secretion via protein glycosylation in mouse islets. Arch Biochem Biophys 2002;405:275–279 [DOI] [PubMed] [Google Scholar]
- 26.Steinberg GR, Watt MJ, Fam BC, et al. Ciliary neurotrophic factor suppresses hypothalamic AMP-kinase signaling in leptin-resistant obese mice. Endocrinology 2006;147:3906–3914 [DOI] [PubMed] [Google Scholar]
- 27.Lamont BJ, Visinoni S, Fam BC, et al. Expression of human fructose-1,6-bisphosphatase in the liver of transgenic mice results in increased glycerol gluconeogenesis. Endocrinology 2006;147:2764–2772 [DOI] [PubMed] [Google Scholar]
- 28.Weis BC, Cowan AT, Brown N, Foster DW, McGarry JD. Use of a selective inhibitor of liver carnitine palmitoyltransferase I (CPT I) allows quantification of its contribution to total CPT I activity in rat heart. Evidence that the dominant cardiac CPT I isoform is identical to the skeletal muscle enzyme. J Biol Chem 1994;269:26443–26448 [PubMed] [Google Scholar]
- 29.Andrikopoulos S, Rosella G, Gaskin E, et al. Impaired regulation of hepatic fructose-1,6-bisphosphatase in the New Zealand obese mouse model of NIDDM. Diabetes 1993;42:1731–1736 [DOI] [PubMed] [Google Scholar]
- 30.Kebede M, Favaloro J, Gunton JE, et al. Fructose-1,6-bisphosphatase overexpression in pancreatic beta-cells results in reduced insulin secretion: a new mechanism for fat-induced impairment of beta-cell function. Diabetes 2008;57:1887–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Liu R, Hawkins M, Barzilai N, Rossetti L. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature 1998;393:684–688 [DOI] [PubMed] [Google Scholar]
- 32.Housley MP, Rodgers JT, Udeshi ND, et al. O-GlcNAc regulates FoxO activation in response to glucose. J Biol Chem 2008;283:16283–16292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song S, Zhang Y, Ma K, et al. Peroxisomal proliferator activated receptor gamma coactivator (PGC-1alpha) stimulates carnitine palmitoyltransferase I (CPT-Ialpha) through the first intron. Biochim Biophys Acta 2004;1679:164–173 [DOI] [PubMed] [Google Scholar]
- 34.Consoli A, Nurjhan N, Capani F, Gerich J. Predominant role of gluconeogenesis in increased hepatic glucose production in NIDDM. Diabetes 1989;38:550–557 [DOI] [PubMed] [Google Scholar]
- 35.Erion MD, van Poelje PD, Dang Q, et al. MB06322 (CS-917): A potent and selective inhibitor of fructose 1,6-bisphosphatase for controlling gluconeogenesis in type 2 diabetes. Proc Natl Acad Sci USA 2005;102:7970–7975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Poelje PD, Potter SC, Chandramouli VC, Landau BR, Dang Q, Erion MD. Inhibition of fructose 1,6-bisphosphatase reduces excessive endogenous glucose production and attenuates hyperglycemia in Zucker diabetic fatty rats. Diabetes 2006;55:1747–1754 [DOI] [PubMed] [Google Scholar]
- 37.Veroni MC, Proietto J, Larkins RG. Evolution of insulin resistance in New Zealand obese mice. Diabetes 1991;40:1480–1487 [DOI] [PubMed] [Google Scholar]
- 38.Baker L, Winegrad AI. Fasting hypoglycaemia and metabolic acidosis associated with deficiency of hepatic fructose-1,6-diphosphatase activity. Lancet 1970;2:13–16 [DOI] [PubMed] [Google Scholar]
- 39.el-Maghrabi MR, Lange AJ, Jiang W, et al. Human fructose-1,6-bisphosphatase gene (FBP1): exon-intron organization, localization to chromosome bands 9q22.2-q22.3, and mutation screening in subjects with fructose-1,6-bisphosphatase deficiency. Genomics 1995;27:520–525 [DOI] [PubMed] [Google Scholar]
- 40.Faiyaz-Ul-Haque M, Al-Owain M, Al-Dayel F, et al. Novel FBP1 gene mutations in Arab patients with fructose-1,6-bisphosphatase deficiency. Eur J Pediatr 2009;168:1467–1471 [DOI] [PubMed] [Google Scholar]
- 41.Kikawa Y, Inuzuka M, Jin BY, et al. Identification of genetic mutations in Japanese patients with fructose-1,6-bisphosphatase deficiency. Am J Hum Genet 1997;61:852–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsuura T, Chinen Y, Arashiro R, et al. Two newly identified genomic mutations in a Japanese female patient with fructose-1,6-bisphosphatase (FBPase) deficiency. Mol Genet Metab 2002;76:207–210 [DOI] [PubMed] [Google Scholar]
- 43.Moon S, Kim JH, Han JH, et al. Novel compound heterozygous mutations in the fructose-1,6-bisphosphatase gene cause hypoglycemia and lactic acidosis. Metabolism 2011;60:107–113 [DOI] [PubMed] [Google Scholar]
- 44.Date Y, Shimbara T, Koda S, et al. Peripheral ghrelin transmits orexigenic signals through the noradrenergic pathway from the hindbrain to the hypothalamus. Cell Metab 2006;4:323–331 [DOI] [PubMed] [Google Scholar]
- 45.Reidelberger RD, Hernandez J, Fritzsch B, Hulce M. Abdominal vagal mediation of the satiety effects of CCK in rats. Am J Physiol Regul Integr Comp Physiol 2004;286:R1005–R1012 [DOI] [PubMed] [Google Scholar]
- 46.Napal L, Marrero PF, Haro D. An intronic peroxisome proliferator-activated receptor-binding sequence mediates fatty acid induction of the human carnitine palmitoyltransferase 1A. J Mol Biol 2005;354:751–759 [DOI] [PubMed] [Google Scholar]
- 47.Hawkins M, Angelov I, Liu R, Barzilai N, Rossetti L. The tissue concentration of UDP-N-acetylglucosamine modulates the stimulatory effect of insulin on skeletal muscle glucose uptake. J Biol Chem 1997;272:4889–4895 [DOI] [PubMed] [Google Scholar]
- 48.Hawkins M, Barzilai N, Liu R, Hu M, Chen W, Rossetti L. Role of the glucosamine pathway in fat-induced insulin resistance. J Clin Invest 1997;99:2173–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Festuccia WT, Blanchard PG, Turcotte V, et al. Depot-specific effects of the PPARgamma agonist rosiglitazone on adipose tissue glucose uptake and metabolism. J Lipid Res 2009;50:1185–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.