Abstract
Gestational diabetes mellitus (GDM) has been shown to be associated with high risk of diabetes in offspring. However, the mechanisms involved and the possibilities of transgenerational transmission are still unclear. We intercrossed male and female adult control and first-generation offspring of GDM (F1-GDM) mice to obtain the second-generation (F2) offspring in four groups: C♂-C♀, C♂-GDM♀, GDM♂-C♀, and GDM♂-GDM♀. We found that birth weight significantly increased in F2 offspring through the paternal line with impaired glucose tolerance (IGT). Regardless of birth from F1-GDM with or without IGT, high risk of IGT appeared as early as 3 weeks in F2 offspring and progressed through both parental lineages, especial the paternal line. IGT in male offspring was more obvious than that in females, with parental characteristics and sex-specific transmission. In both F1 and F2 offspring of GDM, the expression of imprinted genes Igf2 and H19 was downregulated in pancreatic islets, caused by abnormal methylation status of the differentially methylated region, which may be one of the mechanisms for impaired islet ultrastructure and function. Furthermore, altered Igf2 and H19 gene expression was found in sperm of adult F1-GDM, regardless of the presence of IGT, indicating that changes of epigenetics in germ cells contributed to transgenerational transmission.
A growing body of research suggests that exposure to an abnormal environment in the uterus can lead to chronic health problems later in life (1,2). Intrauterine hyperglycemia is a major characteristic of gestational diabetes mellitus (GDM) and has been suggested to be an important determinative factor for the risk of diabetes in adulthood, in addition to the effects of genetic factors (3–6). Although many studies have investigated intrauterine growth retardation and later diabetes, the mechanism involved in the association between intrauterine hyperglycemia and a high risk of diabetes in offspring remains unclear (7,8).
In mammals, epigenetic reprogramming is involved in germ cells and early embryonic development (9,10). Evolutionarily and environmentally acquired genomic susceptibilities can induce epigenomic modulations early in life, which impact on the later development of human disease (11). Moreover, frequent tissue-specific and disease-specific de novo methylation events occur during somatic cell development and differentiation (12). Because erasure and establishment of the genomic imprints for some imprinted genes begin when migratory primordial germ cells (PGCs) enter the embryonic genital ridge through gametogenesis, epigenetic abnormalities that occur during this phase may be involved in transgenerational transmission (13,14). We hypothesized that the hyperglycemic intrauterine environment of GDM could result in a high risk of diabetes in offspring by altering epigenetic modification. In addition to intergenerational transmission (F1 offspring), intrauterine hyperglycemia may also have effects on the second generation (F2 offspring).
Since it is difficult to analyze the comparative parental contributions and the underlying mechanisms that result in F2 offspring outcomes in humans, in the current study we established a GDM mouse model of intrauterine hyperglycemia. The female (♀) and male (♂) F1 adults of control and GDM mice were intercrossed to obtain F2 offspring of four groups: 1) C♂-C♀, 2) C♂-GDM♀, 3) GDM♂-C♀, and 4) GDM♂-GDM♀. Besides the phenotype, we examined the expression of imprinted genes Igf2, H19, and Plagl1 in islets. Abnormal Igf2 production has been shown to develop β-cell dysfunction and result in diabetes and appears to be an early landmark in the pathological sequence leading to apoptosis of β-cells in the fetal Goto-Kakizaki (GK) rat (15–17). H19 is an imprinted gene at 90 kb 3′ of Igf2 and is reciprocally imprinted with respect to Igf2, regulating its imprinting and expression (18,19). Plagl1, known as transcription factor zinc finger protein, is involved in the pathogenesis of transient neonatal diabetes mellitus (20–22). We further analyzed the methylation status of differentially methylated regions (DMRs) in Igf2/H19 in mouse islets to investigate whether intrauterine hyperglycemia affected imprinted gene expression by regulating epigenetic modification. In addition, we examined imprinted genes in sperm of F1-GDM to explore the underlying mechanism of transgenerational transmission by germ cells.
RESEARCH DESIGN AND METHODS
All animal protocols were reviewed and approved by the Zhejiang University Animal Care and Use Committee. At the age of 8 weeks, virgin female ICR mice (n = 60) were mated with normal males. Onset of pregnancy was determined by the presence of a copulation plug after overnight mating (designated as day 0 [D0] of pregnancy). After a 12-h fast, the females were randomly divided into a control group and an intrauterine hyperglycemia group with GDM (GDM group). Mice in the GDM group were injected with a single intraperitoneal injection of streptozotocin (STZ; Sigma, St. Louis, MO) in 0.1 mmol/L citrate buffer (pH 4.5) at a dose of 150 mg/kg body wt. Control pregnant females received an equal volume of citrate buffer. On D3 of pregnancy, diabetes was confirmed by measurement of blood glucose concentration via the tail vein and defined as a glucose level between 14 and 19 mmol/L (252–342 mg/dL), which was also measured on D7 and D20 of pregnancy to confirm the diabetes condition as previously described (23–26).
The pregnant mice were allowed to deliver spontaneously. The litter size was randomly reduced to 10 at birth to assure uniformity. The pups from the GDM group were fostered by normoglycemic females until they were weaned at the age of 3 weeks.
The ♀ and ♂ F1 adults of control (F1-C) and GDM (F1-GDM) mice were intercrossed, and the phenotypes of their F2 offspring were characterized. F2 offspring were obtained from 4 groups: 1) C♂-C♀, 2) C♂-GDM♀, 3) GDM♂-C♀, and 4) GDM♂-GDM♀ (Fig. 1A).
FIG. 1.
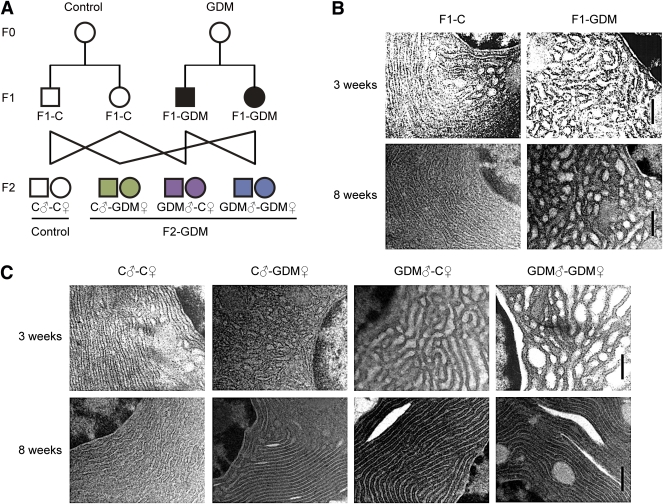
Experimental design and islet ultrastructure of mice. A: Experimental design. Circles designate females and squares designate males. Note that mating pairs were nonsiblings. The pups from the GDM group were fostered by normoglycemic females until weaned. B: Islet ultrastructure of F1 offspring under transmission electron microscopy. Scale bar, 500 nm. C: Islet ultrastructure of F2 offspring under transmission electron microscopy. Scale bar, 500 nm.
In vivo glucose and insulin analysis.
Intraperitoneal glucose injection (2 g/kg body wt) was performed in unrestrained conscious mice after a 12-h overnight fast. The area under the curve (AUC) of glucose against time was calculated for analysis of glucose tolerance as previously described (27). Blood samples of fasted or fed condition were taken for evaluation of serum insulin concentrations by solid-phase enzyme-linked immunosorbent assay using commercially available kits (Linco Research., St. Charles, MI).
Pancreatic islet isolation and insulin secretion.
In anesthetized 8-week-old mice, 2 mg/mL collagenase (Worthington Biochemical, Lakewood, NJ) was injected into the bile duct as previously described (28). The pancreas was dissected out, with static incubation at 38°C for 20 min followed by shaking incubation at 38°C for 8 min. After ficoll gradient separation, freshly isolated islets of similar size were handpicked under a stereomicroscope. Islets (20 per well) were preincubated in RPMI-1640 (Sigma-Aldrich, St. Louis, MO), washed, and incubated in 1 mL fresh RPMI-1640 that contained indicated glucose for 30 min (37°C). Fifty microliters of the medium were removed for insulin analysis. Islets were extracted in acid ethanol (4°C) and stored (−20°C) for insulin content assay. In mice at embryonic day 17 (E17), the pancreas was directly dissected out and incubated in 2 mg/mL collagenase under the same conditions as described above. Isolated islets were incubated in RPMI-1640 containing different glucose concentrations (5 mmol/L or 25 mmol/L) for 24 h (37°C).
Sperm collection.
Sperm were obtained from the caudal epididymis of 8-week-old adult male mice. Then, the sperm were washed in phosphate-buffered saline and centrifuged to pellet.
Gene expression (quantitative PCR).
Total RNA was isolated from islets or sperm using RNeasy (Qiagen, Valencia, CA). The cDNA was synthesized using oligo-dT and random primers (TaKaRa, Dalian, China) for real-time quantitative PCR (ABI Prism 7900HT; Applied Biosystems, Foster City, CA). Glyceraldehyde-3-phosphate dehydrogenase served as an internal control.
DNA methylation (bisulfite genomic sequencing PCR).
Genomic DNA was extracted from islets of 8-week-old mice in the control, F1-GDM, and GDM♂-GDM♀ groups. Bisulfite was converted using the EpiTect bisulfite kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions to deaminate cytosine to uracil; 5-methyl-cytosine was protected from deamination. Analysis of the methylation status of the Igf2 and H19 DMR was determined by cloning and sequencing of bisulfite-treated DNA. The purified PCR products were cloned using the pMD19-T vector system (TaKaRa, Dalian, China). The sequence obtained by cloning was analyzed with 3730 DNA Analyzer polymers (Applied Biosystems, Carlsbad, CA).
Electron microscopy.
Samples were fixed in 2.5% glutaraldehyde and postfixed in 1% osmium tetroxide and then stained with aqueous 2% uranyl acetate for 30 min, dehydrated, and embedded in epoxy resin. Sections of 120 nm were stained with uranyl acetate and lead citrate. Samples were examined at 80 kV under a TECNAI 10 transmission electron microscope (Philips, Amsterdam, the Netherlands).
Statistical analysis.
Data are presented as means ± SE. Statistical analysis was performed by unpaired two-tailed Student t test, one-way ANOVA, or χ2 test as described in the Table and Figure legends (version 16.0; SPSS). P < 0.05 was considered statistically significant.
RESULTS
Intrauterine hyperglycemia induced transgenerational effect on birth weight and islet structure.
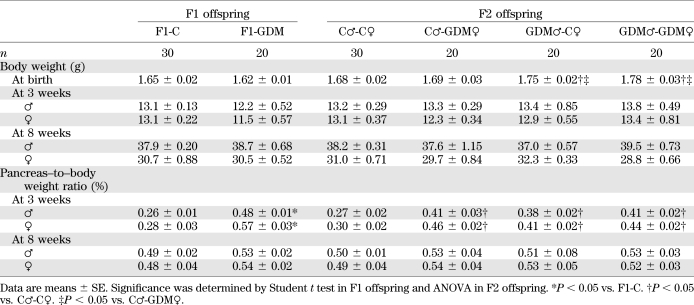
We established a GDM mouse model by inducing moderate hyperglycemia after pregnancy. Female and male F1 adults of control and GDM mice were intercrossed to obtain F2 offspring (Fig. 1A). We found that the birth weight of F1-GDM offspring was not changed significantly (Table 1). After fostering by the normoglycemic mice, the body weight of both F1-GDM males and females at 3 or 8 weeks of age was similar to that of F1-C offspring (Table 1). There was no significant difference of birth weight between control and F2-GDM mice born from F1-GDM with relative normal glucose tolerance (data not shown). After birth from F1-GDM with impaired glucose tolerance (IGT), the birth weight in the GDM♂-C♀ and GDM♂-GDM♀ groups significantly increased compared with that in C♂-C♀ (Table 1). However, the birth weight in the C♂-GDM♀ group did not increase obviously. There was also no significant difference in birth weight between GDM♂-C♀ and GDM♂-GDM♀ groups (Table 1). The ratio of pancreas weight to body weight in F1-GDM offspring at 3 weeks was significantly higher than that in F1-C, although there was no difference at 8 weeks (Table 1). With the same tendency of F1-GDM mice, the pancreas weight–to–body weight ratio in F2-GDM offspring whose one and/or two parents experienced intrauterine hyperglycemia was significantly higher than that in the C♂-C♀ group at 3 weeks (Table 1). There was no significant difference of pancreatic islet morphology, insulin distribution, or glucagon distribution between F1-C and F1-GDM (data not shown). However, analyzed by transmission electron microscopy, the structure of the endoplasmic reticulum in islet cells was obviously swollen and disordered in F1-GDM offspring at 3 weeks of age compared with that of F1-C and progressed at 8 weeks of age (Fig. 1B). In F2 offspring, compared with the C♂-C♀ group, the structure of the endoplasmic reticulum in islet cells was obviously swollen and disordered in F2-GDM groups at 3 weeks of age, especially in GDM♂-C♀ and GDM♂-GDM♀ groups (Fig. 1C). At 8 weeks of age, the structure of endoplasmic reticulum in all the F2-GDM groups was almost recovered, with the exception of some fissures (Fig. 1C).
TABLE 1.
Body weight and the ratio of pancreas weight to body weight in F1 and F2 offspring
Intrauterine hyperglycemia induced transgenerational transmission of glucose intolerance and abnormal insulin level.
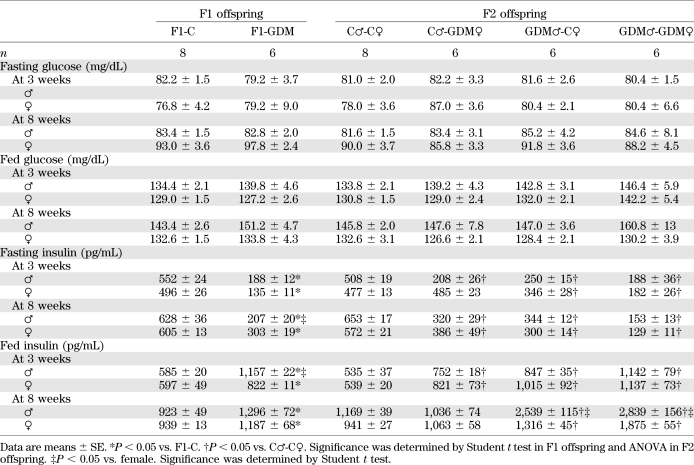
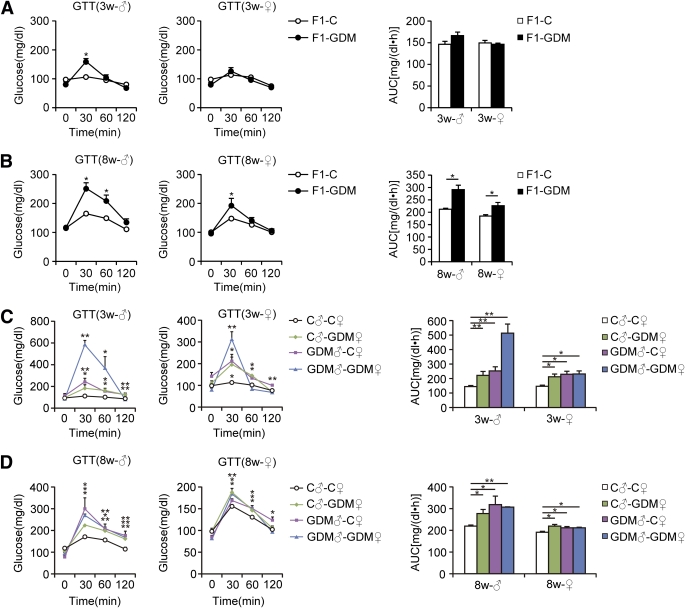
Blood glucose levels of all the 3- and 8-week-old F1-GDM and F2-GDM offspring did not differ from those in the corresponding controls in either the fasting or random-fed condition (Table 2). We further performed glucose tolerance test (GTT) by intraperitoneal injection of glucose (2 g/kg body wt). At 3 weeks, in both males and females, there were no differences in the GTT AUC between F1-GDM and F1-C, although the blood glucose level significantly increased in the males at 30 min after injection (Fig. 2A). However, at 8 weeks, IGT was found in both the male and female mice of the F1-GDM group, whose blood glucose level significantly increased at 30 min after injection (Fig. 2B). F1-GDM males showed more evident IGT than females (Fig. 2B). After GTT was performed by intraperitoneal injection of glucose (2 g/kg body wt), IGT appeared in both 3- and 8-week-old male and female F2-GDM offspring through both parental lineages. The male F2-GDM offspring showed much more IGT than females (Fig. 2C and D). At 3 and 8 weeks of age, in both males and females, although fasting glucose levels were normal, fasting insulin levels were much lower in F1-GDM than in F1-C (Table 2). In contrast, in the random-fed condition, the insulin levels of F1-GDM mice significantly increased. The abnormal insulin level of the F1-GDM males was more obvious than that of the F1-GDM females (Table 2). In F2 offspring, the fasting insulin levels of the F2-GDM males at 3 and 8 weeks were significantly lower than those of the control group, and the fasting insulin levels in 8-week-old F2-GDM females were also significantly decreased compared with the control group (Table 2). In contrast, in the random-fed condition, the insulin levels in all 3-week-old F2-GDM offspring were significantly higher than in controls (Table 2). At 8 weeks, the insulin levels of GDM♂-C♀ and GDM♂-GDM♀offspring were significantly higher than in C♂-C♀ (Table 2).
TABLE 2.
Blood glucose and insulin levels in F1 and F2 offspring
FIG. 2.
GTT of F1 and F2 offspring. A: GTT (2 g/kg body wt) and AUC of 3-week-old F1 offspring. B: GTT and AUC of 8-week-old F1 offspring. C: GTT and AUC of 3-week-old F2 offspring. D: GTT and AUC of 8-week-old F2 offspring. Data are presented as means ± SE (n = 8 in F1-C and C♂-C♀ groups and n = 6 in all other groups). F1 offspring, *P < 0.05, **P < 0.01 vs. F1-C (Student t test); F2 offspring, *P < 0.05, **P < 0.01 vs. C♂-C♀ (ANOVA). w, week.
Intrauterine hyperglycemia impaired insulin secretion.
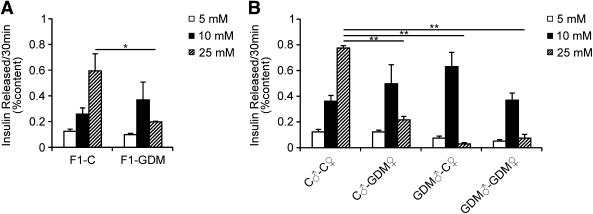
To define further the potential secretory defects that could contribute to glucose intolerance in offspring exposed to intrauterine hyperglycemia, we assessed glucose-stimulated insulin secretion of islets isolated from 8-week-old male mice. In the F1-C group, insulin secretion significantly increased with glucose exposure in a dose-dependent manner. By contrast, islets of the F1-GDM group showed higher insulin secretion in response to moderate glucose concentration but lower insulin secretion in response to high glucose concentration compared with F1-C (Fig. 3A). Likewise, regardless of birth from F1-GDM with or without IGT, in F2 offspring in vitro glucose-stimulated insulin secretion was also impaired in the F2-GDM group (Fig. 3B). Impaired insulin secretion in female mice was similar to that of males, despite not being very significant (data not shown).
FIG. 3.
Glucose-stimulated insulin secretion in vitro. A: Glucose-stimulated insulin secretion in islets isolated from 8-week-old mice of F1 offspring (n = 5 replicates/group in at least two independent isolations). B: Glucose-stimulated insulin secretion in islets isolated from 8-week-old mice of F2 offspring (n = 4 replicates/group in at least two independent isolations). Isolated islets were incubated with the indicated glucose concentration for 30 min. Results are expressed as means ± SE. *P < 0.05; **P < 0.01. Significance was determined by Student t test in F1 offspring and ANOVA in F2 offspring.
Intrauterine hyperglycemia downregulated Igf2 and H19 in islets.
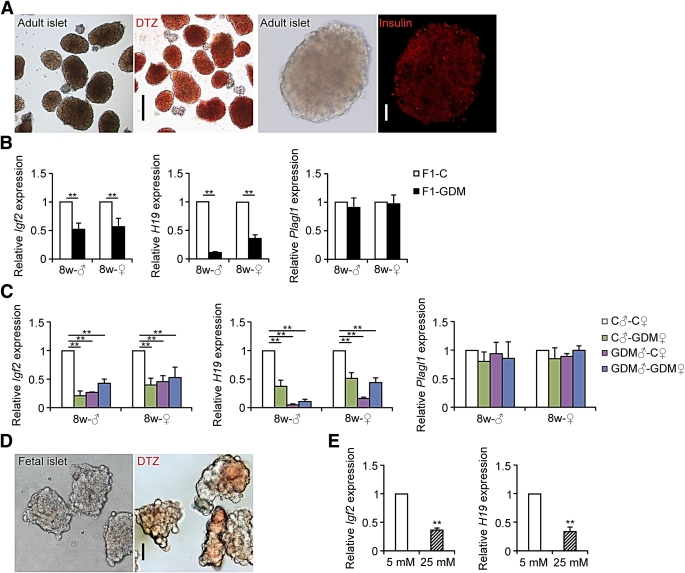
We collected islets from 8-week-old mice and identified the purity and specificity of islets by incubating with dithizone or detecting insulin with immunofluorescence (Fig. 4A). We found that the relative mRNA levels of Igf2 and H19 in islets from 8-week-old males and females were both significantly lower in F1-GDM mice. The abnormal expression of both Igf2 and H19 in F1-GDM males was more obvious than that in females. There were no significant differences in Plagl1 expression levels between F1-GDM and F1-C (Fig. 4B).
FIG. 4.
Igf2, H19, and Plagl1 gene expression assessed by real-time quantitative PCR. A: Adult islets were identified by incubating with dithizone (DTZ) and detecting insulin with immunofluorescence. Black scale bar, 200 μm; white scale bar, 50 μm. B: Igf2, H19, and Plagl1 gene expression in islets of 8-week-old F1 offspring (n = 6 mice in F1-C group; n = 4 mice in F1-GDM group). C: Igf2, H19, and Plagl1 gene expression in islets of 8-week-old F2 offspring (n = 6 mice in C♂-C♀ group; in F2-GDM groups, n = 4 mice per group). D: Fetal islets were identified by incubating with dithizone. Scale bar, 50 μm. E: Igf2 and H19 gene expression in fetal islets cultured in medium containing indicated concentrations of glucose for 24 h (n = 5 replicates/group in at least two independent isolations). Data were analyzed with the Eq. 2−ΔΔCT, where ΔΔCT = ΔCT (treatment group) − ΔCT (control group), and ΔCT = CT (sample) − CT (internal control). The values were presented as relative expression levels of mRNA. In all panels, results are expressed as means ± SE. **P < 0.01. Significance was determined by Student t test in F1 offspring and ANOVA in F2 offspring. (A high-quality digital representation of this figure is available in the online issue.)
In the F2-GDM offspring, regardless of birth from F1-GDM with or without IGT, the relative mRNA levels of Igf2 and H19 in islets from 8-week-old males and females were all significantly lower than those in controls. There were no significant differences in Plagl1 expression levels between F2-GDM offspring and controls. It is interesting that in F2-GDM offspring, Igf2 decreased most significantly in the C♂-GDM♀ group, whereas H19 decreased most significantly in the GDM♂-C♀ group. Showing the same tendency as that in F1-GDM offspring, in F2-GDM offspring, the abnormal expression of Igf2 and H19 in males was also more obvious than that in females (Fig. 4C).
In addition, we collected islets of fetal mice at E17 (Fig. 4D). In order to verify the direct effect of high glucose on islet development, fetal islets were cultured in a medium containing different concentrations of glucose for 24 h. We found that the Igf2 and H19 mRNA levels were significantly lower in fetal mouse islets exposed to a high glucose concentration (25 mmol/L) than to a physiological concentration (5 mmol/L) (Fig. 4E).
Intrauterine hyperglycemia induced hypermethylation of Igf2/H19 DMRs in islets.
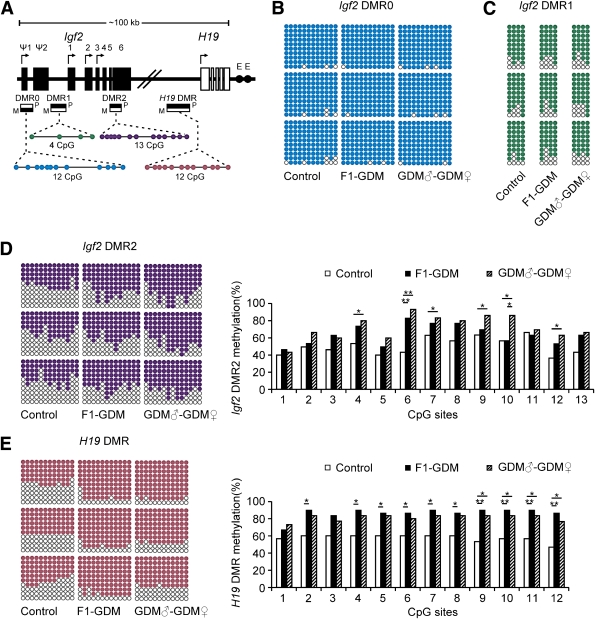
As imprinted genes, Igf2 and H19 allelic expression in mice is regulated by allele-specific methylation at four DMRs (21). We collected islets from 8-week-old male mice of the control, F1-GDM, and GDM♂-GDM♀ groups. We analyzed the methylation levels of 12 cytosine phosphate guanine (CpGs) of the Igf2 DMR0, 4 CpGs of the Igf2 DMR1, and 13 CpGs of the Igf2 DMR2 by bisulfite genomic sequencing PCR. Furthermore, we analyzed the methylation levels of the 12 CpGs located in the CCCTC-binding factor binding sites of the H19 DMR (Fig. 5A).
FIG. 5.
Methylation analysis of Igf2/H19 DMRs by bisulfite genomic sequencing PCR. A: Schematic representation of mouse imprinted locus, showing the relative positions of the Igf2 and H19 genes and indicating the location of the four DMRs known to contribute to Igf2 imprinting. The number and position of upstream exons (Ψ1 and Ψ2 for Igf2) and exons are shown as black (Igf2 gene) and white (H19 gene) rectangles with arrows for transcription start sites. Enhancers (E) are indicated as black circles. The locations of the four DMRs within the Igf2/H19 imprinted locus represented by boxes are shaded to indicate preferential methylation of the maternal (M) or paternal (P) allele in each region. B: Methylation status of individual DNA strands of Igf2 DMR0 containing 12 CpG sites. C: Methylation status of individual DNA strands of Igf2 DMR1 containing 4 CpG sites. D: Methylation status of individual DNA strands of Igf2 DMR2 containing 13 CpG sites and the average methylation ratio in each CpG site. E: Methylation status of individual DNA strands of H19 DMR containing 12 CpG sites and the average methylation ratio in each CpG site. Ten clones per mouse; a total of 30 clones per group were sequenced. Each line represents the sequence of a single clone. CpG sites are shown as blank (unmethylated) or filled (methylated) circles. In the histograms, results are expressed as methylation percentage of each CpG site (n = 3 mice per group). *P < 0.05, **P < 0.01 vs. control (χ2 test).
In Igf2 DMR0 and Igf2 DMR1, the methylation status was all hypermethylated in control, F1-GDM, and GDM♂-GDM♀ groups with no significant differences (Fig. 5B and C). In Igf2 DMR2, we found that the CpGs were moderately methylated in the control group, while the F1-GDM and GDM♂-GDM♀ groups showed significantly higher methylation levels (Fig. 5D). The H19 DMR upstream of the H19 gene (which is 90 kb 3′ of Igf2) acts as a methylation-sensitive boundary element. Analyzed by bisulfite genomic sequencing PCR, H19 DMR in both the F1-GDM and GDM♂-GDM♀ groups was much more highly methylated than that in the control group (Fig. 5E).
Intrauterine hyperglycemia affected Igf2 and H19 expression in sperm of F1 offspring.
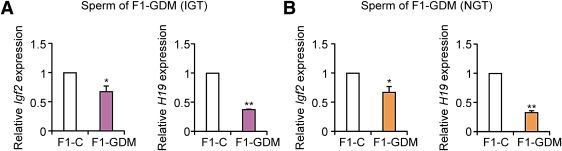
In the GDM♂-C♀ group, without an abnormal intrauterine environment, sperm of F1-GDM was the only factor to affect the offspring. To investigate the underlying mechanism of epigenetic alteration of F2 offspring, we collected sperm of F1-GDM and examined Igf2 and H19 expression. F1-GDM male mice were further divided into the obvious IGT group and relative normal glucose tolerance group. We found that, regardless of whether F1-GDM male mice were with or without IGT, Igf2 and H19 were both downregulated in their sperm. Moreover, the change of H19 was more obvious than that of Igf2 (Fig. 6A and B).
FIG. 6.
Igf2 and H19 gene expression in sperm. A: Igf2 and H19 gene expression in sperm of F1-GDM with obvious IGT. B: Igf2 and H19 gene expression in sperm of F1-GDM with relative normal glucose tolerance (NGT). Data are presented as means ± SE (n = 6 mice per F1-C group, n = 5 mice per F1-GDM group). *P < 0.05, **P < 0.01 vs. F1-C (Student t test).
DISCUSSION
Previous studies have demonstrated that low birth weight (LBW) is associated with adult diabetes (29–32). However, in clinical practice, newborns of mothers with GDM often have normal or higher birth weight, suggesting that the mechanism of high risk of diabetes in GDM offspring may be different from that of LBW-related adult diabetes.
In our study, moderate intrauterine hyperglycemia during pregnancy did not obviously affect birth weight in F1 offspring. Although the body weight was similar, the pancreas weight in 3-week-old F1-GDM was significantly higher than that of controls. Endoplasmic reticulum in pancreatic islets of F1-GDM was swollen and disordered, which suggests that hyperglycemia of intrauterine environment may lead to disorganization of fetal islet cells. The abnormal islet ultrastructure may be related to impaired β-cell activity and the development of IGT, which is primarily caused by insulin deficiency. Actually, although there was no difference in the fasting and fed blood glucose levels, the fasting insulin levels were decreased while random-fed insulin levels were increased in both the male and female F2-GDM offspring. IGT occurred in adults of the F1-GDM group. In vitro experiment also verified reduced glucose-stimulated insulin secretion in F1-GDM islets. Moreover, glucose intolerance of male GDM offspring was more obvious than that of female offspring, suggesting that male fetus might be more sensitive to the intrauterine environment than female.
Human epidemiological investigation indicated a link between mothers exposed to famine in utero with LBW in their offspring (33). Animal models have shown that maternal undernutrition during pregnancy could induce LBW, IGT, and obesity in both first- and second-generation offspring (34,35). In our study, after exposure to intrauterine hyperglycemia, transgenerational transmission was found in F2 offspring with parental characteristics. In F2 offspring born from F1-GDM with IGT, birth weight increased through the paternal line, but not maternal line, indicating that abnormal intrauterine environment had transgenerational effects on the birth weight in a parental-specific manner. For F2 offspring, compared with maternal line, impact factors of the paternal line are much more prone to be transmitted. The pancreas weights in 3-week-old F2 offspring after intrauterine hyperglycemia were all significantly higher than in controls. IGT and abnormal insulin levels appeared as early as 3 weeks and progressed until adulthood in both males and females, through both parental lineages, although the endoplasmic reticulum in adult islets was recovered. Impaired glucose-stimulated insulin secretion in vitro showed in islets of F2-GDM offspring. The intrauterine hyperglycemia induced transgenerational transmission of glucose intolerance and abnormal insulin levels. In all F2-GDM offspring groups, IGT of males was more obvious than in females, suggesting that the effect of intrauterine hyperglycemia on F2 males was more obvious than in F2 females. A number of diabetes-related animal models show sex differences, with males having a more profound phenotype (36). In the Nagoya-Shibata-Yasuda (NSY) mice, there is a marked sex difference, with almost all males developing hyperglycemia but less than one-third of females being affected (37). Many genetically induced forms of insulin resistance have a more severe phenotype in males than in females (38,39). The sex differences in transmission of phenotypes indicate that epigenetic mechanisms may be involved in transgenerational effect.
Reduced glucose-stimulated insulin secretion may contribute to impaired glucose tolerance in both F1 and F2 offspring from intrauterine hyperglycemia. Many factors are related to β-cell dysfunction. In the current study, we focused on the imprinted genes involved in islet development and the pathogenesis of diabetes: Igf2, H19, and Plagl1. Downregulated expression of Igf2 and H19 exhibited not only in F1-GDM but also in F2-GDM offspring through both paternal and maternal lines, confirming that dysregulation of Igf2 leads to inappropriate insulin production and secretion and induces diabetes (15–17). Moreover, in F2-GDM offspring, decreased level of Igf2 and H19 expression was associated with parental characteristics. Igf2 reduced the most through the maternal line, while H19 was reduced the most through the paternal line. In accordance with the tendency of phenotype changes, the change of imprinted gene expression was also more obvious in male offspring than in female.
In vitro culture confirmed the effect of high glucose on Igf2 and H19 expression in fetal islets, providing a probable explanation for intrauterine hyperglycemia directly influencing imprinted genes and inducing β-cell dysfunction. However, during pregnancy period of adult female F1-GDM, the random glucose level was normal, which means both fetus of C♂-GDM♀ and GDM♂-GDM♀ groups developed in a relatively normoglycemic intrauterine environment. Especially in the GDM♂-C♀ group, there was a completely normal maternal metabolic environment during development in utero. The paternal factor of F1 offspring played a more important role in growth and glycometabolism of F2 offspring, suggesting that epigenetic changes occurring in the germ line and inherited through meiosis (13,14,40) may be an explanation for transgenerational transmission. Thus, we further investigated the expression of Igf2 and H19 in sperm of adult F1-GDM male mice. It is interesting that, regardless of whether F1-GDM male mice were with or without IGT, Igf2 and H19 were both downregulated in their sperm. Moreover, the change of H19 was more obvious than that of Igf2. Although relevant epigenetic modification needed further exploration, the results indicated that intrauterine hyperglycemia could alter imprinted gene expression of germ cells and transmitted the risk of disease to next generation.
Parental imprinting is an important genetic mechanism by which some genes are repressed or expressed depending exclusively on their inheritance from mother or father (41). DNA methylation can control gene expression by modulating enhancer access to the gene promoter through regulation of an enhancer boundary. Igf2, an imprinted gene, shares an enhancer with its neighboring imprinted gene H19 (42–45). Igf2 and H19 allelic expression in mice is regulated by allele-specific methylation at four Igf2/H19 DMRs: Igf2 DMR0, Igf2 DMR1, Igf2 DMR2, and H19 DMR (18,46,47). Because IGT and gene alteration were both more obvious in male offspring, we examined all of the Igf2/H19 DMRs in islets of F1-GDM and GDM♂-GDM♀ male mice. Although there were no differences in methylation of Igf2 DMR0 and Igf2 DMR1 compared with controls, in islet of F1-GDM and GDM♂-GDM♀ the methylation levels were significantly higher in Igf2 DMR2, which is a methylation-sensitive activator within the last exon of Igf2 (18). The methylation levels of H19 DMR were also significantly much higher than in controls. These results indicate that intrauterine hyperglycemia could cause hypermethylation at Igf2 DMR2 and H19 DMR in both F1 and F2 offspring, suggesting that altered expression of Igf2 and H19 in islets is associated with the altered modification of Igf2 DMR2 and H19 DMR. Previous studies have also supported that the methylation state at Igf2 DMR2 is especially labile. Maintenance of the methylation status at DMR2 depends on interactions with other Igf2/H19 DMRs; mice carrying a deletion of either the H19 DMR or Igf2 DMR1 show aberrant methylation at Igf2 DMR2, whereas deletion of Igf2 DMR2 does not reciprocally affect the other DMRs (47). Importantly, human genomic regions corresponding to mouse H19 DMR, Igf2 DMR0, and Igf2 DMR2 have been identified and exhibit allele-specific methylation (48–50).
In conclusion, using a GDM mouse model we found that during pregnancy, intrauterine environment with moderate hyperglycemia did not affect birth weight of F1-GDM but increased birth weight of F2 offspring born from F1-GDM with IGT, which showed sex-specific characteristics. Furthermore, intrauterine hyperglycemia induced IGT and abnormal insulin levels in both F1 and F2 offspring regardless of birth from F1-GDM with or without IGT, which are partly due to the deficient islet ultrastructure. Abnormal Igf2 expression related to dysregulation of Igf2/H19 methylation of islet is one of the mechanisms involved in the effects of intrauterine hyperglycemia on F1 and F2 offspring. Altered Igf2 and H19 gene expression was also found in sperm of adult F1-GDM with or without IGT, indicating that changes of epigenetics in germ cells contributed to transgenerational transmission.
ACKNOWLEDGMENTS
This study was supported by the National Basic Research Program of China (2012CB944901), the National Natural Science Foundation of China (30973209, 31171444, 30901616, and 30901604), the Research Fund for the Doctoral Program of Higher Education (20100101110129), and the Key Subjects Group of Reproductive Medicine at Zhejiang Province (XKQ-009-002).
No potential conflicts of interest relevant to this article were reported.
G.-L.D. and F.-F.W. designed and performed experiments, analyzed data, and wrote and edited the manuscript. J.S., S.T., Y.J., D.Z., and N.W. researched data and contributed to discussion. Q.L., Y.Z., F.J., and P.C.K.L. contributed to discussion and edited the manuscript. J.-Z.S. and H.-F.H. designed and supervised the research, contributed to discussion, and edited the manuscript. H.-F.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Qiu-Ping Xie and Yu-Lian Wu (Second Affiliated Hospital, School of Medicine, Zhejiang University) for technical assistance. The authors also thank Hsiao Chang Chan (Epithelial Cell Biology Research Center, School of Biomedical Sciences, Faculty of Medicine, The Chinese University of Hong Kong), Min-Yue Dong, and Xin-Mei Liu (Women’s Hospital, School of Medicine, Zhejiang University) for helpful advice. The authors also thank Professor Iain C. Bruce (School of Medicine, Zhejiang Universit) for reading and editing the manuscript.
REFERENCES
- 1.Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007;30(Suppl. 2):S251–S260 [DOI] [PubMed] [Google Scholar]
- 2.Bateson P, Barker D, Clutton-Brock T, et al. Developmental plasticity and human health. Nature 2004;430:419–421 [DOI] [PubMed] [Google Scholar]
- 3.Dabelea D, Hanson RL, Lindsay RS, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 2000;49:2208–2211 [DOI] [PubMed] [Google Scholar]
- 4.Fetita LS, Sobngwi E, Serradas P, Calvo F, Gautier JF. Consequences of fetal exposure to maternal diabetes in offspring. J Clin Endocrinol Metab 2006;91:3718–3724 [DOI] [PubMed] [Google Scholar]
- 5.Clausen TD, Mathiesen ER, Hansen T, et al. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care 2008;31:340–346 [DOI] [PubMed] [Google Scholar]
- 6.Pettitt DJ, Aleck KA, Baird HR, Carraher MJ, Bennett PH, Knowler WC. Congenital susceptibility to NIDDM. Role of intrauterine environment. Diabetes 1988;37:622–628 [DOI] [PubMed] [Google Scholar]
- 7.Martin-Gronert MS, Ozanne SE. Experimental IUGR and later diabetes. J Intern Med 2007;261:437–452 [DOI] [PubMed] [Google Scholar]
- 8.Park JH, Stoffers DA, Nicholls RD, Simmons RA. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J Clin Invest 2008;118:2316–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science 2001;293:1089–1093 [DOI] [PubMed] [Google Scholar]
- 10.Sasaki H, Matsui Y. Epigenetic events in mammalian germ-cell development: reprogramming and beyond. Nat Rev Genet 2008;9:129–140 [DOI] [PubMed] [Google Scholar]
- 11.Tremblay J, Hamet P. Impact of genetic and epigenetic factors from early life to later disease. Metabolism 2008;57(Suppl. 2):S27–S31 [DOI] [PubMed] [Google Scholar]
- 12.Nagase H, Ghosh S. Epigenetics: differential DNA methylation in mammalian somatic tissues. FEBS J 2008;275:1617–1623 [DOI] [PubMed] [Google Scholar]
- 13.Bartolomei MS, Tilghman SM. Genomic imprinting in mammals. Annu Rev Genet 1997;31:493–525 [DOI] [PubMed] [Google Scholar]
- 14.Thamotharan M, Garg M, Oak S, et al. Transgenerational inheritance of the insulin-resistant phenotype in embryo-transferred intrauterine growth-restricted adult female rat offspring. Am J Physiol Endocrinol Metab 2007;292:E1270–E1279 [DOI] [PubMed] [Google Scholar]
- 15.Serradas P, Goya L, Lacorne M, et al. Fetal insulin-like growth factor-2 production is impaired in the GK rat model of type 2 diabetes. Diabetes 2002;51:392–397 [DOI] [PubMed] [Google Scholar]
- 16.Devedjian JC, George M, Casellas A, et al. Transgenic mice overexpressing insulin-like growth factor-II in beta cells develop type 2 diabetes. J Clin Invest 2000;105:731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calderari S, Gangnerau MN, Thibault M, et al. Defective IGF2 and IGF1R protein production in embryonic pancreas precedes beta cell mass anomaly in the Goto-Kakizaki rat model of type 2 diabetes. Diabetologia 2007;50:1463–1471 [DOI] [PubMed] [Google Scholar]
- 18.Waterland RA, Lin JR, Smith CA, Jirtle RL. Post-weaning diet affects genomic imprinting at the insulin-like growth factor 2 (Igf2) locus. Hum Mol Genet 2006;15:705–716 [DOI] [PubMed] [Google Scholar]
- 19.Hark AT, Schoenherr CJ, Katz DJ, Ingram RS, Levorse JM, Tilghman SM. CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature 2000;405:486–489 [DOI] [PubMed] [Google Scholar]
- 20.Gloyn AL, Mackay DJ, Weedon MN, et al. Assessment of the role of common genetic variation in the transient neonatal diabetes mellitus (TNDM) region in type 2 diabetes: a comparative genomic and tagging single nucleotide polymorphism approach. Diabetes 2006;55:2272–2276 [DOI] [PubMed] [Google Scholar]
- 21.Gardner RJ, Mackay DJ, Mungall AJ, et al. An imprinted locus associated with transient neonatal diabetes mellitus. Hum Mol Genet 2000;9:589–596 [DOI] [PubMed] [Google Scholar]
- 22.Ma D, Shield JP, Dean W, et al. Impaired glucose homeostasis in transgenic mice expressing the human transient neonatal diabetes mellitus locus, TNDM. J Clin Invest 2004;114:339–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breyer MD, Böttinger E, Brosius FC, 3rd, et al. AMDCC Mouse models of diabetic nephropathy. J Am Soc Nephrol 2005;16:27–45 [DOI] [PubMed] [Google Scholar]
- 24.Harris IS, Treskov I, Rowley MW, et al. G-protein signaling participates in the development of diabetic cardiomyopathy. Diabetes 2004;53:3082–3090 [DOI] [PubMed] [Google Scholar]
- 25.Muller KA, Ryals JM, Feldman EL, Wright DE. Abnormal muscle spindle innervation and large-fiber neuropathy in diabetic mice. Diabetes 2008;57:1693–1701 [DOI] [PubMed] [Google Scholar]
- 26.Kim SJ, Nian C, Doudet DJ, McIntosh CH. Inhibition of dipeptidyl peptidase IV with sitagliptin (MK0431) prolongs islet graft survival in streptozotocin-induced diabetic mice. Diabetes 2008;57:1331–1339 [DOI] [PubMed] [Google Scholar]
- 27.Cederholm J, Wibell L. Evaluation of insulin release and relative peripheral resistance with use of the oral glucose tolerance test: a study in subjects with normoglycaemia, glucose intolerance and non-insulin-dependent diabetes mellitus. Scand J Clin Lab Invest 1985;45:741–751 [DOI] [PubMed] [Google Scholar]
- 28.Martinez SC, Tanabe K, Cras-Méneur C, Abumrad NA, Bernal-Mizrachi E, Permutt MA. Inhibition of Foxo1 protects pancreatic islet beta-cells against fatty acid and endoplasmic reticulum stress-induced apoptosis. Diabetes 2008;57:846–859 [DOI] [PubMed] [Google Scholar]
- 29.Oh W, Gelardi NL, Cha CJ. Maternal hyperglycemia in pregnant rats: its effect on growth and carbohydrate metabolism in the offspring. Metabolism 1988;37:1146–1151 [DOI] [PubMed] [Google Scholar]
- 30.Kaijser M, Bonamy AK, Akre O, et al. Perinatal risk factors for diabetes in later life. Diabetes 2009;58:523–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meier JJ. Linking the genetics of type 2 diabetes with low birth weight: a role for prenatal islet maldevelopment? Diabetes 2009;58:1255–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakravarthy MV, Zhu Y, Wice MB, et al. Decreased fetal size is associated with beta-cell hyperfunction in early life and failure with age. Diabetes 2008;57:2698–2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lumey LH. Decreased birthweights in infants after maternal in utero exposure to the Dutch famine of 1944-1945. Paediatr Perinat Epidemiol 1992;6:240–253 [DOI] [PubMed] [Google Scholar]
- 34.Jimenez-Chillaron JC, Hernandez-Valencia M, Reamer C, et al. Beta-cell secretory dysfunction in the pathogenesis of low birth weight-associated diabetes: a murine model. Diabetes 2005;54:702–711 [DOI] [PubMed] [Google Scholar]
- 35.Jimenez-Chillaron JC, Isganaitis E, Charalambous M, et al. Intergenerational transmission of glucose intolerance and obesity by in utero undernutrition in mice. Diabetes 2009;58:460–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rees DA, Alcolado JC. Animal models of diabetes mellitus. Diabet Med 2005;22:359–370 [DOI] [PubMed] [Google Scholar]
- 37.Ueda H, Ikegami H, Yamato E, et al. The NSY mouse: a new animal model of spontaneous NIDDM with moderate obesity. Diabetologia 1995;38:503–508 [DOI] [PubMed] [Google Scholar]
- 38.Corsetti JP, Sparks JD, Peterson RG, Smith RL, Sparks CE. Effect of dietary fat on the development of non-insulin dependent diabetes mellitus in obese Zucker diabetic fatty male and female rats. Atherosclerosis 2000;148:231–241 [DOI] [PubMed] [Google Scholar]
- 39.Trevaskis JL, Meyer EA, Galgani JE, Butler AA. Counterintuitive effects of double-heterozygous null melanocortin-4 receptor and leptin genes on diet-induced obesity and insulin resistance in C57BL/6J mice. Endocrinology 2008;149:174–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chong S, Vickaryous N, Ashe A, et al. Modifiers of epigenetic reprogramming show paternal effects in the mouse. Nat Genet 2007;39:614–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferguson-Smith AC, Surani MA. Imprinting and the epigenetic asymmetry between parental genomes. Science 2001;293:1086–1089 [DOI] [PubMed] [Google Scholar]
- 42.DeChiara TM, Robertson EJ, Efstratiadis A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell 1991;64:849–859 [DOI] [PubMed] [Google Scholar]
- 43.Rainier S, Johnson LA, Dobry CJ, Ping AJ, Grundy PE, Feinberg AP. Relaxation of imprinted genes in human cancer. Nature 1993;362:747–749 [DOI] [PubMed] [Google Scholar]
- 44.Cattanach BM, Beechey CV. Autosomal and X-chromosome imprinting. Dev Suppl 1990;108(Suppl):63–72 [PubMed] [Google Scholar]
- 45.Bell AC, Felsenfeld G. Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature 2000;405:482–485 [DOI] [PubMed] [Google Scholar]
- 46.Feil R, Walter J, Allen ND, Reik W. Developmental control of allelic methylation in the imprinted mouse Igf2 and H19 genes. Development 1994;120:2933–2943 [DOI] [PubMed] [Google Scholar]
- 47.Lopes S, Lewis A, Hajkova P, et al. Epigenetic modifications in an imprinting cluster are controlled by a hierarchy of DMRs suggesting long-range chromatin interactions. Hum Mol Genet 2003;12:295–305 [DOI] [PubMed] [Google Scholar]
- 48.Vu TH, Li T, Nguyen D, et al. Symmetric and asymmetric DNA methylation in the human IGF2-H19 imprinted region. Genomics 2000;64:132–143 [DOI] [PubMed] [Google Scholar]
- 49.Sullivan MJ, Taniguchi T, Jhee A, Kerr N, Reeve AE. Relaxation of IGF2 imprinting in Wilms tumours associated with specific changes in IGF2 methylation. Oncogene 1999;18:7527–7534 [DOI] [PubMed] [Google Scholar]
- 50.Reik W, Brown KW, Schneid H, Le Bouc Y, Bickmore W, Maher ER. Imprinting mutations in the Beckwith-Wiedemann syndrome suggested by altered imprinting pattern in the IGF2-H19 domain. Hum Mol Genet 1995;4:2379–2385 [DOI] [PubMed] [Google Scholar]