Abstract
Overweight characterized by inappropriate expansion of adipose cells (hypertrophic obesity) is associated with the metabolic syndrome and is caused by an inability to recruit and differentiate new precursor cells. We examined the role of bone morphogenetic protein 4 (BMP4) and WNT activation in the regulation of human adipose cell differentiation. Cluster of differentiation (CD)14+/45+ and CD31+ cells were first removed before the remaining stromal vascular cells of human subcutaneous biopsy specimens were differentiated with/without different WNT inhibitors and/or BMP4. Inhibition of WNT and induction of Dickkopf 1 (DKK1) were markers of precursor cells undergoing excellent differentiation. The addition of DKK1 inhibited WNT activation and promoted adipogenesis in cells with a low degree of differentiation. The positive effect of DKK1, inhibiting cellular WNT activation by binding to the Kremen/LDL receptor–related protein receptors, was not seen with inhibitors of secreted WNT ligands. BMP4 increased differentiation, and BMP4 in the presence of DKK1 produced an additive effect. There was an apparent cross-talk between differentiation and commitment because BMP4 expression increased in differentiating adipocytes, and the addition of the BMP4 inhibitor, Noggin, reduced precursor cell differentiation. Thus, differentiated human adipose cells can promote adipogenesis via endogenous BMP4 activation, and the impaired adipogenesis in hypertrophic obesity is mainly due to an inability to suppress canonical WNT and to induce DKK1.
Our current understanding of adipose tissue development in human is that the major pool of preadipocytes develops before puberty, and after this, there is a 10% annual adipose cell turn-over (1). Interestingly, research has also shown that individuals with inappropriately enlarged adipose cells for a given BMI (hypertrophic obesity) in the abdominal subcutaneous tissue are characterized by a reduced recruitment of new cells, suggesting that this is causally related to the development of hypertrophic obesity (2). More important, we have recently shown that adipose cell size in the abdominal subcutaneous region is, for a given BMI, considerably larger in individuals with a genetic predisposition for type 2 diabetes than in subjects lacking a known heredity or in those with a heredity for overweight/obesity (3,4). These findings link heredity for type 2 diabetes to the development of hypertrophic obesity. Furthermore, hypertrophic adipocytes, even in the absence of obesity per se, are associated with several markers of a dysregulated adipose tissue and systemic as well as local insulin resistance (4,5).
In agreement with these in vivo findings, we recently showed that the ability of subcutaneous adipose tissue stromal vascular cells (stromal cells) to undergo adipogenic differentiation was markedly reduced in hypertrophic obesity and that the degree of impairment was positively correlated with adipose cell size of the donor (6). Interestingly, this did not appear to be a consequence of a reduced number of early precursor cells because the number of cluster of differentiation CD133+ cells was actually increased (6). Together, these findings suggest that hypertrophic obesity is due to an apparent genetic impairment in the ability to recruit and differentiate new subcutaneous adipose precursor cells. This, then, promotes inappropriate cell enlargement, inflammation, and a dysregulated adipose tissue that will favor ectopic lipid accumulation and the development of a metabolically obese phenotype (3,4).
Recruitment and differentiation of adipose precursor cells are regulated by the wingless-type mouse mammary tumor virus (MMTV) integration site family (WNT) signaling. Thus, a possible mechanism for the perturbed adipogenesis in hypertrophic obesity is an inability to adequately suppress WNT activation in precursor cells.
Secreted WNT ligands signal through both canonical and noncanonical pathways. The canonical WNT/β-catenin pathway is highly active in precursor cells and directs multipotent mesenchymal stem cells (MSC) toward adipogenic, osteogenic, or myogenic differentiation (7,8). The detailed molecular mechanisms for the commitment of multipotent cells into the adipose lineage are poorly understood (9). However, once committed, preadipocytes can undergo the adipogenic program leading to activation of the dominant adipose regulator peroxisome proliferator-activated receptor (PPAR)-γ as well as the CCAAT/enhancer binding protein (C/EBP) proteins (9,10).
WNT signaling can be inhibited by different secreted antagonists (11) including soluble Frizzled-related proteins (sFRP) 1 and 2, WNT inhibitory factor (WIF) 1 and the Dickkopf (DKK) proteins (12–14). DKK1 inhibits WNT signaling by binding as a high-affinity antagonist to the coreceptors LDL receptor–related proteins (LRPs) 5/6 and Kremen1 and 2, thereby preventing formation of the active LRP/Frizzled complex. sFRPs and WIF1 proteins bind to the secreted WNT ligands and thereby inhibit activation (15). Consistent with the importance of canonical WNT activation, transfection of human MSC isolated from adipose tissue with small interfering RNA (siRNA) for DKK1 reduced adipogenesis (16). We, and others, have shown that Dkk1 is highly expressed in differentiated 3T3-L1 adipocytes and is induced by the PPAR-γ agonists (17–19). Thus, activation and secretion of DKK1 might be a mechanism whereby PPAR-γ can help terminate the WNT signal and promote adipogenesis (16,19).
Bone morphogenetic proteins (BMPs) are members of the transforming growth factor-β superfamily and have been shown to play an important role in the commitment of multipotent precursor cells to the adipocyte lineage (20–22). Most of the effects of the BMPs are mediated through type 1 and type 2 receptors. Interestingly, specific genotypes of the BMPR isoforms BMPR1A and BMPR2 have been shown to associate with obesity in human (23–25). Furthermore, the associated member of the transforming growth factor-β superfamily, inhibin beta A/activin, was recently shown to exert a negative effect on adipogenesis and was induced by macrophages (26).
In the current study, we asked if the reduced adipogenesis in hypertrophic obesity could be overcome by inhibiting WNT activation by specific inhibitors and/or by promoting commitment of residing precursor cells with BMP4.
RESEARCH DESIGN AND METHODS
Human subjects.
Abdominal subcutaneous adipose tissue was obtained from 48 individuals by needle biopsy (n = 46) or bariatric surgery (n = 2). The subjects were aged between 27 and 66 years, had a mean BMI of 27.8 ± 7.1 kg/m2 (range 19.3–54.8), and an adipose cell size of 93.6 ± 17.9 μm (range 52.8–125). With the exception of five patients with known type 2 diabetes (four in Fig. 1 and one in Fig. 6B) all subjects had glucose levels within normal reference ranges and, with the exception of obesity, had no known chronic diseases. Exclusion of the subjects with diabetes from the data in Fig. 1 and Fig. 6B, respectively, did not change the conclusions. The ethical committee of the University of Gothenburg approved this study.
FIG. 1.
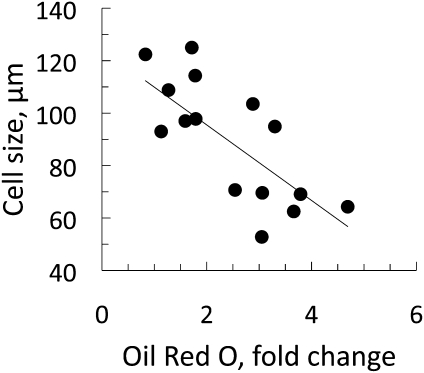
Differentiation of human stromal cells is impaired in hypertrophic obesity. Differentiation of stromal cells was performed with the standard differentiation protocol. The cells were stained with ORO and quantified by dissolving the ORO stain in 2-propanol and measuring optical density at λ-510 nm. Absorbance of the ORO stain was compared with cell size (r2 = 0.53, P < 0.001; BMI mean 30.3 kg/m2 [range 19.3–54.8]; n = 16).
FIG. 6.
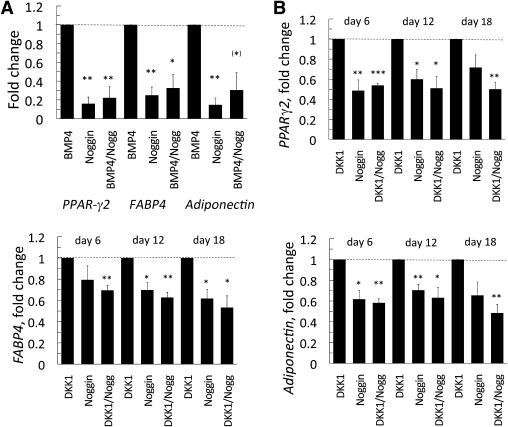
Noggin inhibits the BMP4- and DKK1-induced differentiation of human stromal cells. A: Noggin reduces the differentiation of stromal cells and the effect of BMP4 on adipogenic genes. Stromal cells were differentiated with 3 nmol/L BMP4 and/or 100 ng/mL Noggin. B: The positive effect of DKK1 on differentiation of stromal cells is partly mediated through the associated induction of BMP4 (see Fig. 5). Stromal cells were differentiated with or without Noggin (100 ng/mL) and DKK1 (25 ng/mL). Expression levels of the genes were first normalized to 18S rRNA and then normalized to expression levels in the BMP4 (A) or DKK1 (B) sample (dotted line = 1, n = 4). Data are presented as the mean ± SEM. *P < 0.05, **P < 0.02, and ***P < 0.002 compared with DKK1 or BMP4, respectively.
DKK1-conditioned medium.
3T3-L1 cells expressing DKK1 and control cells with empty vector (a gift from Drs Jazzwinder K. Sethi and Antonio Vidal-Puig, Cambridge, U.K.) were cultured in Dulbecco′s modified Eagle′s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum and puromycin at 37°C and 5% CO2. Cell culture media were repeatedly collected every 3 days. Media from control cells were used in the experiments to validate the specific effects of DKK1. Quantification of the secreted DKK1 protein was performed with a DKK1 enzyme-linked immunosorbent assay (ELISA) (19).
Adipocyte differentiation and measurement of cell size.
The adipose tissue was digested with collagenase, as previously described, and cell size was measured on isolated cells by using a calibrated scale as described (6). Inflammatory cells and endothelial cells were removed with magnetic immune separation using CD14, CD45, and CD31 antibodies (Miltenyi Biotech). The remaining cells were then cultured as described (6). Briefly, cells at passage three to four were induced to differentiate with a cocktail consisting of 850 nmol/L insulin, 10 μmol/L dexamethasone, 0.5 mmol/L isobutyl methylxanthine, 10 μmol/L pioglitazone, 33 μmol/L biotin, and 17 μmol/L pantothenate in DMEM/F12 supplemented with 3% FBS (v/v), 2 mmol/L glutamine, and antibiotics. After 3 days, the medium was changed to DMEM/F12 containing 850 nmol/L insulin, 1 μmol/L dexamethasone, 1 μmol/L pioglitazone, 10% FBS, glutamine, and antibiotics. The medium was changed every third day throughout the differentiation period.
The following WNT and BMP4 inhibitors were added to the differentiation cocktail: DKK1, cell culture supernatant comparable to 25 ng/mL; sFRP1 and sFRP2, 200 ng/mL, and WIF1, 75 ng/mL (5396-SF, 1169-FR, and 1341-WF, R&D Systems); and Noggin, 10–100 ng/mL (SRP4675, Sigma-Aldrich). The inhibitors were maintained during differentiation. Oil Red O (ORO) staining was performed as described (27). The cells were counterstained with Mayer's hematoxylin. Differentiation in percentage was estimated by measuring the area with lipid-containing cells (percentage cell differentiation vs. ORO absorbance; r = 0.827, P < 0.001, n = 42).
Whole-cell extracts and Western blots.
Whole-cell protein lysates were prepared, and Western blots were performed as described (28). Immunoblots were performed with the following antibodies; WIF1, sFRP1, and sFRP2 (sc-25520, sc-13939, and sc-13940 Santa Cruz, Biotechnology); DKK1 (MAB 1765, R&D Systems), β-catenin (C19220, Transduction Laboratories, BD Biosciences), PPAR-γ2 (MAB3630, Chemicon), and DLK1/preadipocyte factor-1 (Pref-1; 2069, Cell Signaling, New England Biolabs).
Quantitative real-time PCR.
Total RNA was isolated from the cells with EZNA total RNA kit (Omega Bio-tek). Real-time PCR with gene-specific primers and probes (Applied Biosystems) was performed as described (18,28). Relative quantification of mRNA levels was plotted as fold-change, generally compared with untreated control cells (= 1). 18S ribosomal RNA was used as an endogenous control (Applied Biosystems). Analyses were performed in duplicates, and all experiments were repeated at least three times.
Statistical analyses.
Conventional statistical methods were used to calculate means ± SEM, and the Student paired or unpaired t test was used, as appropriate, to compare differential gene expression and other parameters shown. Differences were considered statistically significant at P < 0.05.
RESULTS
We initially removed the mature adipose cells as well as the stromal CD14+/CD45+ inflammatory cells and the CD31+ endothelial cells with immunomagnetic separation, leaving stem cells and other noncommitted progenitor cells, committed preadipocytes, and fibroblasts in the cultured cell fraction. In agreement with previous work (15), we confirmed a reduced adipogenesis in hypertrophic obesity and that the ability of the stromal cells to respond to the normal adipogenic cocktail in terms of differentiation and accumulation of lipids was negatively related to the size of the mature adipose cells (Fig. 1). The negative correlation with adipose cell size was not a consequence of obesity because it was also seen in the nonobese individuals and unrelated to BMI (Supplementary Fig. 1A and B).
Induction of DKK1 is a marker of adipogenesis.
We first examined if the ability of committed preadipocytes to differentiate was associated with induction of the WNT inhibitor DKK1. DKK1 expression is upregulated during differentiation of 3T3-L1 and human preadipocytes, and this correlates with inhibition of canonical WNT signaling and β-catenin–dependent gene transcription (17,19). We found DKK1 protein was induced in the stromal cells at approximately differentiation day 8, when the cells also assumed an adipocyte phenotype with expression of PPAR-γ and other adipogenic genes (Fig. 2A, B, and D). DKK1 expression was also related to the degree of differentiation such that it was only clearly seen in stromal cells where many cells underwent adipogenic differentiation measured as ORO accumulation (Fig. 2A and B). Our previous finding that PPAR-γ activation enhances expression and secretion of Dkk1 in 3T3-L1 adipocytes (19) indicates that the stromal cells with a low differentiation have an impaired ability to activate PPAR-γ and undergo adipogenesis rather than a reduced number of precursor cells and that the inability to suppress WNT may play a key role.
FIG. 2.
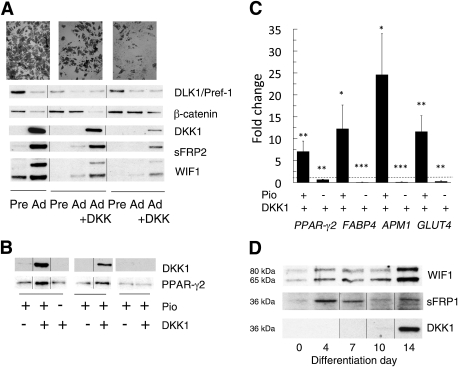
DKK1 expression is related to the degree of differentiation of human stromal cells. A: Differentiation of human abdominal stromal cells was performed with the standard differentiation protocol with and without DKK1 for 21 days. Results are from three representative individuals with different degrees of differentiation, which also relate to the inhibition of β-catenin. Addition of DKK1 to the cell culture medium promotes differentiation of the cells and also induces the WNT inhibitors DKK1, sFRP2, and WIF1. Accumulation of triglycerides was detected by ORO on day 21. B: Differentiation in the presence of DKK1 and pioglitazone (Pio) induces expression of PPAR-γ2 and DKK1 in cells from three different individuals with low degree of differentiation. C: DKK1 enhances the expression of genes related to adipogenesis but not in the absence of pioglitazone. The data were first normalized to 18S rRNA and then normalized to expression levels in the control sample (dotted line = 1). Data indicate means ± SEM from 16 healthy individuals with different BMI (mean 26.1 kg/m2 [range 19.3–44.2]) and cell size (mean 86.1 μm [range 62.5–110.9]). *P < 0.05, **P < 0.02, and ***P < 0.002 compared with untreated. D: Time course for expression of the WNT inhibitors WIF1, sFRP1, and DKK1 during different time points of differentiation of human stromal cells.
We also examined if the low DKK1 expression was a specific event in cells with a low degree of differentiation or if other WNT inhibitors were also insufficiently induced. In fact, cells that differentiated well and induced DKK1 also expressed sFRP2 and WIF1, and this was mirrored by the associated decrease in β-catenin as well as in DLK1/Pref-1 levels, which are consistent with adipocyte differentiation (Fig. 2A).
Addition of DKK1 promotes adipogenesis of human stromal cells with low differentiation.
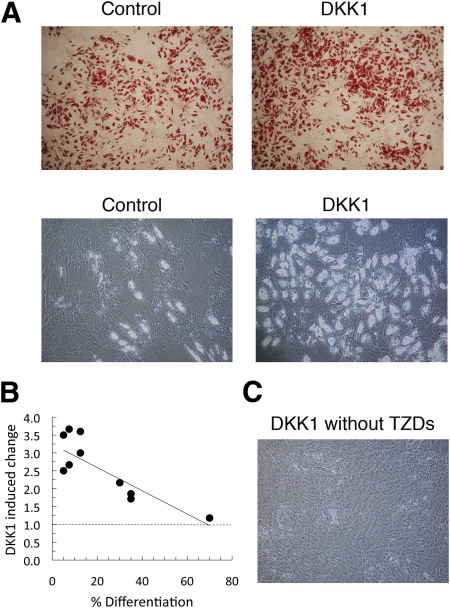
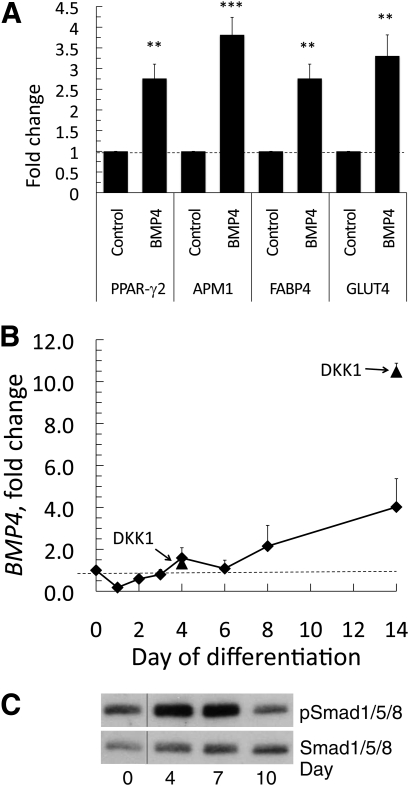
We then examined if it was possible to increase the differentiation of adipose precursor cells from individuals with low degree of differentiation by adding DKK1 for up to 21 days. The addition of DKK1 induced a marked increase in the number of cells acquiring lipids as well as the cellular area with lipid droplets (2.58 ± 0.25-fold, P < 0.001; n = 11; Fig. 3A). More important, stromal cells with a low initial degree of differentiation showed a three- to fourfold increase in lipid accumulation compared with cells with a high degree of differentiation, where DKK1 had much less effect (Fig. 3B). In addition, poorly differentiated stromal cells induced DKK1 when this inhibitor of canonical WNT was added during differentiation (Fig. 2A and B). Taken together, these findings support the concept that the low degree of differentiation of stromal cells in hypertrophic obesity is not due to a small number of precursor cells but rather to an inability to initiate adipogenesis and activate PPAR-γ as a consequence of inappropriate suppression of WNT activation. Consistent with this, cellular β-catenin (Fig. 2A) and Wnt-inducted secreted protein 2 (WISP2) (data not shown) levels were both related to the ability to differentiate. The increased differentiation after the addition of DKK1 was also associated with significant increases in the expression of all tested adipogenic markers, such as PPAR-γ2, fatty acid binding protein 4 (FABP4), adiponectin (APM1), and GLUT4 (Fig. 2C).
FIG. 3.
DKK1 promotes differentiation of adipose tissue stromal cells from individuals with a low degree of differentiation. A: Stromal cells from subcutaneous adipose tissue were differentiated for 21 days with or without DKK1. Results are from two representative individuals. Accumulation of cellular triglyceride was detected with ORO (upper panel) or unstained cells (lower panel). B: Effect of DKK1 on differentiation is more pronounced in stromal cells from individuals with a low degree of differentiation. Differentiation (%) is related to the area of lipid-accumulating cells at day 21 in the cell culture well (r2 = 0.66, P < 0.01, n = 11). C: Differentiation of stromal cells is dependent on the presence of TZDs and cannot be replaced by DKK1. (A high-quality digital representation of this figure is available in the online issue.)
We also examined the potential specific effect of DKK1 versus other secreted inhibitors of canonical WNT (i.e., sFRP1, sFRP2, and WIF1), which inhibit binding of WNT ligands to the receptors. These inhibitors are expressed at different time points during differentiation, and only WIF1 and sFRP2 are highly expressed in adipocytes (Fig. 2A and D). Although these inhibitors have been shown to induce spontaneous differentiation of the highly committed 3T3-L1 preadipocytes in the absence of PPAR-γ ligands (12) and in immortalized MSC from mouse bone marrow (29), the addition of up to 200 ng/mL of these WNT inhibitors did not increase differentiation of the stromal cells from individuals with hypertrophic obesity. Thus, DKK1, by binding to the Kremen and LRP receptors (11), is able to overcome the impaired differentiation in hypertrophic obesity, whereas sFRPs and WIF1 are not. This suggests that increased ligand secretion is not the cause of WNT activation in the adipose precursor cells in hypertrophic obesity.
Human preadipocytes require a PPAR-γ ligand for differentiation.
In contrast to the murine cell line 3T3-L1, human preadipocytes must be differentiated in the continuous presence of a PPAR-γ agonist, such as thiazolidinediones (TZDs). Exclusion of TZDs from the differentiation medium prevents differentiation and lipid accumulation, and withdrawal at day 3, when the initiation medium is replaced by adipocyte medium, diminishes the number and size of the lipid droplets. Furthermore, the need for a PPAR-γ ligand could not be replaced by the addition of DKK1 because this resulted in inhibition of adipogenic gene expression and lipid accumulation (Fig. 2C and Fig. 3C). Together, these data show that induction of DKK1 is an important step to inhibit WNT activation and, thereby, to allow PPAR-γ activation and adipogenesis, but DKK1 cannot replace the need for PPAR-γ agonists in human preadipocytes.
BMP4 promotes commitment and differentiation of human adipose progenitor cells.
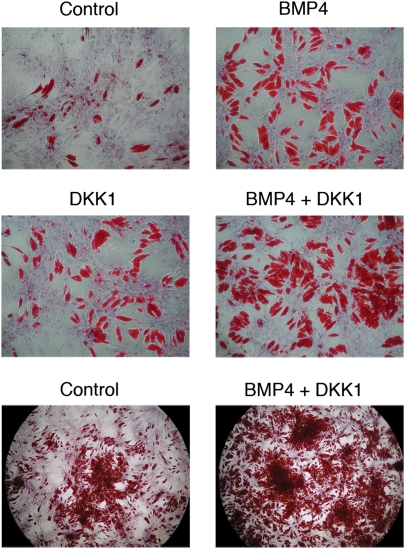
Even in the presence of DKK1, ∼50% of the stromal cells did not undergo differentiation (Fig. 3). We, therefore, examined the possibility that the stromal cells also contained uncommitted precursor cells that require activation by morphogenetic signals. Cells were plated at low density, and the medium was supplemented with 3 nmol/L BMP4 for 5 days before initiation of adipocyte differentiation. This was maintained throughout the entire culture period. BMP4 clearly induced commitment and subsequent differentiation of many cells that had remained undifferentiated after the addition of the regular differentiation cocktail (Fig. 4), and this was also associated with an increased activation of adipogenic genes (Fig. 5A). An important finding was an additive effect of DKK1 and BMP4, whereby ∼80% of the stromal cells could undergo differentiation in the presence of both ligands (Fig. 4).
FIG. 4.
BMP4 induces commitment and differentiation of progenitor cells from adipose tissue stromal cells. Stromal cells were incubated for 5 days with 3 nmol/L (40 ng/mL) BMP4 before initiation of differentiation, and this was maintained during differentiation. Cells were also induced to differentiate with and without DKK1, as described. ORO/hematoxylin staining at day 14. Low magnification shows an overview of the additive effects of DKK1 and BMP4 on differentiation. (A high-quality digital representation of this figure is available in the online issue.)
FIG. 5.
BMP4 promotes differentiation and induction of adipogenic genes. A: mRNA levels of the adipogenic differentiation markers PPAR-γ2, adiponectin (APM1), FABP4, and GLUT4 in control and BMP4-treated stromal cells. Expression levels of the genes were first normalized to 18S rRNA and then normalized to expression levels in the control sample (dotted line = 1) at day 4 (n = 6). Data are presented as means ± SEM. **P < 0.02 and ***P < 0.002 compared with untreated. B: Induction of BMP4 during differentiation and the effect of adding DKK1 to the medium (arrows). C: Phosphorylation of Smad1/5/8 during differentiation of stromal cells.
Adipogenic differentiation leads to induction of BMP4.
Interestingly, differentiation of precursor cells to mature adipose cells was associated with the induction of BMP4 mRNA, and this effect was markedly enhanced by the presence of DKK1 (Fig. 5B), consistent with the increased differentiation seen with this WNT inhibitor. The ability of BMP4 to act as a secreted molecule promoting differentiation of remaining stromal cells was supported by the increased phosphorylation of Smad1/5/8 (Fig. 5C). To further examine the possibility that the induction of BMP4 in mature adipose cells could play a role as a secreted paracrine factor for undifferentiated stromal cells, we differentiated stromal cells in the presence of BMP4 with and without the BMP4 inhibitor Noggin (30). As expected, the presence of Noggin markedly reduced the effect of BMP4 (Fig. 6A). However, the normal differentiation of the stromal cells as well as the positive effect of DKK1 was also inhibited. This was clearly visible at day 6 and was maintained throughout the differentiation period (Fig. 6B). These results strongly suggest that induction of BMP4 in differentiating and/or differentiated adipose cells is able to promote adipogenic differentiation of stromal precursor cells.
To further validate this concept, we added Noggin to fully differentiated adipose cells but saw no inhibition on adipogenic differentiation markers or on lipid accumulation when the differentiated cells were cultured with 100 ng/mL Noggin for up to 72 h without DKK1 (Supplementary Fig. 2). As a positive control, we also performed experiments where fully differentiated adipose cells were incubated with WNT3a because we have previously shown (18) that WNT3a inhibits the expression of PPAR-γ2 and also adipogenic genes in fully differentiated human adipocytes and this was also verified here (Supplementary Fig. 2). Thus, BMP4 is induced during differentiation, and undifferentiated but not differentiated cells are target cells.
We also analyzed Bmp4 induction in differentiating 3T3-L1 cells, but in contrast to human preadipocytes, Bmp4 was inhibited in these cells during differentiation (Supplementary Fig. 3).
DISCUSSION
Hypertrophic obesity is associated with the well-established metabolic complications of obesity (i.e., insulin resistance, dyslipidemia, and other traits of the metabolic syndrome). More important, nonobese individuals with inappropriate expansion of the adipose cells also show these metabolic characteristics, and the degree of insulin sensitivity is negatively correlated with adipose cell size (3). We have shown in several studies that the genetic predisposition for type 2 diabetes is associated with hypertrophic obesity and its metabolic characteristics, including dysregulated adipose tissue with reduced expression of insulin receptor substrate-1, GLUT4, adiponectin, and other PPAR-γ–regulated molecules (4). Furthermore, nonobese individuals with heredity for type 2 diabetes also show several markers of the metabolic syndrome, with dyslipidemia and lower insulin sensitivity, together with inappropriate adipose cell enlargement compared with carefully matched individuals lacking diabetes heredity (3,4). Thus, hypertrophic obesity is associated with a genetic predisposition for type 2 diabetes and can constitute an important link for an increased susceptibility to the environment by inducing insulin resistance and the obesity-linked metabolic complications early and before obesity, as conventionally defined by BMI, develops. Prospective studies have also shown that abdominal adipose cell size is an independent predictor of risk of developing type 2 diabetes (31,32).
Hypertrophic obesity is a consequence of a reduced ability to recruit and differentiate new adipose cells after an increased body weight, and experimental in vivo and in vitro results support this concept (2,4,6). Thus, understanding the mechanisms for this, likely genetic, inability is of great importance because the ability to recruit new adipose cells (hyperplastic obesity) is a more benign metabolic state at the same BMI and prevents ectopic lipid accumulation (3). Several genetically engineered animal models also support this concept; for instance, overexpression of adiponectin in the adipose tissue leads to massive, but hyperplastic, obesity and the animals are perfectly metabolically normal (33).
We here characterized 48 individuals with different BMI and cell size and initially removed inflammatory CD14+/45+ cells and CD31+ endothelial cells from the stromal tissue before induction of adipogenic differentiation. The results clearly show the large differences in ability to undergo differentiation of the remaining stromal cells and that this is negatively related to adipose cell size. In fact, the degree of differentiation varied from ∼5 to 80% after the normal differentiation cocktail, and individuals with hypertrophic obesity had a low degree of adipose cell differentiation, as we also previously have shown (6). This reduction could be due to a reduced number of precursor cells or in their ability to undergo adipogenesis and PPAR-γ activation.
In our previous study (6), we found that the number of CD133+ precursor cells was increased in hypertrophic obesity although overall differentiation was low, suggesting that lack of precursor cells was an unlikely explanation.
In the current study, we show that the ability of the adipogenic precursor cells to undergo differentiation depends on which signals they are provided. In contrast to the highly committed 3T3-L1 cells, human stromal cells require the continuous presence of a PPAR-γ ligand, suggesting that they are unable to secrete such ligands. More important, however, many adipose precursor cells from individuals with hypertrophic obesity are unable to adequately suppress WNT activation to enter into the adipogenic pathway, and DKK1 was found to be a particularly important promotor of adipogenesis. In support of this, we found that adding DKK1 induced a three- to fourfold increase in the number of cells able to undergo adipogenesis, and this effect was particularly pronounced in stromal cells with a low degree of differentiation. These results expand on the work by Park et al. (16) showing a reduced differentiation of cells transfected with siRNA against DKK1. We also have other support for the conclusion of an increased WNT activation in stromal cells in hypertrophic obesity because several markers of canonical WNT activation, including WISP2, are increased and their exprssion is positively correlated with the size of the mature cells (unpublished data).
The reason for the increased WNT activation is unclear, but genetic factors are likely to play an important role and of particular interest are WNT-related genes such as TCF7L2 and Kremen1, where DNA polymorphisms associate with type 2 diabetes and body fat distribution (34,35). Another factor that could contribute is the increased inflammation in the adipose tissue in hypertrophic obesity (36) since proinflammatory cytokines, in particular tumor necrosis factor-α, can promote canonical WNT activation (28). However, a long-term effect of this in the cultured cells is unlikely, and in previous work, we found that the inhibitory effect of tumor necrosis factor-α was transient and dependent on the continuous presence of the cytokine (6).
It is intriguing that the direct inhibitors of canonical WNT ligands, sFRPs and WIF1, did not provide support for the adipogenic differentiation, whereas DKK1 was highly efficient. This indicates that the increased WNT activation is a consequence of endogenous cellular signaling rather than increased secretion of WNT ligands. The molecular mechanisms leading to the activation of DKK1 during adipogenesis are poorly understood. Although PPAR-γ ligands can induce Dkk1 in 3T3-L1 cells, it is unlikely that this is the initial mechanism for DKK1 induction because it is pivotal to inhibit canonical WNT before PPAR-γ can be induced.
The present studies also show that adipose tissue stromal cells contain early precursor cells that can be committed and undergo adipogenesis after the addition of BMP4 (20). We have also found expression of the classic MSC markers CD105 and CD117 in automatic cell sorting analyses of the stromal cells from human adipose tissue; in fact, ∼1–4/1000 cells expressed these markers (unpublished data). A stimulating effect of BMP4 on differentiation of the stromal cells was seen in all tested samples but, in contrast to the effect of DKK1, this effect was not clearly related to initial degree of adipogenesis and cell size like the effect of DKK1. However, our findings of the ability of BMP4 to enhance adipose precursor cell differentiation and lipid accumulation may provide a functional link with the recent observation that BMPR1A and BMPR2 polymorphisms associate with obesity in human (23,25).
An intriguing finding was the induction of BMP4 mRNA levels after differentiation of the human precursor cells. Furthermore, the inhibitory effect of the BMP4 inhibitor, Noggin, in differentiating cells—but not in fully differentiated cells—suggests that mature adipose cells may secrete this morphogenetic factor, which, in turn, can promote commitment and differentiation of ambient precursor cells. Whether such a putative signal is altered in hypertrophic obesity is currently unclear but under examination. Interestingly, induction of BMP4 during differentiation appears specific for human adipose cells because Bmp4 decreases when 3T3-L1 cells undergo differentiation (Supplementary Fig. 3). This emphasizes the importance of studying human stromal cells to understand the pathophysiology of hypertrophic obesity in human.
In conclusion, we have shown that many stromal cells in human adipose tissue are unable to undergo adipogenesis unless specific signals for commitment and differentiation are provided. Of particular importance was the finding that WNT inhibition by DKK1 had a profound positive effect on the differentiation of stromal cells with a low initial degree of adipogenic differentiation, consistent with an inability to adequately suppress this critical regulator of cell differentiation in hypertrophic obesity. Our results also raise the intriguing possibility that differentiated adipose cells can secrete BMP4 and induce a paracrine regulation and commitment of early precursor cells as the mature adipose cells expand.
Supplementary Material
ACKNOWLEDGMENTS
This study received financial support from the Swedish Research Council, the Swedish Diabetes Association, the Novo Nordisk Foundation, the Swedish Foundation for Strategic Research, the European Foundation for the Study of Diabetes, and the Torsten and Ragnar Söderberg Foundation.
No potential conflicts of interest relevant to this article were reported.
B.G. and U.S. designed the research and wrote the manuscript. B.G. performed research. U.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1419/-/DC1.
REFERENCES
- 1.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature 2008;453:783–787 [DOI] [PubMed] [Google Scholar]
- 2.Arner E, Westermark PO, Spalding KL, et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes 2010;59:105–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arner P, Arner E, Hammarstedt A, Smith U. Genetic predisposition for type 2 diabetes, but not for overweight/obesity, is associated with a restricted adipogenesis. PLoS ONE 2011;6:e18284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jansson PA, Pellmé F, Hammarstedt A, et al. A novel cellular marker of insulin resistance and early atherosclerosis in humans is related to impaired fat cell differentiation and low adiponectin. FASEB J 2003;17:1434–1440 [DOI] [PubMed] [Google Scholar]
- 5.Rondinone CM, Wang LM, Lonnroth P, Wesslau C, Pierce JH, Smith U. Insulin receptor substrate (IRS) 1 is reduced and IRS-2 is the main docking protein for phosphatidylinositol 3-kinase in adipocytes from subjects with non-insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A 1997;94:4171–4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isakson P, Hammarstedt A, Gustafson B, Smith U. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes 2009;58:1550–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowers RR, Lane MD. Wnt signaling and adipocyte lineage commitment. Cell Cycle 2008;7:1191–1196 [DOI] [PubMed] [Google Scholar]
- 8.Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest 2006;116:1202–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otto TC, Lane MD. Adipose development: from stem cell to adipocyte. Crit Rev Biochem Mol Biol 2005;40:229–242 [DOI] [PubMed] [Google Scholar]
- 10.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1994;79:1147–1156 [DOI] [PubMed] [Google Scholar]
- 11.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci 2003;116:2627–2634 [DOI] [PubMed] [Google Scholar]
- 12.Bennett CN, Ross SE, Longo KA, et al. Regulation of Wnt signaling during adipogenesis. J Biol Chem 2002;277:30998–31004 [DOI] [PubMed] [Google Scholar]
- 13.Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci 2008;121:737–746 [DOI] [PubMed] [Google Scholar]
- 14.Abdallah BM, Kassem M. New factors controlling the balance between osteoblastogenesis and adipogenesis. Bone 2012;50:540–545 [DOI] [PubMed] [Google Scholar]
- 15.Wang K, Zhang Y, Li X, et al. Characterization of the Kremen-binding site on Dkk1 and elucidation of the role of Kremen in Dkk-mediated Wnt antagonism. J Biol Chem 2008;283:23371–23375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park JR, Jung JW, Lee YS, Kang KS. The roles of Wnt antagonists Dkk1 and sFRP4 during adipogenesis of human adipose tissue-derived mesenchymal stem cells. Cell Prolif 2008;41:859–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christodoulides C, Laudes M, Cawthorn WP, et al. The Wnt antagonist Dickkopf-1 and its receptors are coordinately regulated during early human adipogenesis. J Cell Sci 2006;119:2613–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gustafson B, Smith U. Activation of canonical wingless-type MMTV integration site family (Wnt) signaling in mature adipocytes increases beta-catenin levels and leads to cell dedifferentiation and insulin resistance. J Biol Chem 2010;285:14031–14041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gustafson B, Eliasson B, Smith U. Thiazolidinediones increase the wingless-type MMTV integration site family (WNT) inhibitor Dickkopf-1 in adipocytes: a link with osteogenesis. Diabetologia 2010;53:536–540 [DOI] [PubMed] [Google Scholar]
- 20.Bowers RR, Kim JW, Otto TC, Lane MD. Stable stem cell commitment to the adipocyte lineage by inhibition of DNA methylation: role of the BMP-4 gene. Proc Natl Acad Sci U S A 2006;103:13022–13027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahrens M, Ankenbauer T, Schröder D, Hollnagel A, Mayer H, Gross G. Expression of human bone morphogenetic proteins-2 or -4 in murine mesenchymal progenitor C3H10T1/2 cells induces differentiation into distinct mesenchymal cell lineages. DNA Cell Biol 1993;12:871–880 [DOI] [PubMed] [Google Scholar]
- 22.Tang QQ, Otto TC, Lane MD. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci U S A 2004;101:9607–9611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Böttcher Y, Unbehauen H, Klöting N, et al. Adipose tissue expression and genetic variants of the bone morphogenetic protein receptor 1A gene (BMPR1A) are associated with human obesity. Diabetes 2009;58:2119–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen D, Ji X, Harris MA, et al. Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J Cell Biol 1998;142:295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schleinitz D, Klöting N, Böttcher Y, et al. Genetic and evolutionary analyses of the human bone morphogenetic protein receptor 2 (BMPR2) in the pathophysiology of obesity. PLoS ONE 2011;6:e16155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaragosi LE, Wdziekonski B, Villageois P, et al. Activin a plays a critical role in proliferation and differentiation of human adipose progenitors. Diabetes 2010;59:2513–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang QQ, Lane MD. Activation and centromeric localization of CCAAT/enhancer-binding proteins during the mitotic clonal expansion of adipocyte differentiation. Genes Dev 1999;13:2231–2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gustafson B, Smith U. Cytokines promote Wnt signaling and inflammation and impair the normal differentiation and lipid accumulation in 3T3-L1 preadipocytes. J Biol Chem 2006;281:9507–9516 [DOI] [PubMed] [Google Scholar]
- 29.Taipaleenmäki H, Abdallah BM, AlDahmash A, Säämänen AM, Kassem M. Wnt signalling mediates the cross-talk between bone marrow derived pre-adipocytic and pre-osteoblastic cell populations. Exp Cell Res 2011;317:745–756 [DOI] [PubMed] [Google Scholar]
- 30.Bowers RR, Lane MD. A role for bone morphogenetic protein-4 in adipocyte development. Cell Cycle 2007;6:385–389 [DOI] [PubMed] [Google Scholar]
- 31.Lönn M, Mehlig K, Bengtsson C, Lissner L. Adipocyte size predicts incidence of type 2 diabetes in women. FASEB J 2010;24:326–331 [DOI] [PubMed] [Google Scholar]
- 32.Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia 2000;43:1498–1506 [DOI] [PubMed] [Google Scholar]
- 33.Kim JY, van de Wall E, Laplante M, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 2007;117:2621–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Speliotes EK, Willer CJ, Berndt SI, et al. MAGIC. Procardis Consortium Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 2010;42:937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grant SF, Thorleifsson G, Reynisdottir I, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet 2006;38:320–323 [DOI] [PubMed] [Google Scholar]
- 36.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.