The age of onset of type 1A diabetes is now immunologically predictable (1). Either because of a lack of sufficient understanding of the pathogenesis of type 1A (immune-mediated) diabetes or a lack of effective therapeutics directed at relevant pathogenic pathways (or both), we cannot yet prevent this disease (2). The article by Liu et al. (3) in this issue of Diabetes addresses both issues in a rat model of autoimmune diabetes (Lew.1WR1). In this genetically susceptible model, multiple viral infections or a viral RNA chemical mimic (poly-IC) activate the innate immune system leading to insulitis and β-cell destruction (4). The viruses do not appear to target islets; rather, they act by inducing inflammation such that anti-inflammatory therapy (e.g., prednisone) at the time of infection prevents diabetes (5). This model was discovered when the Kilham rat virus spontaneously infected a colony of “normal” rats with major histocompatibility complex (MHC) and dominant diabetes susceptibility loci on chromosome 4 (Iddm 14) (6). The iddm14 locus encompasses the family of T-cell receptor Vβ segment genes, and in particular, a specific Vβ13 haplotype is associated with diabetes susceptibility (7). Specific T cells can be targeted by monoclonals to their Vβ sequences to prevent disease.
Liu et al. analyzed the percentage and sequences of T cells with Vβ13 in islets of the rat model described above and tested whether a monoclonal antibody to Vβ13 could prevent diabetes. T cells express T-cell receptors (TCRs) composed of two chains (α and β) that recognize peptide-MHC complexes. The TCR α- and β-chain genes (Tcra and Tcrb) contain elements called Vα and Jα (α-chain) and Vβ, Dβ, and Jβ (β-chain). Individual T cells have different α- and β-chains created by recombination of these gene segments. Six complementarity-determining regions (CDRs) molecularly interact with a peptide and an MHC molecule presenting the peptide, and CDR1a, CDR2a, CDR1b, and CDR2b are germline-encoded in the Vα/β element. Each germline-encoded element sequence has a unique number (e.g., Vβ13), and all Vβ13 T cells have identical amino acid sequences including CDR1 and CDR2.
Liu et al. found that triggering of anti-islet autoimmunity in Lew.1WR1 rats induced a significant increase of Vβ13 CD4 T cells within islets. They also found significant skewing of Jβ utilization by T cells invading islets, but those T cells invading islets had a number of different CDR3 sequences. CDR3 as compared with CDR1 and CDR2 has extra sequence diversity due the joining of sequence elements at CDR3. Confirming the importance of Vβ13 (CDR1 and CDR2 sequences in particular) a monoclonal antibody targeting Vβ13 prevented diabetes in both the Lew.1WR1 model and the spontaneous BBDP rat (3,8).
The NOD mouse model also has an invariant germline encoded CDR1 and CDR2 sequence associated with autoimmune diabetes. In the NOD mouse, an insulin B-chain 9–23 peptide (insulin B:9–23) is preferentially recognized by T cells expressing a specific Vα sequence (TRAV5D-4) (9). A single mutation of this peptide (B16:alanine replacing B16:tyrosine) prevents NOD diabetes (10). Multiple murine TRAV5D-4 containing TCR α-chain sequences can induce anti-insulin autoimmunity and, in a subset of mice, diabetes (9,11). Thus for both rats and mice, TCR Vα/β germline-encoded sequences may be critical for the development of autoimmune diabetes.
Nevertheless, linkage of diabetes to TCR loci has not been demonstrated in either the NOD mouse or in humans (12,13). If similar to the Lew.1WR1 model above, either interactions between MHC alleles and V segment sequences have not been studied in enough detail, or critical TCR-germline sequences are not polymorphic. In the latter case, the TCR sequences may contribute to “species” diabetes susceptibility rather than individual susceptibility. Multiple genetic polymorphisms contribute to individual diabetes risk through effects on maintenance of general tolerance (12) and specific organ targeting (MHC alleles [14] and insulin promoter polymorphisms). Similar to the rat model described above, environmental factors may trigger islet autoimmunity as well as set basal population risk (15,16).
Given the findings of Liu et al., two immediate questions arise. What are the sequences of the α-chain of islet invading Vβ13 T cells? What is the target peptide of these Vβ13 bearing T cells? We assume there will be a single dominant peptide recognized by the Vβ13 CD4 T cells. One wants to know not only the sequence of the target peptide, but also its register. That is, how it binds in the MHC groove in which it is recognized (17). Once this is known, it should be possible to create fluorescent tetramers (MHC + peptide) to identify the autoreactive T cells, similar to what has been accomplished for anti-B:9–23 T cells of NOD mice (18). The paradigm that limited germline-encoded T-cell receptor sequences contribute to autoimmune diabetes susceptibility shifts emphasis to invariant sequences of CDR1 and CDR2 of α- and β-chains as essential to pathogenesis. Detailed knowledge of targets and multiple animal models will permit these paradigms to be tested and their therapeutic implications explored. A critical need is the molecular/structural characterization of relevant human anti-islet trimolecular (MHC: peptide: TCR) complexes (19) including relevant TCR sequences.
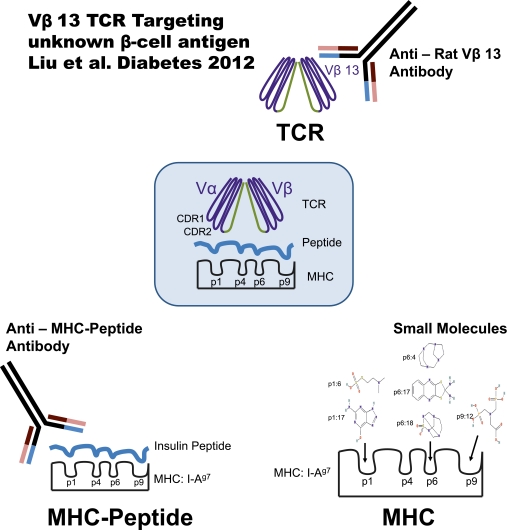
Finally, conserved T-cell receptor elements are only one part of the trimolecular complex that can be targeted to develop novel therapeutics (Fig. 1). Small molecules that bind to pockets of diabetogenic MHC alleles (20) and antibodies that react specifically with MHC with bound peptide (21) provide additional therapeutic pathways. There has been skepticism regarding development of therapeutics directed at the trimolecular complex because it has not been accomplished for any diseases in humans. To quote from the fascinating article by Liu et al.: “We recognize that, based on human and mouse data, it has been assumed that redundancy among cognate rat TCRs would preclude reliance on any one allele of TCR α- or β-chains for disease susceptibility. Our data and data of others strongly suggest that this assumption may be faulty.”
FIG. 1.
Multiple approaches to therapeutically target critical diabetogenic trimolecular recognition complexes (MHC + peptide + TCR). In order to block interactions in the trimolecular complex, anti-Rat Vβ13 antibody, small molecules (20), and anti-I-Ag7-insulin peptide complex antibody (21) target TCR β-chain, MHC peptide-binding grooves, and MHC-peptide complex, respectively.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R00 DK080885 and P30 DK057516), the National Institutes of Health Autoimmune Prevention Center (U19 AI050864), the Juvenile Diabetes Research Foundation (4-2007-1056, 44-2008-913, and 17-2010-744), and the Helmsley Foundation (09AG-118600).
G.S.E. is on two University provisional patents for treating islet autoimmunity targeting MHC-peptide complex with small molecules or antibodies and has research grant from Novartis in the same area. M.N. is on a University provisional patent for treating islet autoimmunity targeting MHC-peptide complex with small molecules. No other potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 1160.
REFERENCES
- 1.Steck AK, Johnson K, Barriga KJ, et al. Age of islet autoantibody appearance and mean levels of insulin, but not GAD or IA-2 autoantibodies, predict age of diagnosis of type 1 diabetes: Diabetes Autoimmunity Study in the Young. Diabetes Care 2011;34:1397–1399 [DOI] [PMC free article] [PubMed]
- 2.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 2010;464:1293–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Z, Cort L, Eberwine R, et al. Prevention of type 1 diabetes in the rat with an allele-specific anti–T-cell receptor antibody: Vβ13 as a therapeutic target and biomarker. Diabetes 2012;61:1160–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tirabassi RS, Guberski DL, Blankenhorn EP, et al. Infection with viruses from several families triggers autoimmune diabetes in LEW*1WR1 rats: prevention of diabetes by maternal immunization. Diabetes 2010;59:110–118 [DOI] [PMC free article] [PubMed]
- 5.Londono P, Komura A, Hara N, Zipris D. Brief dexamethasone treatment during acute infection prevents virus-induced autoimmune diabetes. Clin Immunol 2010;135:401–411 [DOI] [PubMed] [Google Scholar]
- 6.Blankenhorn EP, Cort L, Greiner DL, Guberski DL, Mordes JP. Virus-induced autoimmune diabetes in the LEW.1WR1 rat requires Iddm14 and a genetic locus proximal to the major histocompatibility complex. Diabetes 2009;58:2930–2938 [DOI] [PMC free article] [PubMed]
- 7.Mordes JP, Cort L, Norowski E, et al. Analysis of the rat Iddm14 diabetes susceptibility locus in multiple rat strains: identification of a susceptibility haplotype in the Tcrb-V locus. Mamm Genome 2009;20:162–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuller JM, Bogdani M, Tupling TD, et al. Genetic dissection reveals diabetes loci proximal to the gimap5 lymphopenia gene. Physiol Genomics 2009;38:89–97 [DOI] [PMC free article] [PubMed]
- 9.Kobayashi M, Jasinski J, Liu E, et al. Conserved T cell receptor alpha-chain induces insulin autoantibodies. Proc Natl Acad Sci USA 2008;105:10090–10094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakayama M, Abiru N, Moriyama H, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 2005;435:220–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lennon GP, Bettini M, Burton AR, et al. T cell islet accumulation in type 1 diabetes is a tightly regulated, cell-autonomous event. Immunity 2009;31:643–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. N Engl J Med 2009;360:1646–1654 [DOI] [PubMed] [Google Scholar]
- 13.Leiter EH, Reifsnyder PC, Wallace R, Li R, King B, Churchill GC. NOD x 129.H2(g7) backcross delineates 129S1/SvImJ-derived genomic regions modulating type 1 diabetes development in mice. Diabetes 2009;58:1700–1703 [DOI] [PMC free article] [PubMed]
- 14.Mychaleckyj JC, Noble JA, Moonsamy PV, et al. HLA genotyping in the international Type 1 Diabetes Genetics Consortium. Clin Trials 2010;7(Suppl.):S75–S87 [DOI] [PMC free article] [PubMed]
- 15.Jarosz-Chobot P, Polanska J, Szadkowska A, et al. Rapid increase in the incidence of type 1 diabetes in Polish children from 1989 to 2004, and predictions for 2010 to 2025. Diabetologia 2011;54:508–515 [DOI] [PMC free article] [PubMed]
- 16.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 2002;347:911–920 [DOI] [PubMed] [Google Scholar]
- 17.Stadinski BD, Zhang L, Crawford F, Marrack P, Eisenbarth GS, Kappler JW. Diabetogenic T cells recognize insulin bound to IAg7 in an unexpected, weakly binding register. Proc Natl Acad Sci USA 2010;107:10978–10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crawford F, Stadinski B, Jin N, et al. Specificity and detection of insulin-reactive CD4+ T cells in type 1 diabetes in the nonobese diabetic (NOD) mouse. Proc Natl Acad Sci U S A 2011;108:16729–16734 [DOI] [PMC free article] [PubMed]
- 19.Wucherpfennig KW, Sethi D. T cell receptor recognition of self and foreign antigens in the induction of autoimmunity. Semin Immunol 2011;23:84–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michels AW, Ostrov DA, Zhang L, et al. Structure-based selection of small molecules to alter allele-specific MHC class II antigen presentation. J Immunol 2011;187:5921–5930 [DOI] [PMC free article] [PubMed]
- 21.Zhang L, Stadinski BD, Michels A, Kappler JW, Eisenbarth GS. Immunization with an insulin peptide-MHC complex to prevent type 1 diabetes of NOD mice. Diabetes Metab Res Rev 2011;27:784–789 [DOI] [PubMed] [Google Scholar]