Abstract
The mechanisms by which common genetic variation predisposes to type 2 diabetes remain unclear. The disease-associated variants in TCF7L2 (rs7903146) and WFS1 (rs10010131) have been shown to affect response to exogenous glucagon-like peptide 1 (GLP-1), while variants in KCNQ1 (rs151290, rs2237892, and rs2237895) alter endogenous GLP-1 secretion. We set out to validate these observations using a model of GLP-1–induced insulin secretion. We studied healthy individuals using a hyperglycemic clamp and GLP-1 infusion. In addition, we measured active and total GLP-1 in response to an oral challenge in nondiabetic subjects. After genotyping the relevant single nucleotide polymorphisms, generalized linear regression models and repeated-measures ANCOVA models incorporating potential confounders, such as age and BMI, were used to assess the associations, if any, of response with genotype. These variants did not alter GLP-1 concentrations in response to oral intake. No effects on β-cell responsiveness to hyperglycemia and GLP-1 infusion were apparent. Diabetes-associated variation (T allele at rs7903146) in TCF7L2 may impair the ability of hyperglycemia to suppress glucagon (45 ± 2 vs. 47 ± 2 vs. 60 ± 5 ng/L for CC, CT, and TT, respectively, P = 0.02). In nondiabetic subjects, diabetes-associated genetic variation does not alter GLP-1 concentrations after an oral challenge or its effect on insulin secretion.
Glucagon-like peptide 1 (GLP-1) is an incretin hormone secreted into the portal circulation by enteroendocrine L-cells in response to meal ingestion. It is a powerful insulin secretagogue, and compounds that inhibit its inactivation or act as agonists of the GLP-1 receptor are used to treat diabetes. In addition to its effects on insulin secretion, at least in pharmacologic concentrations, GLP-1 delays gastric emptying, decreases caloric intake, and suppresses glucagon secretion—all powerful antidiabetogenic effects (1). Although a role for defects in postprandial GLP-1 concentrations in the pathogenesis of diabetes has not been demonstrated (2), defects in the insulin secretory response to infused GLP-1 occur in prediabetes (3). It is unlikely that this represents a defect specific to GLP-1, given the blunted insulin secretory response to multiple other secretagogues such as arginine and glucose, evident early in the evolution of disease (4).
More recently, large, well-powered genetic association studies have been used to reproducibly elucidate multiple genetic variants in suspected and unsuspected disease pathways that predispose to type 2 diabetes (5). The identification of such variants, however, has posed difficulties in elucidating the mechanism by which these diabetes-associated loci predispose to disease. Some of these loci could conceivably predispose to disease by altering GLP-1 secretion or action. For example, the product of TCF7L2 had been shown to be a transcription factor regulating the synthesis of glucagon mRNA in the gut but not in the α-cell (6). This led to the suggestion that TCF7L2 predisposed to diabetes via alterations in posttranslational processing of proglucagon to GLP-1 (7).
Schäfer et al. (8) have suggested that individuals with one or more diabetes-associated alleles in this locus have an impaired insulin secretory response to GLP-1 infusion during a hyperglycemic clamp. The same group of investigators has also suggested that WFS1 alters response to infused GLP-1 (9). Moreover, KCNQ1, while not affecting GLP-1 signaling, was nominally associated with decreased GLP-1 concentrations after an oral challenge in nondiabetic subjects (10).
Dahlman et al. (11) proposed criteria to help ensure reliable results from genetic association studies, including the use of large sample sizes with appropriate statistical criteria for accepting an association (12) and replication in an independent cohort. Given the importance of GLP-1 in postprandial glucose metabolism and in the treatment of type 2 diabetes, these data deserve independent replication. We recently developed a model to quantify β-cell responsivity (ϕTotal) to GLP-1 in vivo (13) and have also measured total and active GLP-1 concentrations in nondiabetic subjects undergoing oral glucose tolerance tests (OGTTs), providing the opportunity to examine the role of genetic variation in determining GLP-1 responsiveness (14).
Accordingly, we used data from these experiments to examine whether variants in TCF7L2, KCNQ1, and WFS1 alter GLP-1 concentrations after an OGTT or GLP-1–induced insulin secretion in nondiabetic humans. Our results suggest that these variants have no effect on these parameters in a cohort of similar sample size to previously published studies. This illustrates the necessity of independent replication in appropriately powered studies prior to accepting the conclusions of genotype correlation with complex phenotypes.
RESEARCH DESIGN AND METHODS
After approval from the Mayo Institutional Review Board, a total of 242 healthy, nondiabetic subjects gave informed written consent to participate in one of the two protocols. The study during which GLP-1 was infused was registered at www.clinicaltrials.gov (identifier NCT00588380).
Experimental design—hyperglycemic clamp.
After an overnight fast at 0700 h (0 min), a primed (0.1 g/kg in 4 min), continuous infusion of 50% dextrose maintained peripheral glucose concentrations at ∼8.5 mmol/L. At 0900 h (120 min), GLP-1 (7,36) amide (Bachem, San Diego, CA) was infused (1.5 pmol/kg in 10 min, subsequently 0.75 pmol/kg/min). At 1000 h (180 min), the infusion rate was increased to 1.5 pmol/kg/min and maintained till 1100 h (240 min). Intact GLP-1 concentrations rose from 5.9 + 0.9 to 38.4 + 2.4 pmol/L 10 min after initiation of GLP-1 infusion. Concentrations plateaued at 23.4 + 1.6 pmol/L at 180 min. After 1 h infusion of GLP-1 at the higher rate of 1.5 pmol/kg/min, concentrations were 40.6 + 2.2 pmol/L at 240 min.
Experimental design—OGTT.
After an overnight fast, at 0700 h (time 0), subjects ingested a 75-g glucose drink during a period of 5 min. Blood was drawn for glucose and hormone measurement at seven time points during the study period (120 min).
Measurement of insulin secretion.
The model used to measure β-cell responsivity to glucose and exogenous GLP-1 has previously been described (13,15). It is a modification of the C-peptide minimal model that assumes a nonlinear and derivative action of GLP-1 on both the static (ϕs) and dynamic (ϕd) components of total insulin secretion (ϕTotal) (13). Insulin secretion rate (ISR) was derived from plasma C-peptide concentration by the two-compartment model of C-peptide kinetics originally proposed by Eaton et al. (16). ϕTotal at 120 min represents the mean ϕTotal observed during 110–120 min (i.e., in the presence of hyperglycemia alone), whereas ϕTotal at 180 min represents ϕTotal observed during 160–180 min (hyperglycemia plus GLP-1 infusion at 0.75 pmol/kg/min). ϕTotal at 240 min represents ϕTotal observed during 220–240 min (hyperglycemia plus GLP-1 infusion at 1.5 pmol/kg/min), and peak ϕTotal represents maximum β-cell responsivity during the last hour of the study.
Genotyping.
Genotyping of selected single nucleotide polymorphisms (SNPs) was undertaken using TaqMan (Applied Biosystems Inc., Foster City, CA).
Measurement of active and total GLP-1 concentrations.
Sample tubes used for measurement of GLP-1 had 100 μmol/L dipeptidyl peptidase 4 inhibitor (Linco Research, St. Louis, MO) added. Active GLP-1 and inactive GLP-1 in serum samples were measured off-site using the Theranos System (Theranos, Inc., Palo Alto, CA). The system is a fully automated, chemiluminescence enzyme-linked immunosorbent assay. Duplicate measurements of each analyte were made and averaged. The antibodies selected for the assay were directed to the NH2-terminal (active) and to an epitope in the middle of the peptide, respectively, so that the two forms of the analyte gave equal assay responses. For measurement of inactive GLP-1, the selected antibodies were directed to the NH2-terminal of inactive GLP-1 and to an epitope in the middle of the peptide. Total GLP-1 is reported as the sum of active and inactive GLP-1.
Statistical analysis.
The primary analyses focused on examining the potential association of insulin secretion (characterized by ϕ), glucagon response, and GLP-1 secretion during glucose challenge with specific genotypes. A general genetic model was assumed for each of the genotypes assessed. The univariate association of insulin secretion with genotype was based on the Kruskal-Wallis test. A generalized regression model (assuming a γ-distribution for ϕ and identity link function) incorporating sex, BMI, and fasting glucose as covariates was also used to assess the association of ϕ with genotype. The association of genotype with the time-oriented response profiles for glucagon, GLP-1, ISR, and glucose infusion rate (GIR) concentrations was assessed using repeated-measures ANCOVA models incorporating age, sex, and BMI as covariates. Least squares adjusted mean values, adjusted for age, sex, and BMI, are reported for these analyses. For glucagon response in the hyperglycemic clamp studies, the repeated measures corresponded to the subject-specific mean values during three time periods: basal (−30 min to 0), glucose infusion alone (10–120 min), and 130–240 min, during which GLP-1 was infused at 0.75 pmol/kg/min (160–180 min) and at 1.5 pmol/kg/min (220–240 min). In the OGTT studies, the individual glucagon concentrations (per 10 min during a 10–120-min period) were the repeated measures. For GLP-1 response, the individual concentrations from each serum sample of active and separately total GLP-1, after transforming to log scale, were assessed in the repeated-measures ANCOVA models. When examining ISR and GIR, the repeated measures corresponded to the mean values per subject during two time periods based on the experimental protocol: 160–180 min, and separately, 220–240 min. The SAS procedures (version 9.2) NPAR1WAY, GENMOD, and MIXED were used for these analyses. All reported P values are unadjusted for multiple testing.
RESULTS
Diabetes-associated polymorphisms examined for association with GLP-1 responsiveness and GLP-1 concentrations after an oral challenge.
Five SNPs that were previously associated with either insulin secretion in response to infused GLP-1 or GLP-1 concentrations in response to an oral challenge were studied (Table 1). These were the diabetes-associated polymorphisms in TCF7L2 (8,17,18) and WFS1 (9) as well as three SNPs in KCNQ1. In the case of KCNQ1, the three SNPs studied are in Intron 15 and have been associated with type 2 diabetes and GLP-1 response to an OGTT (10).
TABLE 1.
Diabetes-associated genetic variation studied in experiments
Effects of diabetes-associated genotype on β-cell responsivity in the presence of hyperglycemia and exogenous GLP-1.
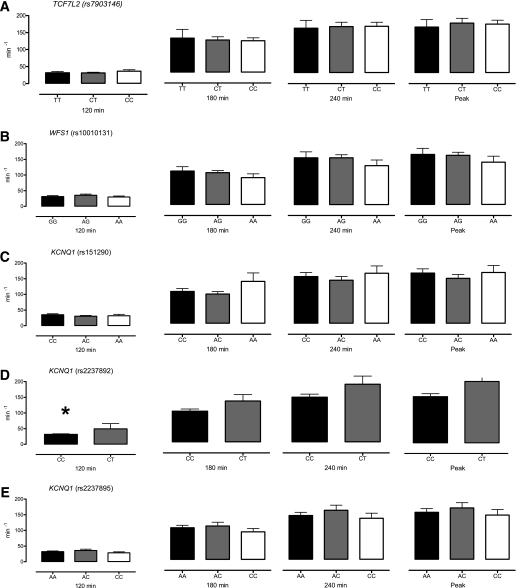
Differences in insulin secretion between genotype groups were quantified by β-cell responsivity (ϕ) measured in response to hyperglycemia (at 120 min) and to two infusion rates of GLP-1 (180 and 240 min, respectively) (Fig. 1). In addition, peak ϕ during GLP-1 infusion (peak) was also quantified. With the exception of differences associated with rs2237892 at 120 min (Fig. 1D), no significant associations were detected with genotype group. Subjects with two copies of the diabetes-associated allele at rs2237892 had lower ϕ at 120 min (32 ± 2 vs. 50 ± 17 × 10−9/min for CC vs. CT, respectively, P = 0.02, adjusting for sex, BMI, and fasting glucose). Similar patterns were observed at the other time points but were not significant (at 180 min, 92 ± 6 vs. 123 ± 19 × 10−9/min, P = 0.08; at 240 min, 134 ± 9 vs. 173 ± 24 × 10−9/min, P = 0.10; and peak response 142 ± 9 vs. 189 ± 28, P = 0.07).
FIG. 1.
A–E: Effect of genotype on β-cell responsivity indices in response to hyperglycemia (120 min), hyperglycemia and 0.75 pmol/kg/min GLP-1 (180 min), hyperglycemia and 1.5 pmol/kg/min GLP-1 (240 min), and peak response to hyperglycemia and GLP-1 infusion (Peak). *Nominal significance for a given time point.
Effects of TCF7L2, WFS1, and KCNQ1 diabetes-associated genotypes on GIR, glucose, C-peptide, ISR, and glucagon concentrations in the presence of hyperglycemia and exogenous GLP-1.
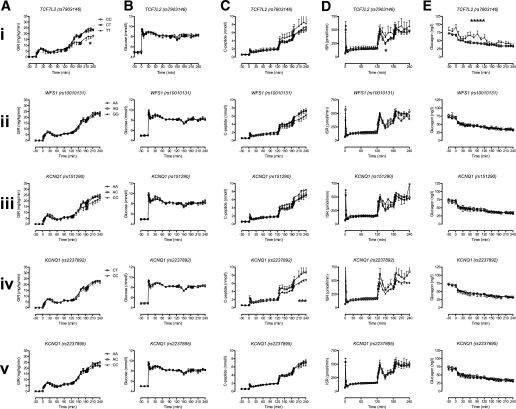
Prior to the infusion of GLP-1, GIRs did not differ significantly between groups (Fig. 2A). After initiation of GLP-1 infusion, a significant difference in GIR was observed for individuals with two copies of the diabetes-associated allele at rs7903146 (TCF7L2). The least squares adjusted mean values adjusted for age, sex, and BMI at 180 min (8.9 ± 0.4 vs. 8.7 ± 0.4 vs. 6.4 ± 1.0 mg/kg/min for CC, CT, and TT, respectively, P = 0.11) did not differ. However, at 240 min, these differences were significant (mean values adjusted for age, sex, and BMI of 12.2 ± 0.4 vs. 11.7 ± 0.4 vs. 9.0 ± 1.0 mg/kg/min for CC, CT, and TT, respectively, P = 0.02). No significant differences in GIRs were observed for other genotypes. Glucose concentrations did not differ, prior to initiation of the experiment, during hyperglycemia alone or during GLP-1 infusion between genotype groups (Fig. 2B). In a similar manner, C-peptide concentrations did not differ, prior to initiation of the experiment, during hyperglycemia alone or during GLP-1 infusion between genotype groups (Fig. 2C).
FIG. 2.
Effect of genotype on GIR (A), glucose concentrations (B), C-peptide (C), ISR (D), and glucagon concentrations (E) during hyperglycemia and GLP-1 infusion. *Nominal significance for a given time point.
Average ISR during infusion of GLP-1 at 0.75 pmol/kg/min did not differ significantly between genotype groups (Fig. 2D). In the case of KCNQ1, individuals with two copies of the diabetes-associated allele at rs2237892 had a lower ISR at 240 min, but this was not statistically significant (mean values adjusted for age, sex, and BMI of 474 ± 24 vs. 6,055 ± 72 pmol/min for CC and CT, respectively, P = 0.08) (Fig. 2D).
Basal glucagon concentrations were not significantly associated with genotype group (Fig. 2E). This was the case for TCF7L2 (least squares adjusted mean values adjusted for age, sex, and BMI of 70 ± 3 vs. 70 ± 5 vs. 83 ± 7 ng/L for CC, CT, and TT, respectively, P = 0.17). However, in the presence of hyperglycemia, the average (10–120 min) glucagon concentrations did not suppress to the same degree in subjects homozygous for the T allele (least squares adjusted mean values adjusted for age, sex, and BMI of 45 ± 2 vs. 47 ± 2 vs. 60 ± 5 ng/L for CC, CT, and TT, respectively, P = 0.02) (Fig. 2E). After initiation of GLP-1, glucagon concentrations suppressed similarly in all groups so that the average (130 to 240 min) glucagon concentrations were not significantly associated with genotype group (mean values adjusted for age, sex, and BMI of 35 ± 2 vs. 36 ± 2 vs. 41 ± 4 ng/L for CC, CT, and TT, respectively, P = 0.38).
Effects of diabetes-associated genotype on GLP-1 concentrations in response to 75-g glucose challenge.
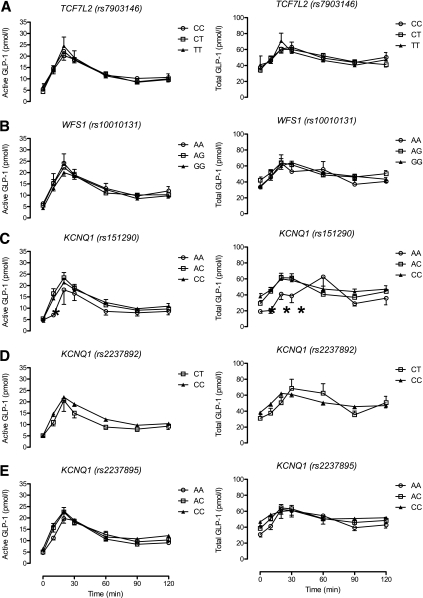
Active GLP-1 concentrations were not associated with genotype group at baseline (Fig. 3). There was a tendency for subjects homozygous for the diabetes protective allele (A) at rs151290 (KCNQ1) to have lower active GLP-1 concentrations at 10 min after initiation of glucose challenge (geometric mean values adjusted for age, sex, and BMI of 6.6 vs. 12.1 vs. 11.0 pmol/L for AA, AC, and CC, respectively, P = 0.005). No other differences in active GLP-1 concentrations after glucose challenge were apparent between genotype groups.
FIG. 3.
A–E: Effect of genotype on active GLP-1 concentrations and total GLP-1 concentrations in response to a 75-g OGTT. *Nominal significance for a given time point.
In a similar manner, concentrations of total GLP-1 at 10 min after initiation of glucose challenge were associated with genotype and were lower in people with the AA genotype at rs151290 (geometric mean values adjusted for age, sex, and BMI of 22.6 vs. 46.2 vs. 43.7 pmol/L for AA, AC, and CC, respectively, P = 0.01). These differences continued to be apparent for the first 30 min after glucose ingestion but did not reach statistical significance after adjusting for covariates. No other significant associations of total GLP-1 concentrations with genotype were apparent.
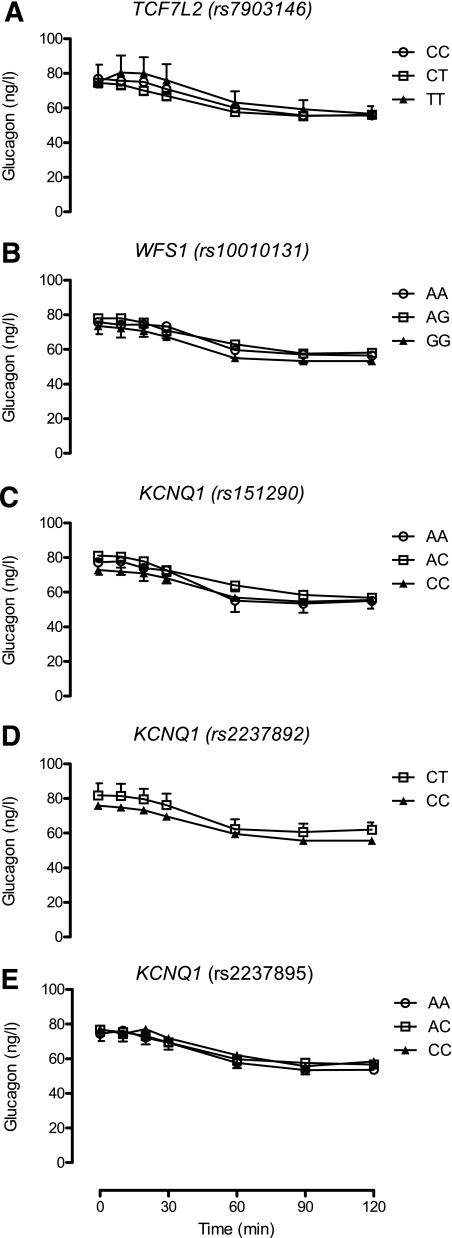
Effects of diabetes-associated genotype on glucagon concentrations in response to 75-g glucose challenge.
With the exception of the rs2237892 SNP in KCNQ1, there was no apparent evidence of association of glucagon concentrations with genotype (Fig. 4). In the case of this SNP, glucagon concentrations at 10 min (mean values adjusted for age, sex, and BMI of 80 ± 5 vs. 75 ± 2 ng/L for CT and CC, respectively) were not significantly lower (P = 0.34) in subjects with two copies of the diabetes-associated allele.
FIG. 4.
A–E: Effect of genotype on glucagon concentrations during a 75-g OGTT.
DISCUSSION
The results of these experiments contrast with prior (similarly powered) reports that TCF7L2 and WFS1 alter β-cell responsiveness to infused GLP-1 and that variants in KCNQ1 alter GLP-1 concentrations after an oral challenge in nondiabetic humans. While far from conclusive, these results highlight the importance of independent replication prior to concluding that a given genotype has a particular effect on a complex phenotype.
Although TCF7L2 regulates proglucagon expression in enteroendocrine cells, diabetes-associated variation in this locus has not been previously associated with a defective rise in GLP-1 concentrations after oral challenge (18). In the same study, 81 healthy male subjects with the diabetes-associated allele of TCF7L2 (at rs7903146) had an impaired insulin secretory response to infused GLP-1 and decreased glucagon secretion during a 24 h period (18). Although this is congruent with the Schäfer et al. (8) study, it differs from our observation that after adjusting for age and sex, no differences in GLP-1–induced changes in β-cell responsiveness were attributable to TCF7L2.
The decrease in TCF7L2 expression observed in the islets of diabetic rats correlates with the downregulation of incretin receptors and decline in islet function (19), thereby suggesting a mechanism by which TCF7L2 might alter β-cell responsiveness to GLP-1 in diabetes. However, this would not necessarily explain why GLP-1 responsiveness is altered in the nondiabetic state. Decreased responsiveness to secretagogues, such as glucose and GLP-1, are hallmarks of the diabetic state and are also present to a lesser degree in people with prediabetes (3). It is interesting to note that in the case of TCF7L2, differences in GIRs were noted, with lower rates observed in subjects with two copies of the (diabetes-associated) T allele at rs7903146. This suggests that these individuals may have decreased glucose clearance under these particular experimental conditions. GLP-1 is not thought to have significant effects on insulin action (the ability of insulin to stimulate glucose uptake and suppress glucose release). Some investigators have suggested that TCF7L2 may adversely affect this parameter (20,21). In this experiment, at identical glucose concentrations, C-peptide concentrations and ISR did not differ significantly by TCF7L2 genotype, although GIR was lower, suggesting an effect on insulin action. In contrast, Pilgaard et al. (18) also reported a negative effect of this allele on insulin-induced suppression of endogenous glucose production. Such differences were not explainable by age, weight, or body composition in our cohort.
Individuals with two copies of the T allele at rs7903146 (TCF7L2) exhibited higher fasting concentrations of glucagon, together with impaired suppression of glucagon by hyperglycemia alone. GLP-1 in the presence of hyperglycemia suppressed glucagon regardless of genotype, suggesting that α-cell responsiveness to GLP-1 is unaffected by TCF7L2 genotype. There was no difference in fasting and postchallenge glucagon concentrations after an OGTT attributable to TCF7L2 genotype as previously reported (17) and in direct contrast to the (paradoxical) suggestion that the diabetes-associated allele of rs7903146 decreases glucagon secretion (18).
WFS1 encodes a transmembrane protein wolframin, located primarily in the endoplasmic reticulum and ubiquitously expressed in the brain, heart, and β-cell. The function of this transmembrane protein is incompletely understood. Mutations in this gene cause the Mendelian disorder (autosomal recessive) Wolfram syndrome (WFS; Online Mendelian Inheritance in Man 222300), which is characterized by diabetes as well as deafness and optic atrophy. The contribution of wolframin to the pathogenesis of diabetes may be mediated via regulation of endoplasmic reticulum stress signaling (22). Its role in GLP-1 signaling, if any, is unclear, although in animal models, GLP-1 modulates endoplasmic reticulum stress, preventing apoptosis and β-cell survival (23). There was no evidence of an effect of rs10010131 in WFS1 on insulin secretion in response to GLP-1 infusion or GLP-1 concentrations in response to a glucose challenge in this series of experiments.
KCNQ1 encodes the α-subunit of the slow inward rectifying potassium channel required for the repolarization phase of cardiac action potentials. Mutations in this gene are associated with hereditary long QT syndromes, and the presence of diabetes-associated variants is associated with decreased insulin secretion in vivo. Although KCNQ1 is expressed in the gastrointestinal epithelium, and is necessary for electrolyte uptake, a physiologic role in the regulation of GLP-1 secretion or action is unclear (24). Despite there being plausible biological reasons to hypothesize that KCNQ1 may alter GLP-1 concentrations in response to a glucose challenge, this was not evident in this particular dataset. If anything, decreased active and total GLP-1 concentrations after glucose challenge associated with variation at rs151290 in KCNQ1 was observed in subjects with two copies of the allele protective against type 2 diabetes—precisely the inverse of the results that have been reported by Müssig et al. (10). Potassium channels may play a role in incretin secretion (25), but the significance of these findings remains uncertain and will require validation in a larger, independent cohort.
Studies aimed at elucidating the function of diabetes-associated variation are a necessary next step in understanding the pathogenesis of type 2 diabetes. It is necessary in such situations to be aware of the power of a given study to detect a difference in quantitative trait(s) based on genotype. Such power depends on the frequency of the disease-associated allele, the effect of such variants on the traits being measured, and the precision with which such traits can be measured (26). The previously cited reports suggesting an effect of TCF7L2 and WFS1 on insulin secretion in response to GLP-1 infusion were powered to detect a 50% change in GLP-1 responsiveness attributable to genotype and GLP-1 secretion. A post hoc analysis of our dataset suggests that we had 80% power (two-sample t test, two-sided α = 0.05) to detect a 35% difference in ϕ during GLP-1 infusion and a 25% difference in total GLP-1 secretion after OGTT. While it is certainly possible that these variants have smaller effects on GLP-1–induced insulin secretion and on postprandial GLP-1 concentrations, in this experiment, the inability to detect an association with GLP-1 concentrations or with GLP-1–stimulated insulin secretion was not a function of an underpowered study compared with the earlier reports of association. It is possible that the use of an OGTT rather than a mixed meal may have obscured a genotype-associated difference in enteroendocrine secretion. However, there is no a priori data to suggest such an interaction. Moreover, there is no evidence of a qualitative difference between the GLP-1 response to a mixed meal or to an OGTT in a given subject (27).
Future studies aiming to elucidate the contribution of diabetes-associated genetic variation to GLP-1 secretion and action will need to take into account the variability of the quantitative trait being measured and account for the likely small effects of these variants on the quantitative trait(s) to ensure that they are adequately powered to reproducibly determine such effects.
ACKNOWLEDGMENTS
This study was supported by Mayo Center for Translational Science Activities Grant RR-24150 and Minnesota Obesity Center Grant DK-50456. A.S. was supported in part by a grant from the Endocrine Fellows Foundation. A.V. was supported by DK-78646.
Genotyping and assay costs were partly defrayed by an investigator-initiated grant funded by Merck to A.V. A.V. has received research grants from Merck and has consulted for sanofi-aventis and Bristol-Myer Squibb. No other potential conflicts of interest relevant to this article were reported.
G.S., M.S., A.S., and F.M. researched data and reviewed and edited the manuscript. C.D.M. researched data, contributed to discussion, and reviewed and edited the manuscript. A.R.Z. performed statistical analysis and reviewed and edited the manuscript. C.C. contributed to discussion and reviewed and edited manuscript. A.V. researched data and wrote the manuscript. A.V. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Monica M. Davis, Mayo Clinic, for help with preparing the manuscript.
REFERENCES
- 1.Drucker DJ. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol 2003;17:161–171 [DOI] [PubMed] [Google Scholar]
- 2.Nauck MA, Vardarli I, Deacon CF, Holst JJ, Meier JJ. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia 2011;54:10–18 [DOI] [PubMed] [Google Scholar]
- 3.Fritsche A, Stefan N, Hardt E, Häring H, Stumvoll M. Characterisation of beta-cell dysfunction of impaired glucose tolerance: evidence for impairment of incretin-induced insulin secretion. Diabetologia 2000;43:852–858 [DOI] [PubMed] [Google Scholar]
- 4.Chen M, Bergman RN, Pacini G, Porte D., Jr Pathogenesis of age-related glucose intolerance in man: insulin resistance and decreased beta-cell function. J Clin Endocrinol Metab 1985;60:13–20 [DOI] [PubMed] [Google Scholar]
- 5.Smushkin G, Vella A. Genetics of type 2 diabetes. Curr Opin Clin Nutr Metab Care 2010;13:471–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yi F, Brubaker PL, Jin T. TCF-4 mediates cell type-specific regulation of proglucagon gene expression by beta-catenin and glycogen synthase kinase-3beta. J Biol Chem 2005;280:1457–1464 [DOI] [PubMed] [Google Scholar]
- 7.Grant SF, Thorleifsson G, Reynisdottir I, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet 2006;38:320–323 [DOI] [PubMed] [Google Scholar]
- 8.Schäfer SA, Tschritter O, Machicao F, et al. Impaired glucagon-like peptide-1-induced insulin secretion in carriers of transcription factor 7-like 2 (TCF7L2) gene polymorphisms [corrected in: Diabetologia 2008;51:208]. Diabetologia 2007;50:2443–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schäfer SA, Müssig K, Staiger H, et al. A common genetic variant in WFS1 determines impaired glucagon-like peptide-1-induced insulin secretion. Diabetologia 2009;52:1075–1082 [DOI] [PubMed] [Google Scholar]
- 10.Müssig K, Staiger H, Machicao F, et al. Association of type 2 diabetes candidate polymorphisms in KCNQ1 with incretin and insulin secretion. Diabetes 2009;58:1715–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahlman I, Eaves IA, Kosoy R, et al. Parameters for reliable results in genetic association studies in common disease. Nat Genet 2002;30:149–150 [DOI] [PubMed] [Google Scholar]
- 12.Thomas DC, Clayton DG. Betting odds and genetic associations. J Natl Cancer Inst 2004;96:421–423 [DOI] [PubMed] [Google Scholar]
- 13.Dalla Man C, Micheletto F, Sathananthan A, Rizza RA, Vella A, Cobelli C. A model of GLP-1 action on insulin secretion in nondiabetic subjects. Am J Physiol Endocrinol Metab 2010;298:E1115–E1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sathananthan A, DallaMan C, Zinsmeister AR, et al. A concerted decline in insulin secretion and action occurs across the spectrum of fasting and postchallenge glucose concentrations. Clin Endocrinol (Oxf) 2012;76:212–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes 2001;50:150–158 [DOI] [PubMed] [Google Scholar]
- 16.Eaton RP, Allen RC, Schade DS, Erickson KM, Standefer J. Prehepatic insulin production in man: kinetic analysis using peripheral connecting peptide behavior. J Clin Endocrinol Metab 1980;51:520–528 [DOI] [PubMed] [Google Scholar]
- 17.Lyssenko V, Lupi R, Marchetti P, et al. Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest 2007;117:2155–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilgaard K, Jensen CB, Schou JH, et al. The T allele of rs7903146 TCF7L2 is associated with impaired insulinotropic action of incretin hormones, reduced 24 h profiles of plasma insulin and glucagon, and increased hepatic glucose production in young healthy men. Diabetologia 2009;52:1298–1307 [DOI] [PubMed] [Google Scholar]
- 19.Shu L, Matveyenko AV, Kerr-Conte J, Cho JH, McIntosh CH, Maedler K. Decreased TCF7L2 protein levels in type 2 diabetes mellitus correlate with downregulation of GIP- and GLP-1 receptors and impaired beta-cell function. Hum Mol Genet 2009;18:2388–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elbein SC, Chu WS, Das SK, et al. Transcription factor 7-like 2 polymorphisms and type 2 diabetes, glucose homeostasis traits and gene expression in US participants of European and African descent. Diabetologia 2007;50:1621–1630 [DOI] [PubMed] [Google Scholar]
- 21.Liu PH, Chang YC, Jiang YD, et al. Genetic variants of TCF7L2 are associated with insulin resistance and related metabolic phenotypes in Taiwanese adolescents and Caucasian young adults. J Clin Endocrinol Metab 2009;94:3575–3582 [DOI] [PubMed] [Google Scholar]
- 22.Fonseca SG, Ishigaki S, Oslowski CM, et al. Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. J Clin Invest 2010;120:744–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yusta B, Baggio LL, Estall JL, et al. GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab 2006;4:391–406 [DOI] [PubMed] [Google Scholar]
- 24.Vallon V, Grahammer F, Volkl H, et al. KCNQ1-dependent transport in renal and gastrointestinal epithelia. Proc Natl Acad Sci U S A 2005;102:17864–17869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gribble FM, Williams L, Simpson AK, Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes 2003;52:1147–1154 [DOI] [PubMed] [Google Scholar]
- 26.Wang WY, Barratt BJ, Clayton DG, Todd JA. Genome-wide association studies: theoretical and practical concerns. Nat Rev Genet 2005;6:109–118 [DOI] [PubMed] [Google Scholar]
- 27.Vollmer K, Holst JJ, Baller B, et al. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes 2008;57:678–687 [DOI] [PubMed] [Google Scholar]