Abstract
Hepatocyte growth factor (HGF) is a mitogen and insulinotropic agent for the β-cell. However, whether HGF/c-Met has a role in maternal β-cell adaptation during pregnancy is unknown. To address this issue, we characterized glucose and β-cell homeostasis in pregnant mice lacking c-Met in the pancreas (PancMet KO mice). Circulating HGF and islet c-Met and HGF expression were increased in pregnant mice. Importantly, PancMet KO mice displayed decreased β-cell replication and increased β-cell apoptosis at gestational day (GD)15. The decreased β-cell replication was associated with reductions in islet prolactin receptor levels, STAT5 nuclear localization and forkhead box M1 mRNA, and upregulation of p27. Furthermore, PancMet KO mouse β-cells were more sensitive to dexamethasone-induced cytotoxicity, whereas HGF protected human β-cells against dexamethasone in vitro. These detrimental alterations in β-cell proliferation and death led to incomplete maternal β-cell mass expansion in PancMet KO mice at GD19 and early postpartum periods. The decreased β-cell mass was accompanied by increased blood glucose, decreased plasma insulin, and impaired glucose tolerance. PancMet KO mouse islets failed to upregulate GLUT2 and pancreatic duodenal homeobox-1 mRNA, insulin content, and glucose-stimulated insulin secretion during gestation. These studies indicate that HGF/c-Met signaling is essential for maternal β-cell adaptation during pregnancy and that its absence/attenuation leads to gestational diabetes mellitus.
β-Cell expansion and enhanced insulin secretion constitute the maternal adaptive response to the increased insulin demand during pregnancy (1,2). Failure to accomplish this adaptation leads to gestational diabetes mellitus (GDM) (3,4). GDM affects ∼135,000 pregnancies annually in the U.S. and greatly increases the risk of developing diabetes later in life (3,5–7). Additional evidence suggests potential trans-generational effects of GDM on progeny leading to offspring with a higher risk of developing obesity and diabetes (4,8–10). Therefore, identifying molecular cues that control β-cell expansion and function during pregnancy is of great importance for future approaches to prevent and treat patients at risk for, or already presenting with, diabetes.
Hepatocyte growth factor (HGF) is a mitogenic, antiapoptotic, and insulinotropic agent for the β-cell (11–15). Importantly, circulating HGF is markedly increased during pregnancy in humans (16). Furthermore, HGF expression is upregulated in rat islet endothelium at gestational day (GD)15, when maximal β-cell proliferation is detected (17). Since the HGF receptor, c-Met, is expressed in the β-cell and HGF is a growth, prosurvival, and insulinotropic agent for the β-cell, circulating and/or locally secreted HGF may participate in the maternal β-cell adaptation during pregnancy. However, no attempt has been made to decipher the role of HGF/c-Met in maternal β-cells during pregnancy.
To address this issue, we generated conditional KO mice lacking c-Met in the pancreas (PancMet KO mice) (15,18,19). Adult PancMet KO mice display normal glucose and β-cell homeostasis under basal physiologic conditions (15). Importantly, however, our current studies indicate that the absence of c-Met in the pancreas of PancMet KO mice during pregnancy results in decreased β-cell proliferation, increased β-cell death, and reduced β-cell mass. In addition, the limited maternal β-cell plasticity together with the lack of adaptive upregulation of GLUT2 and pancreatic duodenal homeobox (Pdx)-1 mRNA, islet insulin content, and glucose-stimulated insulin secretion (GSIS) leads to hypoinsulinemia, higher blood glucose, and impaired glucose tolerance, hallmarks of GDM, in PancMet KO mice. This study provides the first direct evidence indicating an important role for HGF in maternal β-cell adaptation during pregnancy.
RESEARCH DESIGN AND METHODS
Generation of c-Met conditional knockout mice in the pancreas.
PancMet KO mice were generated by crossing mice homozygous for the floxed c-Met allele (18) with Pdx1-Cre transgenic mice (19) as previously described (15). Eight- to ten-week-old female c-Metlox/lox;Pdx1-Cre (PancMet KO) mice and their wild-type littermates c-Metlox/lox without Pdx1-Cre transgene were used in these studies. Cre expression driven by the Pdx-1 promoter does not lead to detrimental effects in β-cells of adult mice (15,20,21). Pregnant PancMet KO and wild-type mice were generated by timed mating. Nonpregnant female littermates were used as controls. All the studies were performed with the approval of, and in accordance with, guidelines established by the University of Pittsburgh Institutional Animal Care and Use Committee.
Glucose homeostasis.
Blood obtained by retroorbital bleed was analyzed for glucose by a portable glucometer (Medisense, Bedford, MA) and plasma insulin by radioimmunoassay (Linco Research, St. Louis, MO) (11). Intraperitoneal glucose tolerance test was performed in mice fasted 16–18 h injected with 2 g glucose/kg body wt i.p., and insulin tolerance test was performed in random-fed mice injected with 1.5 units human insulin/kg body wt i.p. (Humulin; Lilly, Indianapolis, IN) (12).
GSIS and islet insulin content.
Insulin release from 10 islet equivalents (IEs) (1 IE = 125 μm diameter) was measured after incubation in 5 or 20 mmol/L glucose for 30 min (12). Insulin secretion results are expressed as percentage of total islet insulin content. Insulin content was measured in islets homogenized in 0.18 mol/L HCl in 70% ethanol and extracted at −20°C for 24 h. Tubes were centrifuged at 2,500 rpm for 10 min at 4°C, and the supernatant was stored at −20°C until insulin determination by radioimmunoassay (11).
Immunohistochemistry, histomorphometry and β-cell size, apoptosis, and 5-bromo-2-deoxyuridine incorporation.
Paraffin-embedded pancreatic sections were immunostained for insulin and 5-bromo-2-deoxyuridine (BrdU) (Amersham, Piscataway, NJ) as previously described (11,14). β-Cell mass was measured in three insulin-stained pancreas sections per mouse using ImageJ software (National Institutes of Health, Bethesda, MD) (11,14). Islet number was quantified manually in these three insulin-stained sections in a blinded fashion using an Olympus CH2 upright microscope with an intraocular calibrated grid (22).
BrdU incorporation in β-cells was measured in pancreas sections of mice injected intraperitoneally with BrdU, killed 6 h later, and stained for insulin and BrdU (11). β-Cell death was determined in pancreas sections stained for insulin and using the terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) method (Promega, Madison, WI) (15). Images of pancreatic islets were obtained in a Confocal Olympus Fluoview 1000 (Center for Biological Imaging, University of Pittsburgh). β-Cell size was determined as previously described (22). Immunostainings for STAT5 (Cell Signaling, Danvers, MA) and p27 (BD Pharmingen, San Jose, CA) were performed using methods previously described (15).
Islet isolation and real-time PCR.
Mouse islets were isolated following injection of collagenase P through the pancreatic duct as previously described (11). Human islets were provided by the Integrated Islet Distribution Program and Juvenile Diabetes Research Foundation Basic Science Islet Distribution programs. Analysis of c-Met, forkhead box M1 (FoxM1), insulin, GLUT2, glucokinase, and Pdx-1 mRNA expression was performed by real-time PCR using specific primers (primer sequences available on request) (15).
Circulating HGF levels.
HGF levels were measured in serum obtained from pregnant and nonpregnant wild-type mice using ELISA (Abcam, Cambridge, MA) and following the manufacturer’s instructions.
Western blot analysis.
Mouse islet extracts were separated on 7.5–12% SDS-PAGE, transferred to an Immobilon-P membrane (Millipore, Bedford, MA), blocked in 5% nonfat dry milk, and then incubated with primary antibodies against c-Met and prolactin (PRL) receptor (PRLR) (Santa Cruz Biotechnology, Santa Cruz, CA), p27 (BD Pharmingen), tubulin (Calbiochem, La Jolla, CA), and HGF (R&D Systems, Minneapolis, MN). After several washes, blots were incubated with peroxidase-conjugated secondary antibodies followed by chemiluminescence detection (Amersham) (15).
Islet cell cultures and determination of β-cell death.
Mouse and human islet cells were cultured as previously described (14) and incubated with different doses of dexamethasone with or without HGF for 24 h and then fixed in 2% paraformaldehyde. β-Cell death was determined by TUNEL assay and insulin and DAPI staining. At least 2,000 β-cells were counted per well. Images were obtained in the Confocal Olympus Fluoview 1000 microscope.
Statistical analysis.
Data are presented as means ± SE. Statistical analysis was performed using unpaired two-tailed Student t test. P < 0.05 was considered statistically significant.
RESULTS
Upregulation of HGF and c-Met during pregnancy.
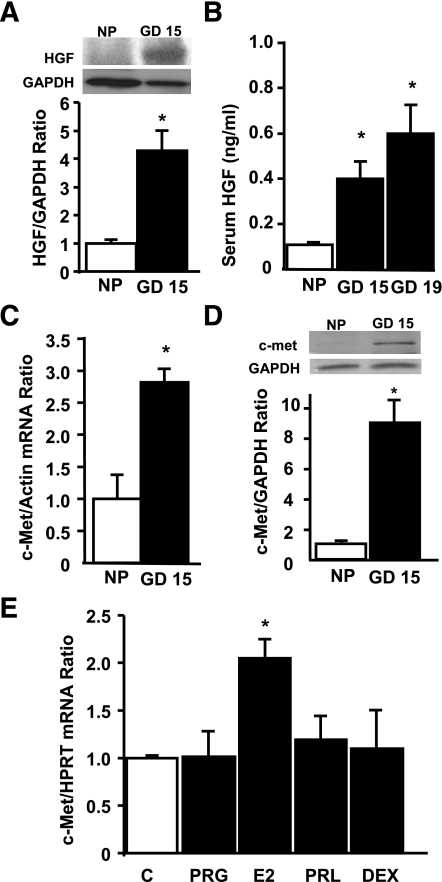
Islets from wild-type pregnant mice displayed significant HGF upregulation at GD15 (Fig. 1A). In addition, circulating HGF was also increased in pregnant wild-type mice at GD15 and -19 (Fig. 1B). The increase in systemic and islet HGF correlated with significant upregulation of islet c-Met mRNA and protein at GD15 (Fig. 1C and D), when maximal maternal β-cell proliferation occurs in rodents (23–27). To analyze whether pregnancy hormones regulate c-Met in islets, we determined the effect of 17β-estradiol, PRL, progesterone, and the synthetic glucocorticoid dexamethasone on islet c-Met mRNA levels by real-time PCR. As shown in Fig. 1E, 17β-estradiol significantly increased islet c-Met mRNA, suggesting a potential role for this hormone in potentiating HGF/c-Met signaling in islets during pregnancy. Taken together, these results indicate that both components of the HGF–c-Met axis are upregulated in the islet during pregnancy and raise the question of whether local or systemic HGF or both have a role in maternal β-cell adaptation during pregnancy.
FIG. 1.
Analysis of HGF and c-Met expression in islets from pregnant mice at GD15. A: Western blot analysis of HGF expression in protein extracts of islets isolated from nonpregnant (NP) and pregnant female wild-type mice at GD15. Results are means ± SEM of nonpregnant (n = 3) and pregnant (n = 3) mice. *P < 0.05 vs. nonpregnant mouse islets. B: Serum HGF levels in nonpregnant or pregnant female wild-type mice at GD15 and -19 measured by ELISA. Results are means ± SEM of nonpregnant (n = 3) and pregnant (n = 5) mice. *P < 0.05 vs. nonpregnant mice. Real-time PCR analysis of c-Met mRNA expression in total RNA (C) and Western blot analysis of c-Met expression in protein extracts from islets obtained from nonpregnant and pregnant wild-type mice at GD15 (D). Results are means ± SEM of nonpregnant (n = 3) and pregnant (n = 3) mice. *P < 0.05 vs. nonpregnant mouse islets. E: Real-time PCR analysis of c-Met mRNA expression in total RNA extracted from islets treated in vitro for 24 h with 50 nmol/L progesterone (PRG), 10 nmol/L 17β-estradiol (E2), 50 ng/mL PRL, or 200 nmol/L dexamethasone (DEX). Results are means ± SEM of n = 4–8 samples analyzed per condition. *P < 0.05 vs. untreated (C) mouse islets. HPRT, hypoxanthine-guanine phosphoribosyltransferase.
HGF/c-Met signaling is required for maternal β-cell expansion during pregnancy.
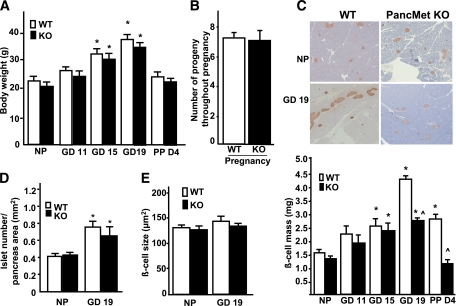
Adult PancMet KO mice display normal glucose homeostasis and β-cell mass and proliferation under basal conditions (15). To address whether HGF/c-Met signaling is important for maternal β-cell expansion during pregnancy, we analyzed β-cell mass in 8- to 10-week-old pregnant and nonpregnant female PancMet KO mice and wild-type littermates. Body weight and the average number of progeny throughout pregnancy were not significantly different between both types of mice (Fig. 2A and B). β-Cell mass was also similar at GD11 and -15 and in nonpregnant conditions (Fig. 2C). In contrast, PancMet KO mice displayed significantly decreased β-cell mass at GD19, when maximal β-cell mass expansion occurs in normal pregnant mice (23–27) (Fig. 2C). This decrease in β-cell mass persisted at postpartum day (PPD)4 (Fig. 2C). Since recent studies indicate that islet number is increased in pregnant mice (27) and PancMet KO mice display decreased β-cell mass at GD19, we quantified this parameter in both types of pregnant mice. No alteration in islet number was observed between wild-type and PancMet KO mice at GD19 (Fig. 2D), suggesting that a decrease in islet size rather than total islet number might be responsible for the decrease in β-cell mass in pregnant PancMet KO mice.
FIG. 2.
Incomplete β-cell mass expansion in pregnant PancMet KO mice. A: Body weight at different gestational days in pregnant, PPD4, and nonpregnant (NP) wild-type (WT) and PancMet KO mice. Results are means ± SEM of nonpregnant (n = 7) and pregnant (n = 4–8) mice. *P < 0.05 vs. nonpregnant mice of the same genotype. B: Number of litters throughout pregnancy in wild-type (n = 24) and PancMet KO (n = 20) mice. No significant differences were found. C: Representative photomicrographs (top panel) of nonpregnant and pregnant (GD19) wild-type and PancMet KO mouse pancreatic sections stained for insulin (brown) and counterstained with hematoxylin-eosin. Quantification of β-cell mass (bottom panel) in pregnant, PPD4, and nonpregnant wild-type (n = 4–8) and PancMet KO (n = 4–7) mice. Results are means ± SEM. *P < 0.05 vs. nonpregnant mice of corresponding genotype; ^P < 0.05 vs. wild type at the same GD or PPD4. D and E: Islet number per pancreatic area (D) and β-cell size in nonpregnant and GD19 pregnant wild-type (n = 6–8) and PancMet KO (n = 5–7) mice (E). Results are means ± SEM. *P < 0.05 vs. nonpregnant mice of corresponding genotype. (A high-quality color representation of this figure is available in the online issue.)
HGF/c-Met signaling is required for maternal β-cell proliferation during pregnancy.
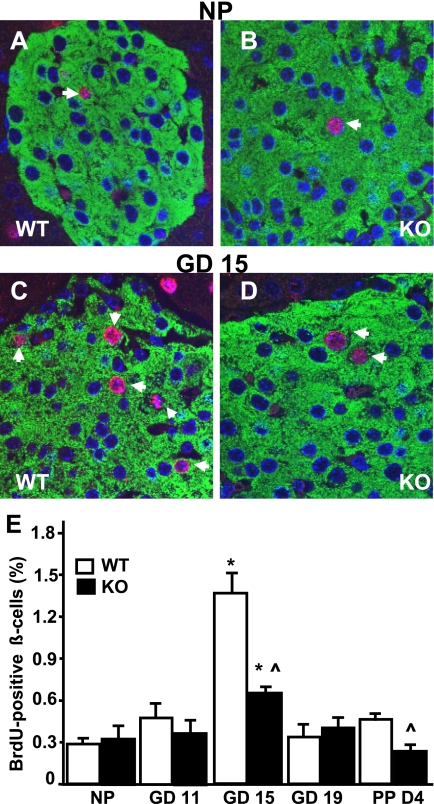
We then explored whether alterations in β-cell size, proliferation, and apoptosis contributed to the impaired β-cell expansion observed in pregnant PancMet KO mice. At GD19, β-cell size was similar in PancMet KO and wild-type mice (Fig. 2E), indicating that a change in β-cell size was not responsible for the incomplete β-cell mass expansion observed in PancMet KO mice at this gestational age. β-Cell proliferation was not significantly different in nonpregnant or pregnant PancMet KO and wild-type littermates at GD11 and -19 (Fig. 3E). However, at GD15, when maximal β-cell proliferation occurs in mice (23–27), PancMet KO mice showed a marked decrease in β-cell replication compared with wild-type mice (Fig. 3A–E). These results indicate that HGF/c-Met signaling in the β-cell is required for adaptive β-cell replication and mass expansion during pregnancy. In addition, β-cell replication was also significantly decreased in PancMet KO mice at PPD4 (Fig. 3E), when β-cell mass was decreased in these mice (Fig. 2C).
FIG. 3.
β-Cell proliferation in pregnant PancMet KO mice. A–D: Representative photomicrographs of nonpregnant (NP) (A and B) and pregnant (C and D) (at GD15) wild-type (WT) and PancMet KO mouse pancreatic sections stained for insulin (green) and BrdU (red). Arrows indicate BrdU-positive β-cell nuclei. E: Quantification of the percentage of BrdU-positive β-cells in pregnant, PPD4, and nonpregnant wild-type (n = 4–8) and PancMet KO (n = 4–7) mice. Results are means ± SEM. *P < 0.05 vs. nonpregnant mice of corresponding genotype; ^P < 0.05 vs. wild type at the same GD or PPD4. (A high-quality digital representation of this figure is available in the online issue.)
HGF/c-Met signaling modulates PRLR levels in islets during pregnancy.
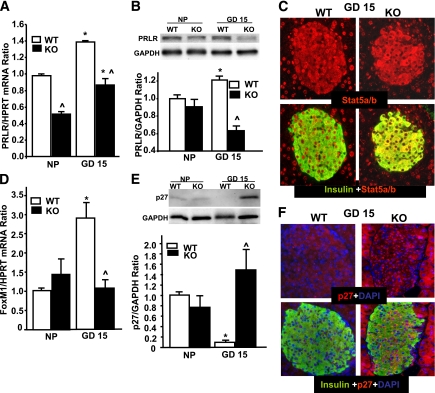
Placental lactogen (PL)/PRL signaling through the PRLR is required for the maternal adaptive increase in β-cell proliferation during pregnancy (1,26). Since pregnant PancMet KO mice displayed decreased β-cell proliferation at GD15, we measured PRLR levels in islets from wild-type and PancMet KO mice at this gestational day. Islet PRLR mRNA was significantly downregulated both in nonpregnant and pregnant PancMet KO mice (Fig. 4A). Importantly, Western blot analysis also showed a decrease in PRLR in PancMet KO mouse islets at GD15, while the levels in nonpregnant mice were not significantly different (Fig. 4B). PRL increases β-cell proliferation through phosphorylation/activation and enhanced nuclear localization of STAT5 (28). Insulin and STAT5 coimmunostaining in pancreas sections from wild-type mice at GD15 clearly showed nuclear localization of this transcription factor in β-cells (Fig. 4C). However, PancMet KO mouse islets at GD15 displayed almost exclusive cytoplasmic STAT5 staining in β-cells (Fig. 4C), suggesting that the decrease in PRLR in pregnant PancMet KO mouse islets could result in diminished islet PL/PRL signaling and reduced STAT5 activation, leading to decreased maternal β-cell proliferation. The transcription factor FoxM1 is a PRL cell cycle target that regulates maternal β-cell expansion during pregnancy (24). Importantly, PancMet KO mouse islets at GD15 failed to upregulate FoxM1 mRNA expression compared with wild-type mouse islets (Fig. 4D). Furthermore, downregulation of the member of the Cip/Kip family of cyclin-dependent kinases inhibitors, p27, is associated with FoxM1 upregulation and enhanced β-cell proliferation during pregnancy (23,24). As previously shown, islets from wild-type mice at GD15 displayed significantly decreased p27 compared with nonpregnant mice (Fig. 4E) (23,24). This decrease was not observed in PancMet KO mice at GD15 (Fig. 4E). Insulin and p27 coimmunostaining of pancreatic sections from wild-type and PancMet KO mice at GD15 showed increased p27 expression in β-cells of PancMet KO mice compared with wild-type littermates (Fig. 4F). Collectively, these results indicate that proliferation, PRLR levels, STAT5 nuclear localization, and cell cycle regulation in β-cells during pregnancy require HGF/c-Met signaling.
FIG. 4.
Pregnant PancMet KO mouse islets displayed altered levels and/or cellular localization of markers involved in cell cycle progression in β-cells. A: Real-Time PCR analysis of PRLR mRNA expression in total islet RNA from islets isolated from nonpregnant (NP) and pregnant (GD15) wild-type (WT) (n = 3–4) and PancMet KO (n = 4–5) mice. Results are means ± SEM. *P < 0.05 vs. nonpregnant mouse islets of their corresponding genotype; ^P < 0.05 vs. wild type at the same GD. HPRT, hypoxanthine-guanine phosphoribosyltransferase. B: Western blot of PRLR in islet protein extracts from nonpregnant and pregnant (GD15) wild-type (n = 4) and PancMet KO (n = 4) mice. Results are means ± SEM. ^P < 0.05 vs. wild type at the same GD. C: Representative photomicrographs of pregnant (GD15) wild-type and PancMet KO mouse pancreatic sections stained for insulin (green) and STAT5 (red). Notice the nuclear localization of STAT5 in the wild-type islet that is absent in the PancMet KO islet. D: Real-time PCR analysis of FoxM1 mRNA expression in total islet RNA from islets isolated from nonpregnant and pregnant (GD15) wild-type (n = 3–4) and PancMet KO (n = 4–5) mice. Results are means ± SEM. *P < 0.05 vs. nonpregnant mouse islets of their corresponding genotype; ^P < 0.05 vs. wild type at the same GD. E: Western blot analysis of p27 expression in islet protein extracts from nonpregnant and pregnant (GD15) wild-type (n = 4–5) and PancMet KO (n = 4–5) mice. Results are means ± SEM. *P < 0.05 vs. nonpregnant mouse islets of their corresponding genotype; ^P < 0.05 vs. wild type at the same GD. F: Representative photomicrographs of pregnant (GD15) wild-type and PancMet KO mouse pancreatic sections stained for insulin (green) and p27 (red). Notice the increased intensity of staining of p27 in the PancMet KO islet. (A high-quality digital representation of this figure is available in the online issue.)
Deletion of pancreatic HGF/c-Met signaling causes premature maternal β-cell apoptosis.
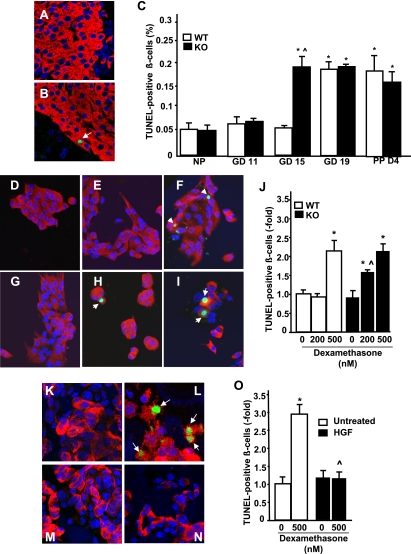
β-Cell death occurs at the end of gestation and the early postpartum period to normalize the expanded maternal β-cell mass (1,29). Based on our previous observations that HGF/c-Met signaling deficiency in β-cells leads to enhanced sensitivity to cell death induced by streptozotocin and cytokines (15), we examined whether β-cell apoptosis was altered in PancMet KO mice during pregnancy. As shown in Fig. 5C, β-cell apoptosis was significantly increased in wild-type and PancMet KO mice at GD19 and PPD4 compared with nonpregnant littermates. However, apoptosis in PancMet KO β-cells was prematurely and significantly increased at GD15 compared with nonpregnant mice or pregnant wild-type mice at the same gestational age (Fig. 5A–C). This suggests that the prosurvival effects of HGF in the β-cell are required for proper maternal β-cell expansion during pregnancy.
FIG. 5.
Increased β-cell death in pregnant PancMet KO mice at GD15. A and B: Representative photomicrographs of islets from pregnant PancMet KO mice and wild-type (WT) littermates at GD15 stained for insulin (red) and TUNEL (green). Arrows indicate TUNEL-positive β-cell nuclei. C: Quantification of the percentage of TUNEL-positive β-cells in pregnant, PPD4, and nonpregnant (NP) wild-type (n = 4–8) and PancMet KO (n = 4–7) mice. Results are means ± SEM. *P < 0.05 vs. nonpregnant mice of their corresponding genotype; ^P < 0.05 vs. wild type at the same GD or PPD4. D–I: Representative photomicrographs of mouse islet cultures from wild-type (D–F) and PancMet KO (G–I) mice treated with 0 nmol/L (D and G), 200 nmol/L (E and H), or 500 nmol/L (F and I) dexamethasone for 24 h and stained for TUNEL (green), insulin (red), and DAPI (blue). Arrows indicate TUNEL-positive β-cell nuclei. J: Quantification of TUNEL-positive β-cell nuclei in four experiments performed in duplicate of mouse islet cell cultures of PancMet KO and wild-type mice treated with 0, 200, or 500 nmol/L dexamethasone. Results are means ± SEM. *P < 0.05 vs. 0 mmol/L of their corresponding genotype; ^P < 0.05 vs. the same dose in wild-type mouse islet cell cultures. K–N: Representative photomicrographs of human islet cultures treated with 0 ng/mL (K and L) or 25 ng/mL (M and N) HGF and 0 nmol/L (K and M) or 500 nmol/L (L and N) dexamethasone for 24 h and stained for TUNEL (green), insulin (red), and DAPI (blue). Arrows indicate TUNEL-positive β-cell nuclei. O: Quantification of TUNEL-positive β-cell nuclei in three experiments performed in duplicate of human islet cell cultures untreated or treated with HGF and 0 or 500 nmol/L dexamethasone. Results are means ± SEM. *P < 0.05 vs. 0 nmol/L and ^P < 0.05 vs. 500 nmol/L without HGF. (A high-quality digital representation of this figure is available in the online issue.)
Circulating steroid hormone levels rise during the last week of gestation in rodents and have been postulated to induce β-cell apoptosis and normalize β-cell mass in late gestation and postpartum (2,30–32). It could be possible that HGF/c-Met signaling deficiency renders β-cells more sensitive to apoptosis induced by low levels of steroid hormones, as we have previously observed with cytokines (15). Indeed, PancMet KO β-cells were significantly more sensitive to the cytotoxic effects of a low dose of the synthetic steroid hormone dexamethasone that did not induce β-cell death in wild-type islets (Fig. 5D–J). A higher dose of dexamethasone was equally cytotoxic in both PancMet KO and wild-type β-cells (Fig. 5J). Interestingly, dexamethasone-induced cytotoxicity was completely abrogated by HGF in human β-cells (Fig. 5K–O). These results indicate that HGF is a protective factor for the β-cell against cytotoxic doses of steroid hormones and that loss of HGF/c-Met signaling in β-cells can confer increased sensitivity to cytotoxicity induced by lower doses of these hormones.
Absence of HGF/c-Met signaling in β-cells leads to GDM.
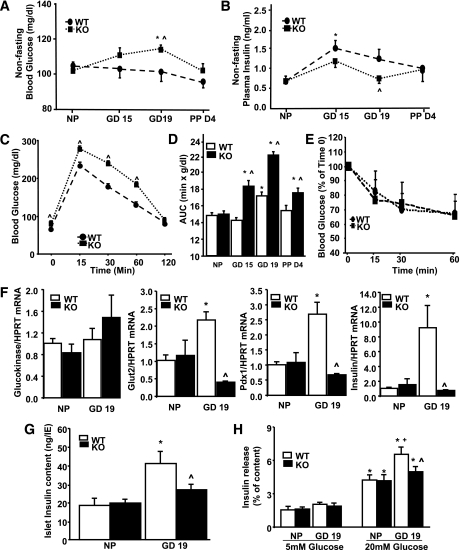
Since loss of c-Met from β-cells leads to changes in maternal β-cell mass expansion, proliferation, and survival, we next analyzed glucose homeostasis in PancMet KO mice during pregnancy. Blood glucose was significantly higher in PancMet KO mice at GD19 compared with nonpregnant mice or pregnant wild-type littermates at this gestational day (Fig. 6A). This increase in blood glucose correlated with diminished plasma insulin (Fig. 6B). In addition, PancMet KO mice also displayed significantly increased fasting blood glucose compared with wild-type mice at GD19 (Fig. 6C).
FIG. 6.
Glucose homeostasis in pregnant PancMet KO mice. Pregnant PancMet KO mice displayed significantly increased blood glucose (A) and significantly decreased plasma insulin (B) at GD19. Results are means ± SEM and were obtained from pregnant, PPD4, and nonpregnant (NP) wild-type (WT) (n = 4–8) and PancMet KO (n = 4–7) mice. *P < 0.05 vs. nonpregnant mice of their corresponding genotype; ^P < 0.05 vs. wild type at the same GD. C: Intraperitoneal glucose tolerance test in wild-type (n = 7) and PancMet KO (n = 6) mice at GD19. Results are means ± SEM. ^P < 0.05 vs. wild type at the same time point. D: Area under the curve (AUC) calculated from the intraperitoneal glucose tolerance test experiments in which nonpregnant and pregnant wild-type (n = 5–7) and PancMet KO (n = 6–8) mice at GD15 and -19 and PPD4 were examined. Results are means ± SEM. *P < 0.05 vs. nonpregnant mice of their corresponding genotype; ^P < 0.05 vs. wild type at the same GD or PP day. E: Insulin tolerance test in pregnant wild-type (n = 5) and PancMet KO (n = 6) mice at GD18. Both types of mice show similar response to insulin administration. F: Real-time PCR analysis of glucokinase, GLUT2, Pdx-1, and insulin mRNA expression in total islet RNA from islets isolated from nonpregnant and pregnant (GD19) wild-type (n = 5–6) and PancMet KO (n = 3–5) mice. Results are means ± SEM. *P < 0.05 vs. wild-type nonpregnant; ^P < 0.05 vs. wild-type GD19. HPRT, hypoxanthine-guanine phosphoribosyltransferase. G: Insulin content in islets isolated from nonpregnant and pregnant (GD19) wild-type (n = 6) and PancMet KO (n = 6) mice. Results are means ± SEM. *P < 0.05 vs. wild-type nonpregnant; ^P < 0.05 vs. wild-type GD19. H: GSIS was performed in groups of 10 islets of similar sizes obtained from nonpregnant and pregnant (GD19) wild-type (n = 5) and PancMet KO (n = 5) mice and incubated for 30 min with 5 or 20 mmol/L glucose. Results are means ± SEM. *P < 0.05 vs. 5 mmol/L glucose of their corresponding genotype; +P < 0.05 vs. nonpregnant wild type at 20 mmol/L glucose; and ^P < 0.05 vs. wild-type GD19 at 20 mmol/L glucose.
Glucose tolerance was impaired in wild-type mice at GD19 compared with nonpregnant mice (Fig. 6D). Importantly, glucose tolerance was further impaired in PancMet KO mice at this gestational day (Fig. 6C and D), when β-cell mass is diminished in these mice (Fig. 2C). The enhanced impairment in glucose tolerance in pregnant PancMet KO mice was not related to changes in insulin sensitivity, since both types of pregnant mice responded similarly to insulin tolerance tests at GD18 (Fig. 6E). The impaired glucose tolerance in PancMet KO mice was maintained at PPD4 (Fig. 6D) and, surprisingly, was also present at GD15 (Fig. 6D), when no change in β-cell mass was observed (Fig. 2C). This suggests that the absence of c-Met from β-cells could also alter insulin secretion during pregnancy independent of its effects on β-cell mass and proliferation.
Absence of HGF/c-Met signaling in β-cells leads to decreased GSIS, diminished islet insulin content, and reduced islet insulin, GLUT2, and Pdx-1 mRNA expression during pregnancy.
We next analyzed mRNA expression of β-cell function markers including glucokinase, GLUT2, insulin, and Pdx-1 in islets from these mice at GD19. No significant alterations in glucokinase mRNA levels were observed between pregnant PancMet KO and wild-type littermates (Fig. 6F). However, GLUT2, insulin, and Pdx-1 mRNA were markedly and significantly increased in wild-type mouse islets but not in islets from pregnant PancMet KO mice at GD19 (Fig. 6F). The increased insulin mRNA in pregnant wild-type mouse islets correlated with a significant increase in islet insulin content; however, this increase was not present in islets from pregnant PancMet KO mice (Fig. 6G). Since pregnant PancMet KO mice display increased blood glucose and decreased plasma insulin, glucose tolerance, and islet insulin content, we analyzed GSIS in isolated islets from these mice. Islets from nonpregnant PancMet KO and wild-type mice displayed similar increases in GSIS (Fig. 6H). Islets from pregnant wild-type mice at GD19 showed significantly enhanced GSIS compared with islets from nonpregnant mice (Fig. 6H). However, this further increase in GSIS was not present in islets from pregnant PancMet KO mice (Fig. 6H). Collectively, these results suggest that HGF/c-Met signaling is required for the functional adaptive response of maternal mouse β-cells to pregnancy.
DISCUSSION
The current study provides the first direct evidence that HGF/c-Met signaling is essential for complete maternal β-cell adaptation during pregnancy. Loss of c-Met in the pancreas diminishes maternal β-cell proliferation and enhances apoptosis, leading to diminished β-cell mass expansion, hyperglycemia, hypoinsulinemia, and glucose intolerance in pregnant mice: hallmarks of GDM. Therefore, these observations indicate that impairment/attenuation of HGF/c-Met signaling might be a pathogenic mechanism of GDM and could be a potential target for detection, prevention, and therapy in diabetes.
Previous studies from our group and others have shown that HGF is a mitogenic, prosurvival, and insulinotropic factor for the β-cell (11–16). Although c-Met loss from the adult β-cell or pancreas does not lead to marked alterations in glucose or β-cell homeostasis under basal conditions (15,33,34), recent studies have shown that c-Met absence facilitates cytokine-mediated β-cell death and accelerates the onset of diabetes in a mouse model of multiple low-dose streptozotocin administration (15). Taken together, these previous results indicate that HGF may have a physiological role in the β-cell in situations of stress-mediated hyperglycemia/diabetes. During pregnancy, insulin resistance develops and leads to an adaptive process in maternal β-cells that includes enhanced insulin secretion and β-cell mass expansion (1,2,35–40). When β-cell expansion or hyperfunction fails to compensate during pregnancy, hyperglycemia/diabetes occurs, suggesting that defective maternal β-cell adaptation can lead to GDM (2–5,23–27). Therefore, there is a growing interest in deciphering the extracellular and intracellular regulators of maternal β-cell mass expansion and hyperfunction to find therapeutic targets not only for preventing/correcting GDM but also for enhancing β-cell regeneration in other diabetic conditions.
HGF expression is markedly enhanced in islet endothelium at GD15, when both islet vascular growth and β-cell proliferation occur in rodents (17). HGF, produced by the villous mesenchyme of the placenta, is essential for placental organogenesis and maintenance of pregnancy (41,42). In addition, circulating HGF is increased in the third trimester of pregnancy in women (16). In the current study, we observed an increase in HGF in both the islet and the circulation at GD15 in mice when maximal β-cell proliferation occurs. More importantly, c-Met mRNA and protein are also upregulated at the same gestational day, suggesting increased HGF/c-Met signaling in islets during gestation. Several hormones such as progesterone, PL, estrogen, glucocorticoids, and cytokines such as tumor necrosis factor-α all present during pregnancy (1,2,30,43) are known to upregulate c-Met expression in ovary, breast, and endometrium (44,45). We have recently shown that cytokines enhance c-Met expression in islets (15), and here we show that 17β-estradiol enhances c-Met mRNA expression as well, suggesting that cytokines and hormones upregulated during gestation can modulate c-Met levels to enhance HGF signaling in β-cells.
In vitro studies have shown that purified proliferating islet endothelial cells stimulate β-cell proliferation through secretion of HGF (17). Since HGF expression is enhanced at GD15 in the islet endothelium, these results suggest a potential role of HGF in maternal β-cell proliferation and expansion. Indeed, our results clearly indicate that the absence of c-Met from β-cells leads to incomplete maternal β-cell mass expansion during pregnancy. This decrease is not due to changes in islet number or β-cell size. However, maternal β-cell proliferation is clearly diminished at GD15 in mice deficient for c-Met in β-cells, a time when maximal β-cell proliferation is observed in wild-type mice. Importantly, since β-cell mass is diminished in PancMet KO mice at GD19 but not at GD15, these results indicate that maternal β-cell growth adaptation is dependent on HGF/c-Met signaling at GD15 and subsequent GDs but is HGF/c-Met signaling independent prior to GD15. Downregulation of p27 potentially contributes to the increase in β-cell replication during pregnancy (23,24). In addition, FoxM1 upregulation is required for the enhanced β-cell proliferation, β-cell mass expansion, and p27 downregulation during gestation in mice (24). Importantly, islets lacking c-Met and with diminished β-cell proliferation at GD15 failed to increase FoxM1 mRNA expression and downregulate p27 levels, suggesting that HGF signaling might regulate these mitogenic intracellular signals. Indeed, HGF has been shown to downregulate p27 in skeletal muscle myoblasts, and this could occur in β-cells as well (46). However, whether HGF regulates FoxM1 in β-cells or any other tissue is unknown and warrants further studies. The transcription factor hepatocyte nuclear factor (HNF)-4α is required for maternal β-cell mass expansion and activation of the Ras/extracellular signal–related kinase (ERK) signaling cascade in islets (25). HGF strongly induces ERK activation in β-cells (14); however, whether HNF-4α is involved in HGF-induced ERK activation or whether HGF requires HNF-4α and ERK activation for β-cell replication during pregnancy is currently unknown and also warrants further studies.
We cannot rule out the possibility that HGF/c-Met plays an indirect role, with the major effect being that the embryonic absence of c-Met leads to alteration of downstream factor(s) primarily responsible for the defect observed in pregnant PancMet KO mice. Another possibility is that HGF regulates the action of hormones known to participate in maternal β-cell adaptation during pregnancy. Accumulating evidence supports the implication of PRL/PL in this gestational adaptive response (1,2,26,47). Interestingly, PancMet KO mouse islets displayed decreased PRLR levels at GD15, suggesting that PRL/PL signaling might be reduced during pregnancy, when circulating levels of these hormones are markedly high. It is important to note that heterozygous PRLR mice displaying a partial decrease in PRLR expression show normal glucose and β-cell homeostasis in basal conditions but impaired β-cell proliferation and expansion during pregnancy, leading to dysregulated glucose homeostasis (26). Recent evidence indicates that PRL/PL induces serotonin synthesis in islets which, in turn, functions in a paracrine-autocrine fashion to stimulate β-cell proliferation during pregnancy (27). Therefore, it is plausible that the decrease in PRLR levels in pregnant PancMet KO islets could affect serotonin synthesis and signaling in the β-cell of these mice during pregnancy, leading to incomplete maternal β-cell adaptation. PRL/PL induces β-cell proliferation through activation and nuclear localization of STAT5 (26,48). Indeed, although STAT5 levels appear unchanged by immunostaining, its intracellular localization is altered in β-cells, being nuclear in pregnant wild-type mice and more diffuse and cytoplasmic in PancMet KO mice, suggesting perhaps diminished PRL/PL signaling. However, c-Met has also been shown to activate STAT proteins and physically associate with STAT5 (49). It is possible that the decreased activation of STAT5 in pregnant PancMet KO mouse β-cells merely reflects the loss of c-Met signaling or a combined decrease in PRLR and c-Met signaling in these cells.
A less explored cellular process in β-cell adaptation during pregnancy is death/apoptosis. It has been shown that apoptosis contributes to the involution of β-cell mass at the end of gestation and the early postpartum period in rodents (29). However, the extracellular and intracellular signals that regulate survival/death in β-cell mass expansion and attrition during gestation/postpartum are unknown. Steroid hormones have been shown to counteract the mitogenic, prosurvival, and functional effects of PRL/PL in β-cells in vitro (31,32,50), suggesting that upregulation of steroid hormones at the end of pregnancy could be responsible for the increase in β-cell death and normalization of β-cell mass and function. In our studies, we have found that the absence of HGF/c-Met signaling in β-cells leads to increased β-cell death at GD15, when β-cell death is not altered in wild-type mice, suggesting enhanced sensitivity to mild increases in steroid hormone levels that could occur at this gestational day (51,52). Indeed, PancMet KO β-cells are more sensitive than wild-type cells to death induced by low doses of dexamethasone, and HGF protects human β-cells from dexamethasone-induced cell death in vitro, further reinforcing the possibility that c-Met deficiency might enhance the susceptibility of β-cells to the negative effects of mild hormonal changes (51,52).
A decrease in β-cell mass and proliferation during pregnancy can lead to negative outcomes in glucose homeostasis and development of GDM (2–5,23–27). Although the decrease in β-cell mass in pregnant PancMet KO mice might contribute to the increased blood glucose, hypoinsulinemia, and glucose intolerance in these mice, alterations in β-cell function due to the absence of HGF/c-Met signaling could also be present. HGF overexpression in β-cells increases insulin secretion, upregulates GLUT2 and glucokinase mRNA, and enhances glucose transport and metabolism in β-cells (12). During pregnancy, it has been reported that upregulation of GLUT2, glucokinase, insulin, and GSIS occurs in rodent islets (36,37). We found that islets from wild-type mice at GD19 display upregulated GLUT2 and Pdx-1 mRNA, islet insulin content, and GSIS. However, these upregulations do not occur in PancMet KO islets. Taken together, these results suggest that HGF/c-Met signaling in β-cells might be required for the hyperfunctional β-cell adaptation during pregnancy. Whether this is a direct effect of HGF or indirect due to decreased PRL/PL signaling is unknown at present. It is important to note that our current studies used Pdx1-Cre transgenic mice to delete c-Met from the pancreas and the β-cell and these mice have been shown to display Cre expression in parts of the brain known to participate in the regulation of glucose homeostasis (53). This could lead to potential elimination of c-Met in different regions of the brain in PancMet KO mice, resulting in alterations in insulin sensitivity and glucose homeostasis. Therefore, β-cell nonautonomous effects could be responsible for the changes in glucose homeostasis in pregnant PancMet KO mice. However, adult PancMet KO mice display normal glucose homeostasis in basal conditions (15). Furthermore, body weight and insulin sensitivity are not different between pregnant wild-type and PancMet KO mice, suggesting that the alterations in blood glucose, plasma insulin, and glucose tolerance in pregnant PancMet KO mice are not related to changes in insulin sensitivity or body weight gains. In addition, ex vivo GSIS is decreased in isolated islets from pregnant PancMet KO mice compared with pregnant wild-type mouse islets. Therefore, these findings suggest a cell-autonomous effect of HGF/c-Met signaling on β-cell function during pregnancy.
In conclusion, these studies are the first to identify HGF as a regulator of maternal β-cell adaptation during pregnancy. Alterations in expression or the presence of mutations/polymorphisms that reduce HGF signaling in the β-cell might be important targets for detection, prevention, and treatment of GDM.
ACKNOWLEDGMENTS
This study was supported by grants from the American Diabetes Association (1-10-BS-59) and the National Institutes of Health (NIH) (DK067351 and DK077096 to A.G.-O. and DK072264 to R.C.V.). C.D. was the recipient of a research fellowship from the Lawson Wilkins Pediatric Endocrine Society. S.E. was a research fellow partly supported by NIH Research Training Grant T32DK07052-32. Human islets were provided by the Juvenile Diabetes Research Foundation and the NIH/National Center for Research Resources– and National Institute of Diabetes and Digestive and Kidney Diseases–supported Integrated Islet Distribution Program.
No potential conflicts of interest relevant to this article were reported.
C.D. and S.E. performed research, analyzed data, designed research, and contributed to discussion. J.C.A.-P. performed research, analyzed data, and contributed to discussion. T.R., S.V., V.S., and G.P.C. performed research. L.C.A. and R.C.V. designed research, analyzed data, and contributed to discussion. A.G.-O. designed research, analyzed data, contributed to discussion, and wrote the manuscript. A.G.-O. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors are grateful to Dr. Douglas A. Melton of the Harvard Stem Cell Institute, Harvard University, and Dr. Snorri Thorgeirsson of the Laboratory of Experimental Carcinogenesis, National Cancer Institute, for providing Pdx-Cre and loxP-c-Met mice, respectively. The authors are also grateful to Andrew F. Stewart, Donald K. Scott, and Nathalie Fiaschi-Taesch from the Division of Endocrinology and Dorothy Becker from the Department of Pediatrics, University of Pittsburgh, for thoughtful discussions.
Footnotes
C.D. is currently affiliated with Pediatric Endocrinology, Connecticut Children's Medical Center, Hartford, Connecticut.
REFERENCES
- 1.Sorenson RL, Brelje TC. Adaptation of islets of Langerhans to pregnancy: beta-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm Metab Res 1997;29:301–307 [DOI] [PubMed] [Google Scholar]
- 2.Ernst S, Demirci C, Valle S, Velazquez-Garcia S, Garcia-Ocaña A. Mechanisms in the adaptation of maternal β-cells during pregnancy. Diabetes Manag (Lond) 2011;1:239–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng YW, Caughey AB. Gestational diabetes: diagnosis and management. J Perinatol 2008;28:657–664 [DOI] [PubMed] [Google Scholar]
- 4.Devlieger R, Casteels K, Van Assche FA. Reduced adaptation of the pancreatic B cells during pregnancy is the major causal factor for gestational diabetes: current knowledge and metabolic effects on the offspring. Acta Obstet Gynecol Scand 2008;87:1266–1270 [DOI] [PubMed] [Google Scholar]
- 5.Löbner K, Knopff A, Baumgarten A, et al. Predictors of postpartum diabetes in women with gestational diabetes mellitus. Diabetes 2006;55:792–797 [DOI] [PubMed] [Google Scholar]
- 6.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009;373:1773–1779 [DOI] [PubMed] [Google Scholar]
- 7.Retnakaran R, Qi Y, Sermer M, Connelly PW, Hanley AJ, Zinman B. Glucose intolerance in pregnancy and future risk of pre-diabetes or diabetes. Diabetes Care 2008;31:2026–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boloker J, Gertz SJ, Simmons RA. Gestational diabetes leads to the development of diabetes in adulthood in the rat. Diabetes 2002;51:1499–1506 [DOI] [PubMed] [Google Scholar]
- 9.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005;115:e290–e296 [DOI] [PubMed] [Google Scholar]
- 10.Silverman BL, Metzger BE, Cho NH, Loeb CA. Impaired glucose tolerance in adolescent offspring of diabetic mothers. Relationship to fetal hyperinsulinism. Diabetes Care 1995;18:611–617 [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Ocaña A, Takane KK, Syed MA, Philbrick WM, Vasavada RC, Stewart AF. Hepatocyte growth factor overexpression in the islet of transgenic mice increases beta cell proliferation, enhances islet mass, and induces mild hypoglycemia. J Biol Chem 2000;275:1226–1232 [DOI] [PubMed] [Google Scholar]
- 12.García-Ocaña A, Vasavada RC, Cebrian A, et al. Transgenic overexpression of hepatocyte growth factor in the beta-cell markedly improves islet function and islet transplant outcomes in mice. Diabetes 2001;50:2752–2762 [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Ocana A, Takane KK, Reddy VT, Lopez-Talavera J-C, Vasavada RC, Stewart AF. Adenovirus-mediated hepatocyte growth factor expression in mouse islets improves pancreatic islet transplant performance and reduces beta cell death. J Biol Chem 2003;278:343–351 [DOI] [PubMed] [Google Scholar]
- 14.Vasavada RC, Wang L, Fujinaka Y, et al. Protein kinase C-zeta activation markedly enhances beta-cell proliferation: an essential role in growth factor mediated beta-cell mitogenesis. Diabetes 2007;56:2732–2743 [DOI] [PubMed] [Google Scholar]
- 15.Mellado-Gil JMD, Rosa TC, Demirci C, et al. Disruption of hepatocyte growth factor/c-Met signaling enhances pancreatic beta-cell death and accelerates the onset of diabetes. Diabetes 2011;60:525–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horibe N, Okamoto T, Itakura A, et al. Levels of hepatocyte growth factor in maternal serum and amniotic fluid. Am J Obstet Gynecol 1995;173:937–942 [DOI] [PubMed] [Google Scholar]
- 17.Johansson M, Mattsson G, Andersson A, Jansson L, Carlsson PO. Islet endothelial cells and pancreatic beta-cell proliferation: studies in vitro and during pregnancy in adult rats. Endocrinology 2006;147:2315–2324 [DOI] [PubMed] [Google Scholar]
- 18.Huh CG, Factor VM, Sánchez A, Uchida K, Conner EA, Thorgeirsson SS. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci USA 2004;101:4477–4482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 2002;129:2447–2457 [DOI] [PubMed] [Google Scholar]
- 20.Johansson JK, Voss U, Kesavan G, et al. N-cadherin is dispensable for pancreas development but required for β-cell granule turnover. Genesis 2010;48:374–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie T, Chen M, Weinstein LS. Pancreas-specific Gsalpha deficiency has divergent effects on pancreatic α- and β-cell proliferation. J Endocrinol 2010;206:261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alonso LC, Yokoe T, Zhang P, et al. Glucose infusion in mice: a new model to induce beta-cell replication. Diabetes 2007;56:1792–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karnik SK, Chen H, McLean GW, et al. Menin controls growth of pancreatic β-cells in pregnant mice and promotes gestational diabetes mellitus. Science 2007;318:806–809 [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Zhang J, Pope CF, et al. Gestational diabetes mellitus resulting from impaired beta-cell compensation in the absence of FoxM1, a novel downstream effector of placental lactogen. Diabetes 2010;59:143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta RK, Gao N, Gorski RK, et al. Expansion of adult beta-cell mass in response to increased metabolic demand is dependent on HNF-4alpha. Genes Dev 2007;21:756–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang C, Snider F, Cross JC. Prolactin receptor is required for normal glucose homeostasis and modulation of beta-cell mass during pregnancy. Endocrinology 2009;150:1618–1626 [DOI] [PubMed] [Google Scholar]
- 27.Kim H, Toyofuku Y, Lynn FC, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med 2010;16:804–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brelje TC, Stout LE, Bhagroo NV, Sorenson RL. Distinctive roles for prolactin and growth hormone in the activation of signal transducer and activator of transcription 5 in pancreatic islets of langerhans. Endocrinology 2004;145:4162–4175 [DOI] [PubMed] [Google Scholar]
- 29.Scaglia L, Smith FE, Bonner-Weir S. Apoptosis contributes to the involution of beta cell mass in the post partum rat pancreas. Endocrinology 1995;136:5461–5468 [DOI] [PubMed] [Google Scholar]
- 30.Nadal A, Alonso-Magdalena P, Soriano S, Ropero AB, Quesada I. The role of oestrogens in the adaptation of islets to insulin resistance. J Physiol 2009;587:5031–5037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinhaus AJ, Bhagroo NV, Brelje TC, Sorenson RL. Dexamethasone counteracts the effect of prolactin on islet function: implications for islet regulation in late pregnancy. Endocrinology 2000;141:1384–1393 [DOI] [PubMed] [Google Scholar]
- 32.Sorenson RL, Brelje TC, Roth C. Effects of steroid and lactogenic hormones on islets of Langerhans: a new hypothesis for the role of pregnancy steroids in the adaptation of islets to pregnancy. Endocrinology 1993;133:2227–2234 [DOI] [PubMed] [Google Scholar]
- 33.Roccisana J, Reddy V, Vasavada RC, Gonzalez-Pertusa JA, Magnuson MA, Garcia-Ocaña A. Targeted inactivation of hepatocyte growth factor receptor c-met in beta-cells leads to defective insulin secretion and GLUT-2 downregulation without alteration of beta-cell mass. Diabetes 2005;54:2090–2102 [DOI] [PubMed] [Google Scholar]
- 34.Dai C, Huh CG, Thorgeirsson SS, Liu Y. Beta-cell-specific ablation of the hepatocyte growth factor receptor results in reduced islet size, impaired insulin secretion, and glucose intolerance. Am J Pathol 2005;167:429–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green IC, Taylor KW. Effects of pregnancy in the rat on the size and insulin secretory response of the islets of Langerhans. J Endocrinol 1972;54:317–325 [DOI] [PubMed] [Google Scholar]
- 36.Weinhaus AJ, Stout LE, Sorenson RL. Glucokinase, hexokinase, glucose transporter 2, and glucose metabolism in islets during pregnancy and prolactin-treated islets in vitro: mechanisms for long term up-regulation of islets. Endocrinology 1996;137:1640–1649 [DOI] [PubMed] [Google Scholar]
- 37.Bone AJ, Taylor KW. Mitabolic adaptation to pregnancy shown by increased biosynthesis of insulin in islets of Langerhans isolated from pregnant rat. Nature 1976;262:501–502 [DOI] [PubMed] [Google Scholar]
- 38.Green IC, Howell SL, Montague W, Taylor KW. Regulation of insulin release from isolated islets of Langerhans of the rat in pregnancy. The role of adenosine 3′:5′-cyclic monophosphate. Biochem J 1973;134:481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Assche FA, Aerts L, De Prins F. A morphological study of the endocrine pancreas in human pregnancy. Br J Obstet Gynaecol 1978;85:818–820 [DOI] [PubMed] [Google Scholar]
- 40.Butler AE, Cao-Minh L, Galasso R, et al. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia 2010;53:2167–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uehara Y, Minowa O, Mori C, et al. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature 1995;373:702–705 [DOI] [PubMed] [Google Scholar]
- 42.Kauma S, Hayes N, Weatherford S. The differential expression of hepatocyte growth factor and met in human placenta. J Clin Endocrinol Metab 1997;82:949–954 [DOI] [PubMed] [Google Scholar]
- 43.Kirwan JP, Hauguel-De Mouzon S, Lepercq J, et al. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes 2002;51:2207–2213 [DOI] [PubMed] [Google Scholar]
- 44.Moghul A, Lin L, Beedle A, et al. Modulation of c-MET proto-oncogene (HGF receptor) mRNA abundance by cytokines and hormones: evidence for rapid decay of the 8 kb c-MET transcript. Oncogene 1994;9:2045–2052 [PubMed] [Google Scholar]
- 45.Satterfield MC, Hayashi K, Song G, Black SG, Bazer FW, Spencer TE. Progesterone regulates FGF10, MET, IGFBP1, and IGFBP3 in the endometrium of the ovine uterus. Biol Reprod 2008;79:1226–1236 [DOI] [PubMed] [Google Scholar]
- 46.Leshem Y, Halevy O. Phosphorylation of pRb is required for HGF-induced muscle cell proliferation and is p27kip1-dependent. J Cell Physiol 2002;191:173–182 [DOI] [PubMed] [Google Scholar]
- 47.Parsons JA, Brelje TC, Sorenson RL. Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology 1992;130:1459–1466 [DOI] [PubMed] [Google Scholar]
- 48.Friedrichsen BN, Richter HE, Hansen JA, et al. Signal transducer and activator of transcription 5 activation is sufficient to drive transcriptional induction of cyclin D2 gene and proliferation of rat pancreatic beta-cells. Mol Endocrinol 2003;17:945–958 [DOI] [PubMed] [Google Scholar]
- 49.Runge DM, Runge D, Foth H, Strom SC, Michalopoulos GK. STAT 1alpha/beta, STAT 3 and STAT 5: expression and association with c-MET and EGF-receptor in long-term cultures of human hepatocytes. Biochem Biophys Res Commun 1999;256:376–381 [DOI] [PubMed] [Google Scholar]
- 50.Fujinaka Y, Takane K, Yamashita H, Vasavada RC. Lactogens promote beta cell survival through JAK2/STAT5 activation and Bcl-XL upregulation. J Biol Chem 2007;282:30707–30717 [DOI] [PubMed] [Google Scholar]
- 51.Coulaud J, Durant S, Homo-Delarche F. Glucose homeostasis in pre-diabetic NOD and lymphocyte-deficient NOD/SCID mice during gestation. Rev Diabet Stud 2010;7:36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lindsay JR, Nieman LK. The hypothalamic-pituitary-adrenal axis in pregnancy: challenges in disease detection and treatment. Endocr Rev 2005;26:775–799 [DOI] [PubMed] [Google Scholar]
- 53.Wicksteed B, Brissova M, Yan W, et al. Conditional gene targeting in mouse pancreatic ß-Cells: analysis of ectopic Cre transgene expression in the brain. Diabetes 2010;59:3090–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]