Abstract
To examine whether the peroxisome proliferator–activated receptor-γ coactivator-1α (PGC-1α), a key regulator linking angiogenesis and metabolism, could enhance the engraftment and angiogenesis of mesenchymal stem cells (MSCs) in diabetic hindlimb ischemia, we engineered the overexpression of PGC-1α within MSCs using an adenoviral vector encoding green fluorescent protein and PGC-1α, and then tested the survivability and angiogenesis of MSCs in vitro and in vivo. Under the condition of hypoxia concomitant with serum deprivation, the overexpression of PGC-1α in MSCs resulted in a higher expression level of hypoxia-inducible factor-1α (Hif-1α), a greater ratio of B-cell lymphoma leukemia-2 (Bcl-2)/Bcl-2–associated X protein (Bax), and a lower level of caspase 3 compared with the controls, followed by an increased survival rate and an elevated expression level of several proangiogenic factors. In vivo, the MSCs modified with PGC-1α could significantly increase the blood perfusion and capillary density of ischemic hindlimb of the diabetic rats, which was correlated to an improved survivability of MSCs and an increased level of several proangiogenic factors secreted by MSCs. We identified for the first time that PGC-1α could enhance the engraftment and angiogenesis of MSCs in diabetic hindlimb ischemia.
Peripheral arterial disease (PAD) affects 25% of diabetic patients all over the world (1). In more severe diseases, critical limb ischemia (CLI) develops, which may owe to diffuse vascular disease, the distal location of obstruction, and the presence of multiple comorbidities (2,3). As a result, incurable ulceration, gangrene, and even limb loss are more likely to occur in diabetic patients than in nondiabetic patients (4). Due to diffuse vascular disease, many diabetic patients are not amenable to revascularization by surgical bypass, endovascular stenting, or balloon dilatation (2,3). Consequently, more and more studies about the administration of autologous stem cells for treating diabetic CLI are being conducted (2).
Mesenchymal stem cells (MSCs), as promising cells for treatment of ischemic disease, are self-renewing and have a huge potential to differentiate into many kinds of angioblasts and secrete a wide array of proangiogenic factors (5,6). Meanwhile, the therapeutic angiogenesis of bone marrow MSCs has been widely proven in many studies (7–9). Recently, MSCs have already been used to treat diabetic patients with CLI by us and other researchers (10,11), but the low survival rate (<1% after 4 days of transplantation) of implanted MSCs (12) may seriously affect the angiogenic potential of cells and then attenuate the effect of this cell therapy.
Peroxisome proliferator–activated receptor-γ coactivator-1α (PGC-1α), a transcriptional coactivator for nuclear receptors (13) that plays a critical role in the biological regulation of mitochondria, is highly responsive to most stimuli from ischemic tissues, including hypoxia, coldness, and lack of nutrients (14). In recent years, some basic studies have shown that PGC-1α can prevent apoptosis of vascular endothelial cells (15,16) and greatly enhance the angiogenic potential of skeletal muscle cells (17). It is implied that PGC-1α serves as a key regulator in linking angiogenesis and metabolism processes (18).
In the current study, to assess the effects of PGC-1α overexpression on the therapeutic angiogenesis of MSCs, we investigated whether the MSCs modified with PGC-1α could be more resistant to apoptosis and improve the perfusion recovery in diabetic hindlimb ischemia more than the controls.
RESEARCH DESIGN AND METHODS
Expansion of rat MSCs.
The expansion of MSCs was conducted with a little modification as described previously (19). All the protocols were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (publication 85-23, revised in 1996) and were approved by the Ethical Committee Board of the Third Military Medical University Southwest Hospital.
In brief, the female SD rats were killed to harvest bone marrow by flushing the cavities of femurs and tibias with complete α-minimum essential medium (α-MEM [GIBCO], supplemented with 10% FBS [GIBCO] and antibiotics). Then after filtration through a 70-mm filter mesh (BD, Falcon), the cell suspension was transferred into a 60-mm dish and incubated in a humidified chamber at 37°C and 5% CO2. After 3 h, the nonadherent cells were removed and replaced with fresh complete medium. After an additional 8-h culture, the medium was replaced again. Thereafter, this step was repeated every 8 h for initial culture of up to 72 h. Then the medium was replaced once every 2 days. Upon ∼70–80% confluence, the cells were resuspended by trypsin-EDTA and the cell density was adjusted to approximately 5,000/cm2. Finally, the MSCs at three to five passages were used in the experiments. In the flow cytometry experiments, the cell-surface antigens were tested and found to be positive for CD105, CD29, and CD90 and negative for CD14, CD34, and CD45.
Adenovirus-mediated gene transfer to MSCs.
Adenovirus-mediated gene transfection was performed as described previously (20). In brief, the MSCs were seeded at a density of 2 × 106 cells per 60-mm plate. One day after adherence, the cells were exposed to the infectious viral particles in 1.5 mL α-minimal essential medium (α-MEM) at 37°C, and infected with Ad–green fluorescent protein (GFP) (Cell Biolabs) or Ad-GFP-PGC-1α (a gift from Daniel P. Kelly, Sanford-Burnham Medical Research Institute, La Jolla, CA) at a multiplicity of infection (MOI) of 0–1,000. The ideal MOI of 100 with high efficiency and low toxicity was detected and then selected in the following experiments.
Apoptosis of MSCs modified with genes in vitro.
The apoptosis of MSCs modified with GFP-PGC-1α or GFP was induced by culture under the condition of hypoxia (5% O2) concomitant with serum deprivation. After 6- and 12-h culture, the survival rate of MSCs was measured by the Annexin V/propidine iodide method. In brief, the cells were washed with ice-cold PBS and then resuspended in 200 μL binding buffer. Thereafter the cells were added with 10 μL Annexin V stock solution (Biolegend) and incubated for 25 min at 4°C. The cells were further incubated with 5 μL propidine iodide (Biolegend) and immediately analyzed on a Fluorescence-activated Cell Sorter LSR (Becton, Dickinson, and Company, San Jose, CA). Approximately 1–2 × 104 cells were analyzed for each of the samples.
The expression levels of caspase 3, procaspase 3, B-cell lymphoma leukemia-2 (Bcl-2), and Bcl-2–associated X protein (Bax) were detected by Western blotting using rabbit polyclonal antibodies (1:500; Abcom, Cambridge, U.K.).
Expression of proangiogenic factors in MSCs modified with genes in vitro.
To identify the protein expression of hypoxia-inducible factor-1α (Hif-1α) and PGC-1α in gene-mediated MSCs under the hypoxia and serum deprivation–conditioned culture (6 h), Western blotting was performed using two rabbit polyclonal antibodies raised against Hif-1α (1:500; Santa Cruz Biotechnology) or PGC-1α (1:500; Santa Cruz Biotechnology).
To evaluate proangiogenic factors secreted by MSCs, the conditioned medium in 60-mm plates (3 mL per 2 × 106 cells) was collected during 3 days normal culture, and the levels of vascular endothelial growth factor (VEGF), fibroblast growth factor-2 (FGF-2), and platelet-derived growth factor subunit B (PDGF-B) were measured using enzyme immunoassay kits (Rat VEGF ELISA Kit RayBio for VEGF; Rat FGF-2 ELISA Kit USCNLIFE for FGF-2; Platelet Derived Growth Factor Subunit B ELISA Kit antibodies-online for PDGF-B).
Animal model and cell transplantation.
SD rats weighing 200–220 g were used in this study. The model of diabetic ischemic limb was established according to the procedures previously published with a little modification (21). In brief, diabetes was induced by intraperitoneal injection of streptozotocin (55 mg/kg; Sigma Chemical, St. Louis, MO). One week after streptozotocin administration, rats with a plasma glucose concentration >16 mmol/L were selected in the subsequent experiments. Twelve weeks after diabetes was induced, the left femoral artery, the distal portion, and all the lateral branches were dissected free and excised under anesthesia with pentobarbital sodium (45 mg/kg i.p.). The right hindlimb was kept intact and served as a control of original blood flow.
Immediately after resection of the left femoral artery, rats were randomized to one of the following four groups: MSC transplantation group (MSC group, n = 21), GFP-modified MSC transplantation group (GFP-MSC group, n = 21), PGC-1α–modified MSC transplantation group (PGC-1α-MSC group, n = 21), or PBS vehicle infusion group (PBS group, n = 21). In each group, 5 × 106 cells or PBS was injected into the ischemic thigh muscles with a 26-gauge needle at five different points. Fourteen days after transplantation, the incidence of hindlimb necrosis was calculated.
Laser Doppler blood flowmetry.
After 0, 7, and 14 days, the serial blood flow was measured by a laser Doppler perfusion image (LDPI) analyzer (Moor Instruments). The highest perfusion was displayed as red, whereas low or no blood perfusion was displayed as dark blue. After blood flow had been scanned three times, the average flow values of the ischemic and nonischemic limbs were counted by computer-assisted quantification using stored images. The LDPI index was defined as the ratio of ischemic to nonischemic hindlimb blood perfusion (22).
Capillary density in diabetic ischemic hindlimb.
To detect capillary endothelial cells, the frozen sections of adductor muscles were stained with alkaline phosphatase according to the procedures previously published 2 weeks after the transplantation (22–24). Six fields from six tissue sections were randomly selected to calculate the number of capillaries in each field. To calculate capillary density correctly, the capillary number adjusted per muscle fiber was used to compare the difference in capillary density among the four groups (22–24).
Survival of transplanted MSCs in diabetic ischemic hindlimb.
For detecting the survival of transplanted MSCs, GFP-positive cell counts per 1,000 nuclei were calculated in the GFP-MSC and PGC-1α-MSC groups 5 days after transplantation (n = 10) (25).
Proangiogenic factors in diabetic ischemic hindlimb.
To detect the protein expression of proangiogenic factors and the colocalization of VEGF or FGF-2 protein, immunofluorescence staining and Western blots for VEGF or FGF-2 were performed 5 days after transplantation. The frozen sections of adductor muscles were fixed in acetone at 4°C, blocked with 10% normal goat serum for 10 min, and incubated with rabbit anti–rat VEGF (1:100; Santa Cruz Biotechnology) or rabbit anti–rat FGF-2 (1:100; Santa Cruz Biotechnology) and then with Cy3-coupled goat anti–rabbit IgG antibody (1:250; Abcom, Cambridge, U.K.). Finally, nucleus staining was performed using DAPI (1:1,000; Sigma Chemical). The stained sections were observed using a laser-scanning confocal fluorescence microscope, and the generated images of GFP (green), growth factors (red), and DAPI (blue) were obtained. With the above antibodies, the protein expression level of VEGF or FGF-2 was detected using Western blotting.
Statistical analysis.
Data were presented as mean ± SD. Statistical comparisons were performed using ANOVA followed by Bonferroni correction/Dunn test. A probability value of P < 0.05 was considered statistically significant.
RESULTS
PGC-1α overexpression decreased apoptosis within MSCs.
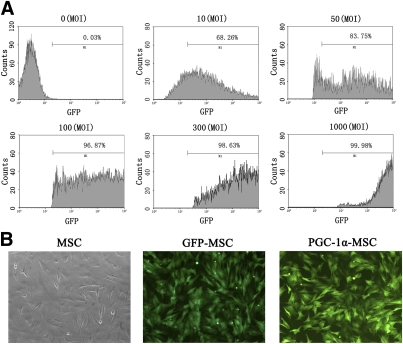
The transfection efficiency of adenoviral vector encoding PGC-1α and GFP (Ad-GFP-PGC-1α) was 96.87% at 100 MOI 2 days after transfection, as quantified by flow cytometry detecting the number of GFP-positive cells (Fig. 1A). After transfection, little change was observed in the morphology of rat MSCs (Fig. 1B). PGC-1α protein expression confirmed by Western blotting was 1.7-fold higher in Ad-GFP-PGC-1α–transfected MSCs than in Ad-GFP–transfected MSCs (P < 0.01, n = 8) (Fig. 2A) and 1.6-fold higher than in MSCs (P < 0.01, n = 8) (Fig. 2A).
FIG. 1.
Optimization of adenovirus transfection efficiency by MSCs. A: Transfection efficiency of MSCs by Ad-GFP-PGC-1α. The MSCs were transfected with Ad-GFP-PGC-1α at different MOI and cultured for 48 h. The transfection efficiency was determined by flow cytometry. B: The morphology of rat MSCs and MSCs transfected with Ad-GFP-PGC-1α (defined as PGC-1α-MSC) or Ad-GFP (defined as GFP-MSC) at an MOI of 100 for 48 h was observed by fluorescence microscope (original magnification ×200). (A high-quality color representation of this figure is available in the online issue.)
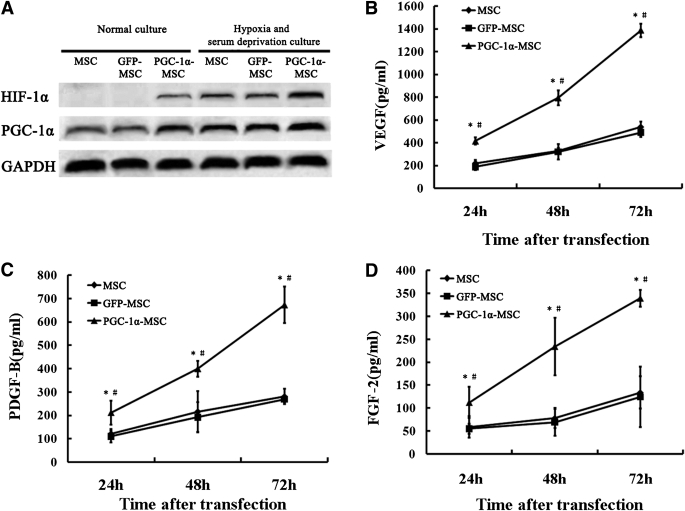
FIG. 2.
Proangiogenic factor expression of MSCs modified with genes in vitro. A: Western blotting analysis for Hif-1α, PGC-1α, and GAPDH. The protein expression of Hif-1α was not detected in MSCs and MSCs transfected with Ad-GFP (defined as GFP-MSC), but could be detected in MSCs transfected with Ad-GFP-PGC-1α (defined as PGC-1α-MSC) under 6 h normal culture. And after 6 h hypoxia and serum deprivation–conditioned culture, PGC-1α-MSCs produced more Hif-1α by 2.8-fold (compared with MSCs; P < 0.001) or by 3.0-fold (compared with GFP-MSCs; P < 0.001). B: Levels of VEGF secreted by MSCs, GFP-MSCs, and PGC-1α-MSCs from 24 to 72 h normal culture. C: Levels of PDGF-B secreted by MSCs, GFP-MSCs, and PGC-1α-MSCs from 24 to 72 h normal culture. D: Levels of FGF-2 secreted by MSCs, GFP-MSCs, and PGC-1α-MSCs from 24 to 72 h normal culture. *P < 0.01, PGC-1α-MSC vs. MSC; #P < 0.01, PGC-1α-MSC vs. GFP-MSC.
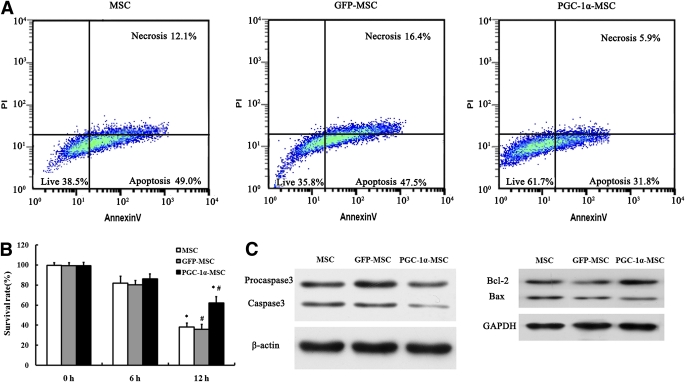
After 12 h hypoxia and serum deprivation–conditioned culture, the survival rate of the PGC-1α-MSC group (62.0 ± 6.5%) was significantly higher than that of the GFP-MSC (35.8 ± 5.0%) and MSC groups (38.2 ± 3.9%) (P < 0.01) (Fig. 3A and B). Protein expression of caspase 3, detected by Western blotting, was 1.9-fold higher in Ad-GFP–transfected MSCs than in Ad-GFP-PGC-1α–transfected MSCs (P < 0.001, n = 5) and 1.8-fold higher in MSCs than in Ad-GFP-PGC-1α–transfected MSCs (P < 0.001, n = 5) (Fig. 3C). The protein expression ratio of Bcl-2/Bax in Ad-GFP-PGC-1α–transfected MSCs was 3.5- and 2.8-fold higher than that separately in Ad-GFP–transfected MSCs (P < 0.001, n = 5) and MSCs (P < 0.001, n = 5) (Fig. 3C).
FIG. 3.
Apoptosis analysis of MSCs modified with genes in vitro. A: Three representative density plots of flow cytometry showed that the survival rate in the PGC-1α-MSC group was 61.8%, 35.8% in the GFP-MSC group, and 38.5% in the MSC group after 12 h hypoxia and serum deprivation culture. B: Survival rate in each group after 0, 6, and 12 h hypoxia and serum deprivation culture. *P < 0.01, PGC-1α-MSC vs. MSC; #P < 0.01, PGC-1α-MSC vs. GFP-MSC. C: Western blotting analysis for caspase 3, procaspase 3, β-actin, Bax, Bcl-2, and GAPDH after 12 h hypoxia and serum deprivation culture. (A high-quality color representation of this figure is available in the online issue.)
PGC-1α overexpression enhanced the expression of proangiogenic factors within MSCs.
Under normal culture conditions, PGC-1α overexpression in MSCs increased the expression of Hif-1α, which could not be detected in MSCs or GFP-MSCs (Fig. 2A). After 6 h hypoxia and serum deprivation–conditioned culture, PGC-1α-MSCs produced more Hif-1α by 2.8-fold (compared with MSCs; P < 0.001, n = 5) or by 3.0-fold (compared with GFP-MSCs; P < 0.001, n = 5) (Fig. 2A). And the increased levels of VEGF, FGF-2, and PDGF-B secreted by PGC-1α-MSCs from 24–72 h normal culture were always higher than those by MSCs (P < 0.01, n = 5) or GFP-MSCs (P < 0.01, n = 5) (Fig. 2B–D).
MSCs modified with PGC-1α promoted perfusion recovery in the diabetic hindlimb ischemia model.
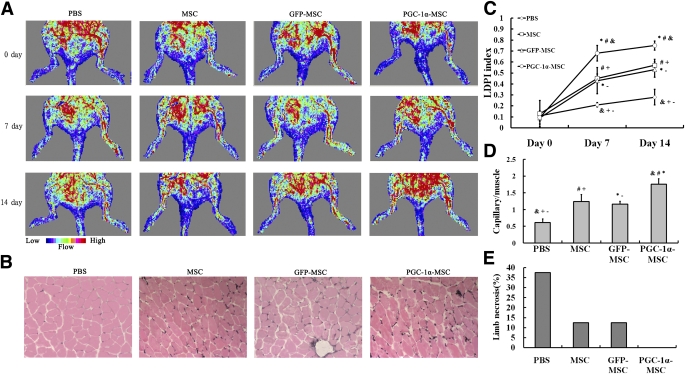
Two weeks after transplantation, the perfusion recovery of diabetic ischemic hindlimb was highest in the PGC-1α-MSC group, followed by the MSC group and GFP-MSC group (Fig. 4A). The perfusion recovery of diabetic ischemic hindlimb in the PBS group was always lowest (Fig. 4A). The quantitative analysis of hindlimb blood perfusion showed that the increase of the LDPI index from 0 to 14 days was significantly higher in the PGC-1α-MSC (0.655 ± 0.051) than in the GFP-MSC (0.438 ± 0.049), MSC (0.440 ± 0.088), and PBS groups (0.170 ± 0.050) (Fig. 4C), and the increase of blood perfusion in the GFP-MSC and MSC groups was significantly higher than that in the PBS group (Fig. 4C). Fourteen days after transplantation, the incidence of limb necrosis in the PBS group was largest, followed by the MSC and GFP-MSC groups (Fig. 4E). There was no necrosis occurring in the PGC-1α-MSC group (Fig. 4E).
FIG. 4.
Perfusion recovery, capillary density, and necrosis incidence of ischemic hindlimb after stem cell–based therapy. A: Representative images of LDPI on 0, 7, and 14 days after therapy. The blood perfusion of ischemic hindlimb was markedly increased in the PGC-1α-MSC group. B: Representative examples of ischemic hindlimb muscles by alkaline phosphatase staining (original magnification ×100). C: Quantitative analysis of hindlimb blood perfusion. The LDPI index was significantly highest in the PGC-1α-MSC group on 7 and 14 days after transplantation, followed by the MSC and GFP-MSC groups, and the lowest was observed in the PBS group. D: Quantitative analysis of capillary density in ischemic hindlimb muscles. Capillary density was shown as capillary/muscle fiber ratio. The capillary/muscle fiber ratio of ischemic hindlimb muscles was highest in the PGC-1α-MSC group, followed by the MSC, GFP-MSC, and PBS groups. E: Incidence of limb necrosis 2 weeks after transplantation. Data in C and D were presented as mean ± S.E.M. &P < 0.01, PGC-1α-MSC group vs. PBS group; *P < 0.05, PGC-1α-MSC group vs. GFP-MSC group; #P < 0.05, PGC-1α-MSC group vs. MSC group; -P < 0.05, PBS group vs. GFP-MSC group; +P < 0.05, PBS group vs. MSC group. (A high-quality color representation of this figure is available in the online issue.)
Capillary density in diabetic ischemic hindlimb.
A large number of capillaries were detected in the diabetic ischemic muscles of the PGC-1α-MSC, GFP-MSC, and MSC groups 2 weeks after transplantation (Fig. 4B). Furthermore, the quantitative analysis demonstrated that the capillary/muscle fiber ratio of ischemic muscle was highest in the PGC-1α-MSC group, followed by the MSC and GFP-MSC groups, and the lowest was observed in the PBS group (Fig. 4D).
Survival of transplanted MSCs modified with genes in diabetic ischemic hindlimb muscles.
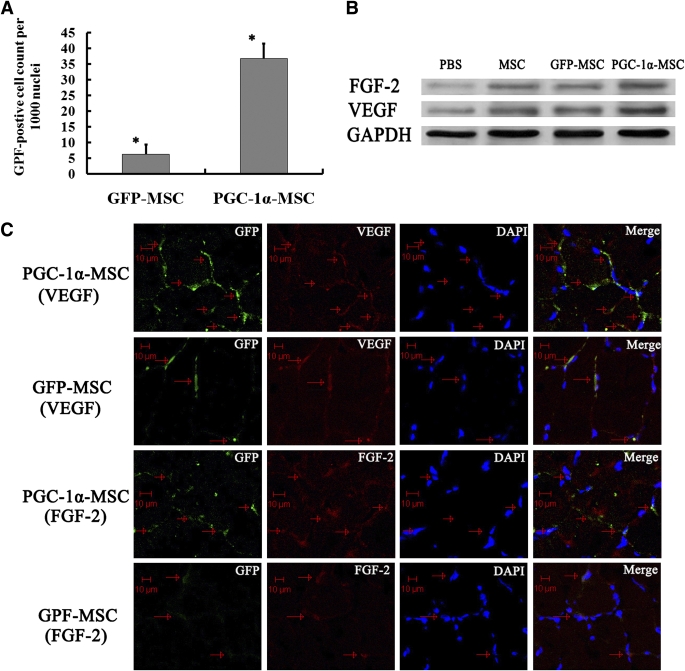
Five days after transplantation, the number of GFP-positive cells per 1,000 nuclei was notably higher in the PGC-1α-MSC group than in the GFP-MSC group (36.8 ± 4.7 vs. 6.2 ± 3.1; P < 0.001) (Fig. 5A).
FIG. 5.
Survival of MSCs modified with genes and secretion of VEGF and FGF-2 by transplanted MSCs in diabetic ischemic hindlimb. A: The GFP-positive MSCs (per 1,000 nuclei) in ischemic muscles of the PGC-1α-MSC group 5 days after transplantation were significantly higher than those in the GFP-MSC group. *P < 0.001. B: Western blotting analysis for VEGF, FGF-2, and GAPDH 5 days after transplantation. C: Secretion of FGF-2 and VEGF by transplanted MSCs that were modified with genes. Colocalization of GFP-positive MSCs (green) with VEGF (red) or GFP-positive MSCs (green) with FGF-2 (red) in ischemic muscles 5 days after transplantation. Scale bars, 10 μm. (A high-quality color representation of this figure is available in the online issue.)
Local production and secretion of FGF-2 and VEGF in diabetic ischemic hindlimb muscles.
Five days after transplantation, the VEGF protein expression level of diabetic ischemia muscles in the PGC-1α-MSC group was 1.86- and 1.91-fold separately of that in the MSC and GFP-MSC groups (P < 0.01) (Fig. 5B). Similarly, the FGF-2 protein expression level was 1.52- and 1.63-fold of that in the MSC and GFP-MSC groups, respectively (P < 0.01) (Fig. 5B).
Immunohistochemically, the green-colored cells indicating GFP-positive MSCs were overlapped or surrounded by a high concentration of red color, suggesting the local secretion of FGF-2 and VEGF from MSCs modified with genes (Fig. 5C).
DISCUSSION
In the current study, the therapeutic angiogenesis of MSCs overexpressing PGC-1α in CLI of diabetic rats was examined. The overexpression of PGC-1α caused an increase of Hif-1α, a higher ratio of Bcl-2/Bax, and a decrease of caspase 3 in MSCs, improved the survival of MSCs under the conditions of hypoxia and serum deprivation, and promoted the secretion of proangiogenic factors by MSCs. In a hindlimb ischemia model of diabetic rat, the injection of MSCs modified with PGC-1α caused significantly greater improvement of blood perfusion than the transplantation of either MSCs or MSCs modified with GFP.
Within the last 10 years, many studies demonstrated that the angiogenesis mechanisms of transplanted MSCs in ischemic tissues mainly included secreting a wide array of proangiogenic factors, differentiating/integrating into angioblasts, and recruiting endogenous stem cells (26), in which the paracrine and autocrine of transplanted MSCs played a major role in the therapeutic angiogenesis of ischemic tissues (9,27–31). In further research, it has been found that MSCs modified with proangiogenic genes, such as VEGF, insulin-like growth factor-1, and angiopoietin-1, could increase the secretion of corresponding proangiogenic factors in ischemic tissues and result in the enhancement of therapeutic angiogenesis of MSCs (32–34). More recently, a study has shown that skeletal muscle cells modified with PGC-1α can secret more proangiogenic factors through estrogen receptor-α (17). Besides, PGC-1α in skeletal muscle cells cannot only increase the stabilization of Hif-1α but also be coupled to Hif-1α–dependent gene expression (35), which implies a PGC-1α/ Hif-1α pathway to promote the secretion of some proangiogenic factors in MSCs (36). Similarly, our study also suggested that overexpressing PGC-1α could increase Hif-1α expression in MSCs, which might contribute to the increase of proangiogenic factors (VEGF, FGF-2, and PDGF-B) secreted by MSCs in part.
On the other hand, another study has also shown that MSCs modified with antiapoptosis genes, such as Bcl-2, can improve the survival of MSCs in ischemic tissues and enhance the effect of MSC therapy (37). In other species of cells, the current reports have shown that PGC-1α may enhance the effect of antiapoptosis and promote cell growth by accelerating the degradation of reactive oxygen species (15), by increasing the activity of ATP/ADP translocase (16), or by interacting with the androgen receptor (38). To our knowledge, the data in this study have shown for the first time that PGC-1α can increase the Bcl-2/Bax ratio notably and inhibit the activated caspase 3 fragments, which may lead to the survival of more cells in the ischemic hindlimb of diabetic rats.
Diabetic PAD is a systemic disease characterized by severe impairment of angiogenesis (39), which may provide a worse living condition for transplanted cells compared with healthy PAD. The downregulation of proangiogenic factors, such as VEGF and FGF-2, plays an important role in the impaired angiogenesis of diabetes (40,41). So the increased level of proangiogenic factors in diabetic ischemic hindlimb can improve blood perfusion greatly (21,40–42). Our data have indicated that the above effects of overexpressing PGC-1α on MSCs may contribute to survival improvement of transplanted MSCs and promote high-level secretion of proangiogenic factors. This may lead to a greater increase of capillary density and blood perfusion in diabetic ischemic hindlimb.
Although the PGC-1α–modified MSCs demonstrated therapeutic angiogenesis by improving the engraftment of transplanted MSCs and secreting a higher level of proangiogenic factors in this study, whether the MSCs can differentiate and incorporate into several tissues or whether they can promote myofiber regeneration is still unknown. Longitudinal and further studies are required to explore these issues and possible related mechanisms.
To our knowledge, the current study demonstrated for the first time that overexpression of PGC-1α enhances the survival and angiogenic potential of MSCs, and transplantation of MSCs modified with PGC-1α can cause significantly greater improvement in diabetic hindlimb ischemia than transplantation of MSCs, or transplantation of MSCs modified with GFP.
ACKNOWLEDGMENTS
B.C. was supported by a grant from Trauma, Burns, and Combined Injury, State Key Laboratory Open Foundation, Third Military Medical University.
No potential conflicts of interest relevant to this article were reported.
D.L. researched data, contributed to discussion, and wrote the manuscript. L.Z., H.W., Y.Z., J.L., J.X., Z.L., W.D., Y.J., Q.W., S.L., and Z.A. researched data. Y.Z., H.L., and F.G. wrote the manuscript. Y.Y. researched data and wrote the manuscript. Z.Z. contributed to discussion and wrote the manuscript. B.C. researched data, contributed to discussion, and reviewed and edited the manuscript. All authors read and approved the final paper. B.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Daniel P. Kelly and Teresa C. Leone (Sanford-Burnham Medical Research Institute) for providing the Ad-GFP-PGC-1α adenovirus.
Footnotes
See accompanying commentary, p. 979.
REFERENCES
- 1.Newton KM, Wagner EH, Ramsey SD, et al. The use of automated data to identify complications and comorbidities of diabetes: a validation study. J Clin Epidemiol 1999;52:199–207 [DOI] [PubMed] [Google Scholar]
- 2.O’Loughlin A, McIntosh C, Dinneen SF, O’Brien T. Review paper: basic concepts to novel therapies: a review of the diabetic foot. Int J Low Extrem Wounds 2010;9:90–102 [DOI] [PubMed] [Google Scholar]
- 3.Kamihata H, Matsubara H, Nishiue T, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation 2001;104:1046–1052 [DOI] [PubMed] [Google Scholar]
- 4.Johannesson A, Larsson GU, Ramstrand N, Turkiewicz A, Wiréhn AB, Atroshi I. Incidence of lower-limb amputation in the diabetic and nondiabetic general population: a 10-year population-based cohort study of initial unilateral and contralateral amputations and reamputations. Diabetes Care 2009;32:275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008;8:726–736 [DOI] [PubMed] [Google Scholar]
- 6.Kinnaird T, Stabile E, Burnett MS, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res 2004;94:678–685 [DOI] [PubMed] [Google Scholar]
- 7.Tang J, Xie Q, Pan G, Wang J, Wang M. Mesenchymal stem cells participate in angiogenesis and improve heart function in rat model of myocardial ischemia with reperfusion. Eur J Cardiothorac Surg 2006;30:353–361 [DOI] [PubMed] [Google Scholar]
- 8.Honma T, Honmou O, Iihoshi S, et al. Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Exp Neurol 2006;199:56–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinnaird T, Stabile E, Burnett MS, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 2004;109:1543–1549 [DOI] [PubMed] [Google Scholar]
- 10.Lu D, Chen B, Liang Z, et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract 2011;92:26–36 [DOI] [PubMed] [Google Scholar]
- 11.Lasala GP, Silva JA, Gardner PA, Minguell JJ. Combination stem cell therapy for the treatment of severe limb ischemia: safety and efficacy analysis. Angiology 2010;61:551–556 [DOI] [PubMed] [Google Scholar]
- 12.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002;105:93–98 [DOI] [PubMed] [Google Scholar]
- 13.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998;92:829–839 [DOI] [PubMed] [Google Scholar]
- 14.Lin JD. Minireview: the PGC-1 coactivator networks: chromatin-remodeling and mitochondrial energy metabolism. Mol Endocrinol 2009;23:2–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valle I, Alvarez-Barrientos A, Arza E, Lamas S, Monsalve M. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc Res 2005;66:562–573 [DOI] [PubMed] [Google Scholar]
- 16.Won JC, Park JY, Kim YM, et al. Peroxisome proliferator-activated receptor-γ coactivator 1-α overexpression prevents endothelial apoptosis by increasing ATP/ADP translocase activity. Arterioscler Thromb Vasc Biol 2010;30:290–297 [DOI] [PubMed] [Google Scholar]
- 17.Arany Z, Foo SY, Ma Y, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1α. Nature. 2008;451:1008–1012 [DOI] [PubMed] [Google Scholar]
- 18.Carmeliet P, Baes M. Metabolism and therapeutic angiogenesis. N Engl J Med 2008;358:2511–2512 [DOI] [PubMed] [Google Scholar]
- 19.Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc 2009;4:102–106 [DOI] [PubMed] [Google Scholar]
- 20.McMahon JM, Conroy S, Lyons M, et al. Gene transfer into rat mesenchymal stem cells: a comparative study of viral and nonviral vectors. Stem Cells Dev 2006;15:87–96 [DOI] [PubMed] [Google Scholar]
- 21.Hirata K, Li T-S, Nishida M, et al. Autologous bone marrow cell implantation as therapeutic angiogenesis for ischemic hindlimb in diabetic rat model. Am J Physiol Heart Circ Physiol 2003;284:H66–H70 [DOI] [PubMed] [Google Scholar]
- 22.Murohara T, Ikeda H, Duan J, et al. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest 2000;105:1527–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shintani S, Murohara T, Ikeda H, et al. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation 2001;103:897–903 [DOI] [PubMed] [Google Scholar]
- 24.Iwase T, Nagaya N, Fujii T, et al. Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovasc Res 2005;66:543–551 [DOI] [PubMed] [Google Scholar]
- 25.Ishii M, Numaguchi Y, Okumura K, et al. Mesenchymal stem cell-based gene therapy with prostacyclin synthase enhanced neovascularization in hindlimb ischemia. Atherosclerosis 2009;206:109–118 [DOI] [PubMed] [Google Scholar]
- 26.Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res 2011;109:923–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mangi AA, Noiseux N, Kong D, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med 2003;9:1195–1201 [DOI] [PubMed] [Google Scholar]
- 28.Noiseux N, Gnecchi M, Lopez-Ilasaca M, et al. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther 2006;14:840–850 [DOI] [PubMed] [Google Scholar]
- 29.Xu M, Uemura R, Dai Y, Wang Y, Pasha Z, Ashraf M. In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function. J Mol Cell Cardiol 2007;42:441–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fedak PW, Szmitko PE, Weisel RD, et al. Cell transplantation preserves matrix homeostasis: a novel paracrine mechanism. J Thorac Cardiovasc Surg 2005;130:1430–1439 [DOI] [PubMed] [Google Scholar]
- 31.Shibata T, Naruse K, Kamiya H, et al. Transplantation of bone marrow-derived mesenchymal stem cells improves diabetic polyneuropathy in rats. Diabetes 2008;57:3099–3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsumoto R, Omura T, Yoshiyama M, et al. Vascular endothelial growth factor-expressing mesenchymal stem cell transplantation for the treatment of acute myocardial infarction. Arterioscler Thromb Vasc Biol 2005;25:1168–1173 [DOI] [PubMed] [Google Scholar]
- 33.Haider HKh, Jiang S, Idris NM, Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1α/CXCR4 signaling to promote myocardial repair. Circ Res 2008;103:1300–1308 [DOI] [PubMed] [Google Scholar]
- 34.Sun L, Cui M, Wang Z, et al. Mesenchymal stem cells modified with angiopoietin-1 improve remodeling in a rat model of acute myocardial infarction. Biochem Biophys Res Commun 2007;357:779–784 [DOI] [PubMed] [Google Scholar]
- 35.O’Hagan KA, Cocchiglia S, Zhdanov AV, et al. PGC-1α is coupled to HIF-1α-dependent gene expression by increasing mitochondrial oxygen consumption in skeletal muscle cells. Proc Natl Acad Sci USA 2009;106:2188–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai Y, Xu M, Wang Y, Pasha Z, Li T, Ashraf M. HIF-1α induced-VEGF overexpression in bone marrow stem cells protects cardiomyocytes against ischemia. J Mol Cell Cardiol 2007;42:1036–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li W, Ma N, Ong L-L, et al. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells 2007;25:2118–2127 [DOI] [PubMed] [Google Scholar]
- 38.Shiota M, Yokomizo A, Tada Y, et al. Peroxisome proliferator-activated receptor γ coactivator-1α interacts with the androgen receptor (AR) and promotes prostate cancer cell growth by activating the AR. Mol Endocrinol 2010;24:114–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benjamin LE. Glucose, VEGF-A, and diabetic complications. Am J Pathol 2001;158:1181–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rivard A, Silver M, Chen D, et al. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol 1999;154:355–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stark J, Baffour R, Garb JL, et al. Basic fibroblast growth factor stimulates angiogenesis in the hindlimb of hyperglycemic rats. J Surg Res 1998;79:8–12 [DOI] [PubMed] [Google Scholar]
- 42.Biscetti F, Straface G, De Cristofaro R, et al. High-mobility group box-1 protein promotes angiogenesis after peripheral ischemia in diabetic mice through a VEGF-dependent mechanism. Diabetes 2010;59:1496–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]