Abstract
High-fat feeding inhibits pyruvate dehydrogenase complex (PDC)–controlled carbohydrate (CHO) oxidation, which contributes to muscle insulin resistance. We aimed to reveal molecular changes underpinning this process in resting and exercising humans. We also tested whether pharmacological activation of PDC overrides these diet-induced changes. Healthy males consumed a control diet (CD) and on two further occasions an isocaloric high-fat diet (HFD). After each diet, subjects cycled for 60 min after intravenous infusion with saline (CD and HFD) or dichloroacetate (HFD+DCA). Quadriceps muscle biopsies obtained before and after 10 and 60 min of exercise were used to estimate CHO use, PDC activation, and mRNAs associated with insulin, fat, and CHO signaling. Compared with CD, HFD increased resting pyruvate dehydrogenase kinase 2 (PDK2), PDK4, forkhead box class O transcription factor 1 (FOXO1), and peroxisome proliferator–activated receptor transcription factor α (PPARα) mRNA and reduced PDC activation. Exercise increased PDC activation and whole-body CHO use in HFD, but to a lower extent than in CD. Meanwhile PDK4 and FOXO1, but not PPARα or PDK2, mRNA remained elevated. HFD+DCA activated PDC throughout and restored whole-body CHO use during exercise. FOXO1 appears to play a role in HFD-mediated muscle PDK4 upregulation and inhibition of PDC and CHO oxidation in humans. Also, pharmacological activation of PDC restores HFD-mediated inhibition of CHO oxidation during exercise.
Several days of high-fat diet (HFD) intake reduces the rate of muscle glycogen degradation and glucose oxidation during low to moderate-intensity exercise in humans (1,2), and has been attributed to the impairment of exercise-mediated activation of the pyruvate dehydrogenase complex (PDC) resulting from increased blood free fatty acid (FFA) availability (3). The PDC controls the rate of skeletal muscle carbohydrate (CHO) oxidation, and therefore this dietary-mediated impairment of PDC activation has been advocated as a causative factor in skeletal muscle insulin resistance and the metabolic syndrome (4).
The activation status of the PDC is controlled by a covalent mechanism involving competing pyruvate dehydrogenase kinase (PDK) and phosphatase (PDP) reactions (5). The resulting interconversion cycle determines the amount of PDC existing in nonphosphorylated (active) form, i.e., PDCa (6). The PDK family is composed of four isoforms (PDK1–4) (7), whereas PDP has two isoforms (PDP1 and 2) (8). Although PDK2 and PDK4 mRNA are expressed in most tissues, including skeletal muscle and heart, the specific activity of PDK4 is eightfold greater than that of PDK2 (7), thereby assigning greater regulatory significance to PDK4. PDK1 and PDK3 appear to be limited to heart, pancreatic islets, and kidney (7). The principal physiological factors that regulate PDK4 expression in resting skeletal muscle are changes in FFA and insulin availability, which explains why PDK4 mRNA is selectively upregulated in response to starvation and hormonal and substrate changes, and in pathologies such as insulin resistance and type 2 diabetes (9–11). Conversely, inhibition of PDK2 using synthetic inhibitors seems to improve blood glucose concentration in obese Zucker rats (12). However, the mechanism by which FFAs upregulate PDK4 expression, thereby inhibiting PDC-controlled CHO oxidation in humans, is still unclear.
Based upon knowledge acquired from mainly cell and animal-based studies, it has been suggested that activation of peroxisome proliferator–activated receptor transcription factors (PPARα, δ, and γ) by ligands, such as FFAs (13), might be a mechanism responsible for the upregulation of muscle PDK4 mRNA expression (3,14–17). However, the more rapid increase in PDK4 mRNA expression compared with PPARα mRNA expression after administration of a PPARα receptor agonist partly speaks against this stance (18). Furthermore, more recently, the clear dissociation between increased plasma FFA levels and muscle PDK4 mRNA expression, together with the lack of any change in muscle PPARα mRNA or protein expression during a 40-h fast in humans (19), suggests other factor(s) could be responsible for the increase in PDK4 mRNA expression under these conditions. Since FFAs can also indirectly induce the translocation of forkhead box class O (FOXO) transcription factors 1 and 3 to the nucleus (20,21), and FOXO1 can bind directly to the promoter region of the PDK4 gene (20), it is plausible that FOXO factors could also play an important role in promoting the upregulation of PDK4 mRNA in response to increased FFA availability.
The activation of PDC during muscle contraction is achieved by the accumulation of mitochondrial calcium and pyruvate (22). They function by activating PDP and inhibiting PDK2 and 4, respectively, and jointly appear to be able to fully activate PDC at exercise intensities of 75% maximal oxygen consumption and above (6,23). However, as outlined above, when exercise at this workload is preceded by several days of HFD intake, calcium and pyruvate seem unable to activate PDC to the same extent as in the control condition (1,2), although they may at lower exercise intensities (24), resulting in reduced CHO oxidation compared with control at exercise intensities where muscle glycogen is an important contributor to energy production. Dichloroacetate (DCA) is a more potent pharmacological inhibitor of PDK2 and 4 protein than pyruvate (7,25), and can fully activate muscle PDC at rest in humans (26). To date, however, no study has determined whether DCA administration at rest can offset the reported HFD-mediated PDK2 and/or PDK4 inhibition of PDC activation and CHO oxidation during subsequent exercise in humans.
The novelty of the current study is that we have concurrently determined changes in PPAR (α, δ, γ) and FOXO (1 and 3) transcription factor mRNA expression at rest and during exercise after 3 days of control or HFD intake. We have also attempted to interpret the significance of these diet-induced changes to muscle PDK2 and 4 mRNA expression, PDC activation, and CHO oxidation during submaximal exercise in human volunteers. Furthermore, we have determined whether the pharmacological inhibition of PDK2 and 4 using DCA could offset any HFD-mediated inhibition of PDC activation and CHO oxidation during exercise. By revealing molecular changes associated with HFD-mediated inhibition of muscle CHO oxidation during exercise in humans, and testing a pharmacological approach to bypass and counteract this diet-induced effect, this work is of clear importance to our understanding and treatment of human muscle insulin resistance.
RESEARCH DESIGN AND METHODS
Subjects.
Six untrained, healthy male volunteers (age, body mass, BMI [mean ± SEM]: 22.0 ± 0.6 years, 79.5 ± 1.6 kg, 24.9 ± 0.8 kg/m2) participated in the current study, which was approved by the University of Nottingham Medical School Ethics Committee in accordance with the Declaration of Helsinki. Before taking part in the study, all subjects underwent routine medical screening and completed a general health questionnaire.
Study protocol.
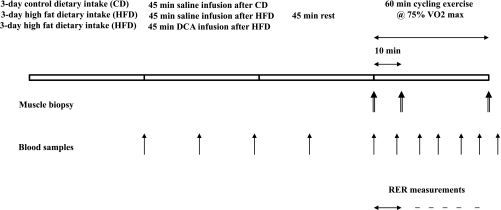
After entry into the study, maximal oxygen consumption (Vo2max) was determined and then verified on a separate occasion in all volunteers. Each subject then completed an exercise familiarization visit consisting of 60 min of cycling at 75% Vo2max, which also allowed confirmation of the accuracy of this workload. Immediately after this, subjects received meal plans for a control diet (CD) for 3 days (55% CHO, 30% fat, and 15% protein) and recorded their dietary intake via diet diaries. Subjects had to weigh and record all food intake, which allowed macronutrient and caloric intake to be calculated using the Microdiet program (Downlee System Ltd., Chapel-en-le-Frith, U.K.). On two further occasions, the volunteers consumed a prescribed isocaloric HFD (10% CHO, 75% fat, 15% protein) (see Fig. 1) each for 3 days. The energy content of this diet was based upon data collected during the 3-day CD. The fat diet trials were administered in a randomized design. All three trials were separated by 2-week washout intervals.
FIG. 1.
Study protocol. Six healthy male subjects consumed a prescribed CD for 3 days (55% CHO, 30% fat, and 15% protein), and on two further occasions a prescribed isocaloric HFD (10% CHO, 75% fat, and 15% protein), each for 3 days. The fat diets were administered in a randomized manner separated by 2-week intervals. The morning after each period of dietary intervention, subjects cycled at 75% Vo2max for 60 min after intravenous infusion with either saline (CD and HFD) or the PDC activator DCA (50 mg/kg body mass; HFD+DCA). Quadriceps muscle biopsy samples were obtained immediately before (0) and after 10 and 60 min of exercise. Throughout the infusion and subsequent exercise periods, blood samples were collected at the time points indicated in the figure.
The morning after each 3-day period of dietary manipulation, subjects reported to the laboratory having fasted overnight. On each occasion, a cannula was inserted into a vein on the dorsum of a heated hand, after administration of local anesthetic (1% lignocaine), to allow infusion of saline or the PDC activator DCA and sampling of arterialized venous blood using a three-way tap. Subjects then rested in a semisupine position while they underwent 45 min of intravenous infusion with either saline (CD and HFD) or DCA (50 mg/kg body mass, HFD+DCA), and then rested for a further 45 min. Arterialized venous blood samples were collected at predetermined intervals throughout this 90-min period (Fig. 1). After this, a resting biopsy sample (27) was obtained from the vastus lateralis muscle, and subjects immediately began exercising at 75% Vo2max on an electrically braked cycle ergometer (Lode, Groningen, the Netherlands). Subjects momentarily stopped cycling after 10 min of exercise, and a second muscle biopsy sample was obtained while the subject remained seated (and supported) on the cycle ergometer. This biopsy was required because the exercise-mediated increase in PDC activation occurs predominantly within the first 10 min of exercise at this workload (6). Subjects then continued cycling at 75% Vo2max for a further 50 min, and a third muscle biopsy was obtained immediately after the termination of exercise while the subject was seated on the cycle ergometer. Expired gas (Vmax Encore; SensorMedics, San Diego, CA) and blood samples were collected at predetermined intervals throughout exercise to determine oxygen consumption and carbon dioxide production rates and blood and plasma metabolites, respectively (Fig. 1).
Blood glucose and lactate.
Immediately after blood sample collection, glucose and lactate concentrations were measured in whole blood using a glucose/lactate analyzer (YSI 2300 STATplus; Yellow Springs Instruments, Yellow Springs, OH).
Plasma FFAs.
After collection into EDTANa2 tubes, whole blood was immediately centrifuged and the plasma snap frozen in liquid nitrogen. This was used to determine plasma FFA concentrations at a later date using an enzymatic colorimetric assay kit (NEFA C kit; Wako Chemicals, Neuss, Germany).
Muscle metabolites and PDC activity.
Snap-frozen vastus lateralis muscle was subsequently divided into two parts while under liquid nitrogen. One part was freeze dried, dissected free from visible connective tissue and blood, and used to extract muscle metabolites (28). Free carnitine and acetylcarnitine were measured in the muscle extract using enzymatic assays as previously described (29). Muscle glycogen and lactate concentrations were measured using a modification of a spectrophotometric method (28). The remainder of the frozen muscle was used to determine PDC activity as previously described (30).
Muscle mRNA expression.
RNA was extracted from the snap-frozen muscle tissue using RNA Plus (Qbiogene). First-strand cDNA was synthesized from a 1-μg RNA sample using Powerscript reverse transcriptase (BD Biosciences). All reactions were performed using the ABI Prism 7000 Sequence Detection System (Applied Biosystems Foster City, CA). The genes analyzed were PDK2 and 4, PDP1 and 2 (PPM2C), insulin receptor substrate 1 (IRS1), v-akt murine thymoma viral oncogene homolog 1 (Akt1), FOXO1 and 3, PPARα, β, and γ, and PPARγ coactivator-1 α isoform (PGC-1α). The duplicate cycle threshold (Ct) values were averaged and the ΔCt was calculated by subtracting the corresponding mean value of the housekeeping gene (porphobilinogen deaminase). 2−ΔΔCt was measured by 2 to the power of the difference in Ct between samples. The control trial (CD) at rest was used as calibrator with a value of 1.
Calculations and statistics.
CHO oxidation rates (g/min) were calculated using the equation of Frayn (31): 4.55 × VCO2 (L/min) – 3.21 × Vo2 (L/min) – 0.459 × Pox, where Pox is the protein oxidation rate, which was calculated using the following equation: Pox (g/min) = 0.12 × energy expenditure (kJ/min)/16.74 kJ/g, assuming that protein oxidation contributed ∼12% of energy expenditure (31). Energy expenditure was calculated from the rates of Vo2 assuming 5 kcal or 22 kJ for each 1 L of oxygen consumed.
One- and two-way repeated ANOVA was used to determine treatment and time main effects. When a significant F ratio was obtained, a least significance difference post hoc test was applied to locate specific differences. Significance was set at the P < 0.05 level of confidence. Wherever indicated, the correlation coefficients between two variables or degree of linear relationship were obtained using Pearson product moment correlation. Unless otherwise stated, all data are expressed as mean ± SEM.
RESULTS
Rates of whole-body CHO oxidation during exercise.
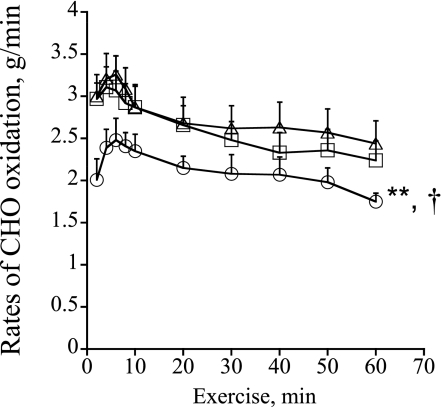
The rates of CHO oxidation during exercise (g/min) are presented in Fig. 2. After an initial increase during the first 10 min of exercise in all treatment groups, the rate of CHO oxidation decreased continuously over time. CHO oxidation after the HFD was significantly lower throughout exercise compared with the CD and HFD+DCA treatments (P < 0.01 and P < 0.05, respectively; treatment effect). There was no difference between CD and HFD+DCA.
FIG. 2.
Rates of CHO oxidation (g/min) during 60 min of exercise at 75% Vo2max. Six healthy male subjects consumed a prescribed CD for 3 days, and on two further occasions a prescribed isocaloric HFD, each for 3 days. The morning after each period of dietary intervention, subjects cycled at 75% Vo2max for 60 min after intravenous infusion with either saline (CD+saline, △; HFD+saline, ○) or DCA (HFD+DCA, □). **Significantly different from the CD trial (P < 0.01). †Significantly different from the HFD+DCA trial (P < 0.05).
Blood and plasma metabolite concentrations.
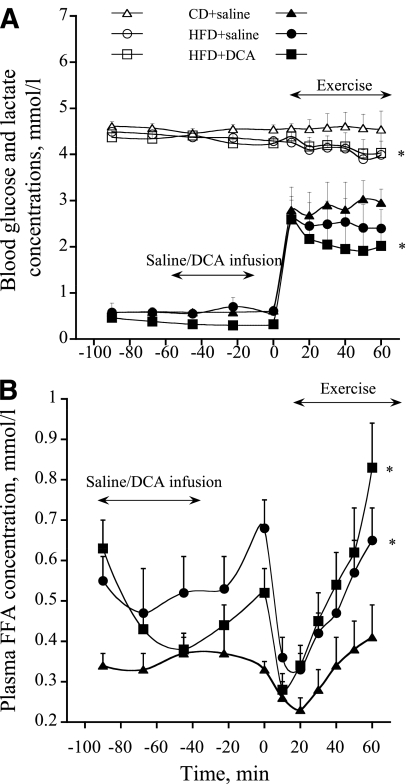
Resting blood glucose concentration during intravenous infusion of saline and DCA was no different between treatment groups (Fig. 3A). During exercise, blood glucose concentration declined progressively with time in HFD and HFD+DCA when compared with CD (P < 0.05; treatment effect). Resting blood lactate concentration declined steadily in HFD+DCA over the course of infusion compared with the other two treatment groups (P < 0.05; treatment effect) (Fig. 3A). During exercise, blood lactate concentration increased in all treatment groups, but the magnitude of increase in HFD+DCA was significantly less than in CD (P < 0.05; treatment effect) (Fig. 3A). Plasma FFA concentration was significantly lower in CD during resting infusion and throughout exercise compared with HFD and HFD+DCA (P < 0.05; treatment effect) (Fig. 3B).
FIG. 3.
A: Whole-blood glucose (white symbols) and lactate (black symbols) concentrations at rest during intravenous infusion of either saline or DCA and during a subsequent 60-min bout of exercise at 75% Vo2max. Six healthy male subjects consumed a prescribed CD for 3 days, and on two further occasions a prescribed isocaloric HFD, each for 3 days. The morning after each period of dietary intervention, subjects cycled at 75% Vo2max for 60 min after intravenous infusion with either saline (CD+saline and HFD+saline) or DCA (HFD+DCA). *Significantly different from the CD trial (P < 0.05). B: Plasma FFA concentrations during 45 min of intravenous infusion of a saline or DCA solution followed by 60 min of exercise at 75%Vo2max. Six healthy male subjects consumed a prescribed CD for 3 days, and on two further occasions a prescribed isocaloric HFD, each for 3 days. The morning after each period of dietary intervention, subjects cycled at 75% Vo2max for 60 min after intravenous infusion with either saline (CD+saline and HFD+saline) or DCA (HFD+DCA). *Significantly different from the CD trial (P < 0.05).
Muscle metabolites and PDC activity.
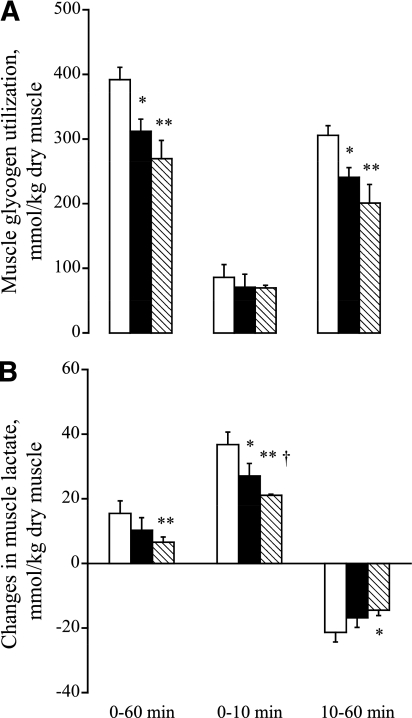
Resting, pre-exercise muscle glycogen content was significantly greater in CD compared with HFD and HFD+DCA (550 ± 31 vs. 402 ± 27 and 368 ± 24 mmol/kg dry muscle (dm), respectively; P < 0.05 and P < 0.01). Muscle glycogen utilization during exercise is shown in Fig. 4A. Glycogen breakdown over 60 min of exercise was significantly lower in the HFD and HFD+DCA than in CD (P < 0.05 and P < 0.01, respectively). There was no difference in muscle glycogen utilization between HFD and HFD+DCA. These between-group differences were mainly accounted for by events that occurred between 10 and 60 min of exercise (Fig. 4A).
FIG. 4.
A: Muscle glycogen use (mmol/kg dm) during 0–60, 0–10, and 10–60 min of exercise at 75% Vo2max. Six healthy male subjects consumed a prescribed CD, and on two further occasions a prescribed isocaloric HFD, each for 3 days. The morning after each period of dietary intervention, subjects cycled at 75% Vo2max for 60 min after intravenous infusion with either saline (CD+saline, □; HFD+saline, ■) or DCA (HFD+DCA, hatched bars). *,**Significantly different from CD trial (P < 0.05 and P < 0.01). B: Rates of lactate accumulation (mmol/kg dm) in human vastus lateralis muscle during 0–60, 0–10, and 10–60 min of exercise at 75% Vo2max. Six healthy male subjects consumed a prescribed CD, and on two further occasions a prescribed isocaloric HFD, each for 3 days. The morning after each period of dietary intervention, subjects cycled at 75% Vo2max for 60 min after intravenous infusion with either saline (CD+saline, □; HFD+saline, ■) or DCA (HFD+DCA, hatched bars). *,**Significantly different from resting value (P < 0.05 and P < 0.01). †Significantly different from the corresponding time point in the CD trial (P < 0.05).
Resting muscle lactate content was lower in HFD+DCA compared with CD (P < 0.05) and HFD (P < 0.05) (5.4 ± 0.8, 7.7 ± 0.8, and 7.5 ± 0.6 mmol/kg dm, respectively). Muscle lactate content increased during the first 10 min of exercise in all groups, although at different rates. Thus, although muscle lactate accumulation during exercise in CD was significantly greater than in HFD and HFD+DCA (P < 0.05 and P < 0.01, respectively), lactate accumulation in HFD+DCA was less than in HFD (P < 0.05). Muscle lactate content declined between 10 and 60 min of exercise in all groups (Fig. 4B), although the decline in HFD+DCA was significantly less than in CD (P < 0.05).
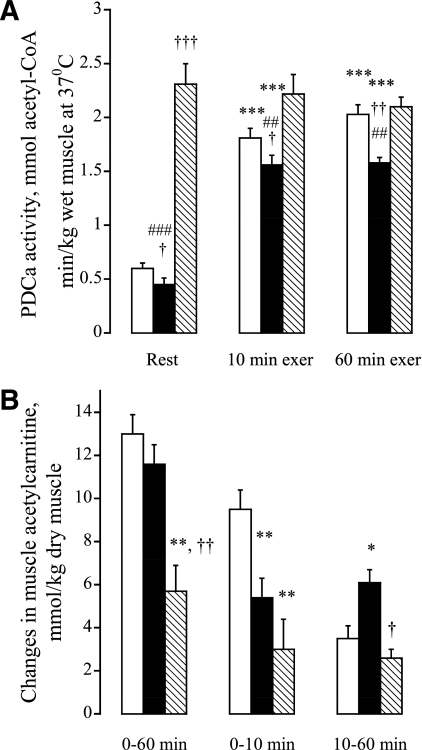
Muscle PDC activity (expressed as amount of active form, PDCa) at rest and during exercise is shown in Fig. 5A. HFD significantly reduced resting muscle PDCa compared with CD (P < 0.05) (Fig. 5A). However, HFD+DCA resulted in PDCa being markedly increased above CD and HFD at rest (P < 0.01 and P < 0.01, respectively). The magnitude of increase in PDCa after 10 min of exercise was significantly less in HFD compared with CD and HFD+DCA (P < 0.05 and P < 0.01, respectively). A further increase in PDCa was observed in CD over the remaining 50 min of exercise, but PDCa remained unchanged over this period in HFD and HFD+DCA, resulting in PDCa remaining significantly lower in HFD compared with CD and HFD+DCA by the end of exercise (P < 0.01 and P < 0.01, respectively).
FIG. 5.
A: Activity of PDCa (mmol/kg dm) in human vastus lateralis muscle at rest, and after 10 and 60 min of exercise at 75% Vo2max. Six healthy male subjects consumed a prescribed CD for 3 days, and on two further occasions a prescribed isocaloric HFD, each for 3 days. The morning after each period of dietary intervention, subjects cycled at 75% Vo2max for 60 min after intravenous infusion with either saline (CD+saline, □; HFD+saline, ■) or DCA (HFD+DCA, hatched bars). ***Significantly different from the resting value within the trial (P < 0.01). †,††,†††Significantly different from the corresponding time point in the CD trial (P < 0.05, P < 0.01, and P < 0.001). ##,###Significantly different from the corresponding time point in the HFD+DCA trial (P < 0.05, P < 0.01, and P < 0.001). B: Rates of acetyl-carnitine accumulation (mmol/kg dm) in human vastus lateralis muscle during 60 min (0–10 and 10–60 min) of exercise at 75% Vo2max. Six healthy male subjects consumed a prescribed CD for 3 days, and on two further occasions a prescribed isocaloric HFD, each for 3 days. The morning after each period of dietary intervention, subjects cycled at 75% Vo2max for 60 min after intravenous infusion with either saline (CD+saline, □; HFD+saline, ■) or DCA (HFD+DCA, hatched bars). *,**Significantly different from the CD trial (P < 0.05 and P < 0.01). †,††Significantly different from the HFD+saline trial (P < 0.05).
Resting muscle acetylcarnitine content in HFD and HFD+DCA was significantly greater than in CD (3.9 ± 0.4, 10.8 ± 0.5, and 2.0 ± 0.5 mmol/kg dm, respectively; P < 0.05 and P < 0.01). Acetylcarnitine accumulation during exercise is presented in Fig. 5B. Although DCA infusion is likely to have acetylated a large portion of cellular free carnitine pool at rest prior to the exercise (32), the magnitude of muscle acetylcarnitine accumulation during exercise in HFD+DCA was significantly less than in CD and HFD (P < 0.01 and P < 0.05, respectively). The exercise-induced increase in muscle acetylcarnitine in CD occurred mainly over the first 10 min of exercise, which was not the case in HFD+saline and HFD+DCA, where the magnitude of accumulation was approximately the same between 0–10 and 10–60 min, albeit approximately twice as high in HFD+saline as in HFD+DCA (P < 0.05).
Muscle mRNA expression.
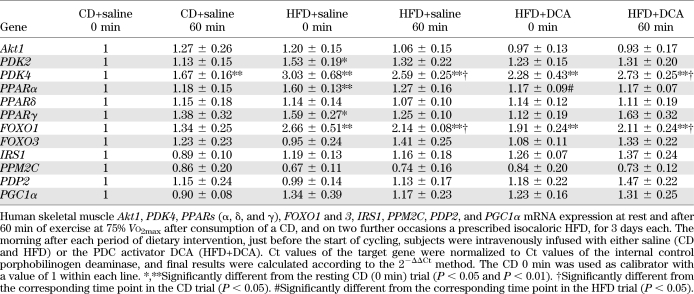
mRNA expression levels of selected signaling proteins from pathways thought to regulate muscle fat and CHO metabolism (PDK2 and 4, PPARα, δ, and γ, Akt1, IRS1, FOXO1 and 3, PDP1 and 2, and PGC-1α) are presented in Table 1. The abundance of PDK4 and FOXO1 mRNA transcripts was significantly increased in muscle at rest after 3 days of HFD (P < 0.01 and P < 0.01, respectively) and HFD+DCA (P < 0.01 and P < 0.01, respectively) compared with CD. Although of lower magnitude, muscle PDK2 (P < 0.05), PPARα (P < 0.01), and PPARγ (P < 0.05) mRNA expression were also significantly increased in HFD compared with CD at rest, but this was not the case for HFD+DCA. Furthermore, the expression of PPARα in resting muscle was significantly lower in HFD+DCA compared with HFD (P < 0.05). After 60 min of exercise, muscle PDK4 and FOXO1, but not PDK2, mRNA expressions were still greater in HFD (P < 0.01 and P < 0.01, respectively) and HFD+DCA (P < 0.01 and P < 0.01, respectively) compared with CD at 0 min. Even when compared with CD at 60 min of exercise, muscle PDK4 and FOXO1 mRNA expressions were still greater in HFD (P < 0.05 and P < 0.05, respectively) and HFD+DCA (P < 0.05 and P < 0.05, respectively). The exercise per se did increase PDK4, but not PDK2 and FOXO1, mRNA expression in CD (P < 0.01). Conversely, mRNA expression levels of all three PPAR isoforms were no different between treatment groups after 60 min of exercise.
TABLE 1.
Human skeletal muscle mRNA expression
DISCUSSION
The current study demonstrated for the first time that consumption of an HFD for 3 days concurrently increased FOXO1 (threefold), PPARα (1.6-fold), PPARγ (1.6-fold), PDK2 (1.5-fold), and PDK4 (threefold) mRNA expression in human skeletal muscle in the resting state compared with an isocaloric CD, while simultaneously reducing muscle PDC activity. In line with these observations, the magnitude of PDC activation and the extent of whole-body CHO oxidation and muscle glycogen use were reduced by the HFD during subsequent exercise at 75% of Vo2max. However, although the HFD-mediated upregulation of FOXO1 and PDK4 mRNA expression seen at rest was maintained during exercise, this was not the case for PPARα and γ expression. Finally, DCA infusion abolished this HFD-mediated inhibition of muscle PDC activation seen at rest and during exercise, and increased the rate of whole-body CHO oxidation during exercise toward that seen after the CD, but did not affect the HFD-mediated changes in muscle gene expression, with the exception of a small, but significant, reduction in PPARα at rest. Jointly, these data point to a role for FOXO1 in HFD-mediated upregulation of muscle PDK4, and thereby the inhibition of PDC activation and CHO oxidation during exercise in humans. Furthermore, pharmacological activation of PDC restores HFD-mediated inhibition of CHO oxidation during exercise, and independently of diet-mediated changes in muscle gene expression.
Previous human volunteer studies have shown that the increase in circulating FFA concentration after short-term (days) HFD intake is associated with increased whole-body fat oxidation and reduced CHO use during subsequent submaximal intensity exercise (1,2). However, despite these changes, consumption of an HFD did not increase exercise performance nor did it have any apparent health benefit because it decreased resting muscle and liver glycogen content compared with a CD (33,34), and increased muscle PDK4 mRNA and protein expression (3), which has been implicated as being a causative factor in muscle insulin resistance and the metabolic syndrome (4). Although we did not measure insulin resistance directly, consumption of an HFD for 3 days has been shown to induce whole-body insulin resistance (3,35). Furthermore, in the current study, we observed changes in CHO oxidation rates (Fig. 2), muscle glycogen use, and PDC activation during exercise (Fig. 4A and Fig. 5A), consistent with the induction of at least peripheral (muscle) insulin resistance.
Short-term increases in circulating insulin concentration under “normal” dietary conditions, together with an increase in muscle Ca2+ and pyruvate availability during exercise, appear to be the main physiological activators of muscle PDC in humans, and they act by controlling PDH kinase and phosphatase activity, respectively. Chronically, they may also control PDC by suppressing muscle PDK4 mRNA and protein expression (6,22,36). Conversely, an increase in systemic circulating FFA availability, as seen after HFD intake and in type 2 diabetes, is associated with an increase in muscle PDK4 mRNA and protein expression, thereby inhibiting activity of PDC. The latter effect could be attributable to either a direct FFA-mediated activation of PPARα, δ, or γ receptors (14,17,37) or an indirect FFA-mediated activation of the forkhead/winged helix box family gene–coded transcription factors, FOXO1 or FOXO3 (20,38), followed by direct binding to the promoter region of the PDK4 gene (20).
Evidence from cell and animal work suggest that molecular responses to increased circulating FFA availability include activation of PPARs and PGC-1, especially PGC-1α (14,39,40). PPARs are members of the nuclear hormone receptor superfamily of ligand-activated transcription factors that interact with FFA and regulate the expression of genes involved in the transport, metabolism, and handling of FFAs within the cells (13). Of the three distinct PPAR subtypes that have been identified so far, i.e., PPARα, PPARδ (also known as PPARβ), and PPARγ (41), the signaling pathways via PPARα and δ have been suggested to be a component of the mechanism that increases PDK4 mRNA and protein expression during starvation and type 2 diabetes (10), and after pharmacological intervention with PPARα and δ agonists (14,17). Nevertheless, based on mRNA data at least, the present results appear to speak against a major involvement of the muscle PPARs in increasing muscle PDK4 mRNA expression, because the HFD was only associated with a modest increase in muscle PPARα and γ expression at rest, which further waned during exercise (Table 1). In line with our finding, Spriet et al. (19) did not see any change in PPARα mRNA and protein expression in human skeletal muscle over 40 h of starvation despite expression of PDK4 mRNA being 14-fold upregulated. However, in the absence of protein measurements in the present experiment, our interpretation cannot be fully substantiated.
Alternatively, rather than being a direct effect of increased circulating FFA availability per se, an increase in muscle PDK4 mRNA expression could also be accounted for by a reduction/impairment of IRS1 signaling after an HFD-induced decrease in insulin availability/sensitivity (42). FOXO1 transcription factor is known to be regulated by the phosphatidylinositol 3-kinase/Akt1 signaling pathway (43), which is a pathway known to be sensitive to circulating insulin and FFA availability in humans (44). Because FOXO1 can sense changes in availability of FFAs or insulin and relay the message downstream by modulating transcription of many skeletal muscle genes, including that of PDK4 (20), it could therefore inhibit PDC-controlled CHO oxidation. Indeed, recent rodent-based evidence suggests a causative link between changes in the expression of muscle PDK4 and levels of FOXO1 protein dephosphorylation in acute insulin-resistant states (45). The current study goes further by offering evidence for an increase in the transcriptional drive of FOXO1, but not FOXO3, and presumably of total protein after 3 days of HFD intake in humans. It is worth noting that when FOXO1 and 3 were transfected in HepG2 cells, marked hPDK4 induction (measured as relative luciferase activity) occurred, and mainly when the cells were transfected with FOXO3 (46).
In the current study, consumption of an isocaloric HFD for 3 days reduced the rate of muscle glycogen use (Fig. 4A) and the rate of whole-body CHO oxidation (Fig. 2) during exercise, which, in keeping with previous work (1–3), can be attributed to a reduction in exercise-induced PDC activation (Fig. 5A). However, infusion of the PDK inhibitor DCA abolished this HFD-mediated inhibition of PDC activity at rest and during exercise (Fig. 5A). It is worth noting that the marked activation of muscle PDC by DCA (Fig. 5A) occurred independently of diet or exercise-induced changes in muscle PDK4 mRNA expression (Table 1), and probably also protein expression (17). Although DCA infusion after the HFD did not increase muscle glycogen use during exercise compared with the HFD alone, it did reduce blood lactate concentration (Fig. 3A) and muscle lactate and acetylcarnitine accumulation during exercise (Fig. 4B and Fig. 5B) more than in any other group, suggesting more efficient oxidative CHO use, i.e., higher flux through the PDC reaction. Additionally, because muscle glycogen use was not altered by DCA compared with HFD alone (Fig. 4A), despite an increase in whole-body CHO oxidation, this suggests that DCA may have increasing PDC-mediated blood glucose extraction and oxidation (47). Alternatively, DCA might have also concurrently inhibited glycolysis (48), thereby resulting in a better matching of glycolytic flux and PDC flux. Finally, DCA may have also increased hepatic glucose oxidation (49). Overall, these events may have accounted for the observed improvement in whole-body CHO use during exercise in the HFD+DCA trial (Fig. 2) that occurred in the face of similar muscle glycogen use during exercise compared with the HFD+saline trial (Fig. 4A). Therefore, what appears to be novel here is that in human skeletal muscle, DCA has rescued the negative effects of HFD intake on CHO oxidation during submaximal exercise. This supports the stance that PDC plays a central role in the regulation of muscle fuel selection, particularly during exercise when fat and CHO oxidation rates are increased. Although, initially, DCA was presented as a potentially novel oral antidiabetic agent that could reduce blood glucose and lactate by inhibiting hepatic glucose synthesis and stimulating glucose clearance (12) and use by peripheral tissues (25), including skeletal muscle, concerns about its lack of tissue specificity and long-term safety has hampered its therapeutic use as an antidiabetic agent.
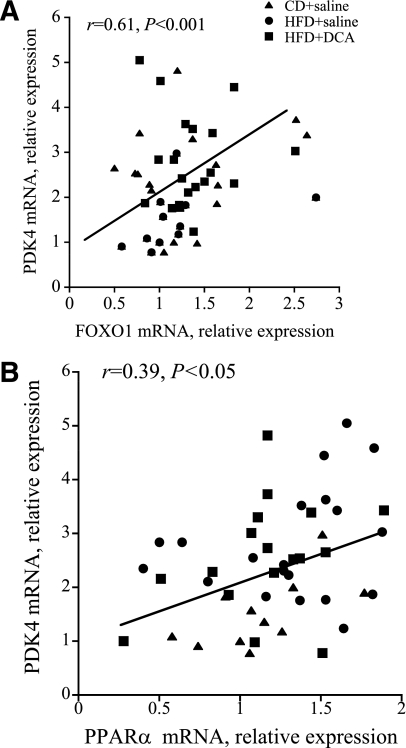
Although the current study cannot offer direct evidence of a causative relationship between changes in either FOXO1 or PPARα and PDK4 mRNA expression in human skeletal muscle, the strong relationship between FOXO1 and PDK4 mRNA expression at rest and during exercise (r = 0.61, P < 0.001) (Fig. 6A), together with the less robust association between PPARα and PDK4 mRNA expression (r = 0.39, P < 0.05) (Fig. 6B), supports the notion that FOXO1 played a more significant role than PPARα in the control of PDK4 expression and muscle CHO oxidation under the present experimental conditions. However, because the amount of FOXO1 protein and its phosphorylation status (covalent modification) are also likely to be important to any association between FOXO1 and PDK4, and also that the message levels of either FOXOs or PPARs will be subject to regulation by more than one mechanism, the present findings warrant further scrutiny by performing measurements at the protein level.
FIG. 6.
Pearson correlation between PDK4 and FOXO1 mRNA expression (A), and PDK4 and PPARα mRNA expression (B) in human vastus lateralis muscle at rest, and after 10 and 60 min of exercise at 75% Vo2max. Six healthy male subjects consumed a prescribed CD for 3 days, and on two further occasions a prescribed isocaloric HFD, each for 3 days. The morning after each period of dietary intervention, subjects cycled at 75% Vo2max for 60 min after intravenous infusion with either saline (CD+saline and HFD+saline) or DCA (HFD+DCA).
In conclusion, these data point to FOXO1 as an important metabolic regulator of muscle CHO and fat oxidation after HFD in humans by regulating muscle PDK4 expression and consequently PDC activity. The finding that DCA infusion prior to exercise overrode this HFD-mediated inhibition of PDC activation and CHO oxidation during exercise points to PDK4 inhibition as a viable pharmacological strategy for treating muscle insulin resistance in obesity and type 2 diabetes.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
D.C.-T. generated the experimental hypothesis and study design, researched data, and wrote and edited the manuscript. D.C. and F.S. researched data and reviewed and edited the manuscript. D.L. researched data. P.L.G. generated the experimental hypothesis and study design and wrote and edited the manuscript. D.C.-T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
See accompanying commentary, p. 983.
REFERENCES
- 1.Jansson E, Kaijser L. Effect of diet on the utilization of blood-borne and intramuscular substrates during exercise in man. Acta Physiol Scand 1982;115:19–30 [DOI] [PubMed] [Google Scholar]
- 2.Putman CT, Spriet LL, Hultman E, et al. Pyruvate dehydrogenase activity and acetyl group accumulation during exercise after different diets. Am J Physiol 1993;265:E752–E760 [DOI] [PubMed] [Google Scholar]
- 3.Peters SJ, Harris RA, Wu P, Pehleman TL, Heigenhauser GJ, Spriet LL. Human skeletal muscle PDH kinase activity and isoform expression during a 3-day high-fat/low-carbohydrate diet. Am J Physiol Endocrinol Metab 2001;281:E1151–E1158 [DOI] [PubMed] [Google Scholar]
- 4.Gorter PM, Olijhoek JK, van der Graaf Y, Algra A, Rabelink TJ, Visseren FL, SMART Study Group Prevalence of the metabolic syndrome in patients with coronary heart disease, cerebrovascular disease, peripheral arterial disease or abdominal aortic aneurysm. Atherosclerosis 2004;173:363–369 [DOI] [PubMed] [Google Scholar]
- 5.Wieland OH. The mammalian pyruvate dehydrogenase complex: structure and regulation. Rev Physiol Biochem Pharmacol 1983;96:123–170 [DOI] [PubMed] [Google Scholar]
- 6.Constantin-Teodosiu D, Cederblad G, Hultman E. PDC activity and acetyl group accumulation in skeletal muscle during prolonged exercise. J Appl Physiol 1992;73:2403–2407 [DOI] [PubMed] [Google Scholar]
- 7.Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J 1998;329:191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang B, Gudi R, Wu P, Harris RA, Hamilton J, Popov KM. Isoenzymes of pyruvate dehydrogenase phosphatase. DNA-derived amino acid sequences, expression, and regulation. J Biol Chem 1998;273:17680–17688 [DOI] [PubMed] [Google Scholar]
- 9.Huang B, Wu P, Bowker-Kinley MM, Harris RA. Regulation of pyruvate dehydrogenase kinase expression by peroxisome proliferator-activated receptor-alpha ligands, glucocorticoids, and insulin. Diabetes 2002;51:276–283 [DOI] [PubMed] [Google Scholar]
- 10.Wu P, Inskeep K, Bowker-Kinley MM, Popov KM, Harris RA. Mechanism responsible for inactivation of skeletal muscle pyruvate dehydrogenase complex in starvation and diabetes. Diabetes 1999;48:1593–1599 [DOI] [PubMed] [Google Scholar]
- 11.Sugden MC, Kraus A, Harris RA, Holness MJ. Fibre-type specific modification of the activity and regulation of skeletal muscle pyruvate dehydrogenase kinase (PDK) by prolonged starvation and refeeding is associated with targeted regulation of PDK isoenzyme 4 expression. Biochem J 2000;346:651–657 [PMC free article] [PubMed] [Google Scholar]
- 12.Mayers RM, Butlin RJ, Kilgour E, et al. AZD7545, a novel inhibitor of pyruvate dehydrogenase kinase 2 (PDHK2), activates pyruvate dehydrogenase in vivo and improves blood glucose control in obese (fa/fa) Zucker rats. Biochem Soc Trans 2003;31:1165–1167 [DOI] [PubMed] [Google Scholar]
- 13.Berger J, Wagner JA. Physiological and therapeutic roles of peroxisome proliferator-activated receptors. Diabetes Technol Ther 2002;4:163–174 [DOI] [PubMed] [Google Scholar]
- 14.Wu P, Peters JM, Harris RA. Adaptive increase in pyruvate dehydrogenase kinase 4 during starvation is mediated by peroxisome proliferator-activated receptor alpha. Biochem Biophys Res Commun 2001;287:391–396 [DOI] [PubMed] [Google Scholar]
- 15.Abbot EL, McCormack JG, Reynet C, Hassall DG, Buchan KW, Yeaman SJ. Diverging regulation of pyruvate dehydrogenase kinase isoform gene expression in cultured human muscle cells. FEBS J 2005;272:3004–3014 [DOI] [PubMed] [Google Scholar]
- 16.Degenhardt T, Saramäki A, Malinen M, et al. Three members of the human pyruvate dehydrogenase kinase gene family are direct targets of the peroxisome proliferator-activated receptor beta/delta. J Mol Biol 2007;372:341–355 [DOI] [PubMed] [Google Scholar]
- 17.Constantin D, Constantin-Teodosiu D, Layfield R, Tsintzas K, Bennett AJ, Greenhaff PL. PPARdelta agonism induces a change in fuel metabolism and activation of an atrophy programme, but does not impair mitochondrial function in rat skeletal muscle. J Physiol 2007;583:381–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato O, Kuriki C, Fukui Y, Motojima K. Dual promoter structure of mouse and human fatty acid translocase/CD36 genes and unique transcriptional activation by peroxisome proliferator-activated receptor alpha and gamma ligands. J Biol Chem 2002;277:15703–15711 [DOI] [PubMed] [Google Scholar]
- 19.Spriet LL, Tunstall RJ, Watt MJ, Mehan KA, Hargreaves M, Cameron-Smith D. Pyruvate dehydrogenase activation and kinase expression in human skeletal muscle during fasting. J Appl Physiol 2004;96:2082–2087 [DOI] [PubMed] [Google Scholar]
- 20.Furuyama T, Kitayama K, Yamashita H, Mori N. Forkhead transcription factor FOXO1 (FKHR)-dependent induction of PDK4 gene expression in skeletal muscle during energy deprivation. Biochem J 2003;375:365–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barreyro FJ, Kobayashi S, Bronk SF, Werneburg NW, Malhi H, Gores GJ. Transcriptional regulation of Bim by FoxO3A mediates hepatocyte lipoapoptosis. J Biol Chem 2007;282:27141–27154 [DOI] [PubMed] [Google Scholar]
- 22.Constantin-Teodosiu D, Peirce NS, Fox J, Greenhaff PL. Muscle pyruvate availability can limit the flux, but not activation, of the pyruvate dehydrogenase complex during submaximal exercise in humans. J Physiol 2004;561:647–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Constantin-Teodosiu D, Carlin JI, Cederblad G, Harris RC, Hultman E. Acetyl group accumulation and pyruvate dehydrogenase activity in human muscle during incremental exercise. Acta Physiol Scand 1991;143:367–372 [DOI] [PubMed] [Google Scholar]
- 24.St Amand TA, Spriet LL, Jones NL, Heigenhauser GJ. Pyruvate overrides inhibition of PDH during exercise after a low-carbohydrate diet. Am J Physiol Endocrinol Metab 2000;279:E275–E283 [DOI] [PubMed] [Google Scholar]
- 25.Stacpoole PW, Greene YJ. Dichloroacetate. Diabetes Care 1992;15:785–791 [DOI] [PubMed] [Google Scholar]
- 26.Timmons JA, Gustafsson T, Sundberg CJ, Jansson E, Greenhaff PL. Muscle acetyl group availability is a major determinant of oxygen deficit in humans during submaximal exercise. Am J Physiol 1998;274:E377–E380 [DOI] [PubMed] [Google Scholar]
- 27.Bergstrom J. Muscle electrolytes in man determined by neutron activation analysis on needle biopsy specimens. A study on normal subjects, kidney patients, and patients with chronic diarrhoea. Scand J Clin Lab Invest 1962;14Suppl. 68:1–110 [Google Scholar]
- 28.Harris RC, Hultman E, Nordesjö LO. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest 1974;33:109–120 [PubMed] [Google Scholar]
- 29.Cederblad G, Carlin JI, Constantin-Teodosiu D, Harper P, Hultman E. Radioisotopic assays of CoASH and carnitine and their acetylated forms in human skeletal muscle. Anal Biochem 1990;185:274–278 [DOI] [PubMed] [Google Scholar]
- 30.Constantin-Teodosiu D, Cederblad G, Hultman E. A sensitive radioisotopic assay of pyruvate dehydrogenase complex in human muscle tissue. Anal Biochem 1991;198:347–351 [DOI] [PubMed] [Google Scholar]
- 31.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 1983;55:628–634 [DOI] [PubMed] [Google Scholar]
- 32.Constantin-Teodosiu D, Simpson EJ, Greenhaff PL. The importance of pyruvate availability to PDC activation and anaplerosis in human skeletal muscle. Am J Physiol 1999;276:E472–E478 [DOI] [PubMed] [Google Scholar]
- 33.Helge JW. Long-term fat diet adaptation effects on performance, training capacity, and fat utilization. Med Sci Sports Exerc 2002;34:1499–1504 [DOI] [PubMed] [Google Scholar]
- 34.Bergström J, Hermansen L, Hultman E, Saltin B. Diet, muscle glycogen and physical performance. Acta Physiol Scand 1967;71:140–150 [DOI] [PubMed] [Google Scholar]
- 35.Bachmann OP, Dahl DB, Brechtel K, et al. Effects of intravenous and dietary lipid challenge on intramyocellular lipid content and the relation with insulin sensitivity in humans. Diabetes 2001;50:2579–2584 [DOI] [PubMed] [Google Scholar]
- 36.Chokkalingam K, Jewell K, Norton L, et al. High-fat/low-carbohydrate diet reduces insulin-stimulated carbohydrate oxidation but stimulates nonoxidative glucose disposal in humans: an important role for skeletal muscle pyruvate dehydrogenase kinase 4. J Clin Endocrinol Metab 2007;92:284–292 [DOI] [PubMed] [Google Scholar]
- 37.Cadoudal T, Distel E, Durant S, et al. Pyruvate dehydrogenase kinase 4: regulation by thiazolidinediones and implication in glyceroneogenesis in adipose tissue. Diabetes 2008;57:2272–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imae M, Fu Z, Yoshida A, Noguchi T, Kato H. Nutritional and hormonal factors control the gene expression of FoxOs, the mammalian homologues of DAF-16. J Mol Endocrinol 2003;30:253–262 [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Roves P, Huss JM, Han DH, et al. Raising plasma fatty acid concentration induces increased biogenesis of mitochondria in skeletal muscle. Proc Natl Acad Sci USA 2007;104:10709–10713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon JC, Xu G, Deeney JT, et al. Suppression of beta cell energy metabolism and insulin release by PGC-1alpha. Dev Cell 2003;5:73–83 [DOI] [PubMed] [Google Scholar]
- 41.Lee CH, Olson P, Evans RM. Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology 2003;144:2201–2207 [DOI] [PubMed] [Google Scholar]
- 42.Lee FN, Zhang L, Zheng D, Choi WS, Youn JH. Insulin suppresses PDK-4 expression in skeletal muscle independently of plasma FFA. Am J Physiol Endocrinol Metab 2004;287:E69–E74 [DOI] [PubMed] [Google Scholar]
- 43.Rena G, Prescott AR, Guo S, Cohen P, Unterman TG. Roles of the forkhead in rhabdomyosarcoma (FKHR) phosphorylation sites in regulating 14-3-3 binding, transactivation and nuclear targetting. Biochem J 2001;354:605–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenhaff PL, Karagounis LG, Peirce N, et al. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab 2008;295:E595–E604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim YI, Lee FN, Choi WS, Lee S, Youn JH. Insulin regulation of skeletal muscle PDK4 mRNA expression is impaired in acute insulin-resistant states. Diabetes 2006;55:2311–2317 [DOI] [PubMed] [Google Scholar]
- 46.Kwon HS, Huang B, Unterman TG, Harris RA. Protein kinase B-alpha inhibits human pyruvate dehydrogenase kinase-4 gene induction by dexamethasone through inactivation of FOXO transcription factors. Diabetes 2004;53:899–910 [DOI] [PubMed] [Google Scholar]
- 47.McAllister A, Allison SP, Randle PJ. Effects of dichloroacetate on the metabolism of glucose, pyruvate, acetate, 3-hydroxybutyrate and palmitate in rat diaphragm and heart muscle in vitro and on extraction of glucose, lactate, pyruvate and free fatty acids by dog heart in vivo. Biochem J 1973;134:1067–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clark AS, Mitch WE, Goodman MN, Fagan JM, Goheer MA, Curnow RT. Dichloroacetate inhibits glycolysis and augments insulin-stimulated glycogen synthesis in rat muscle. J Clin Invest 1987;79:588–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shangraw RE, Rabkin JM, Lopaschuk GD. Hepatic pyruvate dehydrogenase activity in humans: effect of cirrhosis, transplantation, and dichloroacetate. Am J Physiol 1998;274:G569–G577 [DOI] [PubMed] [Google Scholar]