Abstract
Increased myocardial lipid content (MYCL) recently has been linked to the development of cardiomyopathy in diabetes. In contrast to steatosis in skeletal muscle and liver, previous investigations could not confirm a link between MYCL and insulin resistance. Thus, we hypothesized that cardiac steatosis might develop against the background of the metabolic environment typical for prediabetes and early type 2 diabetes: combined hyperglycemia and hyperinsulinemia. Therefore, we aimed to prove the principle that acute hyperglycemia (during a 6-h clamp) affects MYCL and function (assessed by 1H magnetic resonance spectroscopy and imaging) in healthy subjects (female subjects: n = 8, male subjects: n = 10; aged 28 ± 5 years; BMI 22.4 ± 2.6 kg/m2). Combined hyperglycemia (202.0 ± 10.6 mg/dL) and hyperinsulinemia (110.6 ± 59.0 μU/mL) were, despite insulin-mediated suppression of free fatty acids, associated with a 34.4% increase in MYCL (baseline: 0.20 ± 0.17%, clamp: 0.26 ± 0.22% of water signal; P = 0.0009), which was positively correlated with the area under the curve of insulin (R = 0.59, P = 0.009) and C-peptide (R = 0.81, P < 0.0001) during the clamp. Furthermore, an increase in ejection fraction (P < 0.0001) and a decrease in end-systolic volume (P = 0.0002) were observed, which also were correlated with hyperinsulinemia. Based on our findings, we conclude that combined hyperglycemia and hyperinsulinemia induce short-term myocardial lipid accumulation and alterations in myocardial function in normal subjects, indicating that these alterations might be directly responsible for cardiac steatosis in metabolic diseases.
Increased myocardial lipid content (MYCL), or cardiac steatosis, recently has been described as an important feature of disturbed myocardial substrate metabolism (1,2). This most likely contributes to the development of heart failure in patients with diabetes, even in the absence of coronary artery disease and arterial hypertension (diabetic cardiomyopathy) (3,4). In addition, cardiac steatosis, when coexistent with coronary artery disease, might be responsible for adverse outcomes after myocardial infarction, as reported for patients with diabetes (5).
Ectopic lipid deposition in nonadipose tissues is associated with an overstrain of the cellular oxidation capacity. This overstrain leads to insufficient substrate utilization and, consequently, to accumulation of toxic by-products that induce cell damage and organ dysfunction, specifically lipotoxicity (6). However, the precise role of cardiac steatosis in the pathogenesis of myocardial dysfunction remains unclear as yet.
Previous cross-sectional studies applying cardiac magnetic resonance (MR) spectroscopy (MRS) clearly have shown elevated MYCL in metabolic diseases, including type 2 diabetes and obesity (1,2,7), and cardiac steatosis also has been observed in subjects with impaired glucose tolerance (2). However, the exact metabolic mechanisms underlying myocardial lipid accumulation have not yet been identified.
Although a clear relationship between insulin resistance and increased ectopic intracellular lipid content in skeletal muscle and liver has been demonstrated (8–10), we recently could not observe an association between insulin resistance and myocardial lipid accumulation in normoglycemic subjects (11). Thus, cardiac steatosis might develop against the background of the metabolic environment typical for patients with prediabetes or early type 2 diabetes: hyperglycemia, hyperinsulinemia, and elevated plasma concentrations of free fatty acids (FFAs).
Pronounced hyperglycemia, secondary to short-term deprivation of insulin dosage in patients with type 1 diabetes, did not affect MYCL (12). However, recent data suggest that elevated plasma FFAs directly affect myocardial lipid deposition in the presence of low insulin concentrations. Elevation of plasma FFA concentrations during physical exercise is associated with an increase in MYCL and a rise in ejection fraction (EF); these effects can be suppressed by the administration of glucose (and thus a decrease in FFAs) (13). The rise in plasma FFA concentrations secondary to caloric restriction causes an increase in MYCL and a decrease in diastolic function in healthy subjects (14), as well as in patients with type 2 diabetes (15), which can be blunted by suppression of FFA release by pharmacological inhibition of lipolysis. Both studies confirm the short-term flexibility of the myocardial triglyceride pool in healthy subjects and in patients with diabetes. Taken together, excessive FFA supply, secondary to unrestrained lipolysis in the presence of decreased insulin concentrations, likely exceeds myocardial oxidation capacity and leads to the accumulation of unoxidized lipids and lipid intermediates in the cytosol.
However, elevation of plasma FFAs by a high-fat, high-energy diet for 3 days did not increase MYCL but did increase hepatic lipid content (16), suggesting that short-term meal studies preferentially target liver metabolism. It is of note that only plasma insulin, but not glucose concentrations, was increased. Thus, the available data on the potential direct effects of plasma FFAs on MYCL are inconsistent and do not sufficiently explain cardiac steatosis in patients with impaired glucose tolerance or type 2 diabetes (1,2,7).
Given that (postprandial) hyperglycemia and hyperinsulinemia are central and early features of metabolic diseases, we hypothesized that combined hyperglycemia and hyperinsulinemia induce myocardial lipid accumulation.
We previously have shown that insulin infusion in patients with type 2 diabetes leads to lipid accumulation in liver and skeletal muscle, likely resulting from insulin-mediated inhibition of lipolysis and lipid oxidation, stimulation of intracellular triglyceride synthesis, and/or increased uptake of circulating FFAs (17). The resulting competition of glucose and fatty acids for mitochondrial oxidation further decreases myocellular fatty acid utilization (18,19). Reduced fatty acid oxidation, together with insulin-stimulated FFA uptake and trigylceride synthesis, results in net lipid storage. However, whether hyperglycemia and consequent hyperinsulinemia might also affect lipid accumulation in the heart currently is unknown. Therefore, the objective of this study was to investigate the impact of acute hyperglycemia and consequent hyperinsulinemia on MYCL and function in healthy subjects.
RESEARCH DESIGN AND METHODS
Healthy men and women were recruited to participate in this study. Exclusion criteria included the presence of any acute or chronic inflammatory diseases, arterial hypertension, evidence of coronary artery disease, a history of impaired glucose tolerance or diabetes, pregnancy, and intake of oral contraceptives in female subjects, as well as the tendency toward claustrophobia and the existence of metal devices or other magnetic material in or on the subjects. A baseline examination was performed including a complete medical history, physical examination, and blood sampling for electrolytes, kidney, and liver function, as well as blood count to determine subject eligibility. Subjects were included when eligibility criteria were fulfilled and after giving written, informed consent. The protocol was approved by the human ethics committee of the Medical University of Vienna. No subject was taking any medication regularly.
All examinations were performed at the Metabolic Laboratory of the Division of Endocrinology and Metabolism, Department of Internal Medicine III, and the MR-Centre of Excellence, Department of Radiodiagnostics, both at the Medical University of Vienna. Subjects were asked to refrain from vigorous physical activity and to ingest an isocaloric diet (30 kcal/kg/day: carbohydrate/protein/fat: 55/15/30%) for 3 days prior to the experiments. Female subjects were studied between day 3 and day 10 of their menstrual cycle. On the study day, participants arrived in our laboratory at 6:30 a.m. after an overnight fast of at least 10 h. MR examinations took place at baseline, as well as during the sixth hour of the hyperglycemic clamp.
Hyperglycemic clamp.
At baseline, two catheters were placed into an antecubital vein of each arm: one for blood sampling and another for glucose infusion. After the first MR examination, a continuous glucose infusion was started. After the infusion of the priming dose (2 mg glucose/kg body wt for each mg/dL increase in plasma glucose [20]), designed to reach the clamp goal within 15 min, glucose was infused (0–360 min) continuously to maintain blood glucose levels between 180 and 200 mg/dL for 6 h. Blood samples for the measurement of glucose (every 5 min, immediately analyzed with Biosen C_line [EKF Diagnostic, Barleben/Magdeburg, Germany]), insulin, C-peptide, and FFAs (every hour) were regularly drawn. Whole-body insulin sensitivity is presented as the M/I ratio between 120 and 360 min of the clamp test. M/I is calculated for 60-min intervals as (GIR − SC)/insulin concentrations (serum concentrations in μU/mL), where GIR represents glucose infusion rates and SC represents space correction, and given in (mg/kg/min)/(μU/mL) (20).
Analyses of plasma metabolites.
Plasma concentrations of insulin and C-peptide were analyzed by radioimmunoassays (Millipore, Billerica, MA) and plasma concentrations of FFAs by a microfluorimetric assay (Wako, Neuss, Germany) in the endocrinology and metabolism laboratory. Areas under the curve (AUCs) were calculated using the trapezoidal rule.
MR imaging and MRS.
All MR measurements were performed on the 3.0-T Tim Trio System (Siemens, Erlangen, Germany).
Assessment of MYCL.
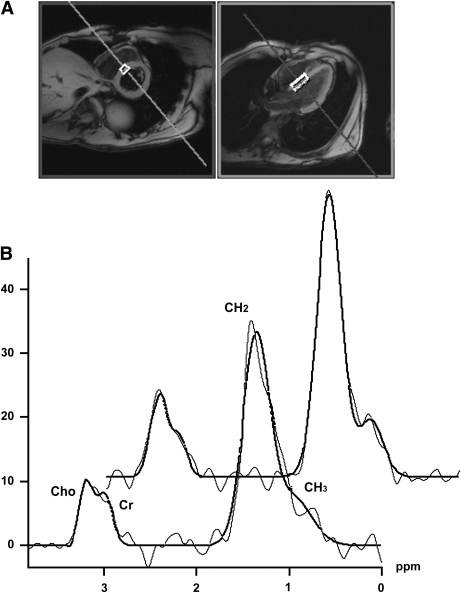
Electrocardiographic (ECG)-gated 1H MRS was used to assess MYCL similarly to previously described protocols (1,11,21), by positioning the volume of interest (~6–8 cm3) in the interventricular septum to avoid signal alterations by the epicardial fat (Fig. 1). Standard B0 field map acquisition for shimming and frequency correction was executed in the operator-driven mode during separate breath holds. A water-suppressed Point RESolved Spectroscopy sequence (echo time = 30 ms, number of scans = 8–12, number of dummy scans = 2, repetition time = 700–1,250 ms according to heartbeat period) was used for spatial localization and signal acquisition within one breath hold. The number of acquisitions of 8–12 was chosen based on the individual length of the heartbeat period to achieve a total acquisition time of <15 s. An additional spectrum without water suppression (number of scans = 4, number of dummy scans = 2, repetition time based on the heart rate period), acquired in a separate breath hold, was used as the internal concentration reference. A similar breath-holding protocol was introduced and evaluated recently (22). The spectra were processed via the spectroscopy processing tool provided by the system manufacturer (Siemens), using appropriate prior-knowledge lipid-methylene (CH2, 1.3 ppm), lipid-methyl (CH3, 0.9 ppm), creatine-CH3 group (3.0 ppm), choline (3.2 ppm), and water (4.7 ppm) resonance lines, with restrictions concerning the line position and line width. A Gaussian line shape was used to fit lipid, creatine, and choline resonances, whereas the water resonance was fitted with a Lorentzian line shape. MYCL is expressed as the ratio of the sum intensities of lipid-methylene and lipid-methyl resonance lines to that of the water signal. Spin-lattice (T1) and spin-spin (T2) relaxation correction was performed using the water and lipid T1 and T2 relaxation time values measured at 3T in skeletal muscle (23) and T1 relaxation of water measured by MR imaging methods (24). The test-retest assessment of variability of our measurement protocol yielded a coefficient of variation of 23%, which is a substantial improvement over the coefficient of variation of 30–39%, as reported earlier (11,21,25).
FIG. 1.
A: Position of the volume of interest for 1H MRS placed in the interventricular septum is depicted in the short- and long-axis MR images of the heart. B: 1H MR spectra acquired before (lower trace) and during the sixth hour of combined hyperglycemia and hyperinsulinemia (upper trace). MRS signal (presented in arbitrary units) is represented by the thin line, and the fit of the data, as performed within the spectroscopy tool of the Syngo VB 17 operating platform, is given in bold.
Assessment of left ventricular function by MR imaging.
Visualization of cardiac function was performed using retrospectively ECG-gated cine true fast imaging with steady-state free precession (True FISP) sequences in two-chamber, four-chamber, and short-axis orientations, with the latter orientation having 10–12 equidistant imaging levels from the apex to the base of the left ventricle. EF, end-systolic volume (ESV) and end-diastolic volume, cardiac output, stroke volume, left ventricular mass (LVM) (1), as well as concentricity [= LVM–to–end-diastolic volume ratio (LVM/LVEDV)] (26) were calculated by dedicated manufacturer’s software (Argus; Siemens Healthcare, Erlangen, Germany) after manually contouring endo- and epicardial borders in end-systolic and end-diastolic short axes cine images of the left ventricle. Papillary muscles were counted as muscle mass, and data were normalized to body surface area using the Dubois formula (body surface area = 0.007184 × height0.725 × weight0.425). After exclusion of the papillary muscles, contours were additionally used for assessment of left ventricular wall thickness (27), according to the 16-segment model of the American Society of Echocardiography. We present median segmental end-systolic and end-diastolic wall thickness.
In addition, a retrospectively ECG-gated, spoiled gradient echo–based phase-contrast sequence (through-plane velocity encoding typically 90 cm/s) was used to assess blood flow across the mitral valve. Biphasic diastolic inflow patterns that consisted of two peaks, representing the peaks of the early filling phase (E) and the atrial contraction (A), were evaluated by the dedicated manufacturer’s software (Argus) and are presented here as the E/A ratio, a marker of diastolic function (28).
Statistical analysis.
Comparisons of quantitative variables were performed using ANOVA. Associations between continuous variables are described by the Pearson correlation coefficient, and Spearman, if appropriate. Regression analyses were performed by applying linear models. Statistical significance was set at P < 0.05. All calculations were performed using SAS Enterprise Guide.
RESULTS
Eighteen healthy subjects (10 male and 8 female) met the inclusion criteria and underwent the study protocol (Table 1). Men and women were matched for age [male subjects: 27.6 ± 3.4 years, female subjects: 29.0 ± 7.2 years; P = not significant (NS)] and BMI (male subjects: 23.2 ± 2.6 kg/m2, female subjects: 21.4 ± 2.3 kg/m2; P = NS).
TABLE 1.
Baseline characteristics of study participants
Metabolic changes.
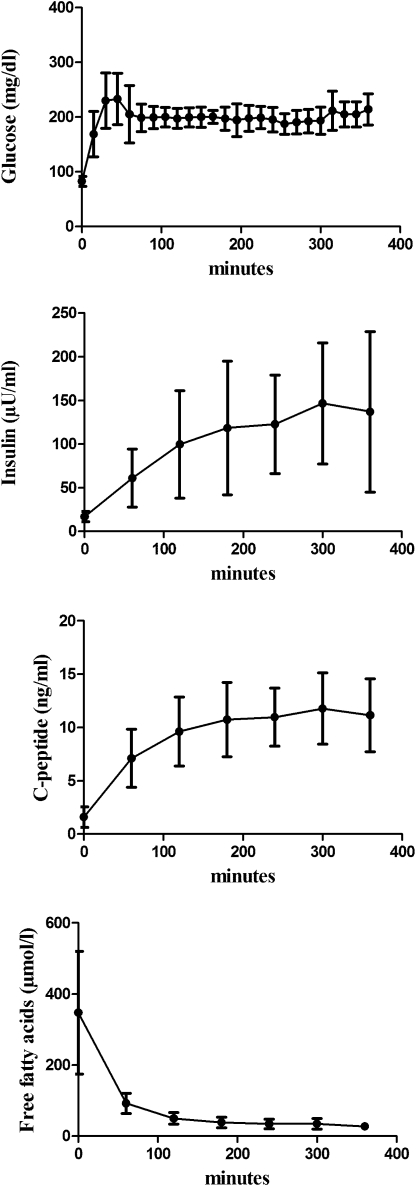
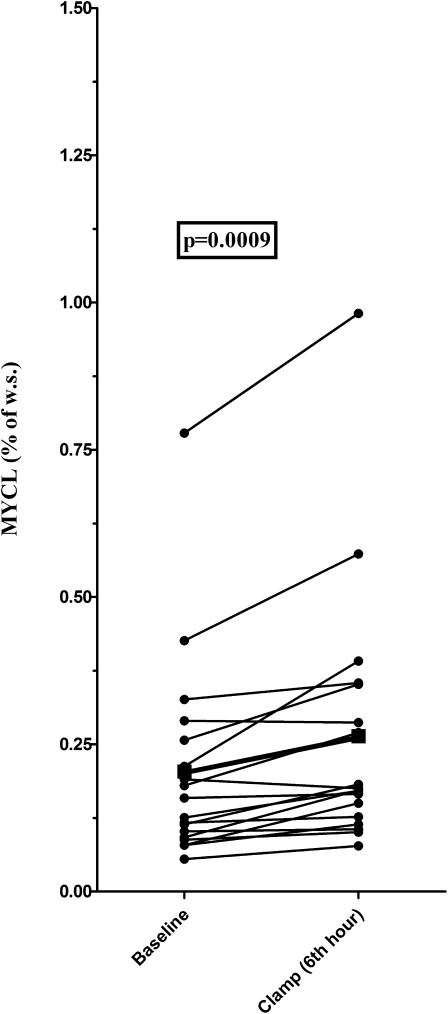
As expected, standardized hyperglycemia (mean glucose: 202.0 ± 10.6 mg/dL) caused an increase in plasma insulin and C-peptide concentrations. The pronounced suppression of circulating FFAs was mediated by hyperinsulinemia (all P < 0.0001) (Fig. 2). In addition, a comparable increase in MYCL (baseline: 0.20 ± 0.17%, clamp: 0.26 ± 0.22%; P = 0.0009) was observed in both male (baseline: 0.22 ± 0.22%, clamp: 0.29 ± 0.29%; P = 0.01) and female (baseline: 0.18 ± 0.09%, clamp 0.23 ± 0.1%; P < 0.02) subjects (Fig. 3).
FIG. 2.
Time course of plasma concentrations of glucose, insulin, C-peptide, and FFAs during the hyperglycemic clamp.
FIG. 3.
Increase in MYCL (ΔMYCL) (given in the percentage of water signal) during combined hyperglycemia and hyperinsulinemia. The boldface line and square describe mean MYCL in the whole study group.
Changes in cardiac function parameters.
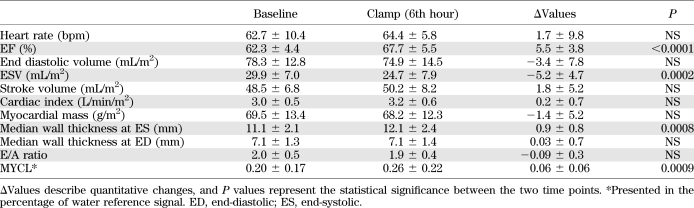
The assessment of myocardial function at baseline, as well as during the sixth hour of the hyperglycemic-hyperinsulinemic clamp, revealed an increase in EF and median end-systolic wall thickness, as well as a significant decrease in ESV (see Table 2). The E/A ratio, a parameter of diastolic function, remained unchanged during the experiment.
TABLE 2.
Parameters of myocardial function and lipid content at baseline and during the 6th hour of sustained hyperglycemia and hyperinsulinemia
Correlation analyses.
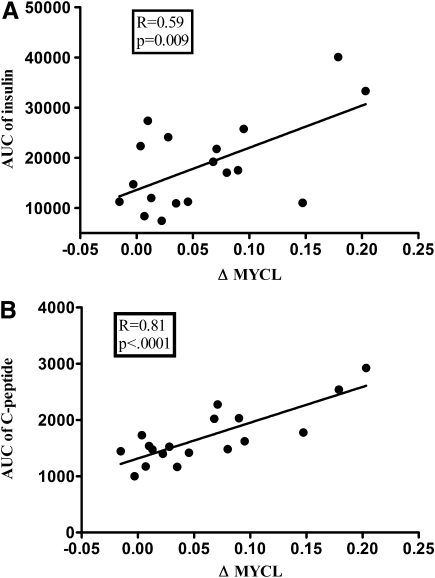
The increase in MYCL (ΔMYCL) was positively correlated with the AUC of plasma insulin and C-peptide concentrations (Fig. 4), whereas no association was found between ΔMYCL and changes in cardiac function parameters. When these variables were integrated into a linear model, the AUC of C-peptide remained predictive for ΔMYCL. No correlation was observed between insulin sensitivity and MYCL (at baseline and during the sixth hour of the clamp) or ΔMYCL.
FIG. 4.
Correlations between the increase in MYCL (ΔMYCL) and the AUCs of insulin (A) and C-peptide (B) during the clamp.
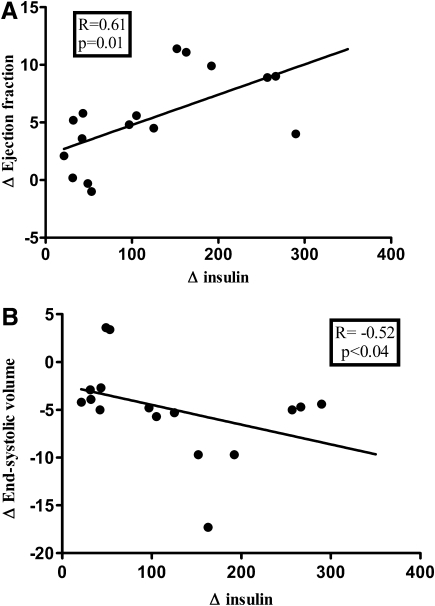
The rise in insulin concentrations during the clamp was positively correlated with ΔEF (Fig. 5A) and inversely associated with the change in ESV (Spearman analysis) (Fig. 5B). The association between ΔEF and Δinsulin remained significant in regression analyses, even after adjustment for heart rate.
FIG. 5.
Correlations between the rise of insulin concentrations and the changes of cardiac volumetric parameters during the clamp. A: ΔEF. B: ΔESV.
Additional interesting associations observed were the inverse correlation between septal wall thickness (at the sixth hour of the clamp test) and the AUC of FFAs (R = −0.63, P < 0.007) and a tendency toward an inverse correlation between the decrease in FFA concentrations and the increase in EF (R = −0.5, P = 0.05). No association was found between the rate of glucose infusion (mean infusion rate: 323.6 ± 127.5 mL/h) and the change in cardiac functional parameters: ΔEF (R = −0.04, P = 0.86), ΔESV (R = 0.02, P = 0.93), and Δcardiac output (R = −0.3, P = 0.24).
DISCUSSION
The current study provides evidence that combined hyperglycemia and hyperinsulinemia induce myocardial lipid accumulation in normal subjects, despite insulin-mediated suppression of plasma FFAs. We also show that the increase in insulin concentrations during sustained hyperglycemia is tightly associated with the increase in MYCL and acute changes in cardiac function (EF and ESV).
It has been convincingly shown that elevation of plasma FFAs is associated with an increase in MYCL (13–15). The underlying mechanism is thought to be an overstrain of the cellular FFA oxidation machinery, which results in accumulation of toxic lipid by-products in the cytosol. Here, we show that even in the presence of suppressed circulating FFAs, combined hyperglycemia and hyperinsulinemia are able to induce myocardial lipid accumulation in normal subjects. The tight association between the increase in MYCL and the extent of glucose-induced insulin release indicates that insulin is the key mediator. This conclusion is supported by a previous study, which showed that hyperglycemia in the presence of low insulin concentrations (attributed to deprivation of insulin dosage) does not affect MYCL in patients with type 1 diabetes (12).
The rise in MYCL could have resulted from 1) an increased uptake of circulating FFAs, 2) an insulin-mediated stimulation of intracellular triglyceride synthesis, and/or 3) an insulin-mediated inhibition of lipolysis and lipid oxidation.
Exposure to insulin in vitro leads to increased uptake and esterification of FFAs, whereas triglyceride hydrolysis and FFA oxidation are reduced in the rodent soleus muscle (29). We previously have shown that insulin infusion, designed to achieve normoglycemia in patients with type 2 diabetes, stimulates lipid accumulation in skeletal muscle and liver (17).
Inhibition of lipid oxidation, as a result of substrate competition between fatty acids and glucose for mitochondrial oxidation (Randle cycle) (18,19), likely contributed to the observed effects. Fatty acids and glucose, the two main substrates of myocardial metabolism, reach the cytosol via transporters, mainly CD36 and GLUT4 (30), respectively. In the presence of hyperglycemia and consequent hyperinsulinemia, insulin forcefully stimulates myocardial glucose uptake via increased GLUT4 translocation to the cellular membrane, thereby fostering substrate competition between fatty acids and glucose. The resulting switch in mitochondrial substrate utilization from fatty acids to glucose is mediated mainly by malonyl-CoA. Malonyl-CoA derives from acetyl-CoA (via carboxylation by acetyl-CoA carboxylase 2) and inhibits carnitine palmitoyltransferase I (19), which controls the rate-limiting step of mitochondrial FFA uptake and, in turn, oxidation. Of note, insulin was reported to be able to directly stimulate acetyl-CoA carboxylase activity and, thus, to suppress mitochondrial lipid oxidation (31). Reduced fatty acid oxidation may result in accumulation of unoxidized lipid intermediates in the cytosol, leading to lipid storage and cardiac steatosis.
We previously have shown that MYCL, in contrast to ectopic lipid accumulation in skeletal muscle and liver, does not relate to systemic insulin resistance in normoglycemic subjects (11). In line with these results, we did not observe any relationship between MYCL and insulin sensitivity (M/I ratio) in the current study of rather insulin-sensitive subjects. This is in contrast to our previous study on the effects of insulin infusion on skeletal muscle lipid content in patients with type 2 diabetes, where intramyocellular lipid content correlated positively with insulin sensitivity after prolonged insulin infusion.
Because women with overt diabetes were reported to display increased cardiac steatosis compared with their male counterparts (7), and sex differences in myocardial blood flow and fatty acid metabolism recently were described (32), we aimed to investigate whether acute changes in MYCL also might be more pronounced in women. In our study, basal MYCL, as well as the change in MYCL, did not differ between male and female participants; therefore, no sex-specific differences in acute myocardial lipid accumulation were observed.
As expected, changes in cardiac function parameters in response to acute glucose-induced hyperinsulinemia were recorded: EF and end-systolic left ventricular wall thickness were increased compared with baseline. In addition, a reduction in ESV was observed. The rise of EF was positively correlated with the increase in insulin concentrations during the clamp, as well as inversely associated with the suppression of FFA concentrations. This is in accordance with previous findings that high-dose insulin infusion increases cardiac output as a result of improving systolic cardiac function (33). Insulin is known to be a stimulator of the sympathetic nervous system (34), which might have contributed to the observed effects. Nevertheless, even when adjusted for heart rate, the change in EF remained significantly related to the changes in insulin concentrations during the clamp. The correlation between the rise in insulin concentrations and the changes in ESV and EF also remained significant after adjustment for the infused volume, indicating that insulin mediated the observed effects on systolic cardiac function. In addition, because FFA oxidation requires more oxygen than glucose utilization, the observed rise in EF could be explained by improved cardiac performance attributed to increased glucose oxidation, as suggested by the observed inverse association between the rise in EF and the suppression of plasma FFA concentrations. However, it cannot be excluded that the observed acute improvements in myocardial function may, in the presence of chronic insulin oversupply in patients with metabolic diseases, result in maladaptive changes and, thus, myocardial dysfunction.
Our study did not address the question of whether myocardial steatosis, per se, alters myocardial function. Based on current evidence and our results, we assume that increased MYCL highlights a state of disturbed myocardial substrate metabolism. Recent findings in Wistar rats showed that both a high-fat and a high-fat/high-carbohydrate (Western) diet lead to a similar gain in body weight; however, only the Western diet induced cardiac contractile dysfunction, which was associated with a decreased palmitoleoyl-CoA content and a decreased unsaturated-to-saturated fatty acids ratio in the myocardium. The disturbances in MYCL/composition were traced back to altered hepatic de novo lipogenesis (35). This indicates that in addition to impaired myocardial lipid metabolism, quantitatively and qualitatively altered hepatic lipid synthesis might contribute to myocardial lipid storage and, consequently, dysfunction. In addition, we agree with these authors that lipotoxicity should be replaced by the term glucolipotoxicity (36) when talking about the cardiometabolic complications in patients with obesity and diabetes.
Some limitations must be considered. The MR methods used in the current study did not allow us to discern the precise alterations in myocardial fuel physiology underlying the observed rise in MYCL. Because biopsies of the human myocardium are not feasible in a research setting, investigations on human myocardial metabolism are limited to noninvasive techniques. Advanced high-field MRS has the potential to facilitate the measurement of qualitative changes in MYCL by allowing the monitoring of the degree of saturation of myocardial lipids (37). However, to our knowledge, these methods have not yet been applied on the beating human myocardium. In addition, positron emission tomography has been used to measure myocardial fatty acid utilization, oxidation, and esterification using radioactively labeled fatty acids. Hence, future studies might combine MR imaging/MRS and positron emission tomography to elucidate the exact mechanisms underlying our current observations.
In summary, insulin release in response to hyperglycemia induces a short-term increase in MYCL in healthy subjects. This effect was observed even in the presence of insulin-mediated suppression of plasma FFA concentrations. These findings support the hypothesis that postprandial and/or chronic hyperinsulinemia and hyperglycemia in prediabetes and early type 2 diabetes enhance myocardial steatosis and might thereby contribute to the development of heart failure.
ACKNOWLEDGMENTS
The study was supported by the “Österreichischer Herzfond” 2010 (to M.Kre.) and by the “Jubiläumsfond” of the Austrian National Bank (project no. 13249; to M.Krš.).
No potential conflicts of interest relevant to this article were reported.
Y.W. researched data and wrote the manuscript. M.Krš., C.-H.A., and G.R. researched data, contributed to the discussion, and reviewed the manuscript. D.J. and A.H. researched data. S.T. and A.L. contributed to the discussion and reviewed the manuscript. M.Kre. researched data, contributed to the discussion, reviewed the manuscript, and was also the supervisor of the project. M.Kre. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Rijzewijk LJ, van der Meer RW, Smit JW, et al. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol 2008;52:1793–1799 [DOI] [PubMed] [Google Scholar]
- 2.McGavock JM, Lingvay I, Zib I, et al. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation 2007;116:1170–1175 [DOI] [PubMed] [Google Scholar]
- 3.Lingvay I, Raskin P, Szczepaniak LS. The fatty hearts of patients with diabetes. Nat Rev Cardiol 2009;6:268–269 [DOI] [PubMed] [Google Scholar]
- 4.Aneja A, Tang WH, Bansilal S, Garcia MJ, Farkouh ME. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med 2008;121:748–757 [DOI] [PubMed] [Google Scholar]
- 5.Stone PH, Muller JE, Hartwell T, et al. The MILIS Study Group The effect of diabetes mellitus on prognosis and serial left ventricular function after acute myocardial infarction: contribution of both coronary disease and diastolic left ventricular dysfunction to the adverse prognosis. J Am Coll Cardiol 1989;14:49–57 [DOI] [PubMed] [Google Scholar]
- 6.McGavock JM, Victor RG, Unger RH, Szczepaniak LS, American College of Physicians and the American Physiological Society Adiposity of the heart, revisited. Ann Intern Med 2006;144:517–524 [DOI] [PubMed] [Google Scholar]
- 7.Iozzo P, Lautamaki R, Borra R, et al. Contribution of glucose tolerance and gender to cardiac adiposity. J Clin Endocrinol Metab 2009;94:4472–4482 [DOI] [PubMed] [Google Scholar]
- 8.Krssak M, Falk Petersen K, Dresner A, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 1999;42:113–116 [DOI] [PubMed] [Google Scholar]
- 9.Krssak M, Roden M. The role of lipid accumulation in liver and muscle for insulin resistance and type 2 diabetes mellitus in humans. Rev Endocr Metab Disord 2004;5:127–134 [DOI] [PubMed] [Google Scholar]
- 10.Krebs M, Roden M. Molecular mechanisms of lipid-induced insulin resistance in muscle, liver and vasculature. Diabetes Obes Metab 2005;7:621–632 [DOI] [PubMed] [Google Scholar]
- 11.Krssak M, Winhofer Y, Gobl C, et al. Insulin resistance is not associated with myocardial steatosis in women. Diabetologia 2011;54:1871–1878 [DOI] [PubMed] [Google Scholar]
- 12.Hammer S, Jonker JT, Lamb HJ, et al. Short-term hyperglycemic dysregulation in patients with type 1 diabetes does not change myocardial triglyceride content or myocardial function. Diabetes Care 2008;31:1613–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilet L, van de Weijer T, Hesselink MK, et al. Exercise-induced modulation of cardiac lipid content in healthy lean young men. Basic Res Cardiol 2011;106:307–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammer S, van der Meer RW, Lamb HJ, et al. Progressive caloric restriction induces dose-dependent changes in myocardial triglyceride content and diastolic function in healthy men. J Clin Endocrinol Metab 2008;93:497–503 [DOI] [PubMed] [Google Scholar]
- 15.Hammer S, van der Meer RW, Lamb HJ, et al. Short-term flexibility of myocardial triglycerides and diastolic function in patients with type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 2008;295:E714–E718 [DOI] [PubMed] [Google Scholar]
- 16.van der Meer RW, Hammer S, Lamb HJ, et al. Effects of short-term high-fat, high-energy diet on hepatic and myocardial triglyceride content in healthy men. J Clin Endocrinol Metab 2008;93:2702–2708 [DOI] [PubMed] [Google Scholar]
- 17.Anderwald C, Bernroider E, Krssak M, et al. Effects of insulin treatment in type 2 diabetic patients on intracellular lipid content in liver and skeletal muscle. Diabetes 2002;51:3025–3032 [DOI] [PubMed] [Google Scholar]
- 18.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle: its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963;1:785–789 [DOI] [PubMed] [Google Scholar]
- 19.Hue L, Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab 2009;297:E578–E591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 21.van der Meer RW, Doornbos J, Kozerke S, et al. Metabolic imaging of myocardial triglyceride content: reproducibility of 1H MR spectroscopy with respiratory navigator gating in volunteers. Radiology 2007;245:251–257 [DOI] [PubMed] [Google Scholar]
- 22.Rial B, Robson MD, Neubauer S, Schneider JE. Rapid quantification of myocardial lipid content in humans using single breath-hold 1H MRS at 3 Tesla. Magn Reson Med 2011;66:619–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krssák M, Mlynárik V, Meyerspeer M, Moser E, Roden M. 1H NMR relaxation times of skeletal muscle metabolites at 3 T. MAGMA 2004;16:155–159 [DOI] [PubMed] [Google Scholar]
- 24.Piechnik SK, Ferreira VM, Dall’Armellina E, et al. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson 2010;12:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reingold JS, McGavock JM, Kaka S, Tillery T, Victor RG, Szczepaniak LS. Determination of triglyceride in the human myocardium by magnetic resonance spectroscopy: reproducibility and sensitivity of the method. Am J Physiol Endocrinol Metab 2005;289:E935–E939 [DOI] [PubMed] [Google Scholar]
- 26.Velagaleti RS, Gona P, Chuang ML, et al. Relations of insulin resistance and glycemic abnormalities to cardiovascular magnetic resonance measures of cardiac structure and function: the Framingham Heart Study. Circ Cardiovasc Imaging 2010;3:257–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waiter GD, McKiddie FI, Redpath TW, Semple SI, Trent RJ. Determination of normal regional left ventricular function from cine-MR images using a semi-automated edge detection method. Magn Reson Imaging 1999;17:99–107 [DOI] [PubMed] [Google Scholar]
- 28.van der Meer RW, Diamant M, Westenberg JJ, et al. Magnetic resonance assessment of aortic pulse wave velocity, aortic distensibility, and cardiac function in uncomplicated type 2 diabetes mellitus. J Cardiovasc Magn Reson 2007;9:645–651 [DOI] [PubMed] [Google Scholar]
- 29.Dyck DJ, Steinberg G, Bonen A. Insulin increases FA uptake and esterification but reduces lipid utilization in isolated contracting muscle. Am J Physiol Endocrinol Metab 2001;281:E600–E607 [DOI] [PubMed] [Google Scholar]
- 30.Schwenk RW, Luiken JJ, Bonen A, Glatz JF. Regulation of sarcolemmal glucose and fatty acid transporters in cardiac disease. Cardiovasc Res 2008;79:249–258 [DOI] [PubMed] [Google Scholar]
- 31.Witters LA, Kemp BE. Insulin activation of acetyl-CoA carboxylase accompanied by inhibition of the 5′-AMP-activated protein kinase. J Biol Chem 1992;267:2864–2867 [PubMed] [Google Scholar]
- 32.Peterson LR, Saeed IM, McGill JB, et al. Sex and type 2 diabetes: obesity-independent effects on left ventricular substrate metabolism and relaxation in humans. Obesity (Silver Spring). 4 August 2011 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 33.Klein LJ, van Campen CM, Sieswerda GT, Kamp O, Visser FC. Effects of high-dose insulin infusion on left ventricular function in normal subjects. Neth Heart J 2010;18:183–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tack CJ, Lenders JW, Goldstein DS, Lutterman JA, Smits P, Thien T. Haemodynamic actions of insulin. Curr Opin Nephrol Hypertens 1998;7:99–106 [DOI] [PubMed] [Google Scholar]
- 35.Harmancey R, Wilson CR, Wright NR, Taegtmeyer H. Western diet changes cardiac acyl-CoA composition in obese rats: a potential role for hepatic lipogenesis. J Lipid Res 2010;51:1380–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taegtmeyer H, Stanley WC. Too much or not enough of a good thing? Cardiac glucolipotoxicity versus lipoprotection. J Mol Cell Cardiol 2011;50:2–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson NA, Walton DW, Sachinwalla T, et al. Noninvasive assessment of hepatic lipid composition: advancing understanding and management of fatty liver disorders. Hepatology 2008;47:1513–1523 [DOI] [PubMed] [Google Scholar]