Abstract
ATP and serotonin (5-HT) are neurotransmitters secreted from taste bud receptor (type II) and presynaptic (type III) cells, respectively. Norepinephrine (NE) has also been proposed to be a neurotransmitter or paracrine hormone in taste buds. Yet, to date, the specific stimulus for NE release in taste buds is not well understood, and the identity of the taste cells that secrete NE is not known. Chinese hamster ovary cells were transfected with α1A adrenoceptors and loaded with fura-2 (“biosensors”) to detect NE secreted from isolated mouse taste buds and taste cells. Biosensors responded to low concentrations of NE (≥10 nm) with a reliable fura-2 signal. NE biosensors did not respond to stimulation with KCl or taste compounds. However, we recorded robust responses from NE biosensors when they were positioned against mouse circumvallate taste buds and the taste buds were stimulated with KCl (50 mm) or a mixture of taste compounds (cycloheximide, 10 μm; saccharin, 2 mm; denatonium, 1 mm; SC45647, 100 μm). NE biosensor responses evoked by stimulating taste buds were reversibly blocked by prazosin, an α1A receptor antagonist. Together, these findings indicate that taste bud cells secrete NE when they are stimulated. We isolated individual taste bud cells to identify the origin of NE release. NE was secreted only from presynaptic (type III) taste cells and not receptor (type II) cells. Stimulus-evoked NE release depended on Ca2+ in the bathing medium. Using dual biosensors (sensitive to 5-HT and NE), we found all presynaptic cells secrete 5-HT and 33% corelease NE with 5-HT.
Keywords: biosensors, taste, serotonin, transmitters, calcium imaging, phospholipase C β2
Introduction
Taste buds are specialized chemosensory organs that transduce chemical stimuli in the oral cavity. Synapses between taste bud cells and primary sensory afferent fibers transmit gustatory signals to the CNS. Several transmitter candidates have been proposed for taste bud synapses, including glutamate (Nagai et al., 1998), serotonin (Huang et al., 2005), acetylcholine (Ogura 2002), neuropeptide Y (Zhao et al., 2005) and ATP (Finger et al., 2005). Of these, only serotonin (5-HT) and ATP have unambiguously been demonstrated to be secreted from taste buds (Huang et al., 2005, 2007; Romanov et al., 2007). There is now direct evidence that 5-HT and ATP are secreted by separate classes of taste cells. Presynaptic (type III) taste cells release 5-HT upon stimulation, and receptor (type II) cells secrete ATP (Huang et al., 2007).
Norepinephrine (NE) has been localized to rodent taste cells and also has been proposed as a candidate neurotransmitter or paracrine hormone in mammalian taste buds (Nagahama and Kurihara, 1985; Herness et al., 2002). Recently, Dvoryanchikov et al. (2007) used reverse transcription-PCR to show that a subset of taste cells expresses the norepinephrine transporter (NET). These authors further showed that lingual epithelium containing taste buds secreted NE when the tissue was depolarized by applying KCl. However, despite these findings there is uncertainty regarding the specific stimuli that evoke NE release from taste buds and ambiguity about which cells secrete NE. Using a novel biosensor cell technique, we have detected NE release in response to taste stimuli and have identified the specific taste cell type that secretes this neurotransmitter.
Materials and Methods
Biosensor cells.
We produced two lines of Chinese hamster ovary (CHO) cells, one stably expressing α1A receptors (hereafter, “NE biosensors”) (Jiao et al., 2002) and another stably coexpressing α1A and 5-HT2c receptors (hereafter, “dual biosensors”). We also used 5-HT biosensors and ATP biosensors as described previously (Huang et al., 2005, 2007). Biosensors were loaded with 5 μm fura-2 AM as described by Huang et al. (2007). An aliquot of fura-2-loaded, suspended biosensor cells was transferred to a recording chamber (Warner Instruments) containing isolated taste buds and/or isolated taste cells and viewed with an inverted microscope (Olympus; IX 70). Immediately after NE-, 5-HT-, or dual biosensors had settled to the bottom of the chamber they were tested with a single application of 5-HT (3 nm) or NE (10 nm). A highly sensitive biosensor cell was identified and drawn onto a fire-polished glass micropipette with gentle suction for use in testing transmitter release from taste cells.
In separate experiments in the absence of taste buds, we verified that NE biosensors and dual biosensors do not respond to bath-applied KCl (50 mm, substituted for equimolar NaCl), or a mixture of taste compounds (cycloheximide, 10 μm; saccharin, 2 mm; SC45647, 0.1 mm; denatonium, 1 mm) (compare Huang et al., 2005, 2007). Prazosin (1 μm), an α1A receptor antagonist, was used to verify NE responses in NE and dual biosensor cells. Similarly, mianserin (1 nm), a 5-HT2c receptor antagonist, was used to verify and distinguish 5-HT responses in dual biosensors. Prazosin and mianserin were obtained from Sigma.
Isolated taste buds and/or taste cells.
Three lines of mice were used in these experiments: C57BL/6J mice (wild-type) of both sexes, transgenic mice expressing enhanced green fluorescent protein (GFP) under control of the PLCβ2 promoter (PLCβ2-GFP mice) (Kim et al., 2006), and transgenic mice expressing GFP under the GAD67 promoter (GAD-GFP mice) (Chattopadhyaya et al., 2004). Taste cells that fluoresce green in PLCβ2-GFP mice identify receptor (type II) cells and those that fluoresce green in GAD-GFP mice identify presynaptic (type III) cells (Kim et al., 2006; Tomchik et al., 2007). Mice were killed following National Institutes of Health guidelines, as approved by the University of Miami Animal Care and Use Committee.
We removed the lingual epithelium containing taste papillae from the tongue by injecting 1 mg/ml collagenase A (Roche), 2.5 mg/ml dispase II (Roche), and 1 mg/ml trypsin inhibitor (Sigma) directly under the epithelium surrounding circumvallate papillae. The peeled epithelium was bathed in Ca2+-free solution 30 min at room temperature and isolated taste cells were drawn into fire-polished glass micropipettes with gentle suction. Taste cells were transferred to a shallow recording chamber having a glass coverslip base and then loaded with 5 μm fura-2 AM. The coverslip was coated with Cell-Tak (BD Biosciences) to hold taste cells firmly attached. Taste buds and/or taste cells were superfused with Tyrode solution (in mm: 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose, 10 Na-pyruvate, 5 NaHCO3, pH7.4, 310–320 Osm). For Ca2+-free Tyrode solution, MgCl2 was substituted for CaCl2 (in mm: 140 NaCl, 5 KCl, 3 MgCl2, 10 HEPES, 10 glucose, 10 Na-pyruvate, 5 NaHCO3, 2 BAPTA, 2 EGTA, pH7.4, 310–320 Osm).
Ca2+ imaging.
For fura-2-loaded biosensor and isolated taste cells, sequential images were recorded at 40× with a long pass emission filter (≥510 nm) when excited at 340 nm followed by 380 nm (Huang et al., 2007). Images were processed with Indec Workbench v5 software. Data shown are the ratios, F340/F380, indicating relative changes in [Ca2+]i.
Stimulation.
Isolated taste cells were stimulated by bath-perfusion of KCl (50 mm substituted equimolar for NaCl), taste mix (cycloheximide, 10 μm; saccharin, 2 mm; SC45647, 0.1 mm; denatonium, 1 mm), or acetic acid (10 mm). All stimuli were made up in Tyrode solution and applied at pH 7.2, except for acetic acid, which was applied at pH 5.0.
Results
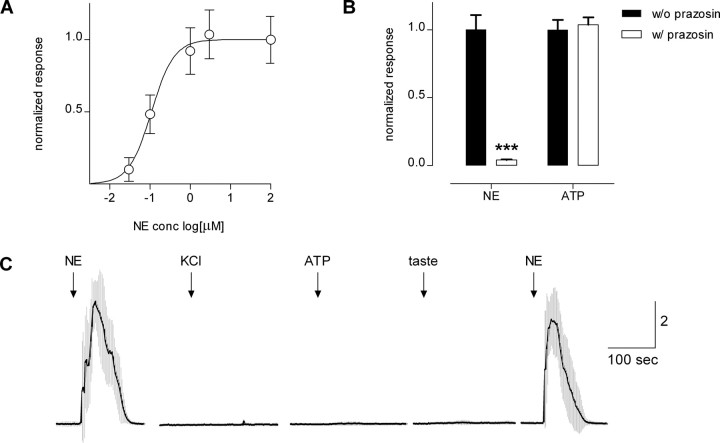
To test whether gustatory stimuli excite taste buds to release NE and if so, to identify which taste cells are responsible for the release, we stably transfected CHO cells with α1A receptors (NE biosensors). NE biosensors were loaded with the Ca2+-sensitive dye fura-2 AM and tested with bath-applied NE (Fig. 1 A). Threshold activation was ∼10 nm NE. Concentration-response relations indicated that EC50 was 117 nm, consistent with published EC50 values (122 nm) for α1A receptors under similar conditions (Jiao et al., 2002). Responses to bath-applied NE were selectively and reversibly abolished by 1 μm prazosin (Fig. 1 B). We prevented signals from endogenous purinoceptors by incubating NE biosensor cells in 500 μm ATP for 30 min before experiments. This desensitized purinoceptors and rendered the NE biosensors unresponsive to ATP (up to 500 μm). NE biosensor cells did not generate Ca2+ responses to bath-applied KCl (50 mm), acetic acid (10 mm, pH5), or a mixture of bitter and sweet taste compounds (10 μm cycloheximide, 1 mm denatonium, 2 mm saccharin, 0.1 mm SC45647, an artificial sweetener) (Fig. 1 C). Last, NE biosensors responded to NE when Ca2+ in the medium was replaced with Mg2+ (data not shown), consistent with the coupling of α1A receptors to intracellular Ca2+ release (Jiao et al., 2002; Horinouchi et al., 2007). In short, NE biosensors were highly sensitive, reliable, and specific detectors for norepinephrine.
Figure 1.
CHO cells stably expressing α1A receptors respond to bath-applied NE and can be used as NE biosensors. Cells were loaded with fura-2 and Ca2+ mobilization was measured. A, Concentration-response relationships for NE. Open circles indicate the mean ± SEM (N = 14 cells). B, NE biosensor responses evoked by 1 μm ATP to stimulate endogenous purinoceptors and 10 nm NE to excite transfected α1A receptors. Filled columns, control responses. Open columns, effect of prazosin (1 μm), an antagonist of α1A receptors. ATP and NE responses were measured in parallel, and responses were normalized to each agonist (in absence of prazosin) separately. As expected, prazosin selectively abolished the responses evoked by NE (***p < 0.0001). C, NE biosensor cells on their own do not respond to KCl depolarization or to stimulation with ATP or taste compounds. Trace shows mean ± SEM (gray bars) of Ca2+ responses from NE biosensor cells (N = 6) after incubating with 500 μm ATP to desensitize endogenous purinoceptors. NE, 10 nm; KCl, 50 mm; ATP, 10 μm; taste, mixture of cycloheximide (10 μm), saccharin (2 mm), SC45647 (0.1 mm), and denatonium (1 mm). Ordinate, calibration in units of F340/F380 in this and subsequent figures.
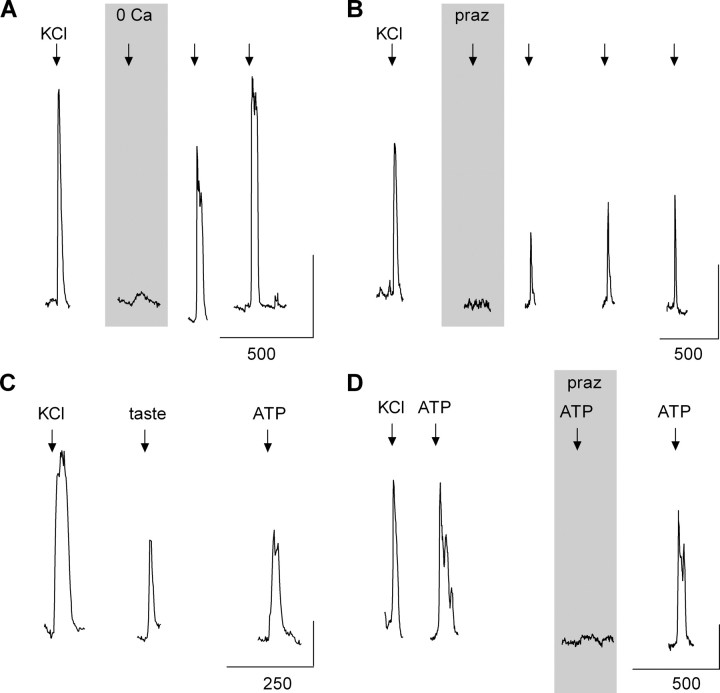
We tested the ability of isolated mouse vallate taste buds to secrete NE when stimulated. NE biosensors were positioned next to isolated taste buds with a glass manipulator pipette. Depolarizing taste buds with KCl (50 mm) generated robust biosensor signals that were abolished in the absence of the extracellular Ca2+ (Fig. 2 A) and blocked by prazosin (Fig. 2 B). These findings indicate that stimulating taste buds evokes NE release and that the release of NE is dependent on Ca2+ influx, consistent with synaptic exocytosis. Furthermore, stimulating isolated taste buds with a mixture of taste compounds also elicited NE biosensor responses (Fig. 2 C). ATP stimulates taste buds to release 5-HT (Huang et al., 2007). Thus, we tested whether ATP would also evoke NE secretion from isolated taste buds. Bath-applied ATP (1 μm) elicited robust responses of NE biosensors (whose endogenous purinoceptors had been desensitized, see above) indicating that ATP also stimulates taste bud cells to secrete NE (Fig. 2 C). Prazosin blocked ATP-evoked NE biosensor responses, verifying that the responses were produced by NE (Fig. 2 D). These data indicate that KCl depolarization, taste stimulation, or ATP excitation of taste buds evokes NE secretion.
Figure 2.
Stimulating taste buds evokes Ca-dependent release of norepinephrine. A, An NE biosensor cell positioned against a taste bud responds when the taste bud was depolarized by 50 mm KCl. Removing Ca2+ from the bathing medium (“0 Ca”) abolished stimulus-evoked NE release from the taste bud. B, Applying prazosin (“praz,” 1 μm) reversibly reduced NE biosensor responses evoked by depolarizing taste buds with KCl, verifying that biosensor responses originated from α1A receptors and thus confirming that NE was the transmitter being released. C, Taste stimuli (“taste,” cycloheximide, 10 μm; saccharin, 2 mm; SC45647, 0.1 mm; denatonium, 1 mm) and ATP (1 μm) also evoked NE release from taste buds. D, Prazosin blocked ATP-evoked biosensor responses, verifying that biosensor responses were generated by NE release and not elicited by activating endogenous biosensor cell purinoceptors. In all cases, endogenous biosensor cell purinoceptors were desensitized before the experiments, as described in Materials and Methods. Calibration: vertical, 0.5; horizontal, seconds, as marked.
Isolated taste buds also release serotonin (5-HT) when stimulated (Huang et al., 2005). We surveyed isolated taste buds for the relative incidence of 5-HT versus NE release. Using 5-HT biosensors, we found that KCl evoked 5-HT release in 39% of isolated taste buds (213 of 540). However, under identical conditions, KCl depolarization evoked NE release in only 12% of isolated taste buds (6 of 49), or ∼1/3 the incidence of 5-HT secretion. This difference is highly significant (p < 0.0001, Fisher's exact test).
We next investigated which taste cell type(s) secreted NE. We isolated individual taste cells, loaded them with fura-2, and measured their responses to taste stimulation and to KCl depolarization (Huang et al., 2007). Taste bud cells can be differentiated by whether they respond to taste compounds (receptor, type II, cells) or whether they respond to KCl depolarization (presynaptic, type III, cells) (DeFazio et al., 2006; Huang et al., 2007; Tomchik et al., 2007). We also isolated taste cells from PLC2-GFP mice which express GFP in receptor cells (Kim et al., 2006) and from GAD-GFP mice which express GFP selectively in presynaptic cells (Tomchik et al., 2007) to determine which cells release NE. NE biosensor cells were positioned against identified receptor or presynaptic taste cells to test for NE secretion.
We did not detect NE secretion from receptor cells, despite prominent taste-evoked Ca2+ responses in those cells (0 of 17 cells tested). However, NE biosensors showed marked responses when they were positioned against presynaptic cells and the presynaptic cells were stimulated with KCl or acetic acid (Fig. 3) [KCl and acetic acid alike are effective stimuli for presynaptic taste cells (Huang et al., 2005, 2008; Tomchik et al., 2007)]. Although we recorded NE secretion from isolated presynaptic cells, the incidence was low compared with that for 5-HT release. Depolarizing presynaptic cells with KCl evoked 5-HT release in 84% of cells (43 of 51 cells), but only 28% (8 of 29 cells) for NE release, or ∼1/3 as frequent. These data are consistent with the above findings on NE versus 5-HT release from whole taste buds.
Figure 3.
Presynaptic (type III) taste cells, but not receptor (type II) cells, secrete NE. GAD-GFP mice were used to isolate and identify individual presynaptic taste cells; PLCβ2-GFP mice were used to identify receptor taste cells. A, Receptor cells do not secrete NE. The pair of traces shows simultaneous Ca2+ recordings in a receptor taste cell (Rec) and an apposed NE biosensor cell (NE bio). NE (10 nm) was applied first to verify biosensor sensitivity. Taste stimulation was a mixture, as in Figure 2. B, Presynaptic cells secrete NE. Stimulus-evoked responses are from the presynaptic cell shown in C2 (next). Stimulating with KCl (50 mm) or acetic acid (“HOAc,” 10 mm, pH 5.0) triggered Ca2+ responses in the presynaptic cell (Pre) and NE release (NE bio). As in A, 10 nm NE was applied initially to validate the NE biosensor. C, Fluorescence and interference contrast optics micrographs showing a NE biosensor (“NE-bio”) apposed to identified presynaptic cell (i.e., expressing GFP, “pre”). Traces in B were taken from the presynaptic cell shown in C 2. Note that debris attached to cells in C2 did not interfere with responses.
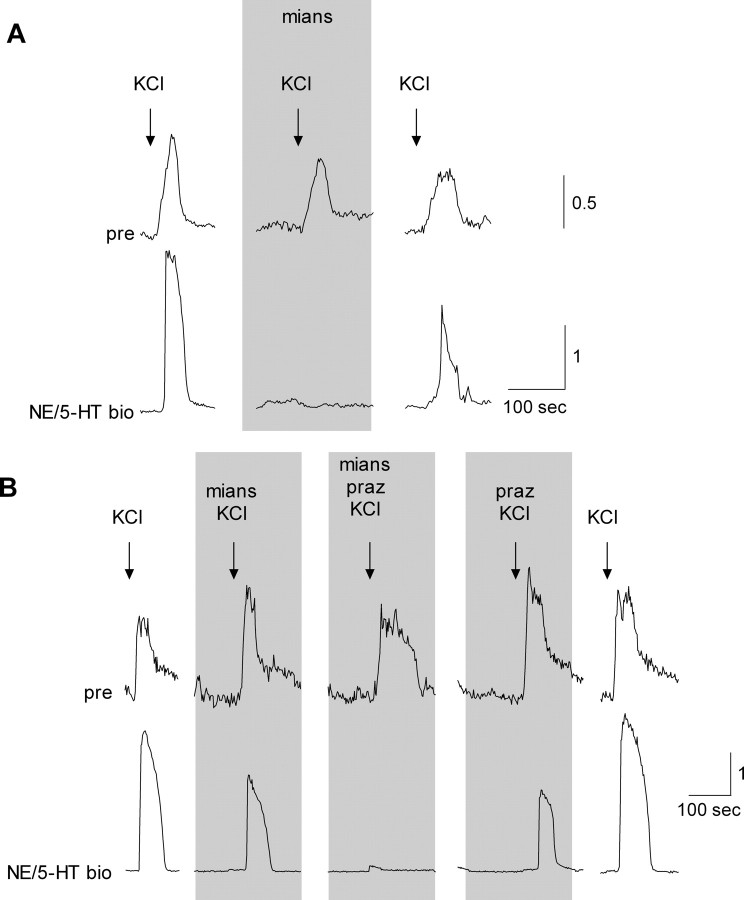
Last, we asked whether presynaptic cells that secreted NE might corelease NE with 5-HT. To study corelease, we used dual biosensor cells that coexpressed serotonergic and adrenergic receptors and thus could report the secretion of 5-HT, or NE, or both. Selective antagonists allowed us to distinguish which biosensor receptors were activated and thus identify the source of the responses. Dual biosensors were positioned next to presynaptic cells and the presynaptic cells were depolarized with KCl, as above. When we observed a dual biosensor responding to this procedure, we applied mianserin to block the biosensor's serotonergic receptors. In most cases, mianserin totally abolished KCl-evoked dual biosensor signals, indicating that KCl stimulation had evoked 5-HT release (Fig. 4 A) (see also Huang et al., 2005, their Fig. 1 C). However, in 4 of 13 experiments, mianserin only partially blocked the dual biosensor response. In these experiments, adding prazosin (in presence of manserin) to block biosensor adrenoceptors completely eliminated the biosensor responses (Fig. 4 B). We also conducted the reverse sequence: prazosin followed by prazosin plus mianserin. Prazosin alone did not eliminate biosensor responses to presynaptic cell stimulation in any experiments. Collectively, these findings suggest that when depolarized with KCl, all presynaptic cells secrete 5-HT but ∼1/3 corelease NE and 5-HT.
Figure 4.
A subset of presynaptic (type III) taste cell corelease 5-HT and NE. Individual presynaptic taste cells were isolated and identified and tested for transmitter release with a dual biosensor, sensitive to either NE or 5-HT (NE- and 5-HT biosensor), as described in Materials and Methods. As in Figure 3, simultaneous recordings were made from a presynaptic cell and an apposed dual biosensor. A, KCl depolarization triggered Ca2+ responses in the presynaptic cell (pre; top) and evoked NE release (NE/5-HT bio; bottom). Mianserin (“mian,” 1 nm), a 5-HT2c receptor antagonist, reversibly blocked the dual biosensor cell responses, indicating that in this case, the presynaptic cell only released 5-HT. B, In other cases (4 of 13), mianserin or prazosin (“praz,” 1 μm) alone only partially blocked responses from the dual biosensor, but adding mianserin together with prazosin strongly inhibited dual biosensor responses. This finding indicates that in some cases, stimulating presynaptic taste cells coreleases 5-HT and NE.
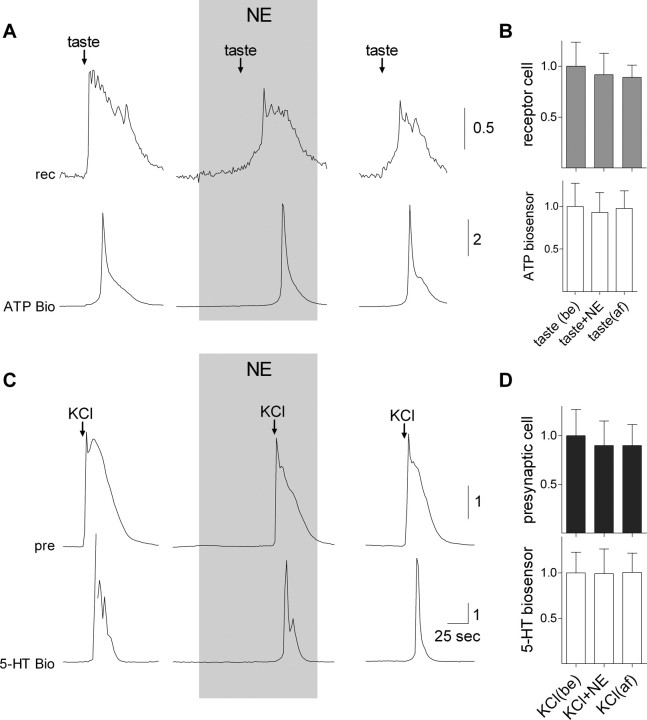
Recently, Heath et al. (2006) reported that human taste thresholds were lowered by circulating NE. Accordingly, we tested whether bath applying NE to isolated mouse taste bud cells affected excitation and transmitter secretion from receptor (type II) or presynaptic (type III) cells. We isolated and identified taste cells, used ATP biosensors (for receptor cells) or 5-HT biosensors [for presynaptic cells, as described by Huang et al. (2007)], and applied NE. Based on our observations that applying 10 nm NE calibration doses to NE biosensors evoked responses equal to or much larger than those generated by NE release from presynaptic cells (Fig. 3 C), we estimated that stimulus-evoked NE in interstitial spaces within taste buds in situ might reach or exceed 10 nm. Nevertheless, we tested bath-applied NE up to 100 μm. In no case were receptor or presynaptic cell responses (Ca2+ mobilization) altered by NE. Further, NE did not alter stimulus-evoked secretion of ATP or 5-HT (Fig. 5). We conclude that NE acts on targets in the taste bud other than receptor and presynaptic taste cells, perhaps sensory nerve terminals or type I taste cells. This question remains to be tested.
Figure 5.
Norepinephrine does not affect stimulus-evoked transmitter release (ATP, 5-HT) from receptor (type II) or presynaptic (type III) taste cells. A, Simultaneous recordings from an isolated taste receptor cell (top) and an apposed ATP-biosensor to monitor transmitter secretion during taste stimulation (Fig. 2). Taste-evoked responses in the receptor cell and ATP secretion were unaffected in the presence of even relatively high concentrations of NE (100 μm; shaded area). B, Summary of the effects of NE on taste receptor cells and taste-evoked ATP secretion. Error bars represent mean ± SEM of taste-evoked responses in receptor cells (gray bars) and in ATP biosensors (white bars) (N = 6 cells). Individual responses were normalized to the (pooled) average taste evoked responses for all experiments in this series. No significant effects were of applying NE. C, Similarly, comparable recordings taken from an isolated presynaptic taste cell, and an apposed 5-HT biosensor cell showed no effects of applying 100 μm NE. D, As in B, summary of data from eight cells showing the lack of effect of NE on KCl-evoked responses in presynaptic cells and in stimulus-evoked 5-HT release.
Discussion
This report shows that NE is a neurotransmitter in mammalian taste buds. Isolated taste buds cells secrete NE in response to taste stimulation. The cells responsible for NE release comprise a subset of presynaptic (type III) taste cells. NE appears to be coreleased with 5-HT.
We previously reported that taste cells that respond to sweet and bitter taste compounds, i.e., receptor (type II) cells, secrete ATP through pannexin 1 hemichannels in response to taste stimulation (Huang et al., 2007). Moreover, taste-evoked secretion of ATP excites presynaptic (type III) cells and causes them to release 5-HT, indicating cell-to-cell communication between receptor and presynaptic cells within taste buds. Thus, the likely explanation for taste-evoked NE secretion from isolated taste buds (Fig. 2 B) is that NE release is secondary to taste stimulation of receptor cells, release of ATP, and subsequent excitation of presynaptic cells, just as the case for 5-HT release.
The findings reported by Heath et al. (2006) on NE modulation of human taste threshold emphasize the importance of noradrenergic mechanisms in the peripheral sensory organs of taste. However, the specific postsynaptic target(s) for NE remain to be determined. Herness and Sun (1999) and Herness et al. (2002) reported that NE inhibits K+ currents and enhances Cl− currents in taste cells from rats. They also reported that applying NE evoked intracellular Ca2+ transients in ∼17% of taste cells, but without identifying cell type. Herness et al. (2002) concluded that NE may play a modulatory role in taste buds. However, we found no evidence for effects of NE on stimulus-evoked neurotransmitter (ATP, 5-HT) release in mouse taste buds nor any evidence for NE-evoked Ca2+ mobilization in taste cells. If NE modulates taste bud function in mouse taste buds as reported for rat (Herness et al., 2002), NE must act on mechanisms other than ATP and 5-HT release, or act on cells other than receptor (type II) and presynaptic (type III) cells. NE was reported to enhance gustatory nerve responses in frogs (Morimoto and Sato, 1982; Nagahama and Kurihara, 1985), raising the possibility that gustatory afferent fibers are the targets for taste-stimulated NE secretion in taste buds. Our experiments were not designed to test this possibility and this remains an intriguing possibility.
Based on the RT-PCR and immunohistochemical data, Dvoryanchikov et al. (2007) reported that biosynthetic enzymes for NE, tyrosine hydroxylase and dopamine β-hydroxylase, are not present in mouse taste buds. They concluded that the source of NE in taste buds is cellular uptake from interstitial spaces. Those workers also reported that KCl stimulation of lingual tissues elicited NE release, presumably from NE uptake into taste cells. Ultrastructural studies have shown that there are adrenergic fibers in and around taste buds (Yoshie et al., 1996, rat; Reutter and Witt, 2004, fish), possibly representing efferent sympathetic innervation and a potential source of NE for taste buds. Dvoryanchikov et al. (2007) found that a subset of presynaptic cells in mouse taste buds express the NET, thus providing a mechanism for NE uptake. Our findings are consistent with those results, namely that a subset of presynaptic cells take up and secrete NE upon stimulation.
Our data indicate that the majority or all presynaptic (type III) taste cells secrete 5-HT upon stimulation but only ∼1/3 of them corelease NE with 5-HT. We found no instance of NE secretion alone. One interpretation is that there are 3–5 times as many serotonergic presynaptic cells as there are NE/5-HT-secreting cells. Another interpretation is that 5-HT is secreted diffusely over the entire presynaptic cell surface but NE is released only at discrete foci, such as at the synapses that these cells, and only these cells, possess (Yang et al., 2000; Yee et al., 2001; Clapp et al., 2006). According to this interpretation, a biosensor cell positioned anywhere near a presynaptic cell would be likely to detect 5-HT release, but NE secretion would only be recorded when the biosensor was positioned in close proximity to a synaptic release site. Such a possibility, as well as further information about the molecular mechanisms of 5-HT and NE release is currently under investigation.
Footnotes
This work was supported by National Institute on Deafness and Other Communication Disorders–National Institutes of Health Grant 5R01DC007630. We thank Dr. William Jeffries (Creighton University School of Medicine) for the generous donation of α1A receptor DNA.
References
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp TR, Medler KF, Damak S, Margolskee RF, Kinnamon SC. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol. 2006;4:7. doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci. 2006;26:3971–3980. doi: 10.1523/JNEUROSCI.0515-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoryanchikov G, Tomchik SM, Chaudhari N. Biogenic amine synthesis and uptake in rodent taste buds. J Comp Neurol. 2007;505:302–313. doi: 10.1002/cne.21494. [DOI] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Heath TP, Melichar JK, Nutt DJ, Donaldson LF. Human taste thresholds are modulated by serotonin and noradrenaline. J Neurosci. 2006;26:12664–12671. doi: 10.1523/JNEUROSCI.3459-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herness MS, Sun XD. Characterization of chloride currents and their noradrenergic modulation in rat taste receptor cells. J Neurophysiol. 1999;82:260–271. doi: 10.1152/jn.1999.82.1.260. [DOI] [PubMed] [Google Scholar]
- Herness S, Zhao FL, Kaya N, Lu SG, Shen T, Sun XD. Adrenergic signalling between rat taste receptor cells. J Physiol. 2002;543:601–614. doi: 10.1113/jphysiol.2002.020438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi T, Miyake Y, Nishiya T, Nishimoto A, Yorozu S, Jinno A, Kajita E, Miwa S. Characterization of noradrenaline-induced increases in intracellular Ca2+ levels in Chinese hamster ovary cells stably expressing human alpha1A-adrenoceptor. J Pharmacol Sci. 2007;105:103–111. doi: 10.1254/jphs.fp0070891. [DOI] [PubMed] [Google Scholar]
- Huang YA, Maruyama Y, Stimac R, Roper SD. Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. J Physiol. 2008;586:2903–2912. doi: 10.1113/jphysiol.2008.151233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Lu KS, Pereira E, Plonsky I, Baur JE, Wu D, Roper SD. Mouse taste buds use serotonin as a neurotransmitter. J Neurosci. 2005;25:843–847. doi: 10.1523/JNEUROSCI.4446-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao X, Gonzalez-Cabrera PJ, Xiao L, Bradley ME, Abel PW, Jeffries WB. Tonic inhibitory role for cAMP in alpha(1a)-adrenergic receptor coupling to extracellular signal-regulated kinases 1/2. J Pharmacol Exp Ther. 2002;303:247–256. doi: 10.1124/jpet.102.037747. [DOI] [PubMed] [Google Scholar]
- Kim JW, Roberts C, Maruyama Y, Berg S, Roper S, Chaudhari N. Faithful expression of GFP from the PLCbeta2 promoter in a functional class of taste receptor cells. Chem Senses. 2006;31:213–219. doi: 10.1093/chemse/bjj021. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Sato M. Role of monoamines in afferent synaptic transmission in frog taste organ. Jpn J Physiol. 1982;32:855–871. doi: 10.2170/jjphysiol.32.855. [DOI] [PubMed] [Google Scholar]
- Nagahama S, Kurihara K. Norepinephrine as a possible transmitter involved in synaptic transmission in frog taste organs and Ca dependence of its release. J Gen Physiol. 1985;85:431–442. doi: 10.1085/jgp.85.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Delay RJ, Welton J, Roper SD. Uptake and release of neurotransmitter candidates, [3H]serotonin, [3H]glutamate, and [3H]gamma-aminobutyric acid, in taste buds of the mudpuppy, Necturus maculosus. J Comp Neurol. 1998;392:199–208. [PubMed] [Google Scholar]
- Ogura T. Acetylcholine increases intracellular Ca2+ in taste cells via activation of muscarinic receptors. J Neurophysiol. 2002;87:2643–2649. doi: 10.1152/jn.2002.87.6.2643. [DOI] [PubMed] [Google Scholar]
- Reutter K, Witt M. Are there efferent synapses in fish taste buds? J Neurocytol. 2004;33:647–656. doi: 10.1007/s11068-005-3333-z. [DOI] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 2007;26:657–667. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci. 2007;27:10840–10848. doi: 10.1523/JNEUROSCI.1863-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Crowley HH, Rock ME, Kinnamon JC. Taste cells with synapses in rat circumvallate papillae display SNAP-25-like immunoreactivity. J Comp Neurol. 2000;424:205–215. doi: 10.1002/1096-9861(20000821)424:2<205::aid-cne2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Yee CL, Yang R, Böttger B, Finger TE, Kinnamon JC. “Type III” cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J Comp Neurol. 2001;440:97–108. doi: 10.1002/cne.1372. [DOI] [PubMed] [Google Scholar]
- Yoshie S, Kanazawa H, Fujita T. A possibility of efferent innervation of the gustatory cell in the rat circumvallate taste bud. Arch Histol Cytol. 1996;59:479–484. doi: 10.1679/aohc.59.479. [DOI] [PubMed] [Google Scholar]
- Zhao FL, Shen T, Kaya N, Lu SG, Cao Y, Herness S. Expression, physiological action, and coexpression patterns of neuropeptide Y in rat taste-bud cells. Proc Natl Acad Sci U S A. 2005;102:11100–11105. doi: 10.1073/pnas.0501988102. [DOI] [PMC free article] [PubMed] [Google Scholar]