Abstract
To examine whether abnormal pancreatic alpha-cell function found in human diabetes mellitus may represent a selective insensitivity to glucose, plasma glucagon responses to hyperglycemia and elevation of plasma free fatty acid levels (both known suppressors of glucagon secretion) were compared in juvenile-onset, insulin-requiring diabetic subjects, and in normal nondiabetic subjects. In the latter, both elevation of plasma free fatty acid levels induced by heparin administration of hyperglycemia produced by intravenous infusion of glucose resulted in a comparable 30--40% suppression of circulating glucagon levels (P less than 0.01). In the diabetic subjects, glucagon suppression by hyperglycemia (less than 20%) was less than that occurring in normal subjects (P less than 0.01), even when accompanied by infusion of supraphysiologic amounts of insulin. However, suppression of glucagon levels by elevation of plasma free fatty acids in the diabetic group was similar to that found in normal subjects and of comparable magnitude to that due to hyperglycemia in the normal subjects. These results thus demonstrate a selective impairment of the diabetic alpha-cell response to glucose and provide further evidence for the presence of an abnormal alpha-cell glucoreceptor in human diabetes mellitus.
Full text
PDF
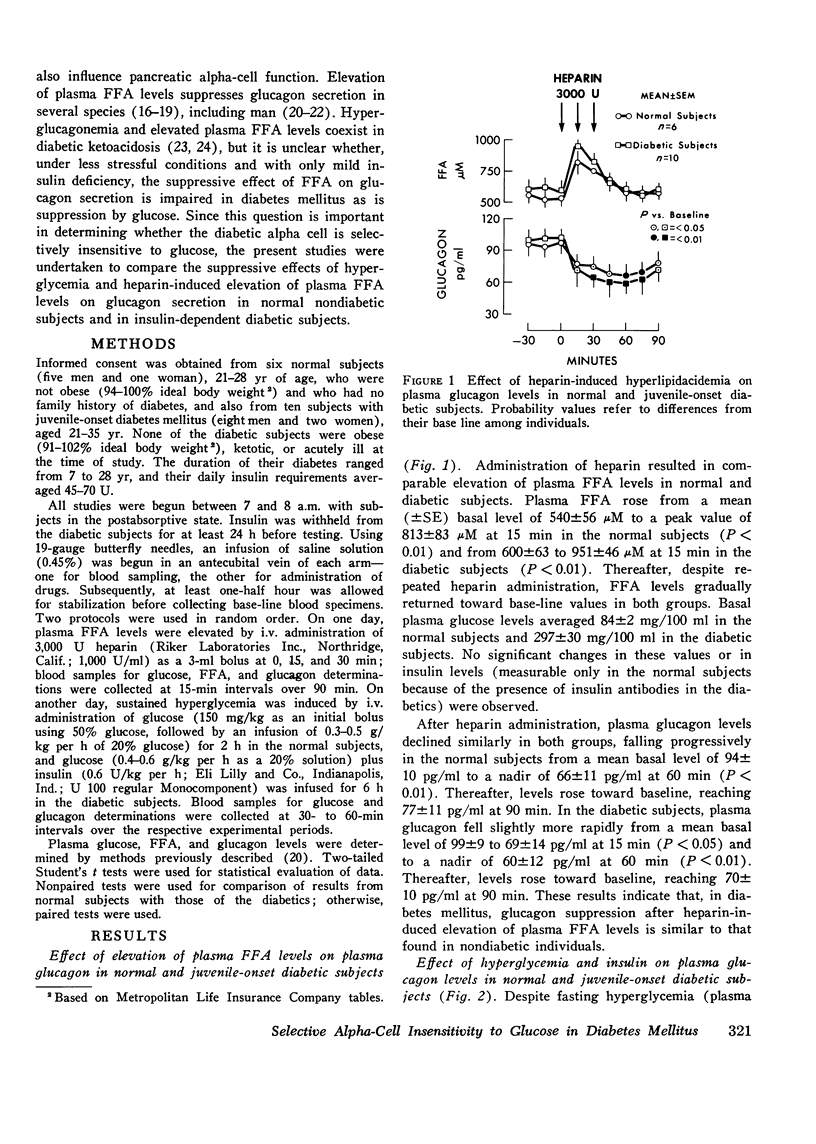
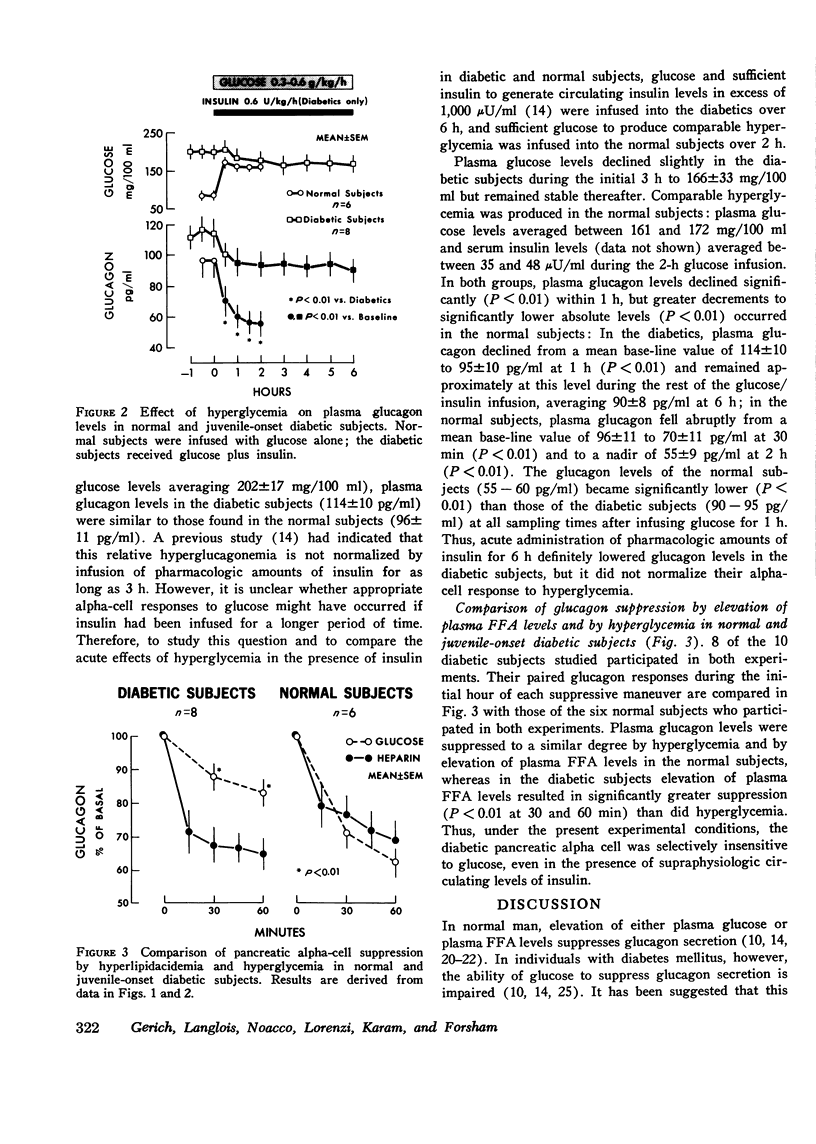



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberti K. G. Role of glucagon and other hormones in development of diabetic ketoacidosis. Lancet. 1975 Jun 14;1(7920):1307–1311. doi: 10.1016/s0140-6736(75)92315-6. [DOI] [PubMed] [Google Scholar]
- Andrews S. S., Lopez-S A., Blackard W. G. Effect of lipids on glucagon secretion in man. Metabolism. 1975 Jan;24(1):35–44. doi: 10.1016/0026-0495(75)90005-0. [DOI] [PubMed] [Google Scholar]
- Assan R., Hautecouverture G., Guillemant S., Dauchy F., Protin P., Derot M. Evolution de paramètres hormonaux (glucagon, cortisol, hormone somatotrope) et énergétiques (glucose, acides gras, glycérol libre) dans dix acido-cétoses diabétiques graves traitées. Pathol Biol (Paris) 1969 Dec;17(23):1095–1105. [PubMed] [Google Scholar]
- Balasse E. O., Neef M. A. Operation of the "glucose-fatty acid cycle" during experimental elevations of plasma free fatty acid levels in man. Eur J Clin Invest. 1974 Aug;4(4):247–252. doi: 10.1111/j.1365-2362.1974.tb00400.x. [DOI] [PubMed] [Google Scholar]
- Braaten J. T., Faloona G. R., Unger R. H. The effect of insulin on the alpha-cell response to hyperglycemia in long-standing alloxan diabetes. J Clin Invest. 1974 Apr;53(4):1017–1021. doi: 10.1172/JCI107638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan K. D., McCarroll A. M. Abnormalities of glucagon metabolism in untreated diabetes mellitus. Lancet. 1972 Dec 30;2(7792):1394–1395. doi: 10.1016/s0140-6736(72)92964-9. [DOI] [PubMed] [Google Scholar]
- Cerasi E., Luft R., Efendic S. Decreased sensitivity of the pancreatic beta cells to glucose in prediabetic and diabetic subjects. A glucose dose-response study. Diabetes. 1972 Apr;21(4):224–234. doi: 10.2337/diab.21.4.224. [DOI] [PubMed] [Google Scholar]
- Deckert T., Lauridsen U. B., Madsen S. N., Mogensen P. Insulin response to glucose, tolbutamide, secretin, and isoprenaline in maturity-onset diabetes mellitus. Dan Med Bull. 1972 Oct;19(7):222–226. [PubMed] [Google Scholar]
- Edwards J. C., Taylor K. W. Fatty acids and the release of glucagon from isolated guinea-pig islets of Langerhans incubated in vitro. Biochim Biophys Acta. 1970 Aug 14;215(2):310–315. doi: 10.1016/0304-4165(70)90029-2. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Langlois M., Noacco C., Karam J. H., Forsham P. H. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973 Oct 12;182(4108):171–173. doi: 10.1126/science.182.4108.171. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Langlois M., Schneider V., Karam J. H., Noacco C. Effects of alternations of plasma free fatty acid levels on pancreatic glucagon secretion in man. J Clin Invest. 1974 May;53(5):1284–1289. doi: 10.1172/JCI107675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich J. E., Lorenzi M., Hane S., Gustafson G., Guillemin R., Forsham P. H. Evidence for a physiologic role of pancreatic glucagon in human glucose homeostasis: studies with somatostatin. Metabolism. 1975 Feb;24(2):175–182. doi: 10.1016/0026-0495(75)90018-9. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Tsalikian E., Lorenzi M., Karam J. H., Bier D. M. Plasma glucagon and alanine responses to acute insulin deficiency in man. J Clin Endocrinol Metab. 1975 Mar;40(3):526–529. doi: 10.1210/jcem-40-3-526. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Tsalikian E., Lorenzi M., Schneider V., Bohannon N. V., Gustafson G., Karam J. H. Normalization of fasting hyperglucagonemia and excessive glucagon responses to intravenous arginine in human diabetes mellitus by prolonged infusion of insulin. J Clin Endocrinol Metab. 1975 Dec;41(06):1178–1180. doi: 10.1210/jcem-41-6-1178. [DOI] [PubMed] [Google Scholar]
- Grodsky G. M., Fanska R., West L., Manning M. Anomeric specificity of glucose-stimulated insulin release: evidence for a glucoreceptor? Science. 1974 Nov 8;186(4163):536–538. doi: 10.1126/science.186.4163.536. [DOI] [PubMed] [Google Scholar]
- Gross R., Mialhe P. Free fatty acid-glucagon feed-back mechanism. Diabetologia. 1974 Aug;10(4):277–283. doi: 10.1007/BF02627728. [DOI] [PubMed] [Google Scholar]
- Kalk W. J., Vinik A. I., Bank S., Buchanan K. D., Keller P., Jackson W. P. Glucagon responses to arginine in chronic pancreatitis. Possible pathogenic significance in diabetes. Diabetes. 1974 Apr;23(4):257–263. doi: 10.2337/diab.23.4.257. [DOI] [PubMed] [Google Scholar]
- Luyckx A. S., Gerard J., Gaspard U., Lefebvre P. J. Plasma glucagon levels in normal women during pregnancy. Diabetologia. 1975 Dec;11(6):549–554. doi: 10.1007/BF01222105. [DOI] [PubMed] [Google Scholar]
- Luyckx A. S., Lefebvre P. J. Arguments for a regulation of pancreatic glucagon secretion by circulating plasma free fatty acids. Proc Soc Exp Biol Med. 1970 Feb;133(2):524–528. doi: 10.3181/00379727-133-34511. [DOI] [PubMed] [Google Scholar]
- Matschinsky F. M., Pagliara A. S., Hover B. A., Haymond M. W., Stillings S. N. Differential effects of alpha- and beta-D- glucose on insulin and glucagon secretion from the isolated perfused rat pancreas. Diabetes. 1975 Apr;24(4):369–372. doi: 10.2337/diab.24.4.369. [DOI] [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Unger R. H. Hyperglucagonemia in diabetic ketoacidosis. Its prevalence and significance. Am J Med. 1973 Jan;54(1):52–57. doi: 10.1016/0002-9343(73)90083-1. [DOI] [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Unger R. H. The effect of experimental insulin deficiency on glucagon secretion. J Clin Invest. 1971 Sep;50(9):1992–1999. doi: 10.1172/JCI106691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki A., Niki H. Letter: Is diabetes mellitus a disorder of the glucoreceptor? Lancet. 1975 Oct 4;2(7936):658–658. doi: 10.1016/s0140-6736(75)90137-3. [DOI] [PubMed] [Google Scholar]
- Pagliara A. S., Stillings S. N., Haymond M. W., Hover B. A., Matschinsky F. M. Insulin and glucose as modulators of the amino acid-induced glucagon release in the isolated pancreas of alloxan and streptozotocin diabetic rats. J Clin Invest. 1975 Feb;55(2):244–255. doi: 10.1172/JCI107928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin P., Fujita Y., Unger R. H. Effect of insulin-glucose infusions on plasma glucagon levels in fasting diabetics and nondiabetics. J Clin Invest. 1975 Nov;56(5):1132–1138. doi: 10.1172/JCI108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson R. P., Porte D., Jr The glucose receptor. A defective mechanism in diabetes mellitus distinct from the beta adrenergic receptor. J Clin Invest. 1973 Apr;52(4):870–876. doi: 10.1172/JCI107251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyffert W. A., Jr, Madison L. L. Physiologic effects of metabolic fuels on carbohydrate metabolism. I. Acute effect of elevation of plasma free fatty acids on hepatic glucose output, peripheral glucose utilization, serum insulin, and plasma glucagon levels. Diabetes. 1967 Nov;16(11):765–776. doi: 10.2337/diab.16.11.765. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Aguilar-Parada E., Müller W. A., Eisentraut A. M. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970 Apr;49(4):837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H. Glucagon physiology and pathophysiology. N Engl J Med. 1971 Aug 19;285(8):443–449. doi: 10.1056/NEJM197108192850806. [DOI] [PubMed] [Google Scholar]



