Abstract
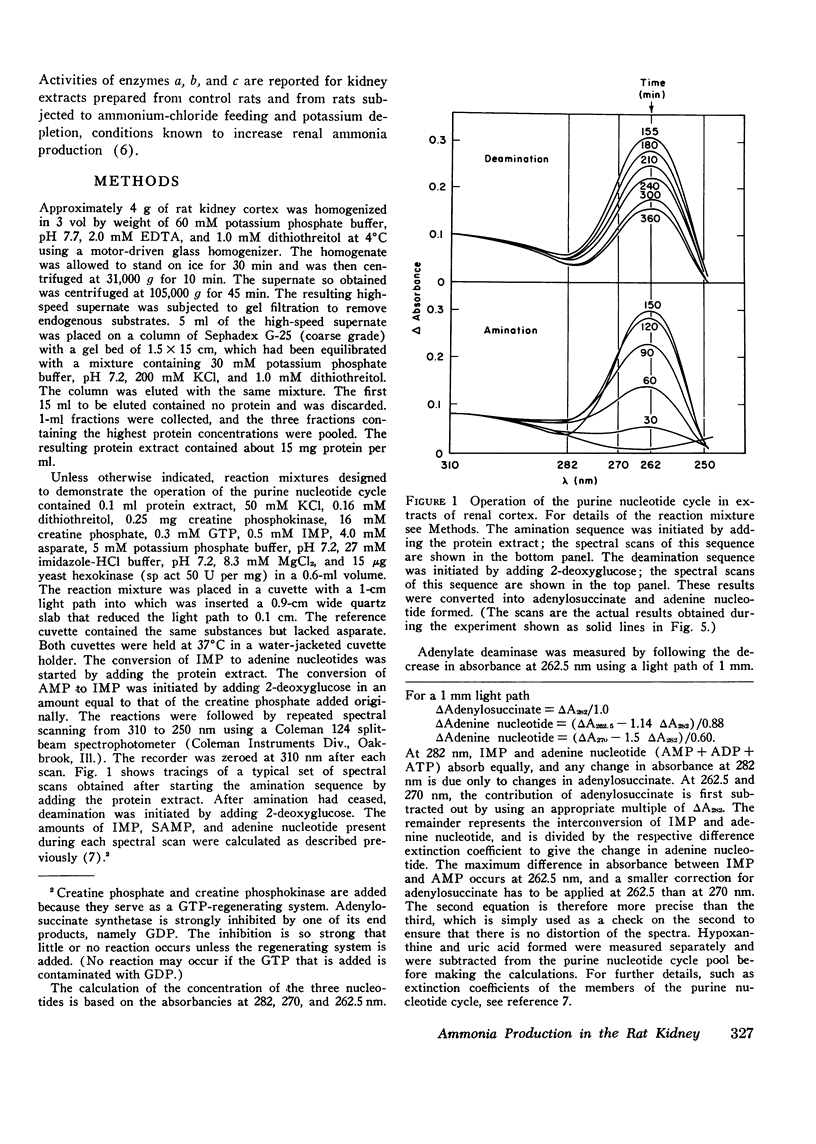
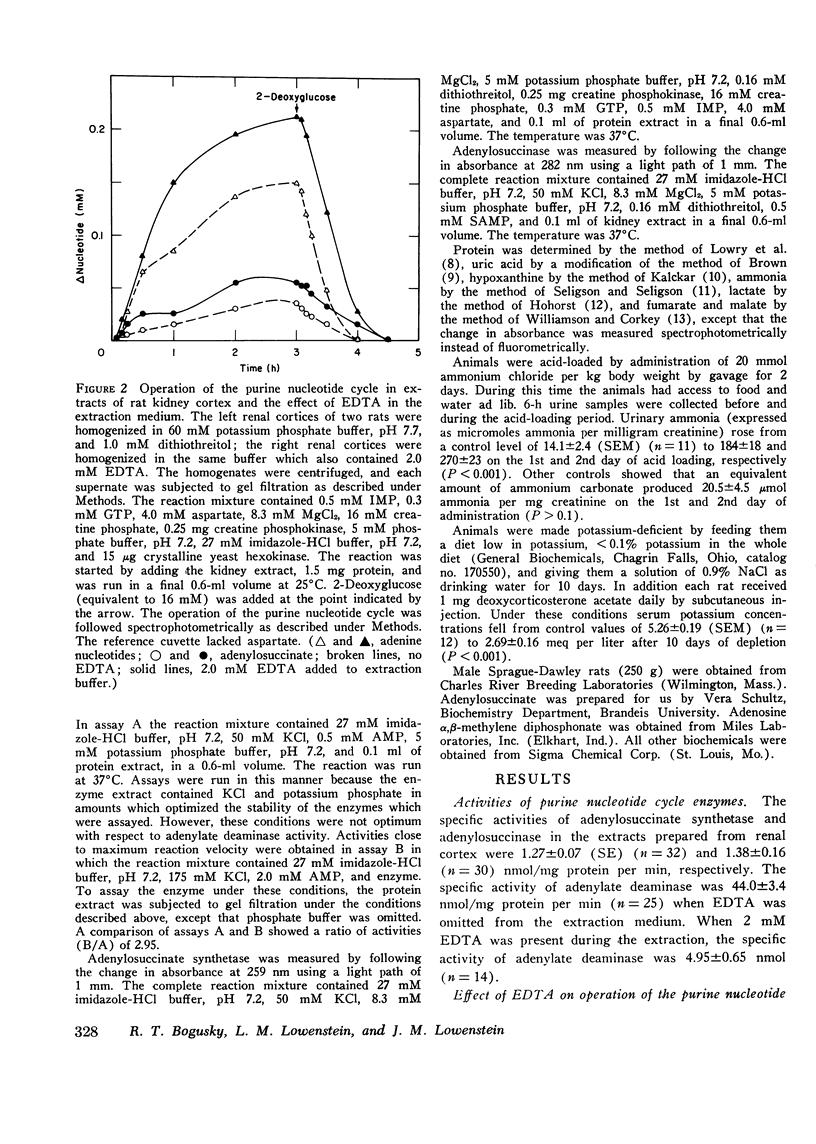
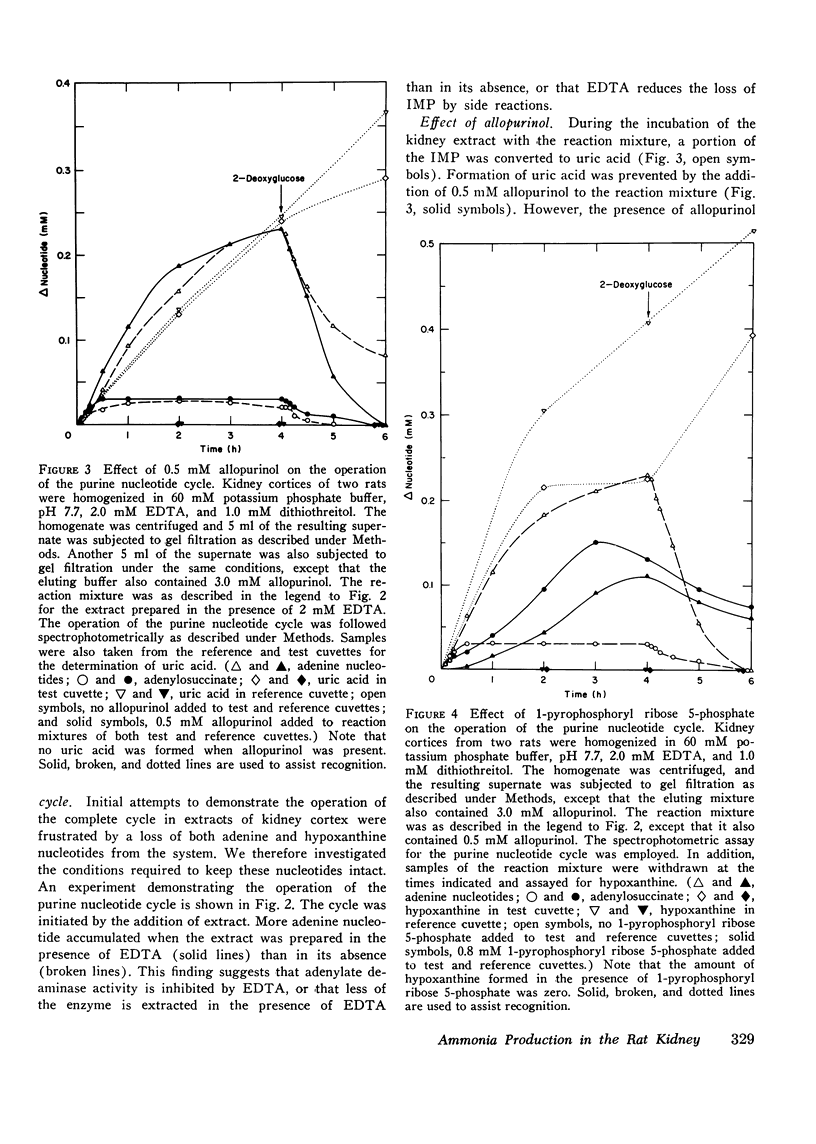
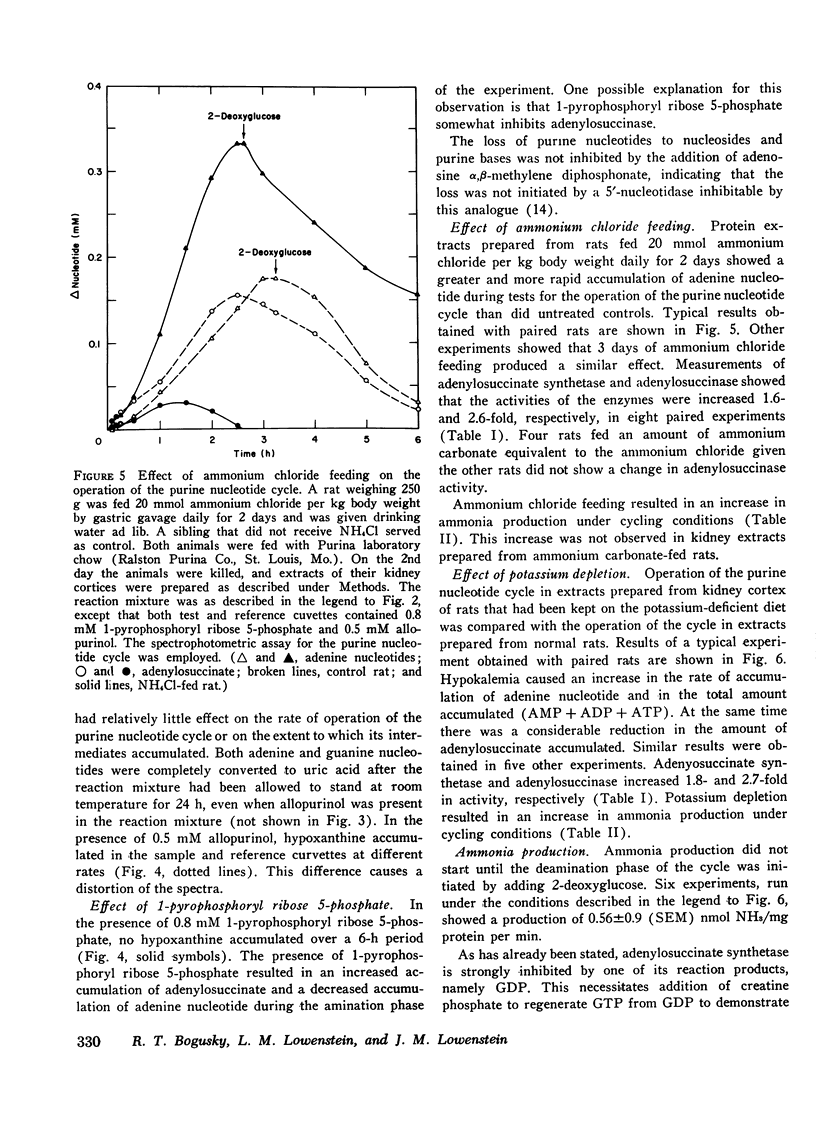
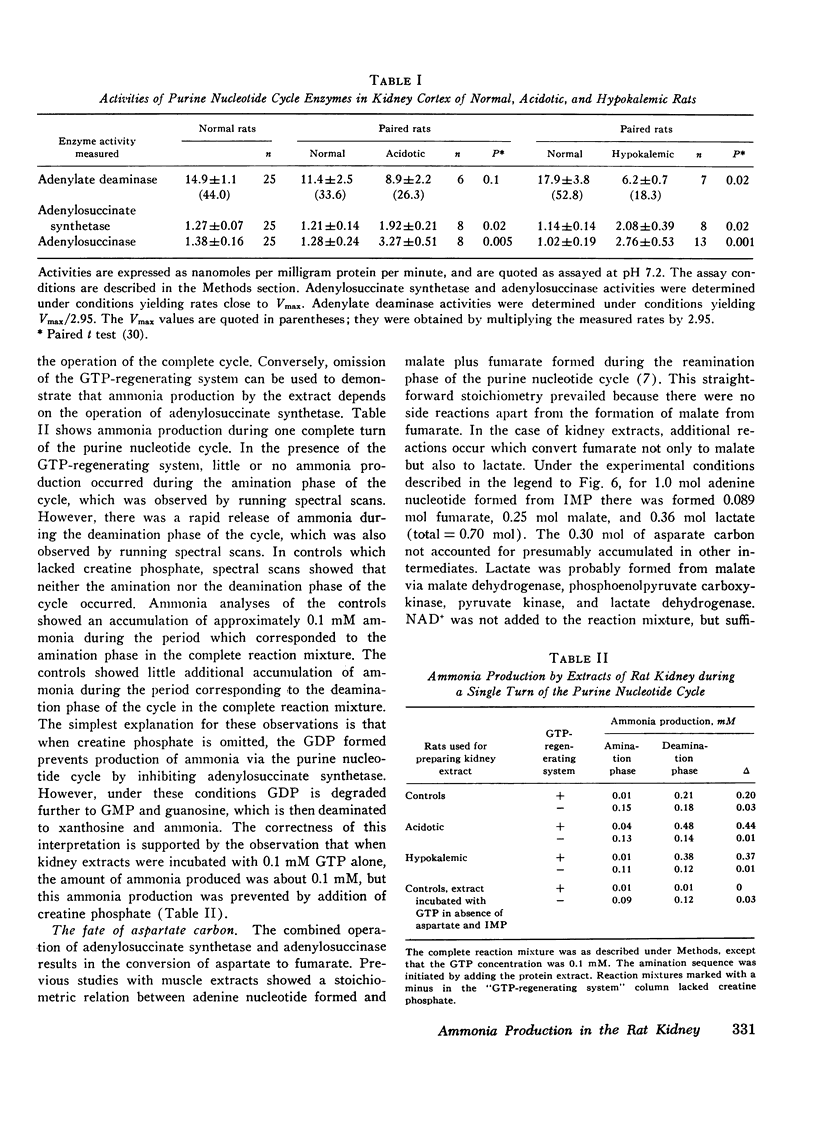
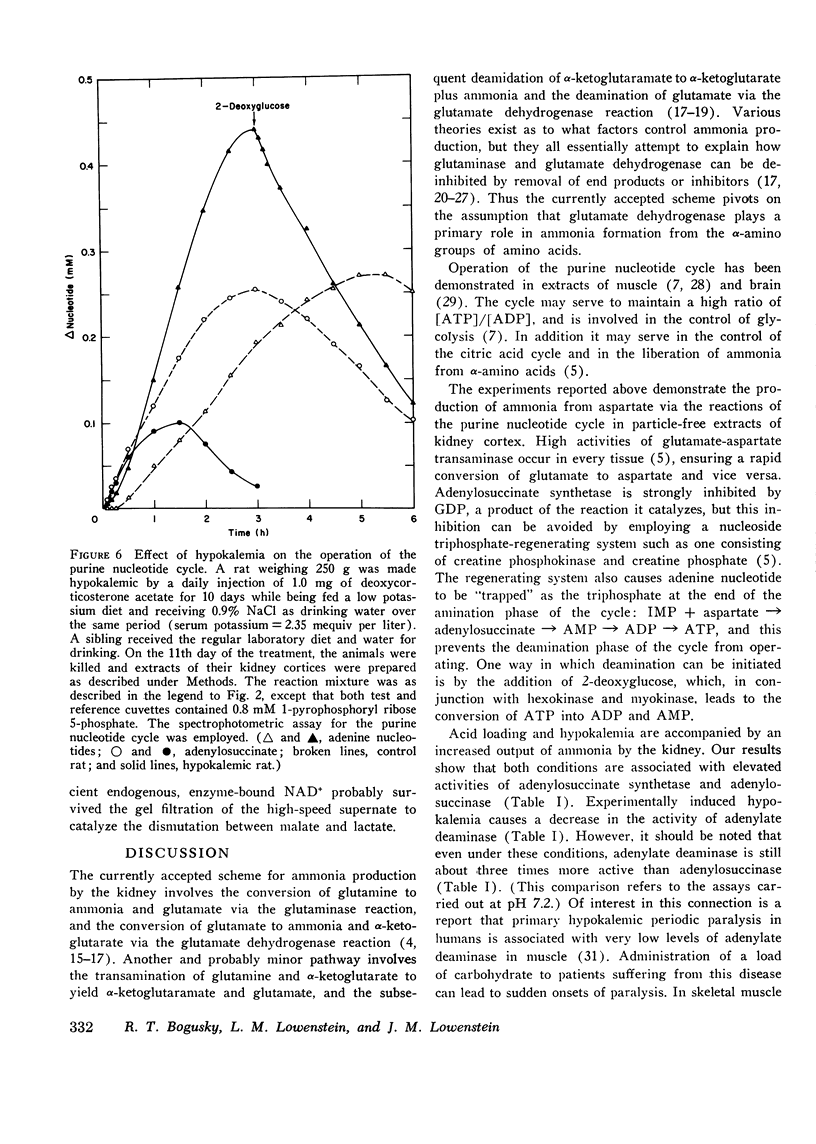
Particle-free extracts prepared from kidney cortex of rat catalyze the formation of ammonia via the purine nucleotide cycle. The cycle generates ammonia and fumarate from aspartate, using catalytic amounts of inosine monophosphate, adenylosuccinate, and adenosine monophosphate. The specific activities of the enzymes of the cycle are 1.27+/-0.27 nmol/mg protein per min (SE) for adenoylosuccinate synthetase, 1.38+/-0.16 for adenylosuccinase, and 44.0+/-3.3 for AMP deaminase. Compared with controls, extracts prepared from kidneys of rats fed ammonium chloride for 2 days show a 60% increase in adenylosuccinate synthetase and a threefold increase in adenylosuccinase activity, and a greater and more rapid synthesis of ammonia and adenine nucleotide from aspartate and inosine monophosphate. Extracts prepared from kidneys of rats fed a potassium-deficient diet show a twofold increase in adenylosuccinate synthetase and a threefold increase in adenylosuccinase activity. In such extracts the rate of synthesis of ammonia and adenine nucleotide from aspartate and inosine monophosphate is also increased. These results show that the reactions of the purine nucleotide cycle are present and can operate in extracts of kidney cortex. The operational capacity of the cycle is accelerated by ammonium chloride feeding and potassium depletion, conditions known to increase renal ammonia excretion. Extracts of kidney cortex convert inosine monophosphate to uric acid. This is prevented by addition of allopurinol of 1-pyrophosphoryl ribose 5-phosphate to the reaction mixture.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alleyne G. A., Scullard G. H. Renal metabolic response to acid base changes. I. Enzymatic control of ammoniagenesis in the rat. J Clin Invest. 1969 Feb;48(2):364–370. doi: 10.1172/JCI105993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger R. M., Lowenstein J. M. 5'-Nucleotidase from smooth muscle of small intestine and from brain. Inhibition of nucleotides. Biochemistry. 1975 Jun 3;14(11):2362–2366. doi: 10.1021/bi00682a014. [DOI] [PubMed] [Google Scholar]
- ENGEL A. G., POTTER C. S., ROSEVEAR J. W. NUCLEOTIDES AND ADENOSINE MONOPHOSPHATE DEAMINASE ACTIVITY OF MUSCLE IN PRIMARY HYPOKALAEMIC PERIODIC PARALYSIS. Nature. 1964 May 16;202:670–672. doi: 10.1038/202670a0. [DOI] [PubMed] [Google Scholar]
- Falls W. F., Jr Comparison of urinary acidification and ammonium excretion in normal and gouty subjects. Metabolism. 1972 May;21(5):433–445. doi: 10.1016/0026-0495(72)90055-8. [DOI] [PubMed] [Google Scholar]
- Freed K. H., Bowie C., Vavatsi-Manos O., Preuss H. G. Oxygen consumption and ammoniagenesis in rat kidney slices. Am J Physiol. 1973 Feb;224(2):268–270. doi: 10.1152/ajplegacy.1973.224.2.268. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN L., RICHTERICH-VANBAERLE R., DEARBORN E. H. Kidney glutaminases. II. The glutamine-alpha-keto acid transamination-deamidation system of the guinea pig. Enzymologia. 1957 Jun 15;18(4):261–270. [PubMed] [Google Scholar]
- GUTMAN A. B., YUE T. F. AN ABNORMALITY OF GLUTAMINE METABOLISM IN PRIMARY GOUT. Am J Med. 1963 Dec;35:820–831. doi: 10.1016/0002-9343(63)90244-4. [DOI] [PubMed] [Google Scholar]
- Goldstein L. Pathways of glutamine deamination and their control in the rat kidney. Am J Physiol. 1967 Oct;213(4):983–989. doi: 10.1152/ajplegacy.1967.213.4.983. [DOI] [PubMed] [Google Scholar]
- Goodman A. D., Fuisz R. E., Cahill G. F., Jr Renal gluconeogenesis in acidosis, alkalosis, and potassium deficiency: its possible role in regulation of renal ammonia production. J Clin Invest. 1966 Apr;45(4):612–619. doi: 10.1172/JCI105375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorno W. E., Rector F. C., Jr, Seldin D. W. Relation of renal gluconeogenesis to ammonia production in the dog and rat. Am J Physiol. 1967 Oct;213(4):969–974. doi: 10.1152/ajplegacy.1967.213.4.969. [DOI] [PubMed] [Google Scholar]
- Gutman A. B., Yu T. F. Hyperglutamatemia in primary gout. Am J Med. 1973 Jun;54(6):713–724. doi: 10.1016/0002-9343(73)90057-0. [DOI] [PubMed] [Google Scholar]
- Hems D. A. Metabolism of glutamine and glutamic acid by isolated perfused kidneys of normal and acidotic rats. Biochem J. 1972 Dec;130(3):671–680. doi: 10.1042/bj1300671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm D. E., Strope G. L. The effects of acidosis and alkalosis on the metabolism of glutamine and glutamate in renal cortex slices. J Clin Invest. 1972 May;51(5):1251–1263. doi: 10.1172/JCI106920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lowenstein J. M. Ammonia production in muscle and other tissues: the purine nucleotide cycle. Physiol Rev. 1972 Apr;52(2):382–414. doi: 10.1152/physrev.1972.52.2.382. [DOI] [PubMed] [Google Scholar]
- LéJohn H. B. Unidirectional inhibition of glutamate dehydrogenase by metabolites. A possible regulatory mechanism. J Biol Chem. 1968 Oct 10;243(19):5126–5131. [PubMed] [Google Scholar]
- PITTS R. F., PILKINGTON L. A., DEHAAS J. C. N15 TRACER STUDIES ON THE ORIGIN OF URINARY AMMONIA IN THE ACIDOTIC DOG, WITH NOTES ON THE ENZYMATIC SYNTHESIS OF LABELED CLUTAMIC ACID AND GLUTAMINES. J Clin Invest. 1965 May;44:731–745. doi: 10.1172/JCI105186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliara A. S., Goodman A. D. Relation of renal cortical gluconeogenesis, glutamate content, and production of ammonia. J Clin Invest. 1970 Nov;49(11):1967–1974. doi: 10.1172/JCI106416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts R. F. Metabolism of amino acids by the perfused rat kidney. Am J Physiol. 1971 Apr;220(4):862–867. doi: 10.1152/ajplegacy.1971.220.4.862. [DOI] [PubMed] [Google Scholar]
- Pitts R. F. Symposium on acid-base homeostasis. Control of renal production of ammonia. Kidney Int. 1972 May;1(5):297–305. doi: 10.1038/ki.1972.42. [DOI] [PubMed] [Google Scholar]
- Preuss H. G. Pyridine nucleotides in renal ammonia metabolism. J Lab Clin Med. 1968 Sep;72(3):370–382. [PubMed] [Google Scholar]
- Preuss H. G., Weiss F. R., Adler S. Renal ammonia production in the presence of citric acid cycle blockade. Proc Soc Exp Biol Med. 1971 Mar;136(3):738–741. doi: 10.3181/00379727-136-35354. [DOI] [PubMed] [Google Scholar]
- Preuss H. G., Weiss F. R. Rate-limiting factor in rat kidney slice ammoniagenesis. Am J Physiol. 1971 Aug;221(2):458–464. doi: 10.1152/ajplegacy.1971.221.2.458. [DOI] [PubMed] [Google Scholar]
- RICHTERICH R. W., GOLDSTEIN L. Distribution of glutamine metabolizing enzymes and production of urinary ammonia in the mammalian kidney. Am J Physiol. 1958 Nov;195(2):316–320. doi: 10.1152/ajplegacy.1958.195.2.316. [DOI] [PubMed] [Google Scholar]
- Relman A. S., Narins R. G. The control of ammonia production in the rat. Med Clin North Am. 1975 May;59(3):583–593. doi: 10.1016/s0025-7125(16)32010-7. [DOI] [PubMed] [Google Scholar]
- SELIGSON D., SELIGSON H. A microdiffusion method for the determination of nitrogen liberated as ammonia. J Lab Clin Med. 1951 Aug;38(2):324–330. [PubMed] [Google Scholar]
- Schultz V., Lowenstein J. M. Purine nucleotide cycle. Evidence for the occurrence of the cycle in brain. J Biol Chem. 1976 Jan 25;251(2):485–492. [PubMed] [Google Scholar]
- Simpson D. P. Pathways of glutamine and organic acid metabolism in renal cortex in chronic metabolic acidosis. J Clin Invest. 1972 Aug;51(8):1969–1978. doi: 10.1172/JCI107003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer A., Corman L., Lowenstein J. M. Citrate and the conversion of carbohydrate into fat. A comparison of citrate and acetate incorporation into fatty acids. Biochem J. 1964 Nov;93(2):378–388. doi: 10.1042/bj0930378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornheim K., Lowenstein J. M. The purine nucleotide cycle. Control of phosphofructokinase and glycolytic oscillations in muscle extracts. J Biol Chem. 1975 Aug 25;250(16):6304–6314. [PubMed] [Google Scholar]
- Tornheim K., Lowenstein J. M. The purine nucleotide cycle. The production of ammonia from aspartate by extracts of rat skeletal muscle. J Biol Chem. 1972 Jan 10;247(1):162–169. [PubMed] [Google Scholar]
- Vavatsi-Manos O., Roxe D. M., Schreiner G. E., Preuss H. G. -Aminobutyric acid shunt in renal ammoniagenesis. Am J Physiol. 1973 Jan;224(1):154–157. doi: 10.1152/ajplegacy.1973.224.1.154. [DOI] [PubMed] [Google Scholar]
- Welbourne T. C. Ammonia production and pathways of glutamine metabolism in the isolated perfused rat kidney. Am J Physiol. 1974 Mar;226(3):544–548. doi: 10.1152/ajplegacy.1974.226.3.544. [DOI] [PubMed] [Google Scholar]
- Welbourne T. C., Balagura-Baruch S. Renal metabolism of glutamine in dogs during infusion of -ketoglutaric acid. Am J Physiol. 1972 Mar;222(3):663–666. doi: 10.1152/ajplegacy.1972.222.3.663. [DOI] [PubMed] [Google Scholar]



