Abstract
CD4+CD25+Foxp3+ regulatory T cells (Tregs) are required to restrain the immune system from mounting an autoaggressive systemic inflammatory response, but why their activity can prevent (or allow) organ-specific autoimmunity remains poorly understood. We have examined how TCR specificity contributes to Treg activity using a mouse model of spontaneous autoimmune arthritis, in which CD4+ T cells expressing a clonotypic TCR induce disease by an IL-17-dependent mechanism. Administration of polyclonal Tregs suppressed Th17 cell formation and prevented arthritis development; notably, Tregs expressing the clonotypic TCR did not. These clonotypic Tregs exerted antigen-specific suppression of effector CD4+ T cells using the clonotypic TCR in vivo, but failed to mediate bystander suppression and did not prevent Th17 cells using nonclonotypic TCRs from accumulating in joint-draining lymph nodes of arthritic mice. These studies indicate that the availability of Tregs with diverse TCR specificities can be crucial to their activity in autoimmune arthritis.
INTRODUCTION
Both mice and humans that congenitally lack Foxp3 develop a severe autoimmune disorder characterized by widespread lymphocytic infiltration, and elimination of CD4+Foxp3+ T cells from adult mice similarly precipitates a fatal systemic pathology (1-3). These findings have indicated that CD4+CD25+Foxp3+ regulatory T cells (Tregs)2 can play a dominant role in regulating autoreactive lymphocytes. However, many autoimmune diseases develop despite the presence of Tregs, and why disease develops despite their presence is currently unknown (4, 5). Tregs can be generated intrathymically based on interactions between the thymocyte TCR and self-peptides, and one possibility is that affected individuals produce Treg TCR repertoires lacking specificities that are required for recognition of critical disease-related self-peptides (6). TCR specificity can play a role in determining Treg function, since targeting Tregs to particular autoantigens could suppress disease in mouse models of diabetes, experimental autoimmune encephalitis, and autoimmune ovarian disease (7-10). However, there is also evidence that Tregs can exert regulatory activity via bystander mechanisms (11), and the extent to which Treg recognition of disease-related autoantigens is required to prevent autoimmune disease remains poorly understood. One reason that it has been difficult to assess the role of TCR specificity in directing Treg activity in autoimmune settings is that in many cases the antigen(s) that are recognized are poorly defined.
Studies in rheumatoid arthritis (RA) patients have made the seemingly paradoxical observation that arthritic individuals typically possess elevated frequencies of CD4+CD25+ Tregs at an inflammatory site (i.e. in the synovial fluid of arthritic joints) (12-14). In vitro suppression studies have shown that exposure to TNF-α (which is elevated in RA patients) can impede CD4+CD25+ Treg function, and Tregs isolated from RA patients undergoing Infliximab (anti-TNF-α) treatment exhibited improved suppressor function (15-17). These studies have led to the suggestion that Tregs might be incapable of mediating suppression in the inflammatory environment of RA as an explanation for their ineffectiveness in preventing disease despite their high frequency. Consistent with the findings in RA patients, CD4+CD25+ Tregs are present in the synovial fluid, joints, and joint-draining LNs in murine models of inflammatory arthritis (18, 19). Murine models have also been used to show that Treg deficiency can impact arthritis development; thus, K/B×N mice that had been crossed to scurfy mice to eliminate Foxp3-expressing cells developed an accelerated and more severe arthritis than conventional K/B×N mice (19). In the collagen-induced arthritis model, antibody depletion of CD4+CD25+ Tregs results in increased disease severity, and prophylactic transfer of exogenous CD4+CD25+ Tregs could ameliorate arthritis (20, 21). While these mouse models have provided evidence that Treg activity can affect arthritis development in some settings, it remains unclear why the Tregs that are present in diseased mice and humans fail to prevent arthritis from developing. Whether and how the Treg repertoire might be able to be manipulated to prevent arthritis development is similarly not understood.
In this report, we examine how TCR specificity affects the ability of Tregs to suppress autoimmune arthritis in a mouse model in which CD4+ T cell recognition of a systemically expressed neo-self peptide drives disease (22). We show that exogenously administered polyclonal Tregs suppress arthritis, demonstrating that increasing the representation of Tregs in pre-arthritic mice can prevent disease. Unexpectedly, however, Tregs that are specific for the surrogate autoantigen do not prevent arthritis; even though they mediate effective antigen-specific suppression in vivo, they fail to exert bystander suppression and do not prevent the accumulation of effector cells using non-clonotypic TCRs in the draining LNs of arthritic mice. These studies provide novel insights into the role that TCR specificity plays in directing Treg activity in vivo, and may shape their use as therapeutic approaches to autoimmune disease treatment.
MATERIALS AND METHODS
Mouse strains
TS1, HA28, HACII, TS1×HA28, and TS1×HACII mice have been previously described (22-27) and backcrossed with BALB/c mice for at least 10 generations. Foxp3EGFP mice (28) are on a BALB/c background and were purchased from Jackson Laboratories. Tcrα−/− mice (29) were bred to homozygosity for the H-2d haplotype and then backcrossed at least four generations with BALB/c mice before breeding with HACII transgenic mice. All mice were maintained in specific pathogen free conditions in the Wistar Institute Animal Facility, and experiments were conducted under protocols approved by the Wistar Institute Institutional Animal Care and Use Committee.
Assessment of arthritis
Mice were assessed weekly for signs of arthritis for a minimum of 9 to 10 wks. Distal joints were examined for limb swelling (visual assessment and measurement with micrometer caliper) and mice were assigned a score based on one of two indices: [A] (0) - no arthritic limbs, (1) - one arthritic limb, (2) -two arthritic limbs, (3) - three arthritic limbs, and (4) - arthritis in all four limbs or [B] (0) – no visible swelling or discoloration, (1) visible swelling but no discoloration, and (2) severe swelling accompanied by skin discoloration. In scoring model [B], the minimum score per mouse is 0 (no affected limbs) and the maximum score per mouse is 8 (four limbs with a value of 2).
Flow cytometry
Single cell suspensions of LNs (either popliteal, or pooled axillary, brachial, cervical, and mesenteric LNs) or spleens were stained with antibodies at 4° C for 30 minutes. The following antibodies were purchased from eBioscience: anti-CD4 -PECy7, -APC, -APCeF780 (L3T4); anti-CD25 PE, PerCpCy5.5 (PC61); anti-CD44 Alexa700 (IM7); anti-CD62L PerCpCy5.5 (MEL14); anti-Foxp3 PE, eF450 (FJK-16s); anti-IFN-γ PE, PECy7, APC (XMG1.2); anti-IL-17 PE, APC (eBio17B7). Anti-CD69 PE (H1.2F3) was purchased from BD Pharmingen. Anti 6.5-biotin (27) was detected using Streptavidin-APC (eBioscience) or Streptavidin-Qdot655 (Invitrogen). Intracellular Foxp3 was detected according to the eBioscience protocols. Samples were collected on FACS Calibur or FACS LSR II flow cytometers (BD Biosciences), and data was analyzed using FlowJo software (Treestar).
Cell sorting
Single cell suspensions from LNs or spleens were stained with antibodies at 4° C for 30 minutes and sorted using a DakoCytomation MoFlo (DakoCytomation) or a BD FACSAria cell sorter (BD Biosciences).
Adoptive transfers
0.5-1×106 purified cells in sterile PBS were injected into the tail veins of 5 to 6 wk-old recipient mice, or i.p into 2 d-old neonatal mice.
Intracellular cytokine staining
Cells were incubated in supplemented Iscove’s Modified Dulbecco’s Medium (IMDM) with 10% FBS, 50 ng of PMA (Sigma-Aldrich), 1μM ionomycin (Sigma-Aldrich), and a 1:1000 dilution of Brefeldin A (eBioscience) for 4 hours. Cells were then stained with cell surface antibodies, fixed, permeabilized, and stained with anti-cytokine antibodies. In cases where intracellular cytokine staining was analyzed in conjunction with surface staining for the 6.5 TCR, the amounts of PMA and ionomycin used were titrated to achieve optimal cytokine staining with minimal TCR downmodulation.
Anti-IL-17 antibody treatment
Mice were treated from 5 to 14 wks of age with three i.p. injections per wk of 150 μg of anti-IL-17A antibody (clone M210, generously provided by Amgen) or rat IgG2a isotype control antibody.
Statistical analysis
The numbers of arthritic limbs among cohorts were analyzed using the Mann-Whitney test. Other analyses were conducted using the unpaired two-tailed Student’s t-test.
RESULTS
Arthritic TS1×HACII mice contain CD4+CD25+Foxp3+ Tregs
TS1×HACII mice express a TCR transgene (detected by the mAb 6.5) that recognizes the major I-Ed-restricted T cell determinant (termed S1) from the influenza virus PR8 hemagglutinin (HA) (27), and co-express PR8 HA as a self-antigen under the control of a MHC class II I-Eα promoter (25). We previously reported that thymocytes expressing the clonotypic 6.5+ TCR undergo extensive deletion in TS1×HACII mice, and that despite this deletion, a population of 6.5+CD4+ T cells expressing activated phenotypes can be found in the spleens and lymph nodes (LNs) (Fig. 1A) (22). Moreover, the majority of TS1×HACII mice develop spontaneous inflammatory arthritis with overt articular manifestations becoming apparent in 10 to 12 wk-old mice (Fig. 1B, 1C and (22)). Importantly, we also showed that arthritis develops in TS1×HACII.JH−/− mice (which congenitally lack B cells), indicating that neither B cells nor antibody is required (22). Arthritis also develops despite the presence of CD4+CD25+Foxp3+ Tregs, which comprise a similar or greater proportion of CD4+ T cells as are present in control TS1 mice (Fig. 1D), and are found in similar proportions in young (pre-arthritic) and older (arthritic) TS1×HACII mice (Fig. 1E). The CD4+CD25+Foxp3+ T cells in arthritic TS1×HACII mice included a population that expresses the 6.5 TCR, although the proportion of CD4+Foxp3+ cells expressing the 6.5 TCR was substantially lower than in TS1 mice (Fig. 1F). Thus TS1×HACII mice spontaneously develop inflammatory arthritis despite the presence of CD4+CD25+Foxp3+ Tregs, although Tregs expressing the 6.5 TCR (which confers specificity for the surrogate autoantigen S1) appear to be relatively under-represented.
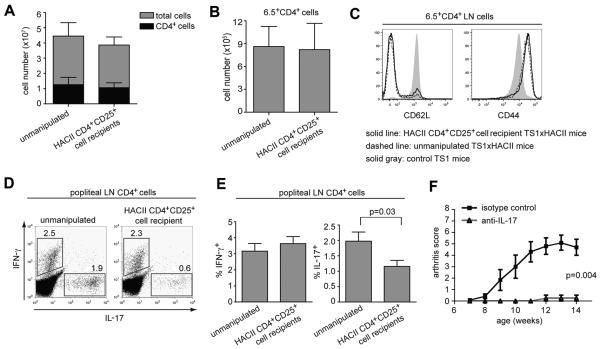
Figure 1.
Arthritic TS1×HACII mice contain CD4+CD25+Foxp3+ Tregs. (A) 6.5 staining on CD4+ cells, and CD62L and CD44 staining on 6.5+CD4+ cells from joint-draining LNs of TS1 (filled) or arthritic TS1×HACII (line) mice. Numbers (mean of 3 mice) indicate percentages of 6.5+CD4+ or CD62Llo6.5+CD4+ cells, or MFI of CD44. (B) Representative images of limbs from non-arthritic TS1 and arthritic TS1×HACII mice. (C) Left graph shows average number of arthritic limbs per mouse (±SEM). Right graph shows number of arthritic limbs per mouse in 16 wk-old TS1 and TS1×HACII mice, line represents mean. (D) Foxp3 and CD25 expression by CD4+ LN cells from TS1×HACII and TS1 mice. Numbers indicate percentage. (E) Average percentage (±SEM) of Foxp3+CD25+ cells in CD4+ LN cells from TS1×HACII mice (gray bars) and TS1 mice (black bars); n ≥ 3 mice per age group. (F) 6.5 and Foxp3 staining of CD4+ T cells from the joint-draining LNs of arthritic TS1×HACII or TS1 mice. Numbers indicate mean percentages (n=7).
Polyclonal Tregs prevent arthritis development in TS1×HACII mice
Since TS1×HACII mice develop autoimmune disease and appear to have limited representation of Treg cells expressing the clonotypic 6.5 TCR, we wondered if increasing the representation of Tregs in pre-arthritic mice could prevent disease development. We first examined whether transfer of CD4+CD25+ T cells that are enriched in specificity for the S1 peptide could modulate arthritis development, because S1 is the target peptide that is recognized by autoreactive 6.5+CD4+ T cells in TS1×HACII mice. As a source of S1-specific Tregs we used TS1×HA28 mice, which also co-express the HA self-antigen (at lower levels than in TS1×HACII mice), and which contain a large population of 6.5+CD4+CD25+Foxp3+ T cells that develop intrathymically in response to the S1 peptide (24). CD4+CD25+ T cells from TS1×HA28 mice are heavily enriched for 6.5+Foxp3+ cells and can exert regulatory function in response to S1 peptide both in vitro (Fig. S1) and in vivo (30, 31). Accordingly, 1×106 CD4+CD25+ T cells from TS1×HA28 mice were transferred into 5 to 6 wk-old prearthritic TS1×HACII mice; however, no effects on arthritis development were observed, since the average number of arthritic limbs per mouse did not differ from unmanipulated TS1×HACII mice at any of the examined timepoints, and in both cohorts the majority of mice developed arthritis (Fig. 2A).
Figure 2.
Polyclonal, but not antigen-specific, CD4+CD25+ T cells prevent arthritis development in TS1×HACII mice. (A) 6.5 and Foxp3 staining among TS1×HA28 CD4+CD25+ cells that were transferred into pre-arthritic TS1×HACII mice. Left graph shows the average number of arthritic limbs per mouse (±SEM), right graph shows the number of arthritic limbs per mouse, lines represent mean. (B) As for (A), except that CD4+CD25+ cells from HACII mice were transferred into pre-arthritic TS1×HACII mice. (C) As for (B), except that CD4+CD25+ cells from BALB/c mice were transferred into pre-arthritic TS1×HACII mice.
We also examined whether the administration of polyclonal CD4+CD25+ Tregs (i.e. Tregs that are not enriched in specificity for the S1 peptide) to pre-arthritic TS1×HACII mice might modify arthritis development. We obtained these cells from two sources; HACII mice, which express the same HA transgene as TS1×HACII mice in the context of an otherwise unmanipulated T cell repertoire (and which are isogenic with respect to the self-peptides that are present in TS1×HACII mice), and BALB/c mice. Strikingly, TS1×HACII mice that had received 1×106 polyclonal CD4+CD25+ T cells from either HACII or BALB/c mice possessed significantly fewer arthritic limbs per mouse, and a significantly lower fraction of these mice developed arthritis than was the case for unmanipulated TS1×HACII mice (Fig. 2B, 2C). Collectively these data demonstrate that arthritis development in TS1×HACII mice can be prevented by administration of exogenous polyclonal CD4+CD25+ T cells, including those from BALB/c mice that cannot have undergone any form of repertoire selection in response to S1 as a self-peptide. Moreover, similar numbers of CD4+CD25+ T cells enriched in specificity for the S1 antigen were ineffective.
Polyclonal Tregs inhibit an arthritogenic Th17 response
To examine how polyclonal CD4+CD25+ Tregs modulate arthritis development in TS1×HACII mice, we first compared the number and phenotype of CD4+ T cells from unmanipulated arthritic TS1×HACII mice with those from TS1×HACII mice that had been protected from arthritis development by receipt of polyclonal CD4+CD25+ T cells. There were no differences in the numbers of CD4+ T cells, or of 6.5+CD4+ T cells in LNs of arthritic versus protected TS1×HACII mice (Fig. 3A, B). The levels of CD62L and CD44 were also similar on 6.5+CD4+ T cells from protected versus arthritic mice (Fig. 3C). Since IL-17 has been implicated in several models inflammatory arthritis (32-34), we also examined the production of IL-17 and IFN-γ by popliteal LN cells from treated and untreated mice. Notably, the percentage of CD4+ popliteal LN cells that were able to produce IL-17 following direct ex-vivo activation was significantly lower in TS1×HACII mice that had received protective HACII CD4+CD25+ T cells than in arthritic TS1×HACII mice; by contrast, the percentages of cells that could secrete IFN-γ did not differ (Fig. 3D, 3E). To determine whether IL-17 production is required for arthritis development, TS1×HACII mice were treated either with an anti-IL-17A mAb or an isotype control three times weekly, beginning at 5 to 6 wks of age. Indeed, treatment with an anti-IL-17A mAb abrogated arthritis development in TS1×HACII mice (Fig. 3F). Thus, the provision of polyclonal CD4+CD25+ Tregs did not appear to affect either the systemic accumulation or overall activation state of CD4+ or 6.5+CD4+ LN cells in TS1×HACII mice, but did prevent local accumulation (in the joint draining LNs) of CD4+ T cells that can secrete the arthritogenic cytokine IL-17.
Figure 3.
Polyclonal CD4+CD25+ Tregs inhibit an arthritogenic Th17 response in TS1×HACII mice. (A) Numbers (±SEM) of total cells and of CD4+ cells in LNs of TS1×HACII mice (n=7) and of TS1×HACII mice that received protective CD4+CD25+ Tregs (n=9). (B) As (A) except for 6.5+CD4+ T cells. (C) CD62L and CD44 expression on 6.5+CD4+ LN cells from TS1×HACII, protected TS1×HACII, or TS1 mice. (D) Intracellular IL-17 and IFN-γ staining (numbers indicate percentages) of popliteal CD4+ popliteal LN cells from arthritic and protected TS1×HACII mice. (E) Percentages (±SEM) of IFN-γ and IL-17 producing CD4+ cells in popliteal LNs from arthritic (n=8) and protected TS1×HACII mice (n=11). (F) Average arthritis score (±SEM) in anti-IL-17 (n=6) and isotype control (n=6) treated TS1×HACII mice.
S1-specific Tregs fail to suppress arthritis development in TS1×HACII mice
Since CD25 is also expressed on activated T cells, we crossed TS1×HA28 mice with Foxp3EGFP mice (28), and used GFP expression as an additional parameter to more stringently purify CD4+CD25+Foxp3+ Tregs. Administration of CD4+CD25+GFP+ T cells from TS1×HA28.Foxp3EGFP mice (half of which express the 6.5 TCR) to 5 to 6 wk-old pre-arthritic TS1×HACII mice affected neither the incidence nor the severity of arthritis, as had been seen in mice that received CD4+CD25+ T cells from TS1×HA28 mice (Fig. 4A). Moreover, GFP-expressing CD4+Foxp3+ T cells were present in the LNs and spleens of the recipient mice 9 to 10 wks after the initial transfer, indicating that the donor Tregs had engrafted the recipient TS1×HACII mice (Fig. 4A). We next examined whether administering 6.5+CD4+CD25+GFP+ T cells from TS1×HA28.Foxp3EGFP mice to 2 d-old neonatal TS1×HACII mice might affect arthritis development; by purifying cells based on 6.5 expression we ensured that the Tregs were heavily enriched for specificity for the S1 peptide, yet here too, the provision of Tregs failed to affect arthritis development, and the degree of engraftment with GFP-expressing CD4+Foxp3+ T cells 15 wks after Treg transfer was similar to that seen in the 5 to 6 wk-old recipient TS1×HACII mice (Fig. 4B). Lastly, we generated HACII.Foxp3EGFP mice, and found that administration of polyclonal CD4+CD25+GFP+ Tregs from these mice to 5 to 6 wk-old TS1×HACII mice caused a significant reduction in the incidence and severity of arthritis relative to untreated TS1×HACII mice (Fig. 4C). Interestingly, CD4+CD25+GFP+ Tregs from HACII.Foxp3EGFP mice also achieved a greater degree of engraftment of the Foxp3+ cells in the LNs of TS1×HACII mice than was the case for mice receiving CD4+CD25+GFP+ Tregs from TS1×HA28.Foxp3EGFP mice (Fig. 4C and 4A). Collectively, these analyses indicated that arthritis development in TS1×HACII mice can be prevented by administration of exogenous CD4+CD25+Foxp3+ Tregs, while a population that is enriched in specificity for the S1 peptide was ineffective.
Figure 4.
Polyclonal and antigen-specific Foxp3+ Tregs engraft TS1×HACII mice but have differing abilities to prevent arthritis development. (A) 1×106 CD4+CD25+GFP+ T cells from TS1×HA28.Foxp3EGFP mice were transferred into 5-6 wk-old pre-arthritic TS1×HACII mice. Graph shows the arthritis score per mouse, lines represent means. Dot plots show GFP expression versus anti-Foxp3 mAb staining (numbers indicate percentages) by CD4+ cells in recipient mice, and in a TS1×HACII mouse that did not receive GFP+ cells. Average percentages (±SEM) of GFP+Foxp3+ cells are shown in graph. (B) As for (A), except that 0.5×106 6.5+CD4+CD25+GFP+ T cells from TS1×HA28.Foxp3EGFP mice were transferred into 2 d-old neonatal TS1×HACII mice. (C) As for (A), except that 1×106 CD4+CD25+GFP+ cells from HACII.Foxp3EGFP mice were transferred into 5-6 wk-old pre-arthritic TS1×HACII mice.
S1-specific Tregs can mediate antigen-specific suppression in vivo
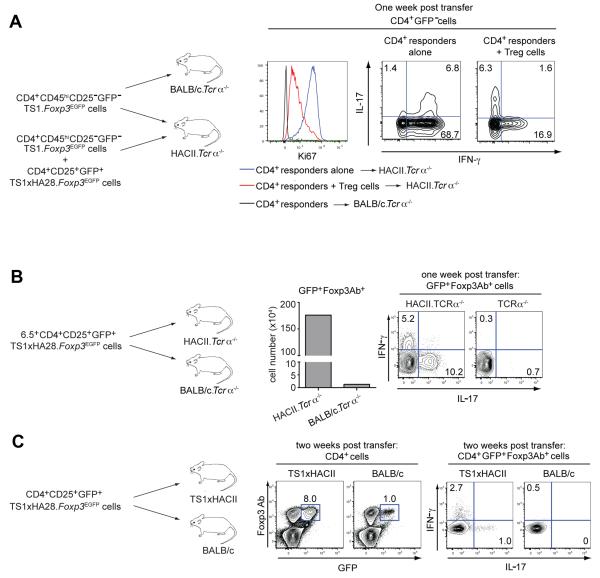
CD4+CD25+ T cells from TS1×HA28 mice are enriched in specificity for the S1 peptide, and it was unexpected that these cells would be unable to suppress arthritis development in TS1×HACII mice. One reason could be that these cells are for some reason incapable of modulating CD4+ T cell responses to the S1 self-peptide that is expressed in HACII mice, even though they express Foxp3 and can suppress S1-specific T cell responses in vitro (Fig. S1), and in other settings in vivo (30, 31). To examine this, we analyzed their ability to suppress the proliferation and differentiation of S1-specific effector CD4+ T cells (obtained from TS1.Foxp3EGFP mice) following transfer into HACII.Tcrα−/− mice (which do not contain T cells). CD4+CD45hiCD25−GFP− T cells from TS1.Foxp3EGFP mice expressed high levels of the cell proliferation marker Ki-67 1 wk after transfer into HACII.Tcrα−/− mice (but not in BALB/c.Tcrα−/− mice), and the majority of these proliferating responder cells produced IFN-γ upon re-stimulation (Fig. 5A). When these responder cells were mixed with equal numbers of CD4+CD25+GFP+ T cells from TS1×HA28.Foxp3EGFP mice prior to transfer, there were substantial decreases in both the level of Ki-67 expression and in the production of IFN-γ by the effector cells in response to the S1 self-peptide in HACII.Tcrα−/− mice (Fig. 5A). Although there appeared to be a modest increase in the percentages of effector cells that could produce IL-17 (without co-producing IFN-γ) in the mice that received Tregs, the absolute number of these cells was quite small (because of reduced proliferation; data not shown). Thus, the Tregs from TS1×HA28 mice are capable of modulating the in vivo proliferation and differentiation of effector CD4+ T cells in response to the S1 self-peptide that is expressed in HACII mice.
Figure 5.
Limited differentiation of autoantigen-specific CD4+CD25+ Tregs in TS1×HACII mice. (A) 1×106 CD4+CD45hiCD25−GFP− responder cells from TS1.Foxp3EGFP mice were transferred with or without 1×106 CD4+CD25+GFP+ Tregs from TS1×HA28.Foxp3EGFP mice into HACII.Tcrα−/− or BALB/c.Tcrα−/− mice. Histogram shows Ki67 staining of CD4+GFP− responder T cells transferred alone or in the presence of GFP+ Tregs 1 wk after transfer. Contour plots show intracellular IL-17 and IFN-γ staining (numbers indicate percentages) among responder CD4+GFP− T cells transferred without or with GFP+ Tregs into HACII.Tcrα−/− mice. (B) 1×106 6.5+CD4+CD25+GFP+ cells from TS1×HA28.Foxp3EGFP mice were transferred into HACII.Tcrα−/− or BALB/c.Tcrα−/− mice. Graph shows the number of GFP+Foxp3+ cells in the spleens 1 wk post-transfer. Contour plots show IL-17 and IFN-γ production (numbers indicate percentages) by GFP+Foxp3+ cells. (C) 1×106 CD4+CD25+GFP+ cells from TS1×HA28.Foxp3EGFP were transferred into TS1×HACII and BALB/c mice. Contour plots show GFP and Foxp3 antibody expression in CD4+ splenocytes 2 wks post transfer, and IL-17 and IFN-γ production by CD4+GFP+Foxp3+ cells (numbers indicate percentages). All results are representative of three independent experiments.
Limited acquisition of cytokine production by S1-specific Tregs in TS1×HACII mice
Tregs can under certain conditions acquire the ability to produce T helper cell-associated cytokines, including IL-17 (35-37), so we examined whether the Tregs from TS1×HA28 mice might fail to suppress arthritis because they undergo differentiation to become cytokine-producing effector cells in response to S1 peptide in vivo. Substantially higher numbers of CD4+ GFP+Foxp3+ T cells were recovered 1 wk after transfer of CD4+CD25+GFP+ Tregs from TS1×HA28.Foxp3EGFP into HACII.Tcrα−/− mice than from BALB/c.Tcrα−/− mice, reflecting expansion of these cells in response to the S1 peptide (Fig. 5B). Notably, the GFP+Foxp3+ Tregs isolated from HACII.Tcrα−/− mice, but not those obtained from BALB/c.Tcrα−/− recipients, produced both IL-17 and IFN-γ following stimulation, with preferential production of IL-17 (Fig. 5B). This acquisition of IL-17 production appears to be a propensity of CD4+Foxp3+ T cells, because conventional CD4+ T cells isolated from TS1 mice (and also expressing the 6.5 TCR) were strongly polarized to produce IFN-γ, rather than IL-17, upon transfer into HACII.Tcrα−/− mice (Fig. 5A).
Since we had obtained evidence that S1-specific Tregs from TS1×HA28.Foxp3EGFP mice are capable of becoming IL-17 producing CD4+Foxp3+ T cells in response to S1 peptide in vivo, we examined whether a similar process could be occurring when these cells are transferred into TS1×HACII mice. Accordingly, CD4+CD25+GFP+ T cells from TS1×HA28.Foxp3EGFP mice were transferred into prearthritic TS1×HACII mice; 2 wks later, higher numbers of donor-derived GFP+Foxp3+ T cells were found in TS1×HACII mice than in BALB/c controls, again reflecting expansion in response to the S1 peptide (Fig. 5C). In this case, however, a much smaller fraction of the GFP+Foxp3+ cells had acquired the ability to produce cytokines than had been observed in the HACII.Tcrα−/− recipients, and of those that were cytokine producers, a greater proportion produced IFN-γ than IL-17 (contrasting the bias toward IL-17 production observed in HACII.Tcrα−/− recipients) (Fig. 5C, 5A). Indeed, the frequency of cytokine-producing Foxp3+ T cells that were isolated from TS1×HACII mice was only modestly higher than can be found in Foxp3+ cells from TS1×HA28 mice, before transfer into the recipient mice (data not shown). Thus, despite a capacity to differentiate into IL-17 secreting T cells in response to S1 peptide that was observed in HACII.Tcrα−/− recipients, there appeared to be only limited formation of Foxp3+ cytokine secreting cells when CD4+CD25+GFP+ T cells from TS1×HA28.Foxp3EGFP mice were transferred into pre-arthritic TS1×HACII mice.
S1-specific Tregs do not suppress the formation of non-clonotypic Th17 cells in TS1×HACII mice
Lastly, we wanted to determine whether the CD4+ T cell responses that develop in TS1×HACII mice were being modified by the S1-specific Tregs from TS1×HA28.Foxp3EGFP mice, even though arthritis development was not being suppressed. Accordingly, we compared the popliteal LNs of arthritic TS1×HACII mice that had received 6.5+CD4+CD25+GFP+ T cells from TS1×HA28.Foxp3EGFP mice as neonates (see Fig. 4B) with unmanipulated controls. The mice that had received Tregs as neonates contained increased frequencies of 6.5+CD4+Foxp3+ cells than were present in unmanipulated mice (Fig. 6A), consistent with the preceding studies showing that GFP-positive cells could be recovered from the recipient mice (see Fig. 4B). There was also very little acquisition of cytokine production by 6.5+CD4+Foxp3+ T cells in TS1×HACII mice that had received S1-specific Tregs as neonates (data not shown), consistent with the limited acquisition of cytokine production observed 1 wk after transfer into TS1×HACII mice (see Fig. 5B). There was a smaller increase in the representation of 6.5+CD4+Foxp3− effector T cells in the LNs of mice that had received the S1-specific Tregs, and notably, the frequency of IL-17-secreting cells in this subset was substantially reduced relative to mice that had not received Treg cells, while the frequency of IFN-γ secretors was increased (Fig. 6B). Interestingly, this effect of the S1-specific Treg cells was only observed among the CD4+Foxp3− cells expressing the clonotypic 6.5 TCR; when we examined the non-clonotypic 6.5−CD4+Foxp3− cells (which comprise approximately 75% of the CD4+ cells in the popliteal LNs of arthritic mice; Fig. 6A), no differences were seen in the frequencies of IFN-γ or IL-17 secreting cells between mice that had or had not received Treg cells (Fig. 6C).
Figure 6.
S1-specific Tregs do not suppress the formation of non-clonotypic Th17 cells in TS1×HACII mice. (A) 6.5 and Foxp3 staining of CD4+ cells (numbers indicate percentages) from popliteal LNs of 15 wk-old TS1×HACII mice that were unmanipulated (n=11) or had received 5×105 6.5+CD4+CD25+GFP+ Tregs from TS1×HA28.Foxp3EGFP mice at 2 d of age (n=15)(from experiment in Fig. 4B). Graphs show the percentages of 6.5+CD4+Foxp3+ and 6.5+CD4+Foxp3− cells in unmanipulated and Treg recipient TS1×HACII mice (bars indicate mean). (B) Dot plots show IFN-γ and IL-17 production (numbers indicate percentages) by 6.5+CD4+Foxp3− popliteal LN cells from unmanipulated and Treg recipient TS1×HACII mice. Graphs show the percentages of IFN-γ- and IL-17-producing 6.5+CD4+Foxp3− cells (bars indicate mean). (C) Dot plots show IFN-γ and IL-17 production (numbers indicate percentages) by 6.5+CD4+Foxp3+ popliteal LN cells from unmanipulated and Treg recipient TS1×HACII mice. Graphs show the percentages of IFN-γ- and IL-17-producing 6.5+CD4+Foxp3+ cells (bars indicate mean).
Collectively, these data argue against the possibility that the 6.5+CD4+CD25+GFP+ T cells from TS1×HA28.Foxp3EGFP mice fail to suppress arthritis because they undergo abundant differentiation into CD4+Foxp3−IL-17+ effectors. Indeed, the representation of 6.5+CD4+Foxp3−IL-17+ T cells was significantly reduced in mice that had received 6.5+CD4+CD25+GFP+ T cells from TS1×HA28.Foxp3EGFP mice, indicating that the formation of Th17 cells expressing the clonotypic 6.5 TCR had been suppressed by the 6.5+CD4+CD25+GFP+ T cells from TS1×HA28.Foxp3EGFP mice. Notably, however, these Tregs did not prevent non-6.5-expressing CD4+IL-17+ T cells from accumulating in the joint draining LNs of arthritic TS1×HACII mice.
DISCUSSION
The majority of TS1×HACII mice spontaneously develop an IL-17-driven inflammatory arthritis. Since arthritis develops despite the presence of CD4+CD25+Foxp3+ Tregs, it was possible that some aspect of the inflammatory environment in TS1×HACII mice prevents Foxp3+ Tregs from suppressing this disease. Using adoptive transfer approaches, we showed that Tregs can modulate immune responses in this environment; however, the specificity of the Tregs was critical, since polyclonal Tregs could prevent arthritis development, while Tregs enriched in specificity for the S1 self-peptide were ineffective, even when administered to neonatal mice.
The failure of Tregs from TS1×HA28 mice to suppress arthritis development despite their enrichment in specificity for the S1 peptide is notable in several respects. First, the S1 peptide is a defined target antigen that is recognized by a major population of autoreactive CD4+ T cells in arthritic mice, since despite extensive thymic deletion, ~7% of the CD4+ T cells in TS1×HACII mice express the 6.5 TCR (which confers specificity for the S1 peptide), and many of these cells are capable of cytokine production. Interestingly, although 6.5+CD4+ T cells in the popliteal LNs of TS1×HACII mice that had received TS1×HA28 Tregs as neonates produced significantly lower levels of the arthritogenic cytokine IL-17 compared to 6.5+CD4+ T cells in untreated mice (indicating that the TS1×HA28 Tregs did indeed modulate the effector 6.5+CD4+ T cell response), there was no effect on arthritis development. By contrast, the presence of TS1×HA28 Tregs had no effect on cytokine production by CD4+ T cells expressing allelically-included (i.e. 6.5−) TCRs in TS1×HACII mice. Second, the failure of Tregs from TS1×HA28 mice to suppress arthritis development did not appear to be due to their differentiation into IL-17-secreting effector T cells. CD4+CD25+Foxp3+ Tregs from TS1×HA28 mice produced limited amounts of IL-17 following transfer into TS1×HACII mice, and as noted above, the popliteal LNs of TS1×HACII mice that had received TS1×HA28 Tregs actually contained significantly fewer 6.5+ CD4+IL-17+ effector T cells than untreated controls. Interestingly, 6.5+CD4+CD25+Foxp3+ Tregs from TS1×HA28 mice were capable of producing IL-17 following transfer into HACII.Tcrα−/− mice, consistent with reports indicating that Tregs can under certain conditions acquire effector properties (38-40). Since a low frequency of IL-17 producing cells is present among the CD4+CD25+GFP+ population from TS1×HA28.Foxp3EGFP mice when analyzed directly ex vivo (data not shown), it remains possible that these cells underwent preferential expansion, rather than being generated de novo following transfer into HACII.Tcrα−/− mice. Whatever the basis, however, this process did not occur to any great extent when these cells were transferred into intact TS1×HACII mice, perhaps because resources necessary for driving such an accumulation were unavailable or limiting. Third, since the S1 self-peptide (whose synthesis is under control of an I-E promoter) is expressed directly by MHC Class II+ APCs (including B cells and dendritic cells), the Tregs from TS1×HA28 mice should be readily able to interact with APCs in TS1×HACII mice. Although the mechanisms by which Tregs can suppress immune responses in vivo remain poorly understood, there is evidence that they might do so at least in part by bystander mechanisms, such as modulating APC function (11, 41, 42). However, the administration of Tregs that are enriched in specificity for a self-antigen that is abundantly expressed by MHC class II positive cells was insufficient to prevent arthritis from developing in this model, arguing against the possibility that Treg cells act via their ability to modulate APC function in this system.
Since Tregs from TS1×HA28 mice were ineffective, why then were polyclonal Tregs from HACII and/or BALB/c mice able to suppress arthritis development in TS1×HACII mice? It may be that the recognition of the systemically expressed S1 peptide by 6.5+CD4+ T cells initiates the autoimmune response, but that the effector CD4+ T cells that cause joint inflammation primarily use non-6.5 TCRs. Notably, the large majority of IL-17-secreting CD4+ T cells in the popliteal LNs of arthritic TS1×HACII mice do not express the 6.5 TCR. Furthermore, TS1×HACII mice on a RAG−/− background exhibit less severe ankle pathology than do TS1×HACII mice on a RAG-sufficient background, suggesting that CD4+ T cells that do not express the 6.5 TCR may be required for the manifestation of severe arthritis in TS1×HACII mice (22). In this regard, the endogenous Treg repertoire that develops in TS1×HACII mice, and which fails to prevent arthritis development, may be qualitatively insufficient because it lacks certain requisite specificities that are necessary to suppress the formation of these IL-17 secreting effector CD4+ T cells. The endogenous Treg repertoire in TS1×HACII mice is necessarily generated using non-transgene-encoded TCRs that arise through allelic inclusion principally of the alpha chain locus (data not shown). Since there is also substantial thymocyte deletion in young TS1×HACII mice, one can imagine that the range of specificities that is present in Tregs from TS1×HACII mice might be more constrained than that of either BALB/c or HACII mice, neither of which carry TCR transgenes that would restrict or modify their Treg repertoires. Along these lines, it is noteworthy that the Tregs that develop in TS1×HA28 mice are also likely limited in the range of specificities that are produced, because we have previously shown that non-S1-specific Tregs in TS1×HA28 mice are generated through use of allelically-included TCR α-chains paired with the transgenic Vβ8.2 chain (26). Thus, even though approximately half of the CD4+Foxp3+ T cells from TS1×HA28 mice do not express the clonotypic 6.5 TCR, these non-clonotypic Treg cells are likely to possess much less TCR sequence diversity than is the case for the polyclonal Treg cells from BALB/c or HACII mice because they all utilize the same TCR Vβ chain. We further note that we have not examined how administration of varying doses of Tregs from the different sources might affect their ability to suppress arthritis development, which may be significant if, for example, the different populations can exert regulatory effects by different mechanisms. However, it is clear that polyclonal Treg cells from BALB/c or HACII mice are more effective than are Treg cells from TS1×HA28 mice when administered in similar numbers to pre-arthritic TS1×HACII mice. These polyclonal Tregs may be protective because they provide a more diverse repertoire of TCR specificities than are present among Tregs from either TS1×HACII or TS1×HA28 mice, and because they are able to suppress disease by recognizing a variety of target peptides that are also recognized by the effector T cells.
The conclusion that TS1×HACII mice develop arthritis at least partly because their Treg repertoire contains limited specificities is notable because CD4+IL-17+ T cells in the popliteal LNs of arthritic mice also appear to have been generated predominantly through use of allelically-included TCR chains. Thus, under conditions in which both the effector and Treg populations were constrained, the effector population appeared to retain sufficient specificities to cause disease, and it was necessary to augment the Treg population with cells derived from unmanipulated mice to prevent arthritis development. A similar situation arises in mice that receive a d-3 thymectomy (d3Tx) and develop a systemic autoimmune disease that can be rescued by provision of polyclonal Tregs from unmanipulated mice (43). The frequencies of CD4+CD25+ and CD4+CD25− T cells are reduced approximately 10-fold in d3Tx mice relative to controls, and here too, the TCR repertoires are likely constrained because they must be generated from only those cells that had populated the periphery in the first days of life (43). In this case also then, restriction of both the effector and Treg repertoires allows for the spontaneous development of autoimmunity, implying that conditions that limit the formation of both effector and Treg specificities can lead to an imbalance that favors autoimmunity.
An alternative explanation for the ability of polyclonal Tregs to protect against arthritis is that they contain a greater phenotypic heterogeneity than is found in the Tregs from TS1×HA28 mice. There is increasing evidence that Foxp3+ T cells can exhibit distinct phenotypes (e.g. through expression of transcription factors and/or chemokine receptors) (44), and the polyclonal Tregs from HACII and BALB/c mice might contain populations expressing phenotypic characteristics that make them better able to populate TS1×HACII mice and/or suppress the development of effector CD4+ T cells. In support of this, the polyclonal Tregs appeared to be better able to engraft the LNs (but not the spleens) of recipient TS1×HACII mice than was the case for Tregs from TS1×HA28 mice. If this is the case, however, the formation of these putative Treg populations appears to be at least partly linked to TCR specificity, because Tregs from TS1×HA28 mice (in which the TCR repertoire has been manipulated to produce elevated numbers of 6.5+ Tregs) must lack the appropriate phenotypic heterogeneity, since they fail to prevent arthritis.
As has been observed in other models of inflammatory arthritis (32-34), IL-17 is a critical effector cytokine in TS1×HACII mice. Treatment with an anti-IL-17A mAb could prevent arthritis development, and moreover, there were significant reductions in IL-17-producing CD4+ T cells in the LNs of mice that were protected from arthritis with polyclonal Tregs. However, how the specificity of IL-17-secreting effector cells contributes to arthritis development is less clear. IL-17-secreting 6.5+CD4+ T cells appeared to be under-represented in the popliteal LNs, and Tregs from TS1×HA28 mice could inhibit the accumulation of such cells without preventing disease. A process of “epitope spreading” has been described in a number of autoimmune settings (45), and it is possible that IL-17-secreting 6.5−CD4+ T cells cause disease because they utilize endogenous TCRs that recognize joint-specific self-peptides, including cryptic self-peptides that might be presented by unconventional APCs such as synoviocytes that become MHC class II-positive in the inflamed joint (46). However, a recent study suggested that any Th17 cells that are able to traffic to the joints (regardless of antigen specificity) can induce arthritis development by acting as a local source of IL-17, and that direct recognition of joint antigens may not be required for arthritis to develop in such a setting (47). In this case, then, the key role of the 6.5 TCR in disease development could be in establishing conditions that favor Th17 cell formation; these could include the lymphopenic environment that develops in young TS1×HACII mice as a consequence of severe thymocyte deletion (22), since lymphopenic environments have been found to contribute to Th17 cell formation in the SKG model of arthritis (34). In either event, the findings here extend studies showing that specificity for defined autoantigens can be a critical parameter in determining Treg function in autoimmune settings. Notably, however, whereas studies in diabetes, experimental autoimmune encephalitis, and autoimmune ovarian disease demonstrated that targeting a single autoantigen can result in disease suppression (7-10), our findings suggest that there can be disease settings, such as inflammatory arthritis, in which a diverse set of autoantigens may be involved in pathogenesis. In such a setting, suppressing the immune response to a single major autoantigen may not be sufficient to prevent arthritis development; rather, targeting a wide range of autoantigens may be required for disease suppression.
Supplementary Material
Footnotes
This work was supported by NIH grants AI-24541, AI-59166, and CA-010815, by The Commonwealth of Pennsylvania, and by Sibley Memorial Hospital. S. Oh and D. Simons were supported by NIH-T32-CA09171.
Abbreviations used in this paper: Treg, regulatory T cell; RA, rheumatoid arthritis; HA, hemagglutinin; LN, lymph node
REFERENCES
- 1.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genetics. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 2.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat. Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 3.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 4.Suri-Payer E, Fritzsching B. Regulatory T cells in experimental autoimmune disease. Springer Semin. Immunopathol. 2006;28:3–16. doi: 10.1007/s00281-006-0021-8. [DOI] [PubMed] [Google Scholar]
- 5.Valencia X, Lipsky PE. CD4+CD25+FoxP3+ regulatory T cells in autoimmune diseases. Nat. Clinic. Pract. Rheumatol. 2007;3:619–626. doi: 10.1038/ncprheum0624. [DOI] [PubMed] [Google Scholar]
- 6.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 7.Hori S, Haury M, Coutinho A, Demengeot J. Specificity requirements for selection and effector functions of CD25+4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proc. Natl. Acad. Sci. USA. 2002;99:8213–8218. doi: 10.1073/pnas.122224799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy J, Illes Z, Zhang X, Encinas J, Pyrdol J, Nicholson L, Sobel RA, Wucherpfennig KW, Kuchroo VK. Myelin proteolipid protein-specific CD4+CD25+ regulatory cells mediate genetic resistance to experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA. 2004;101:15434–15439. doi: 10.1073/pnas.0404444101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J. Exp. Med. 2004;199:1467–1477. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samy ET, Parker LA, Sharp CP, Tung KS. Continuous control of autoimmune disease by antigen-dependent polyclonal CD4+CD25+ regulatory T cells in the regional lymph node. J. Exp. Med. 2005;202:771–781. doi: 10.1084/jem.20041033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 12.Mottonen M, Heikkinen J, Mustonen L, Isomaki P, Luukkainen R, Lassila O. CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clinic. Exp. Immunol. 2005;140:360–367. doi: 10.1111/j.1365-2249.2005.02754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao D, Borjesson O, Larsson P, Rudin A, Gunnarsson I, Klareskog L, Malmstrom V, Trollmo C. FOXP3 identifies regulatory CD25bright CD4+ T cells in rheumatic joints. Scand. J. Immunol. 2006;63:444–452. doi: 10.1111/j.1365-3083.2006.001755.x. [DOI] [PubMed] [Google Scholar]
- 14.Ruprecht CR, Gattorno M, Ferlito F, Gregorio A, Martini A, Lanzavecchia A, Sallusto F. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J. Exp. Med. 2005;201:1793–1803. doi: 10.1084/jem.20050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J. Exp. Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadkarni S, Mauri C, Ehrenstein MR. Anti-TNF-alpha therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-beta. J. Exp. Med. 2007;204:33–39. doi: 10.1084/jem.20061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan ME, Flierman R, van Duivenvoorde LM, Witteveen HJ, van Ewijk W, van Laar JM, de Vries RR, Toes RE. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005;52:2212–2221. doi: 10.1002/art.21195. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen LT, Jacobs J, Mathis D, Benoist C. Where FoxP3-dependent regulatory T cells impinge on the development of inflammatory arthritis. Arthritis Rheum. 2007;56:509–520. doi: 10.1002/art.22272. [DOI] [PubMed] [Google Scholar]
- 20.Morgan ME, Sutmuller RP, Witteveen HJ, van Duivenvoorde LM, Zanelli E, Melief CJ, Snijders A, Offringa R, de Vries RR, Toes RE. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003;48:1452–1460. doi: 10.1002/art.11063. [DOI] [PubMed] [Google Scholar]
- 21.Kelchtermans H, De Klerck B, Mitera T, Van Balen M, Bullens D, Billiau A, Leclercq G, Matthys P. Defective CD4+CD25+ regulatory T cell functioning in collagen-induced arthritis: an important factor in pathogenesis, counter-regulated by endogenous IFN-gamma. Arthritis Res. Ther. 2005;7:R402–415. doi: 10.1186/ar1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rankin AL, Reed AJ, Oh S, Cozzo Picca C, Guay HM, Larkin J, III, Panarey L, Aitken MK, Koeberlein B, Lipsky PE, Tomaszewski JE, Naji A, Caton AJ. CD4+ T cells recognizing a single self-peptide expressed by APCs iinduce spontaneous autoimmune arthritis. J. Immunol. 2008;180:833–841. doi: 10.4049/jimmunol.180.2.833. [DOI] [PubMed] [Google Scholar]
- 23.Cerasoli DM, McGrath J, Carding SR, Shih FF, Knowles BB, Caton AJ. Low avidity recognition of a class II-restricted neo-self peptide by virus-specific T cells. Int. Immunol. 1995;7:935–945. doi: 10.1093/intimm/7.6.935. [DOI] [PubMed] [Google Scholar]
- 24.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 25.Reed AJ, Noorchashm H, Rostami SY, Zarrabi Y, Perate AR, Jeganathan AN, Caton AJ, Naji A. Alloreactive CD4 T cell activation in vivo: an autonomous function of the indirect pathway of alloantigen presentation. J. Immunol. 2003;171:6502–6509. doi: 10.4049/jimmunol.171.12.6502. [DOI] [PubMed] [Google Scholar]
- 26.Larkin J, III, Rankin AL, Picca CC, Riley MP, Jenks SA, Sant AJ, Caton AJ. CD4+CD25+ Regulatory T cell repertoire formation shaped by differential presentation of peptides from a self-antigen. J. Immunol. 2008;180:2149–2157. doi: 10.4049/jimmunol.180.4.2149. [DOI] [PubMed] [Google Scholar]
- 27.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J. Exp. Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haribhai D, Lin W, Relland LM, Truong N, Williams CB, A T. Regulatory T cells dynamically control the primary immune response to foreign antigen. J. Immunol. 2007;178:2961–2972. doi: 10.4049/jimmunol.178.5.2961. [DOI] [PubMed] [Google Scholar]
- 29.Mombaerts P, Clarke AR, Rudnicki MA, Iacomini J, Itohara S, Lafaille JJ, Wang L, Ichikawa Y, Jaenisch R, Hooper ML, Tonegawa S. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 30.Seo SJ, Fields ML, Buckler JL, Reed AJ, Mandik-Nayak L, Nish SA, Noelle RJ, Turka LA, Finkelman FD, Caton AJ, Erikson J. The impact of T helper and T regulatory cells on the regulation of anti-double-stranded DNA B cells. Immunity. 2002;16:535–546. doi: 10.1016/s1074-7613(02)00298-4. [DOI] [PubMed] [Google Scholar]
- 31.Lee M. K. t., Moore DJ, Jarrett BP, Lian MM, Deng S, Huang X, Markmann JW, Chiaccio M, Barker CF, Caton AJ, Markmann JF. Promotion of allograft survival by CD4+CD25+ regulatory T cells: evidence for in vivo inhibition of effector cell proliferation. J. Immunol. 2004;172:6539–6544. doi: 10.4049/jimmunol.172.11.6539. [DOI] [PubMed] [Google Scholar]
- 32.Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc. Natl. Acad. Sci. USA. 2003;100:5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu HJ, Ivanov, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirota K, Hashimoto M, Yoshitomi H, Tanaka S, Nomura T, Yamaguchi T, Iwakura Y, Sakaguchi N, Sakaguchi S. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J. Exp. Med. 2007;204:41–47. doi: 10.1084/jem.20062259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3-T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J. Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 36.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng XH, Jetten AM, Dong C. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, Dos Santos L, O’Brien S, Blank R, Lamb E, Natarajan S, Kastenmayer R, Hunter C, Grigg ME, Belkaid Y. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Komatsu N, Mariotti-Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc. Natl. Acad. Sci. USA. 2009;106:1903–1908. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 40.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kryczek I, Wei S, Zou L, Zhu G, Mottram P, Xu H, Chen L, Zou W. Cutting edge: induction of B7-H4 on APCs through IL-10: novel suppressive mode for regulatory T cells. J. Immunol. 2006;177:40–44. doi: 10.4049/jimmunol.177.1.40. [DOI] [PubMed] [Google Scholar]
- 42.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 43.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat. Rev. Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat. Rev. Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- 46.Fox DA, Gizinski A, Morgan R, Lundy SK. Cell-cell interactions in rheumatoid arthritis synovium. Rheum. Dis. Clin. North Am. 2010;36:311–323. doi: 10.1016/j.rdc.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murakami M, Okuyama Y, Ogura H, Asano S, Arima Y, Tsuruoka M, Harada M, Kanamoto M, Sawa Y, Iwakura Y, Takatsu K, Kamimura D, Hirano T. Local microbleeding facilitates IL-6- and IL-17-dependent arthritis in the absence of tissue antigen recognition by activated T cells. J. Exp. Med. 2011;208:103–114. doi: 10.1084/jem.20100900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.